The Role of Indoor Microbiome and Metabolites in Shaping Children’s Nasal and Oral Microbiota: A Pilot Multi-Omic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Sample Collection
2.2. DNA Extraction, High-Throughput Sequencing and Microbiome Analysis
2.3. Indoor Dust LC/MS for Metabolomics Profiling
2.4. Environmental Characteristics and Association Analysis
- (1)
- Personal and family data, including, but not limited to, gender, age to start kindergarten, breastfeeding duration, age of child, premature delivery, type of delivery (premature or cesarean section), presence of siblings, and parental income and education level. These variables were self-reported and treated as categorical (e.g., gender) or continuous (e.g., age, income).
- (2)
- Living environment characteristics, including room cleaning frequency, the age of the residential building, number of cohabitants, proximity to heavy traffic, rivers, parks, or gardens, presence of indoor pets, presence of indoor plants, maternal and child exposure to smoking, visible mold/dampness, and family history of Helicobacter pylori infection. Data were self-reported and categorized based on pre-defined criteria.
- (3)
- Food intake frequency, including intake of meat, milk, eggs, seafood, fruits, salad, cooked vegetables, juice, soft drinks, fries, rice/pasta/bread. The data were self-reported and treated as ordinal variables based on intake frequency.
- (4)
- The concentration of annual average outdoor air pollutants, including SO2, NO2, CO, O3, PM10, and PM2.5. The daily average values of atmospheric pollutants were collected from the environmental monitoring station closest to the children’s residences over a period of one year preceding the collection of biological samples.
- (5)
- Indoor microbial exposure, including indoor microbial abundance and diversity, including the alpha diversity of the indoor microbiome (Shannon index, Chao1, observed a number of species), microbial virulence factors, antimicrobial resistance genes, and NIAID-defined pathogen species https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogens (accessed on 26 September 2023). These data were calculated from indoor shotgun metagenomics sequencing.
- (6)
- Indoor metabolites come from four classes, including keto acids, indoles, flavonoids, and mycotoxins. These data were calculated from indoor metabolomic profiling. To address the compositional nature of the microbiome and metabolome data, we employed a centered log-ratio (CLR) transformation prior to conducting regression analyses.
2.5. Potential Microbial Transfer between Indoor Environment to Nasal/Oral Cavities
3. Results
3.1. Personal Information, Environmental Characteristics and Dietary Frequency
3.2. Indoor Microbiome, VFs, ARGs, and Metabolites
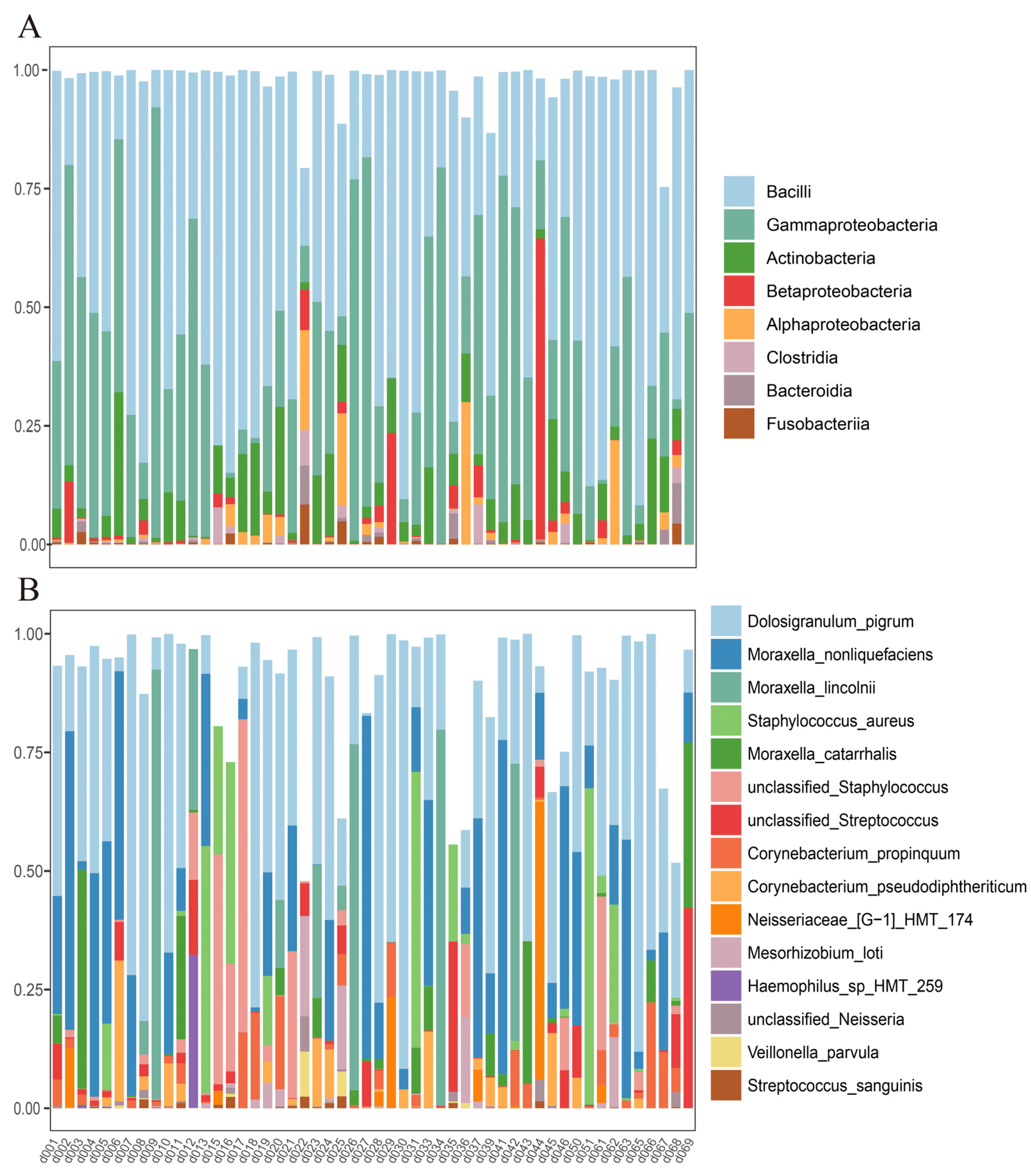
