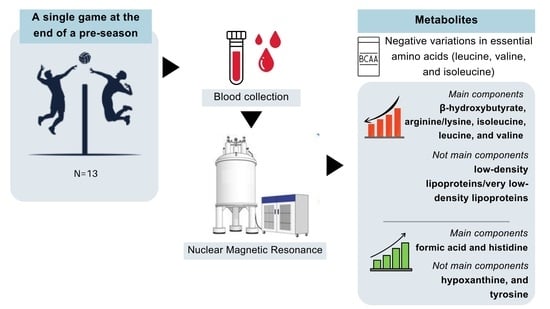
A Decrease in Branched-Chain Amino Acids after a Competitive Male Professional Volleyball Game—A Metabolomic-Based Approach
Abstract
1. Introduction
2. Materials and Methods
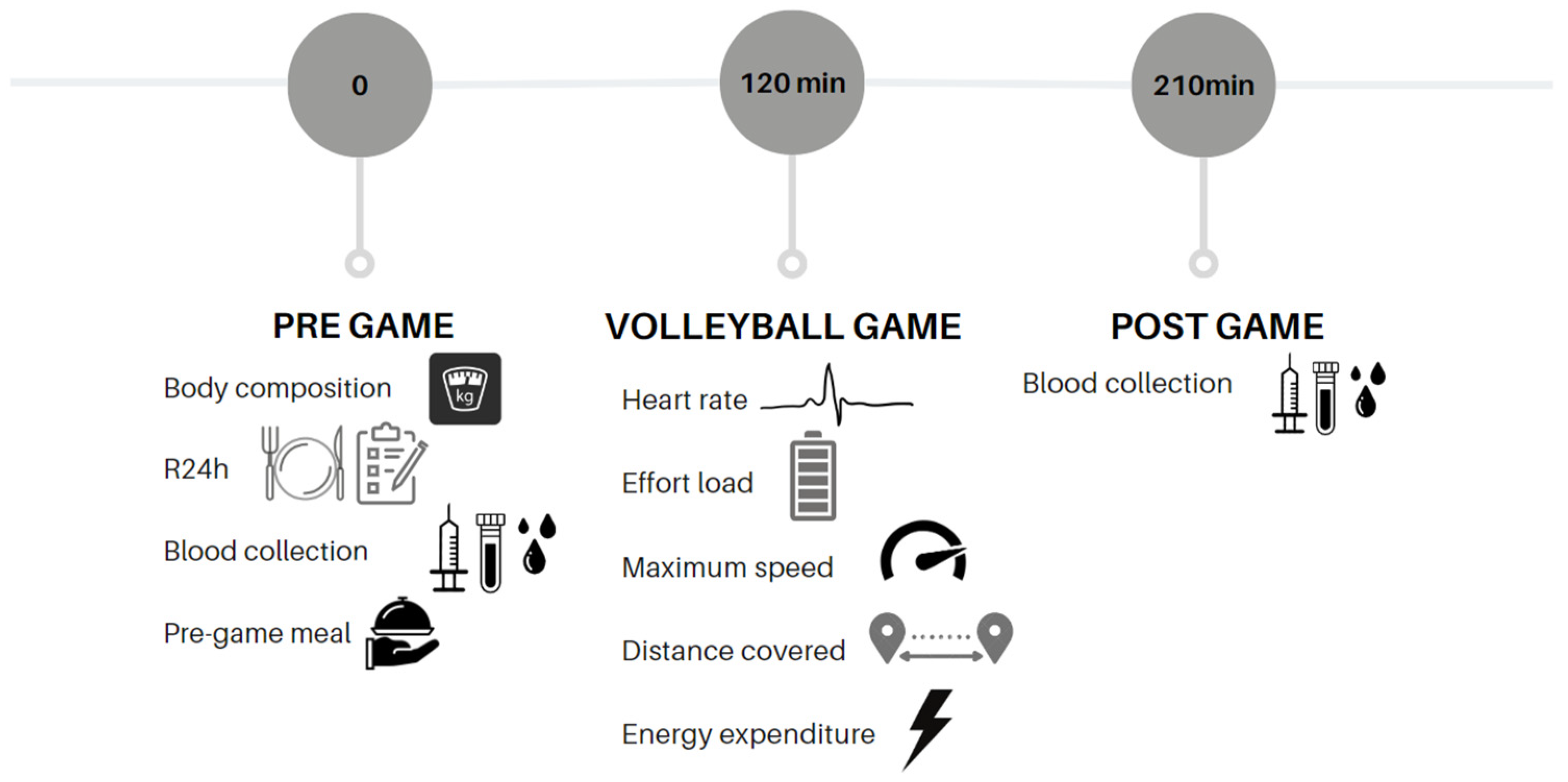
2.1. Study Design and Participants
2.2. Food Intake
2.3. Anthropometric and Body Composition Evaluation
2.4. Monitoring of Physiological Variables during the Game
2.5. Blood Collection
2.6. Biochemical Analysis
2.7. NMR Spectroscopy
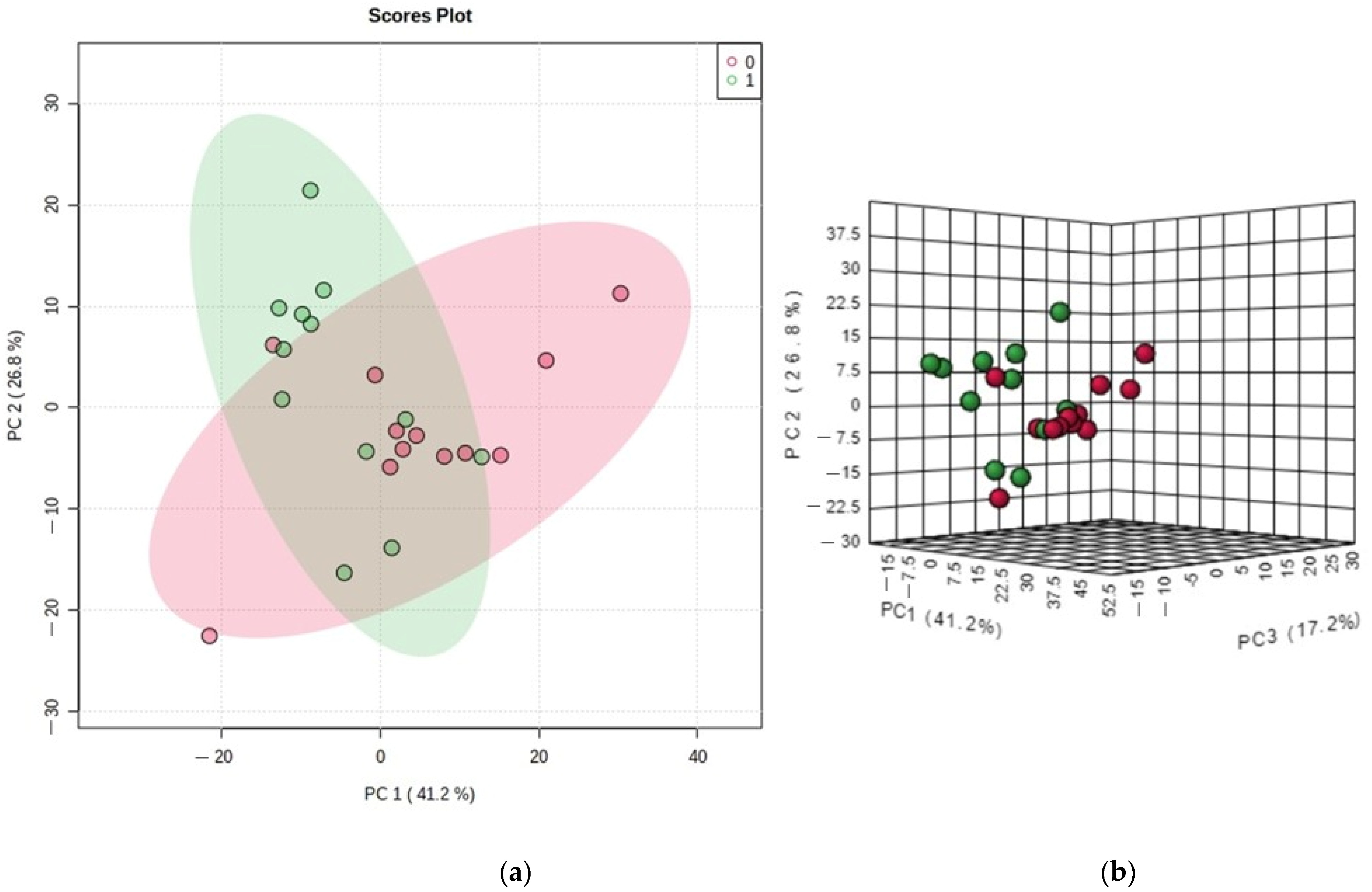
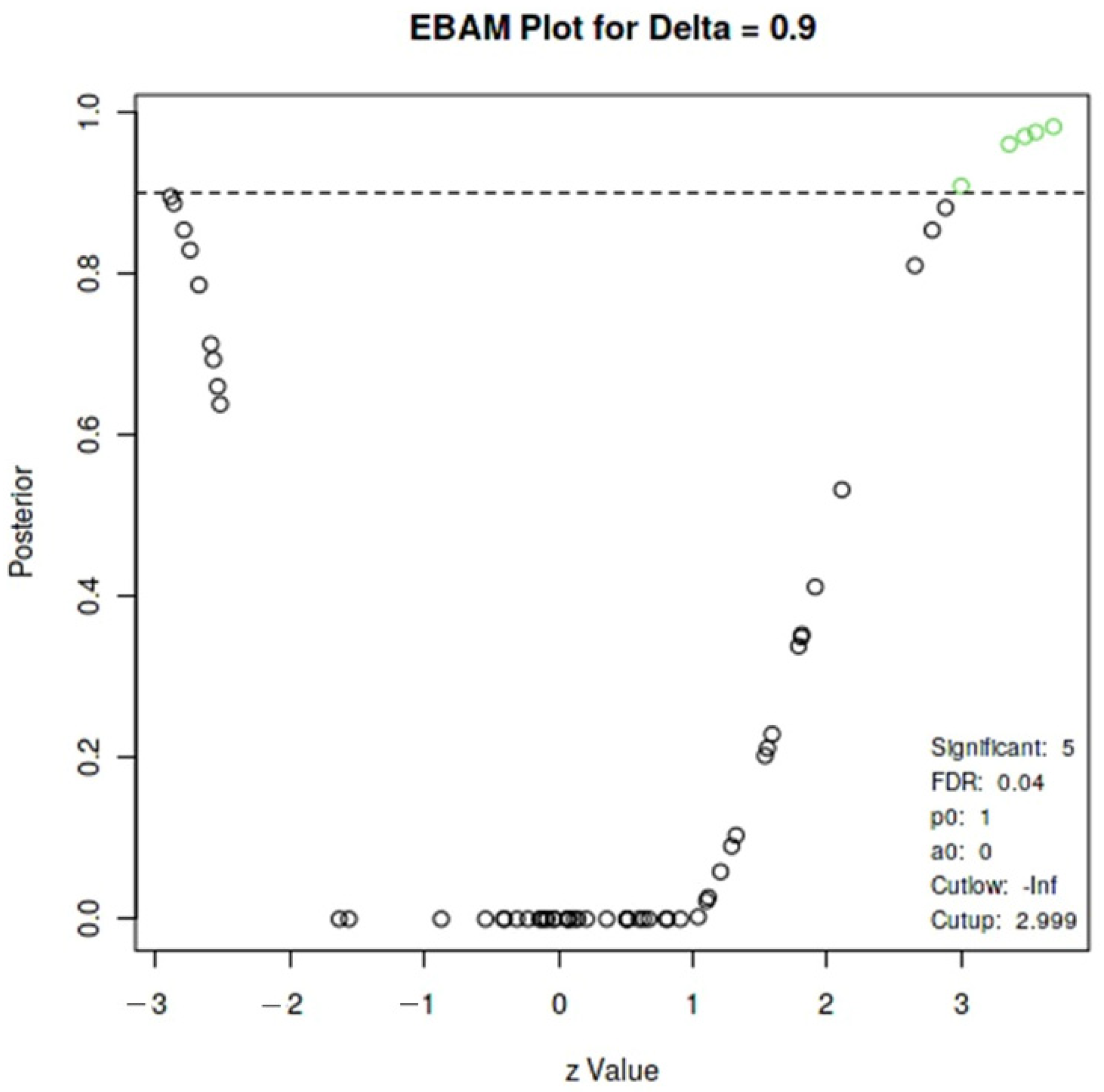
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tianyu, L. On the Core Elements of Volleyball Players’ Special Physical Training. Appl. Educ. Psychol. 2021, 2, 92–95. [Google Scholar]
- Kunstlinger, U.; Ludwig, H.G.; Stegemann, J. Metabolic Changes During Volleyball Matches. Int. J. Sports Med. 1987, 8, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, D.; Mroczek, D. Fatigue and Training Load Factors in Volleyball. Int. J. Environ. Res. Public Health 2022, 19, 11149. [Google Scholar] [CrossRef]
- Taylor, J.B.; Wright, A.A.; Dischiavi, S.L.; Townsend, M.A.; Marmon, A.R. Activity Demands During Multi-Directional Team Sports: A Systematic Review. Sports Med. 2017, 47, 2533–2551. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.C.; Van Den Tillaar, R.; Gabbett, T.J.; Reis, V.M.; González-Badilho, J.J. Physical fitness qualities of professional volleyball players: Determination of positional differences. J. Strength Cond. Res. 2009, 23, 1106–1111. [Google Scholar] [CrossRef]
