Metabolic Alterations of Short-Chain Organic Acids in the Elderly Link Antibiotic Exposure with the Risk for Depression
Abstract
1. Introduction
2. Methods and Materials
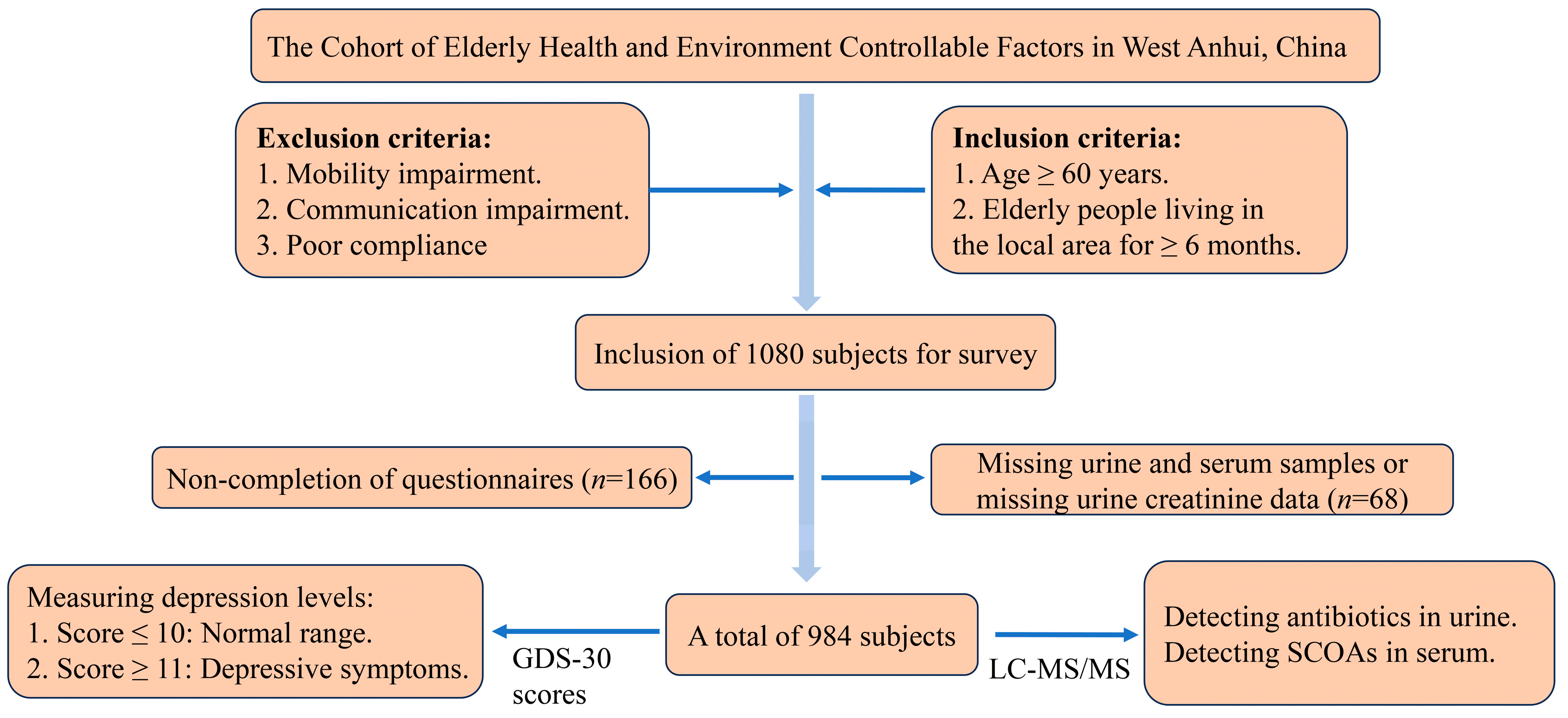