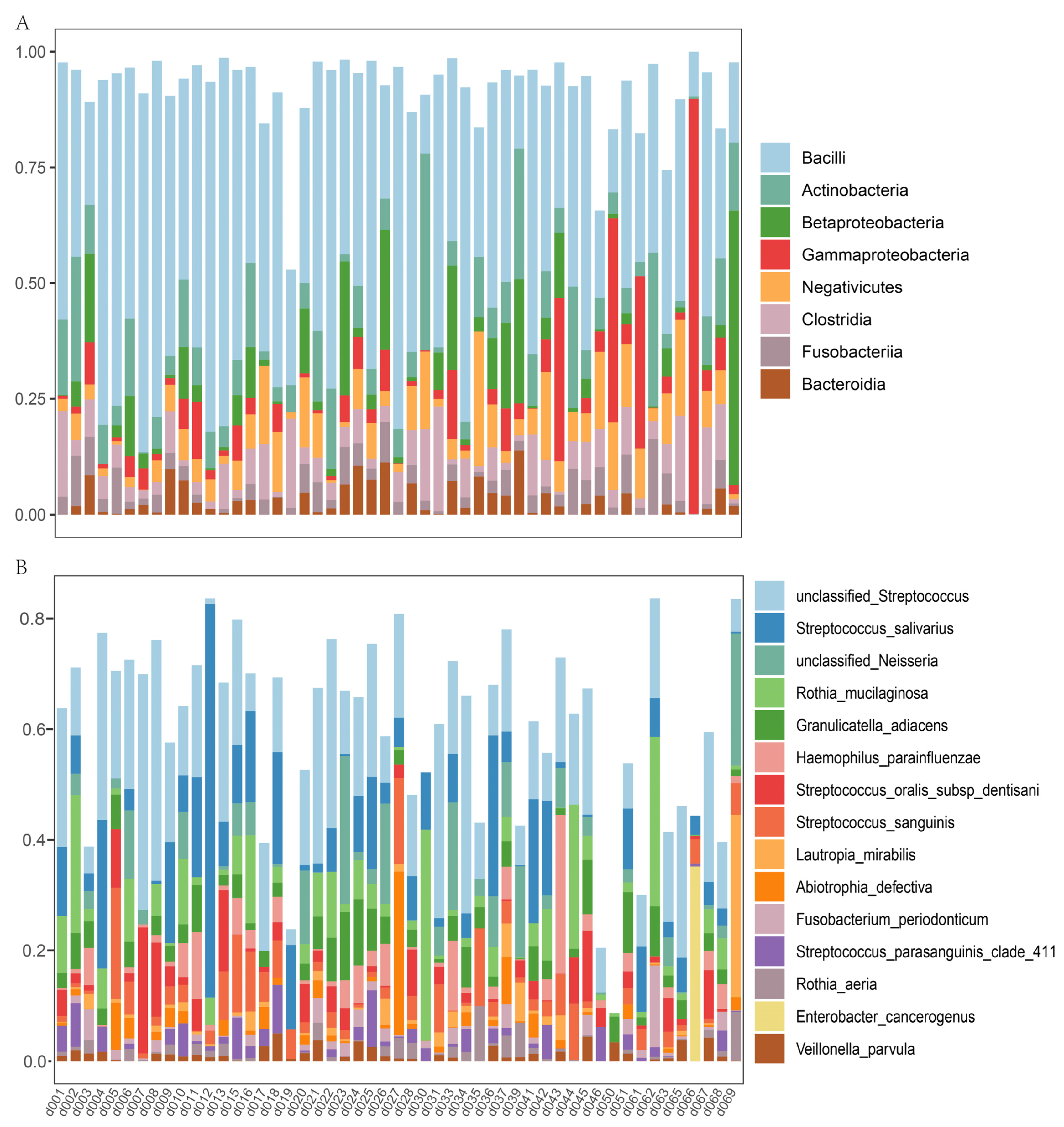
3.3. Nasal and Oral Microbial Composition
3.4. Impact of Environmental Variables on Overall Nasal and Oral Microbial Composition
3.5. Impact of Environmental Variables on Alpha Diversity and the Abundance of Risky/Protective Nasal and Oral Microorganisms
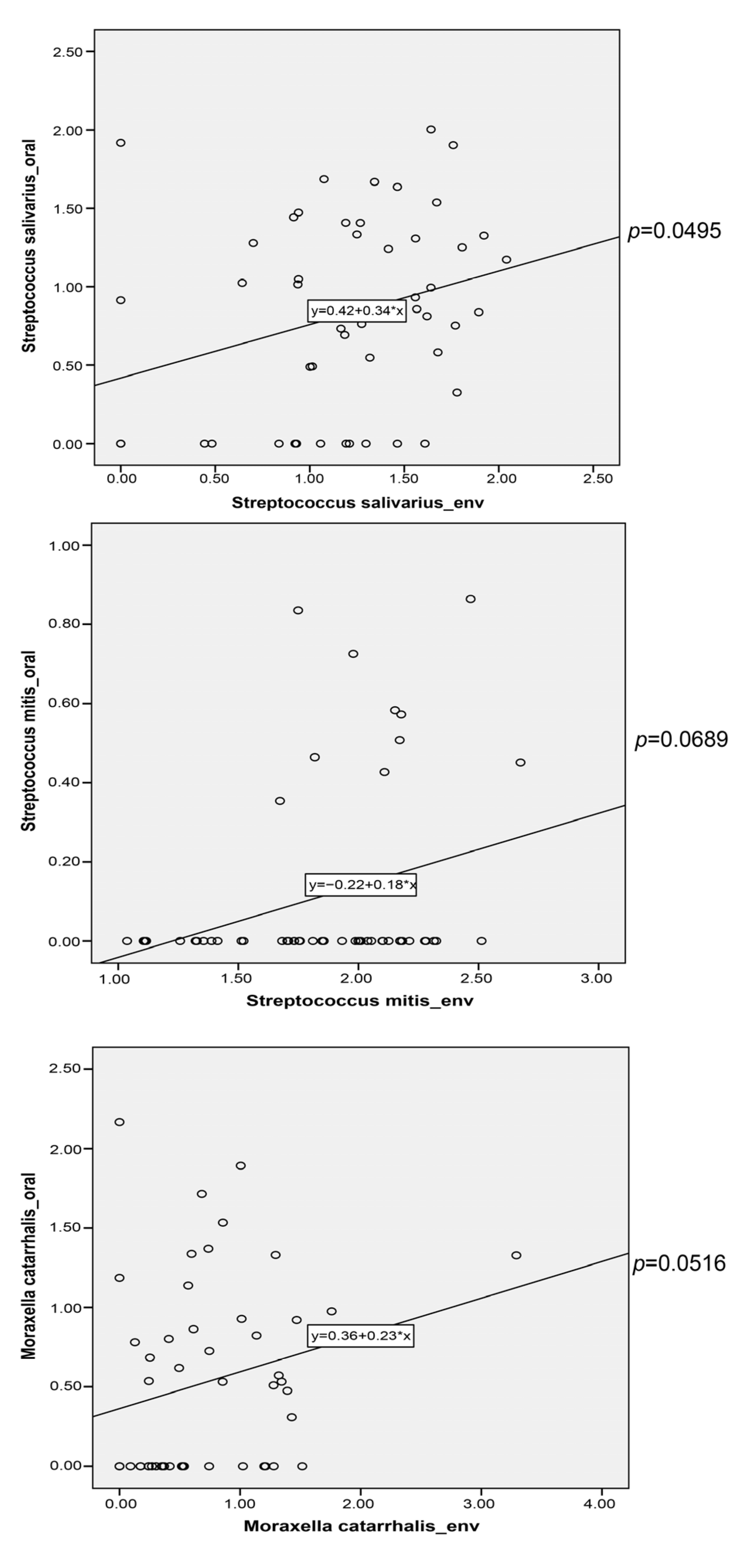
3.6. Potential Microbial Transfer from Indoor Environment to Nasal/Oral Cavity
4. Discussion
4.1. Strengths and Limitations of the Study
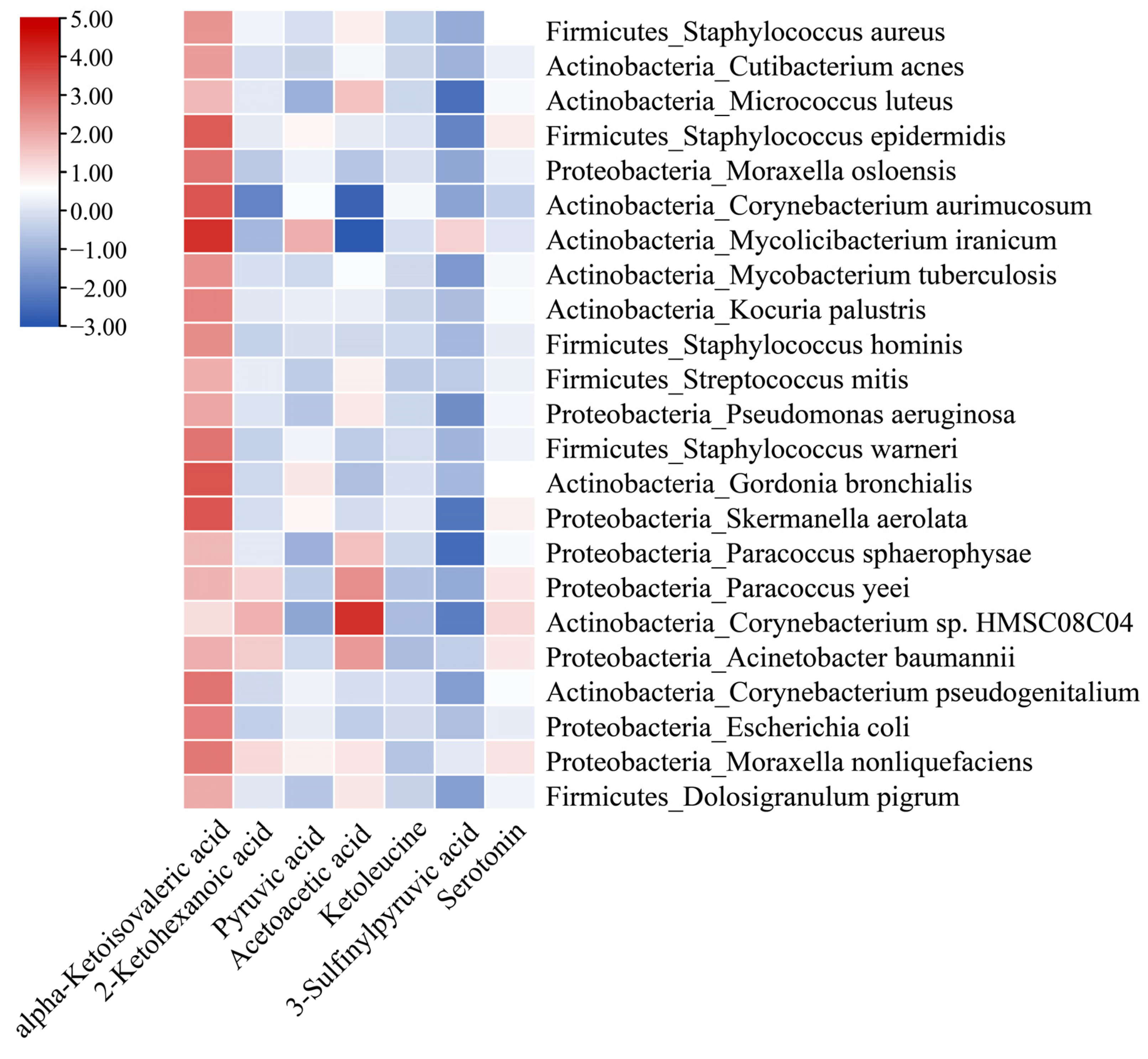
4.2. Indoor Metabolites and Nasal/Oral Microbiota
4.3. Other Environmental and Personal Characteristics and Nasal/Oral Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemon, K.P. Human nasal microbiota. Curr. Biol. 2020, 30, R1118–R1119. [Google Scholar] [CrossRef] [PubMed]
- De Steenhuijsen Piters, W.A.; Sanders, E.A.; Bogaert, D. The role of the local microbial ecosystem in respiratory health and disease. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140924. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.Y.; Hunter, R.C.; Ramakrishnan, V.R. The Microbiome and Chronic Rhinosinusitis. Immunol. Allergy Clin. N. Am. 2020, 40, 251–263. [Google Scholar] [CrossRef]
- Shah, R.; Bunyavanich, S. The airway microbiome and pediatric asthma. Curr. Opin. Pediatr. 2021, 33, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Boushey, H.A. The microbiome in asthma. J. Allergy Clin. Immunol. 2015, 135, 25–30. [Google Scholar] [CrossRef]
- Heinrich, A.; Heyl, K.A.; Klaile, E.; Müller, M.M.; Klassert, T.E.; Wiessner, A.; Fischer, K.; Schumann, R.R.; Seifert, U.; Riesbeck, K.; et al. Moraxella catarrhalis induces CEACAM3-Syk-CARD9-dependent activation of human granulocytes. Cell. Microbiol. 2016, 18, 1570–1582. [Google Scholar] [CrossRef]
- Hoggard, M.; Waldvogel-Thurlow, S.; Zoing, M.; Chang, K.; Radcliff, F.J.; Wagner Mackenzie, B.; Biswas, K.; Douglas, R.G.; Taylor, M.W. Inflammatory Endotypes and Microbial Associations in Chronic Rhinosinusitis. Front. Immunol. 2018, 9, 2065. [Google Scholar] [CrossRef]
- Bomar, L.; Brugger, S.D.; Yost, B.H.; Davies, S.S.; Lemon, K.P. Corynebacterium accolens Releases Antipneumococcal Free Fatty Acids from Human Nostril and Skin Surface Triacylglycerols. mBio 2016, 7, e01725-15. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.; Wang, X.; Zhang, L.; Li, L.; Fu, X.; Sun, Z.; Li, Z.; Zhang, X.; Zhang, M. Characteristics and Clinical Implications of the Nasal Microbiota in Extranodal NK/T-Cell Lymphoma, Nasal Type. Front. Cell. Infect. Microbiol. 2021, 11, 686595. [Google Scholar] [CrossRef]