- Issurin, V.B. New Horizons for the Methodology and Physiology of Training Periodization. Sports Med. 2010, 40, 189–206. [Google Scholar] [CrossRef]
- Debien, P.B.; Mancini, M.; Coimbra, D.R.; De Freitas, D.G.S.; Miranda, R.; Bara Filho, M.G. Monitoring Training Load, Recovery, and Performance of Brazilian Professional Volleyball Players During a Season. Int. J. Sports Physiol. Perform. 2018, 13, 1182–1189. [Google Scholar] [CrossRef]
- Horta, T.A.G.; Mauri’, M.; Filho, M.G.B.; Coimbra, D.R.; Miranda, R.; Werneck, F.Z. Training Load, Physical Performance, Biochemical Markers, and Psychological Stress during a Short Preparatory Period in Brazilian Elite Male Volleyball Players. J. Strength. Cond. Res. 2019, 33, 3392–3399. [Google Scholar] [CrossRef]
- Toledo, H.C.; Miloski, B.; Campos, Y.D.A.C.; Guerrero, R.V.D.; Miranda, R.; Vianna, J.M.; Bara Filho, M. Distribuição Da Carga Interna de Treinamento Durante Uma Temporada No Voleibol Profissional. Rev. Bras. Educ. Física Esporte 2020, 34, 567–575. [Google Scholar] [CrossRef]
- Eliakim, E.; Doron, O.; Meckel, Y.; Nemet, D.; Eliakim, A. Pre-Season Fitness Level and Injury Rate in Professional Soccer—A Prospective Study. Sports Med. Int. Open 2018, 2, E84. [Google Scholar] [CrossRef]
- Holway, F.E.; Spriet, L.L. Sport-Specific Nutrition: Practical Strategies for Team Sports. J. Sports Sci. 2011, 29, 115–125. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Yoshikawa, T. Exercise and Functional Foods. Nutr. J. 2006, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Sesbreno, E.; Dziedzic, C.E.; Sygo, J.; Blondin, D.P.; Haman, F.; Leclerc, S.; Brazeau, A.S.; Mountjoy, M. Elite Male Volleyball Players Are at Risk of Insufficient Energy and Carbohydrate Intake. Nutrients 2021, 13, 1435. [Google Scholar] [CrossRef] [PubMed]
- Pons, V.; Riera, J.; Capó, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Drobnic, F.; Pons, A. Calorie Restriction Regime Enhances Physical Performance of Trained Athletes. J. Int. Soc. Sports Nutr. 2018, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength. Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Horta, T.A.G.; de Lima, P.H.P.; Matta, G.G.; de Freitas, J.V.; Dias, B.M.; Vianna, J.M.; Toledo, H.C.; Miranda, R.; Timoteo, T.F.; Filho, M.G.B. Training Load Impact on Recovery Status in Professional Volleyball Athletes. Rev. Bras. Med. Esporte 2020, 26, 158–161. [Google Scholar] [CrossRef]