- Escapa, I.F.; Huang, Y.; Chen, T.; Lin, M.; Kokaras, A.; Dewhirst, F.E.; Lemon, K.P. Construction of habitat-specific training sets to achieve species-level assignment in 16S rRNA gene datasets. Microbiome 2020, 8, 65. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Mahurkar, A.; Rahnavard, G.; Crabtree, J.; Orvis, J.; Hall, A.B.; Brady, A.; Creasy, H.H.; McCracken, C.; Giglio, M.G.; et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017, 550, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Simon-Soro, A.; Curtis, M.A. Role of microbial communities in the pathogenesis of periodontal diseases and caries. J. Clin. Periodontol. 2017, 44, S23–S38. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontology 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D.; Cai, Q.; Hua, X.; Long, J.; Shi, J.; Wan, Y.; Yang, Y.; Abnet, C.; Bassig, B.A.; Hu, W.; et al. Variation in oral microbiome is associated with future risk of lung cancer among never-smokers. Thorax 2021, 76, 256. [Google Scholar] [CrossRef]
- Mariani, J.; Favero, C.; Spinazzè, A.; Cavallo, D.M.; Carugno, M.; Motta, V.; Bonzini, M.; Cattaneo, A.; Pesatori, A.C.; Bollati, V. Short-term particulate matter exposure influences nasal microbiota in a population of healthy subjects. Environ. Res. 2018, 162, 119–126. [Google Scholar] [CrossRef]
- Gisler, A.; Korten, I.; de Hoogh, K.; Vienneau, D.; Frey, U.; Decrue, F.; Gorlanova, O.; Soti, A.; Hilty, M.; Latzin, P.; et al. Associations of air pollution and greenness with the nasal microbiota of healthy infants: A longitudinal study. Environ. Res. 2021, 202, 111633. [Google Scholar] [CrossRef]
- Irizar, H.; Chun, Y.; Arditi, Z.; Do, A.; Grishina, G.; Grishin, A.; Vicencio, A.; Bunyavanich, S. Examination of host genetic effects on nasal microbiome composition. J. Allergy Clin. Immunol. 2022, 150, 1232–1236. [Google Scholar] [CrossRef]
- Zeineldin, M.; Aldridge, B.; Blair, B.; Kancer, K.; Lowe, J. Microbial shifts in the swine nasal microbiota in response to parenteral antimicrobial administration. Microb. Pathog. 2018, 121, 210–217. [Google Scholar] [CrossRef]
- Ma, G.; Qiao, Y.; Shi, H.; Zhou, J.; Li, Y. Comparison of the Oral Microbiota Structure among People from the Same Ethnic Group Living in Different Environments. BioMed Res. Int. 2022, 2022, 6544497. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.K.; Chan, J.Y.K.; Wong, M.C.S.; Wong, P.Y.; Lei, P.; Cai, L.; Lan, L.; Ho, W.C.S.; Yeung, A.C.M.; Chan, P.K.S.; et al. Determinants and Interactions of Oral Bacterial and Fungal Microbiota in Healthy Chinese Adults. Microbiol. Spectr. 2022, 10, e0241021. [Google Scholar] [CrossRef] [PubMed]
- Bomar, L.; Brugger, S.D.; Lemon, K.P. Bacterial microbiota of the nasal passages across the span of human life. Curr. Opin. Microbiol. 2018, 41, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Liao, Y.; He, Y.Q.; Zheng, M.Q.; Tong, X.T.; Xue, W.Q.; Zhang, J.B.; Yuan, L.L.; Zhang, W.L.; Jia, W.H. Association Between Oral Microbiota and Cigarette Smoking in the Chinese Population. Front. Cell. Infect. Microbiol. 2021, 11, 658203. [Google Scholar] [CrossRef]