- Prado, E.; Souza, G.H.M.F.; Pegurier, M.; Vieira, C.; Lima-Neto, A.B.M.; Assis, M.; Guedes, M.I.F.; Koblitz, M.G.B.; Ferreira, M.S.L.; Macedo, A.F.; et al. Non-Targeted Sportomics Analyses by Mass Spectrometry to Understand Exercise-Induced Metabolic Stress in Soccer Players. Int. J. Mass Spectrom. 2017, 418, 1–5. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Dessì, A.; Noto, A.; Sardo, S.; Finco, G.; Corsello, G.; Fanos, V.; Pintus, R. Sportomics: Metabolomics Applied to Sports. The New Revolution? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11011–11019. [Google Scholar] [PubMed]
- Jaguri, A.; Al Thani, A.A.; Elrayess, M.A. Exercise Metabolome: Insights for Health and Performance. Metabolites 2023, 13, 694. [Google Scholar] [CrossRef]
- Zhou, W.; Zeng, G.; Lyu, C.; Kou, F.; Zhang, S.; Wei, H. The Effect of Strength-Endurance Training on Serum and Urine Metabolic Profiles of Female Adolescent Volleyball Athletes. Physiol. Int. 2021, 108, 285–302. [Google Scholar] [CrossRef]
- Pinheiro, A.B.V.; Lacerda, E.M.D.A.; Benzecry, E.H.; Gomes, M.C.D.S.; Costa, V.M.D. Tabela Para Avaliação de Consumo Alimentar Em Medidas Caseiras; Atheneu: Itaquaquecetuba, Brazil, 2008; p. 131. [Google Scholar]
- U.S. Department of Agriculture. Agricultural Research Service USDA National Nutrient Database for Standard Reference, Legacy Release|Ag Data Commons. Available online: https://data.nal.usda.gov/dataset/usda-national-nutrient-database-standard-reference-legacy-release (accessed on 7 November 2023).