- Vallès, Y.; Inman, C.K.; Peters, B.A.; Wareth, L.A.; Abdulle, A.; Alsafar, H.; Anouti, F.A.; Dhaheri, A.A.; Galani, D.; Haji, M.; et al. Incense Burning is Associated with Human Oral Microbiota Composition. Sci. Rep. 2019, 9, 10039. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, Y.; Ou, Z.; Li, Y.; Zhang, M.; Chen, Y.; Zhang, Z.; Chen, X.; Mu, P.; Norbäck, D.; et al. Indoor microbiome, air pollutants and asthma, rhinitis and eczema in preschool children—A repeated cross-sectional study. Environ. Int. 2022, 161, 107137. [Google Scholar] [CrossRef]
- Fu, X.; Ou, Z.; Sun, Y. Indoor microbiome and allergic diseases: From theoretical advances to prevention strategies. Eco-Environ. Health 2022, 1, 133–146. [Google Scholar] [CrossRef]
- Fu, X.; Ou, Z.; Zhang, M.; Meng, Y.; Li, Y.; Chen, Q.; Jiang, J.; Zhang, X.; Norbäck, D.; Zhao, Z.; et al. Classroom microbiome, functional pathways and sick-building syndrome (SBS) in urban and rural schools—Potential roles of indoor microbial amino acids and vitamin metabolites. Sci. Total Environ. 2021, 795, 148879. [Google Scholar] [CrossRef]
- Fu, X.; Yuan, Q.; Zhu, X.; Li, Y.; Meng, Y.; Hashim, J.H.; Hashim, Z.; Ali, F.; Zheng, Y.W.; Lai, X.X.; et al. Associations between the indoor microbiome, environmental characteristics and respiratory infections in junior high school students of Johor Bahru, Malaysia. Environ. Sci. Process. Impacts 2021, 23, 1171–1181. [Google Scholar] [CrossRef]
- Fu, X.; Norbäck, D.; Yuan, Q.; Li, Y.; Zhu, X.; Hashim, J.H.; Hashim, Z.; Ali, F.; Zheng, Y.-W.; Lai, X.-X.; et al. Indoor microbiome, environmental characteristics and asthma among junior high school students in Johor Bahru, Malaysia. Environ. Int. 2020, 138, 105664. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Meng, Y.; Yuan, Q.; Zhang, Z.; Norbäck, D.; Deng, Y.; Zhang, X.; Sun, Y. Associations between respiratory infections and bacterial microbiome in student dormitories in Northern China. Indoor Air 2020, 30, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Ou, Z.; Meng, Y.; Chen, Y.; Lin, R.; Hashim, J.H.; Hashim, Z.; Wieslander, G.; Chen, Q.; et al. Indoor microbiome, microbial and plant metabolites, chemical compounds and asthma symptoms in junior high school students: A multicentre association study in Malaysia. Eur. Respir. J. 2022, 60, 2200260. [Google Scholar] [CrossRef] [PubMed]
- Crane, J.; Mallol, J.; Beasley, R.; Stewart, A.; Asher, M.I. Agreement between written and video questions for comparing asthma symptoms in ISAAC. Eur. Respir. J. 2003, 21, 455–461. [Google Scholar] [CrossRef]