- IOM (Institute of Medicine). Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Stallings, V.A.; Harrison, M.; Oria, M. Dietary Reference Intakes for Sodium and Potassium; National Academies Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Norton, K.I. Standards for Anthropometry Assessment. Kinanthropometry Exerc. Physiol. 2019, 4, 68–137. [Google Scholar]
- Ministério da Saúde. Brasil Técnicas Para Coleta de Sangue, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2001. [Google Scholar]
- De Oliveira, L.R.P.; Martins, C.; Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; De Oliveira Torres, R.; Soares, A.L.; Almeida, F.C.L.; Valente, A.P.; De Souza, I.P.R. Salivary Metabolite Fingerprint of Type 1 Diabetes in Young Children. J. Proteome Res. 2016, 15, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Nagana Gowda, G.A.; Raftery, D. Quantitating Metabolites in Protein Precipitated Serum Using NMR Spectroscopy. Anal. Chem. 2014, 86, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Tibshirani, R.; Storey, J.D.; Tusher, V. Empirical Bayes Analysis of a Microarray Experiment. J. Am. Stat. Assoc. 2001, 96, 1151–1160. [Google Scholar] [CrossRef]
- León-Guereño, P.; Urdampilleta, A.; Zourdos, M.C.; Mielgo-Ayuso, J. Anthropometric Profile, Body Composition and Somatotype in Elite Traditional Rowers: A Cross-Sectional Study. Rev. Española Nutr. Humana Dietética 2018, 22, 279–286. [Google Scholar] [CrossRef]
- Fields, J.B.; Merrigan, J.J.; White, J.B.; Jones, M.T. Body Composition Variables by Sport and Sport-Position in Elite Collegiate Athletes. J. Strength. Cond. Res. 2018, 32, 3153–3159. [Google Scholar] [CrossRef]
- Saunders, M.J. Coingestion of Carbohydrate-Protein during Endurance Exercise: Influence on Performance and Recovery. Int. J. Sport. Nutr. Exerc. Metab. 2007, 17 (Suppl. S1), S87–S103. [Google Scholar] [CrossRef]
- Yeo, W.K.; Carey, A.L.; Burke, L.; Spriet, L.L.; Hawley, J.A. Fat Adaptation in Well-Trained Athletes: Effects on Cell Metabolism. Appl. Physiol. Nutr. Metab. 2011, 36, 12–22. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Blomstrand, E.; Andersson, S.; Hassmen, P.; Ekblom, B.; Newsholme, E.A. Effect of Branched-Chain Amino Acid and Carbohydrate Supplementation on the Exercise-Induced Change in Plasma and Muscle Concentration of Amino Acids in Human Subjects. Acta Physiol. Scand. 1995, 153, 87–96. [Google Scholar] [CrossRef] [PubMed]
- EssÉn-Gustavsson, B.; Blomstrand, E. Effect of Exercise on Concentrations of Free Amino Acids in Pools of Type I and Type II Fibres in Human Muscle with Reduced Glycogen Stores. Acta Physiol. Scand. 2002, 174, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Veselkov, K.; Mikros, E.; Mougios, V.; Theodoridis, G.A. 1H NMR Study on the Short- and Long-Term Impact of Two Training Programs of Sprint Running on the Metabolic Fingerprint of Human Serum. J. Proteome Res. 2013, 12, 470–480. [Google Scholar] [CrossRef]