- Bell, M.L. The use of ambient air quality modeling to estimate individual and population exposure for human health research: A case study of ozone in the Northern Georgia Region of the United States. Environ. Int. 2006, 32, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Norbäck, D.; Lu, C.; Zhang, Y.; Li, B.; Zhao, Z.; Huang, C.; Zhang, X.; Qian, H.; Sun, Y.; Sundell, J.; et al. Onset and remission of childhood wheeze and rhinitis across China—Associations with early life indoor and outdoor air pollution. Environ. Int. 2019, 123, 61–69. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, X.; Li, Y.; Yuan, Q.; Ou, Z.; Lindgren, T.; Deng, Y.; Norbäck, D. Shotgun Metagenomics of Dust Microbiome from Flight Deck and Cabin in Civil Aviation Aircraft. Indoor Air 2020, 30, 1199–1212. [Google Scholar] [CrossRef]
- Wang, Y.; Song, F.; Zhu, J.; Zhang, S.; Yang, Y.; Chen, T.; Tang, B.; Dong, L.; Ding, N.; Zhang, Q.; et al. GSA: Genome Sequence Archive. Genom. Proteom. Bioinform. 2017, 15, 14–18. [Google Scholar] [CrossRef]
- Li, D.; Luo, R.; Liu, C.M.; Leung, C.M.; Ting, H.F.; Sadakane, K.; Yamashita, H.; Lam, T.W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lomsadze, A.; Borodovsky, M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010, 38, e132. [Google Scholar] [CrossRef]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN analysis of metagenomic data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Morton, J.T.; Aksenov, A.A.; Nothias, L.F.; Foulds, J.R.; Quinn, R.A.; Badri, M.H.; Swenson, T.L.; Van Goethem, M.W.; Northen, T.R.; Vazquez-Baeza, Y.; et al. Learning representations of microbe–metabolite interactions. Nat. Methods 2019, 16, 1306–1314. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Sato, M.; Fujiwara, S.; Tsuchiya, H.; Fujii, T.; Iinuma, M.; Tosa, H.; Ohkawa, Y. Flavones with antibacterial activity against cariogenic bacteria. J. Ethnopharmacol. 1996, 54, 171–176. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Cheang, W.S. Isoflavones daidzin and daidzein inhibit lipopolysaccharide-induced inflammation in RAW264.7 macrophages. Chin. Med. 2022, 17, 95. [Google Scholar] [CrossRef]
- Chen, H.; Xie, S.; Gao, J.; He, L.; Luo, W.; Tang, Y.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Yang, D. Flavonoid Baicalein Suppresses Oral Biofilms and Protects Enamel Hardness to Combat Dental Caries. Int. J. Mol. Sci. 2022, 23, 10593. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Eiteman, M.A. Recent Progress in the Microbial Production of Pyruvic Acid. Fermentation 2017, 3, 8. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F. Cytoprotection of pyruvic acid and reduced beta-nicotinamide adenine dinucleotide against hydrogen peroxide toxicity in neuroblastoma cells. Neurochem. Res. 2003, 28, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.H.; Lee, J.-Y.; Lee, S.E.; Koh, J.-Y.; Yoon, Y.H. Protection by Pyruvate of Rat Retinal Cells against Zinc Toxicity In Vitro, and Pressure-Induced Ischemia In Vivo. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1523–1530. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef]
- Lin, R.; Sun, Y.; Mu, P.; Zheng, T.; Mu, H.; Deng, F.; Deng, Y.; Wen, J. Lactobacillus rhamnosus GG supplementation modulates the gut microbiota to promote butyrate production, protecting against deoxynivalenol exposure in nude mice. Biochem. Pharmacol. 2020, 175, 113868. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Hur, H.J.; Wu, X.; Yang, H.J.; Kim, M.J.; Lee, K.-H.; Hong, M.; Park, S.; Kim, M.-S. Beneficial Effects of a Low-Glycemic Diet on Serum Metabolites and Gut Microbiota in Obese Women with Prevotella and Bacteriodes Enterotypes: A Randomized Clinical Trial. Front. Nutr. 2022, 9, 861880. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.-F.; He, S.; Chen, X.; Wang, J.; Li, J.; Zhu, Q.; Zhang, Z.; Li, L.; Alam, M.S. Effects of High Carbohydrate Diet-Modulated Microbiota on Gut Health in Chinese Perch. Front. Microbiol. 2020, 11, 575102. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Wei, G.; Ye, Y.; Yan, X.; Chao, X.; Yang, F.; Wang, M.; Zhang, W.; Yuan, C.; Zeng, Q. Effect of banana pulp dietary fibers on metabolic syndrome and gut microbiota diversity in high-fat diet mice. J. Food Biochem. 2020, 44, e13362. [Google Scholar] [CrossRef] [PubMed]

| Nasal | Oral | ||||
|---|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | ||
| Personal characteristics | Q1–Q3 percentile | ||||
| Age (year) | 4–6 | 4.20 | 0.008 | 0.82 | 0.57 |
| Breastfeeding duration (year) | 0.5–1 | 0.82 | 0.49 | 1.02 | 0.38 |
| Number of cohabitants | 3–5 | 0.92 | 0.42 | 1.44 | 0.16 |
| Age start kindergarten | 3–4 | 1.45 | 0.21 | 1.23 | 0.25 |
| Girl | 59% | 1.86 | 0.13 | 1.36 | 0.17 |
| Preterm delivery | 12% | 0.38 | 0.82 | 0.58 | 0.75 |
| Cesarean section | 45% | 0.33 | 0.84 | 0.95 | 0.46 |
| Presence of siblings | 51% | 1.23 | 0.28 | 0.53 | 0.92 |
| High parents income | 14% | 0.26 | 0.90 | 1.44 | 0.18 |
| High education level for mother (graduate and postgraduate) | 50% | 1.00 | 0.38 | 0.51 | 0.83 |
| High education level for father (graduate and postgraduate) | 54% | 2.82 | 0.04 | 0.51 | 0.89 |
| Food intake frequency | Weekly/daily | ||||
| Juice and soda drink | 86%/14% | 2.10 | 0.09 | 0.98 | 0.41 |
| Fries | 90%/10% | 0.90 | 0.43 | 0.49 | 0.81 |
| Rice/pasta/bread | 37%/63% | 1.92 | 0.12 | 0.94 | 0.47 |
| Fruits, vegetables | 4%/96% | 1.42 | 0.23 | 0.56 | 0.83 |
| Eggs, milk, fish, meat, and sea foods | 22%/78% | 0.17 | 0.97 | 0.82 | 0.57 |
| Outdoor air pollution | |||||
| SO2 (μg/m3) | 4.75–10.78 | 1.40 | 0.23 | 0.57 | 0.78 |
| NO2 (μg/m3) | 29.35–43.10 | 1.66 | 0.17 | 1.20 | 0.25 |
| CO (mg/m3) | 0.59–0.76 | 1.15 | 0.32 | 0.67 | 0.74 |
| O3 (μg/m3) | 63.15–81.92 | 1.34 | 0.25 | 0.63 | 0.72 |
| PM10 (μg/m3) | 38.53–48.10 | 2.47 | 0.06 | 0.81 | 0.59 |
| PM2.5 (μg/m3) | 22.03–32.87 | 0.58 | 0.64 | 1.05 | 0.34 |