- Berton, R.; Conceição, M.S.; Libardi, C.A.; Canevarolo, R.R.; Gáspari, A.F.; Chacon-Mikahil, M.P.T.; Zeri, A.C.; Cavaglieri, C.R. Metabolic Time-Course Response after Resistance Exercise: A Metabolomics Approach. J. Sports Sci. 2017, 35, 1211–1218. [Google Scholar] [CrossRef]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef]
- Sale, C.; Artioli, G.G.; Gualano, B.; Saunders, B.; Hobson, R.M.; Harris, R.C. Carnosine: From Exercise Performance to Health. Amino Acids 2013, 44, 1477–1491. [Google Scholar] [CrossRef]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Jiye, A.; Wang, G.; Lu, H.; Huang, X.; Liu, Y.; Zha, W.; Hao, H.; Zhang, Y.; Liu, L.; et al. Metabolomic Investigation into Variation of Endogenous Metabolites in Professional Athletes Subject to Strength-Endurance Training. J. Appl. Physiol. 2009, 106, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Ławiński, J.; Rysz, J. The Impact of Aerobic Exercise on HDL Quantity and Quality: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4653. [Google Scholar] [CrossRef] [PubMed]
- George, F.C. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar]
- Faccin, A.P.; Alves, M.K.; Macedo, R.C.O. Perfil Antropométrico e Alimentar e o Conhecimento Nutricional de Atletas de Voleibol. Rev. Bras. Nutr. Esportiva 2017, 11, 259–264. [Google Scholar]
- Zieliński, J.; Kusy, K. Hypoxanthine: A Universal Metabolic Indicator of Training Status in Competitive Sports. Exerc. Sport. Sci. Rev. 2015, 43, 214–221. [Google Scholar] [CrossRef]
- Stathis, C.G.; Febbraio, M.A.; Carey, M.F.; Snow, R.J. Influence of Sprint Training on Human Skeletal Muscle Purine Nucleotide Metabolism. J. Appl. Physiol. 1994, 76, 1802–1809. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63. [Google Scholar] [CrossRef]
| Variables | Mean ± SD |
|---|---|
| Height (cm) | 202.00 ± 3.74 |
| Total body mass (kg) | 87.21 ± 2.86 |
| BMI (m/kg2) | 23.47 ± 1.71 |
| Fat Mass (kg) | 18.41 ± 3.26 |
| Fat-free mass (kg) | 68.02 ± 8.00 |
| Fat mass (%) | 21.26 ± 2.86 |
| Fat-free mass (%) | 78.78 ± 21.18 |
| Variables | Mean ± SD | Recommendation [Reference] |
|---|---|---|
| Energy (kcal/day) | 3752.26 ± 1388.38 | |
| PTN (g/kg) | 1.96 ± 0.89 | 1,2 a 2 g/kg [15] |
| PTN (%) | 18.19 ± 4.17 | |
| CHO (g/kg) | 4.52 ± 1.14 | 5 a 10 g/kg [15] |
| CHO (%) | 44.54 ± 8.21 | |
| LIP (g/kg) | 1.85 ± 0.95 | |
| LIP (%) | 37.26 ± 8.03 | 20 a 35% [15] |
| Calcium (mg) | 1246.78 ± 678.70 | 1000 [25] |
| Iron (mg) | 34.70 ± 15.21 | 8 [25] |
| Potassium (mg) | 3897.32 ± 926.70 | 3.400 [25] |
| Zinc (mg) | 24.10 ± 12.51 | 11 [25] |
| Selenium (µg) | 246.38 ± 123.44 | 55 [25] |
| Sodium (mg) | 4923.95 ± 2141.12 | 1500 [25] |
| Vitamin A (µg) | 1682.032 ± 1918.29 | 900 [24] |
| Vitamin D (µg) | 182.46 ± 195.14 | 15 [24] |
| Vitamin E (mg) | 21.93 ± 22.94 | 15 [24] |
| Vitamin C (mg) | 207.73 ± 122.19 | 90 [24] |
| Thiamine (mg) | 3.68 ± 1.77 | 1.2 [24] |