| Living environment characteristics | |||||
| Adjacent to heavy traffic | 41% | 0.68 | 0.56 | 1.92 | 0.04 |
| Adjacent to river/park/garden | 22% | 0.74 | 0.52 | 0.47 | 0.91 |
| ETS mother—pregnancy | 18% | 4.63 | 0.005 | 1.74 | 0.09 |
| ETS children—early childhood (<1 year) | 14% | 0.96 | 0.40 | 2.43 | 0.03 |
| ETS children—previous 10 months | 18% | 1.87 | 0.13 | 1.17 | 0.30 |
| Presence of pets/plants indoors—early childhood (<1 year old) | 27% | 1.55 | 0.19 | 1.29 | 0.22 |
| Presence of pets/plants indoors—previous 10 months | 59% | 1.06 | 0.36 | 1.48 | 0.13 |
| Visible mold/dampness—pregnancy | 20% | 1.41 | 0.22 | 1.39 | 0.18 |
| Visible mold/dampness—early childhood (<1 year) | 27% | 1.01 | 0.37 | 0.77 | 0.65 |
| Visible mold/dampness—previous 10 months | 25% | 1.06 | 0.35 | 0.80 | 0.61 |
| Frequent room cleaning | 38% | 1.57 | 0.19 | 0.73 | 0.67 |
| Building age (years) | 10–40 | 0.40 | 0.79 | 0.42 | 0.96 |
| Abundance of potential pathogens indoor | 0.05–0.32 | 1.28 | 0.27 | 0.67 | 0.73 |
| Clostridium perfringens | 0.01% | 0.92 | 0.42 | 0.35 | 0.86 |
| Salmonella enterica | 0.50% | 0.47 | 0.73 | 0.88 | 0.48 |
| Listeria monocytogenes | 0.01% | 1.14 | 0.31 | 1.15 | 0.28 |
| Toxoplasma gondii | 0.01% | 0.52 | 0.69 | 0.74 | 0.56 |
| Mycobacterium tuberculosis | 1.47% | 1.57 | 0.18 | 0.38 | 0.95 |
| Total abundance of VFs indoors (RPKM) | 2.3 × 103–5.2 × 103 | 0.92 | 0.42 | 0.61 | 0.81 |
| Total abundance of ARGs indoors (RPKM) | 2.4 × 103–5.5 × 103 | 0.91 | 0.42 | 0.67 | 0.75 |
| Abundance of flavonoids indoors | 3.91 | 0.01 | 1.08 | 0.35 | |
| Baicalein | 0–2.86 × 1010 | 0.84 | 0.46 | 1.10 | 0.34 |
| Daidzein | 2.46 × 105–1.18 × 108 | 1.96 | 0.11 | 3.88 | 0.03 |
| Tangeritin | 1.92 × 106–5.01 × 108 | 1.58 | 0.18 | 0.69 | 0.60 |
| Isoliquiritigenin | 5.03 × 106–2.85 × 108 | 0.79 | 0.49 | 1.07 | 0.37 |
| Apigenin | 2.65 × 106–5.31 × 108 | 2.38 | 0.06 | 0.55 | 0.62 |
| (2S)-Liquiritigenin | 1.59 × 107–2.22 × 109 | 0.37 | 0.82 | 1.19 | 0.25 |
| Hesperidin | 2.31 × 106–1.12 × 109 | 2.45 | 0.07 | 1.78 | 0.09 |
| Eupatilin | 3.41 × 105–1.32 × 108 | 0.58 | 0.64 | 1.49 | 0.16 |
| Abundance of indoles indoors | 0.78 | 0.50 | 0.95 | 0.46 | |
| 3-Methylindole | 7.33 × 107–1.64 × 109 | 3.57 | 0.01 | 0.39 | 0.86 |
| Serotonin | 8.37 × 105–6.52 × 108 | 0.58 | 0.65 | 0.41 | 0.84 |
| Indole | 1.04 × 109–5.03 × 109 | 1.05 | 0.35 | 0.39 | 0.93 |
| L-Tryptophan | 1.67 × 109–3.95 × 1010 | 0.73 | 0.54 | 2.14 | 0.04 |
| Indole-3-carboxylic acid | 7.50 × 105–1.51 × 107 | 1.04 | 0.36 | 0.28 | 0.99 |
| Abundance of keto acids indoors | 0.74 | 0.53 | 0.49 | 0.89 | |
| Pyruvic acid | 3.51 × 107–1.41 × 109 | 0.75 | 0.52 | 0.99 | 0.37 |
| Ketoleucine | 1.43 × 107–4.40 × 108 | 2.74 | 0.04 | 1.17 | 0.26 |
| 2-Ketohexanoic acid | 3.20 × 106–2.95 × 108 | 1.69 | 0.16 | 3.49 | 0.04 |