| Riboflavin (mg) | 4.91 ± 2.60 | 1.3 [24] |
| Niacin (mg) | 58.96 ± 25.69 | 16 [24] |
| Ac. Pantothenic (mg) | 15.05 ± 11.88 | 5 [24] |
| Pyridoxine (mg) | 3.68 ± 2.51 | 1.3 [24] |
| Folate (µg) (mg) | 931.79 ± 558.26 | 400 [24] |
| Cobalamin (µg) | 15.40 ± 11.27 | 2.4 [24] |
| Metabolites | ppm | Mean Pre-Game Intensity | Mean Post-Game Intensity | ∆T2-T1 | p-Value | FDR |
|---|---|---|---|---|---|---|
| 3-Hydroxybutyrate | 1.08 | 1.02 | 0.46 | −0.56 | 0.01 | 0.06 |
| Formic Acid | 8.46 | 1.40 | 3.12 | +1.72 | <0.01 | 0.06 |
| Arginine/lysine a | 1.89 | 1.23 | 0.78 | −0.45 | 0.01 | 0.06 |
| Arginine/lysine b | 1.71 | 2.41 | 1.80 | −0.61 | <0.01 | 0.06 |
| Formic Acid | 8.43 | 1.57 | 3.28 | +1.71 | <0.01 | 0.06 |
| Hypoxanthine | 8.16 | 1.94 | 3.52 | +1.57 | 0.01 | 0.06 |
| Histidine a | 7.41 | 2.63 | 4.39 | +1.76 | 0.01 | 0.07 |
| Histidine b | 7.38 | 2.34 | 4.19 | +1.85 | 0.01 | 0.07 |
| Histidine c | 7.32 | 3.02 | 4.74 | +1.72 | 0.01 | 0.07 |
| Histidine d | 7.05 | 2.50 | 4.51 | +2.01 | 0.01 | 0.06 |
| Isoleucine a | 1.02 | 5.75 | 4.00 | −1.74 | <0.01 | 0.04 |
| Isoleucine b | 0.93 | 9.61 | 7.01 | −2.60 | <0.01 | 0.04 |
| LDL/VLDL | 1.20 | 2.18 | 1.77 | −0.41 | 0.04 | 0.15 |
| Leucine | 0.96 | 8.56 | 6.22 | −2.34 | <0.01 | 0.04 |
| Tyrosine a | 7.17 | 3.71 | 5.36 | −1.65 | 0.01 | 0.07 |
| Tyrosine b | 6.87 | 3.55 | 5.28 | −1.73 | 0.01 | 0.07 |
| Valine a | 1.05 | 1.45 | 0.83 | −0.62 | <0.01 | 0.04 |
| Valine b | 0.99 | 2.48 | 1.74 | −0.73 | <0.01 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.M.; Ferreira, T.J.; Franca, P.A.P.; da Cruz, R.R.; Bara-Filho, M.G.; Cahuê, F.L.C.; Valente, A.P.; Pierucci, A.P.T.R. A Decrease in Branched-Chain Amino Acids after a Competitive Male Professional Volleyball Game—A Metabolomic-Based Approach. Metabolites 2024, 14, 115. https://doi.org/10.3390/metabo14020115
Oliveira TM, Ferreira TJ, Franca PAP, da Cruz RR, Bara-Filho MG, Cahuê FLC, Valente AP, Pierucci APTR. A Decrease in Branched-Chain Amino Acids after a Competitive Male Professional Volleyball Game—A Metabolomic-Based Approach. Metabolites. 2024; 14(2):115. https://doi.org/10.3390/metabo14020115
Chicago/Turabian StyleOliveira, Taillan Martins, Tathiany Jéssica Ferreira, Paula Albuquerque Penna Franca, Rudson Ribeiro da Cruz, Mauricio Gattás Bara-Filho, Fábio Luiz Candido Cahuê, Ana Paula Valente, and Anna Paola Trindade Rocha Pierucci. 2024. "A Decrease in Branched-Chain Amino Acids after a Competitive Male Professional Volleyball Game—A Metabolomic-Based Approach" Metabolites 14, no. 2: 115. https://doi.org/10.3390/metabo14020115
APA StyleOliveira, T. M., Ferreira, T. J., Franca, P. A. P., da Cruz, R. R., Bara-Filho, M. G., Cahuê, F. L. C., Valente, A. P., & Pierucci, A. P. T. R. (2024). A Decrease in Branched-Chain Amino Acids after a Competitive Male Professional Volleyball Game—A Metabolomic-Based Approach. Metabolites, 14(2), 115. https://doi.org/10.3390/metabo14020115