| Acetoacetic acid | 8.16 × 107–1.28 × 1010 | 0.15 | 0.97 | 0.59 | 0.58 |
| alpha-Ketoisovaleric acid | 2.87 × 107–9.61 × 108 | 1.20 | 0.30 | 0.97 | 0.40 |
| Abundance of mycotoxins indoors | 1.83 | 0.14 | 1.41 | 0.17 | |
| Vomitoxin (deoxynivalenol) | 7.30 × 104–5.49 × 107 | 3.26 | 0.02 | 1.34 | 0.19 |
| Nivalenol | 2.38 × 105–1.81 × 107 | 0.42 | 0.78 | 2.77 | 0.03 |
| Tentoxin | 8.60 × 107–1.56 × 109 | 2.14 | 0.09 | 1.78 | 0.10 |
| Diacetoxyscirpenol | 4.83 × 104–1.75 × 107 | 1.24 | 0.28 | 1.54 | 0.15 |
| Coefficient | p-Value | 95%CI | ||
|---|---|---|---|---|
| Nasal | ||||
| Shannon index | ||||
| Presence of siblings | 0.69 | 0.008 | 0.19 | 1.19 |
| Baicalein (flavonoid) | 0.72 | 0.004 | 0.24 | 1.20 |
| Observed number of species | ||||
| Presence of siblings | 29.9 | 0.01 | 7.49 | 52.3 |
| Eupatilin (flavonoid) | 0.68 | 0.004 | 0.23 | 1.12 |
| Protective microorganisms | ||||
| Rice/pasta/bread | −0.06 | 0.009 | −0.11 | −0.02 |
| Isoliquiritigenin (flavonoid) | 0.002 | 0.002 | 0.001 | 0.003 |
| Serotonin (indole) | 0.62 | 0.0005 | 0.03 | 0.95 |
| Oral | ||||
| Shannon index | ||||
| Age start kindergarten | 0.48 | 0.002 | 0.18 | 0.77 |
| Observed_OTU_env | −0.0009 | 0.011 | −0.002 | −0.0002 |
| Observed number of species | ||||
| Tangeritin (flavonoid) | 2.29 | 0.014 | 0.49 | 4.09 |
| Hesperidin (flavonoid) | 1.40 | 0.007 | 0.39 | 2.40 |
| Risk microorganisms | ||||
| ETS children—early childhood (<1 year) | 0.005 | 0.013 | 0.001 | 0.009 |
| Fries | 0.005 | 0.019 | 0.001 | 0.01 |
| Pyruvic acid (keto acid) | −0.056 | 0.017 | −0.10 | −0.01 |
| Protective microorganisms | ||||
| Total keto acid | 2.30 | 0.015 | 0.47 | 4.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Tang, H.; Yuan, Y.; Ou, Z.; Chen, Z.; Xu, Y.; Fu, X.; Zhao, Z.; Sun, Y. The Role of Indoor Microbiome and Metabolites in Shaping Children’s Nasal and Oral Microbiota: A Pilot Multi-Omic Analysis. Metabolites 2023, 13, 1040. https://doi.org/10.3390/metabo13101040
Zhang M, Tang H, Yuan Y, Ou Z, Chen Z, Xu Y, Fu X, Zhao Z, Sun Y. The Role of Indoor Microbiome and Metabolites in Shaping Children’s Nasal and Oral Microbiota: A Pilot Multi-Omic Analysis. Metabolites. 2023; 13(10):1040. https://doi.org/10.3390/metabo13101040
Chicago/Turabian StyleZhang, Mei, Hao Tang, Yiwen Yuan, Zheyuan Ou, Zhuoru Chen, Yanyi Xu, Xi Fu, Zhuohui Zhao, and Yu Sun. 2023. "The Role of Indoor Microbiome and Metabolites in Shaping Children’s Nasal and Oral Microbiota: A Pilot Multi-Omic Analysis" Metabolites 13, no. 10: 1040. https://doi.org/10.3390/metabo13101040
APA StyleZhang, M., Tang, H., Yuan, Y., Ou, Z., Chen, Z., Xu, Y., Fu, X., Zhao, Z., & Sun, Y. (2023). The Role of Indoor Microbiome and Metabolites in Shaping Children’s Nasal and Oral Microbiota: A Pilot Multi-Omic Analysis. Metabolites, 13(10), 1040. https://doi.org/10.3390/metabo13101040