2.1. Study Population and Design
2.2. Detection of Urinary Antibiotics
2.3. Measurement of Short-Chain Organic Acids in Serum
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association Between Antibiotic Use and Depression
3.3. Short-Chain Organic Acids Levels
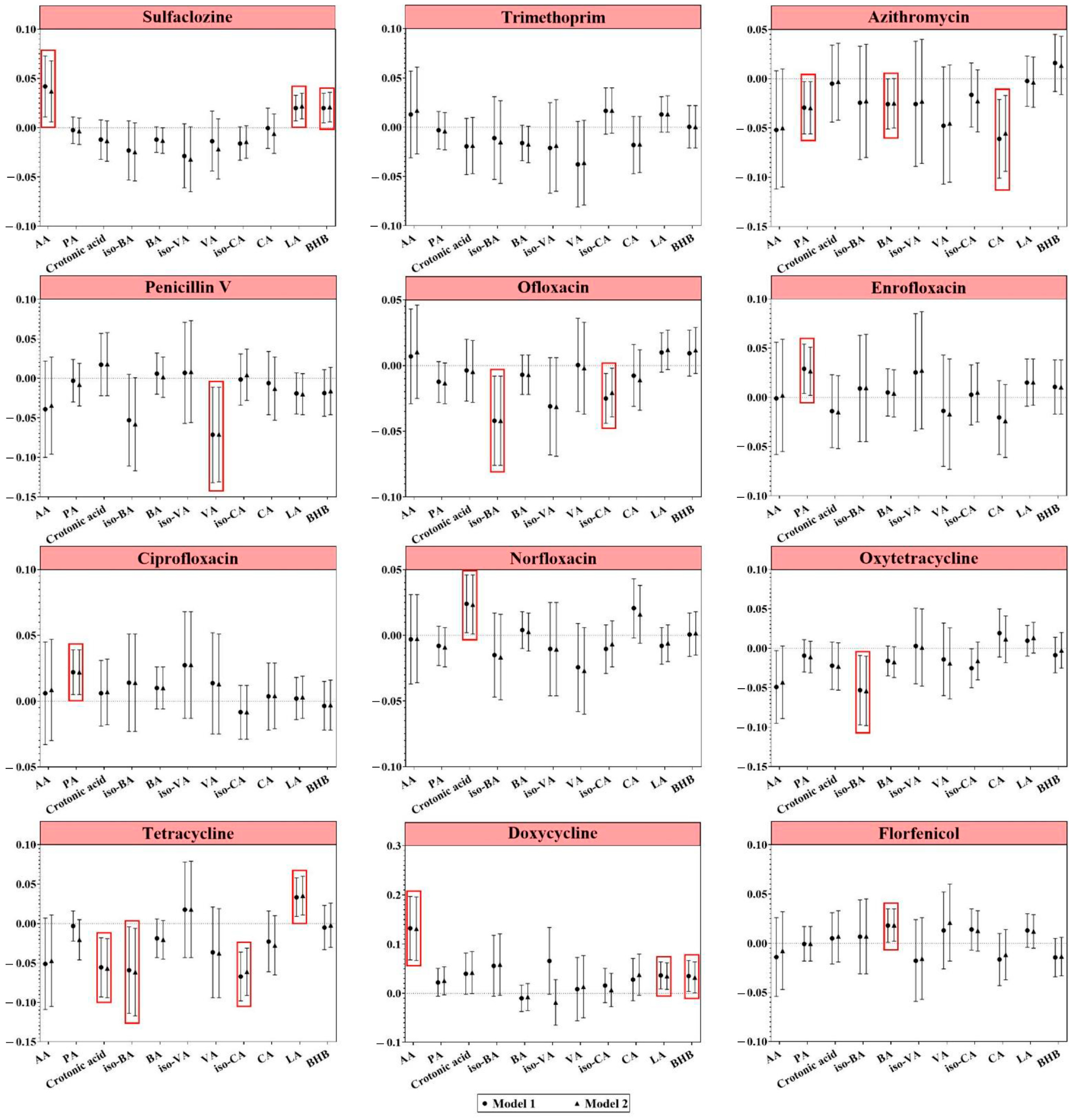
3.3.1. Antibiotic Exposure and Short-Chain Organic Acids
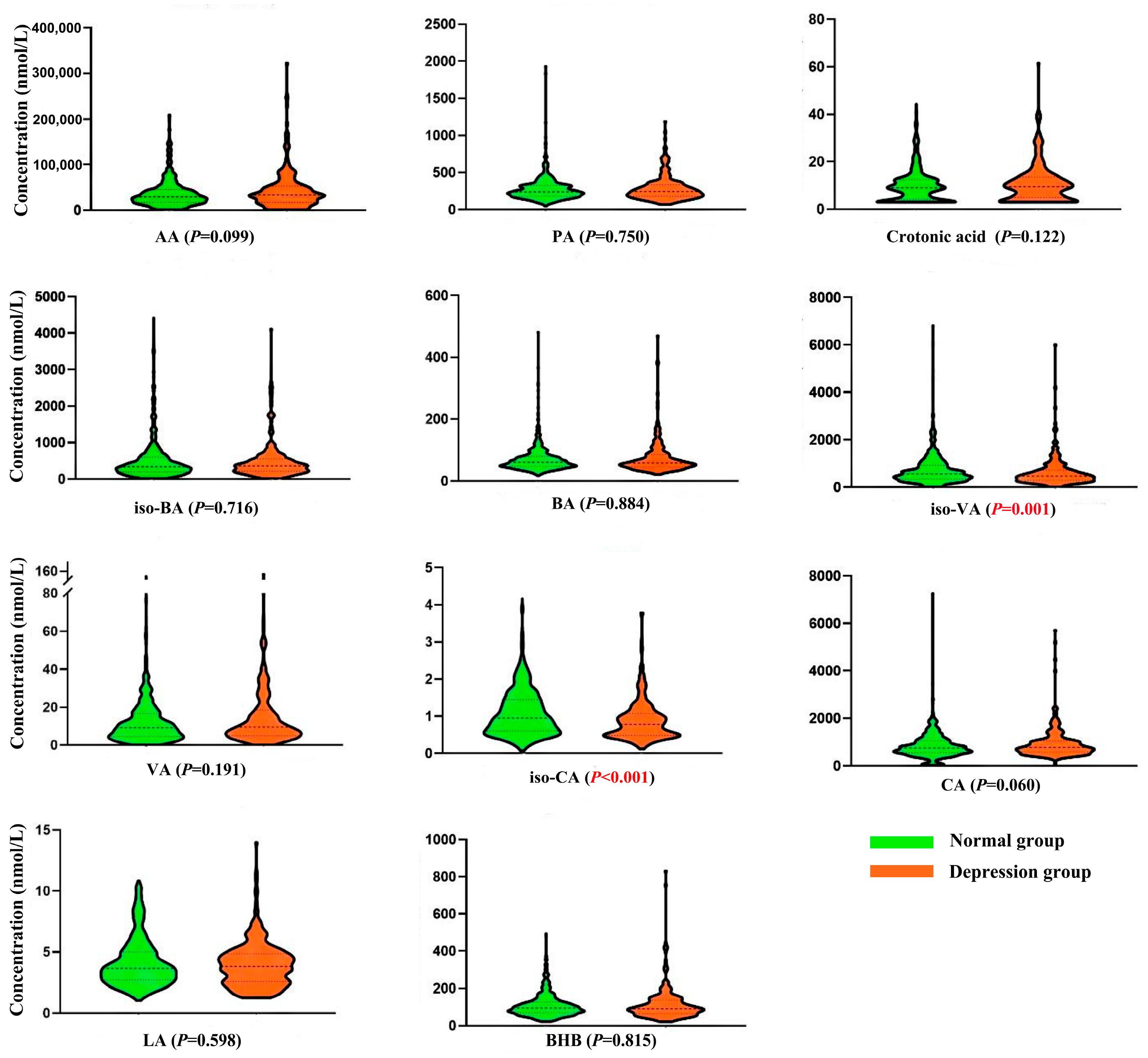
3.3.2. Association of Short-Chain Organic Acids with Depression
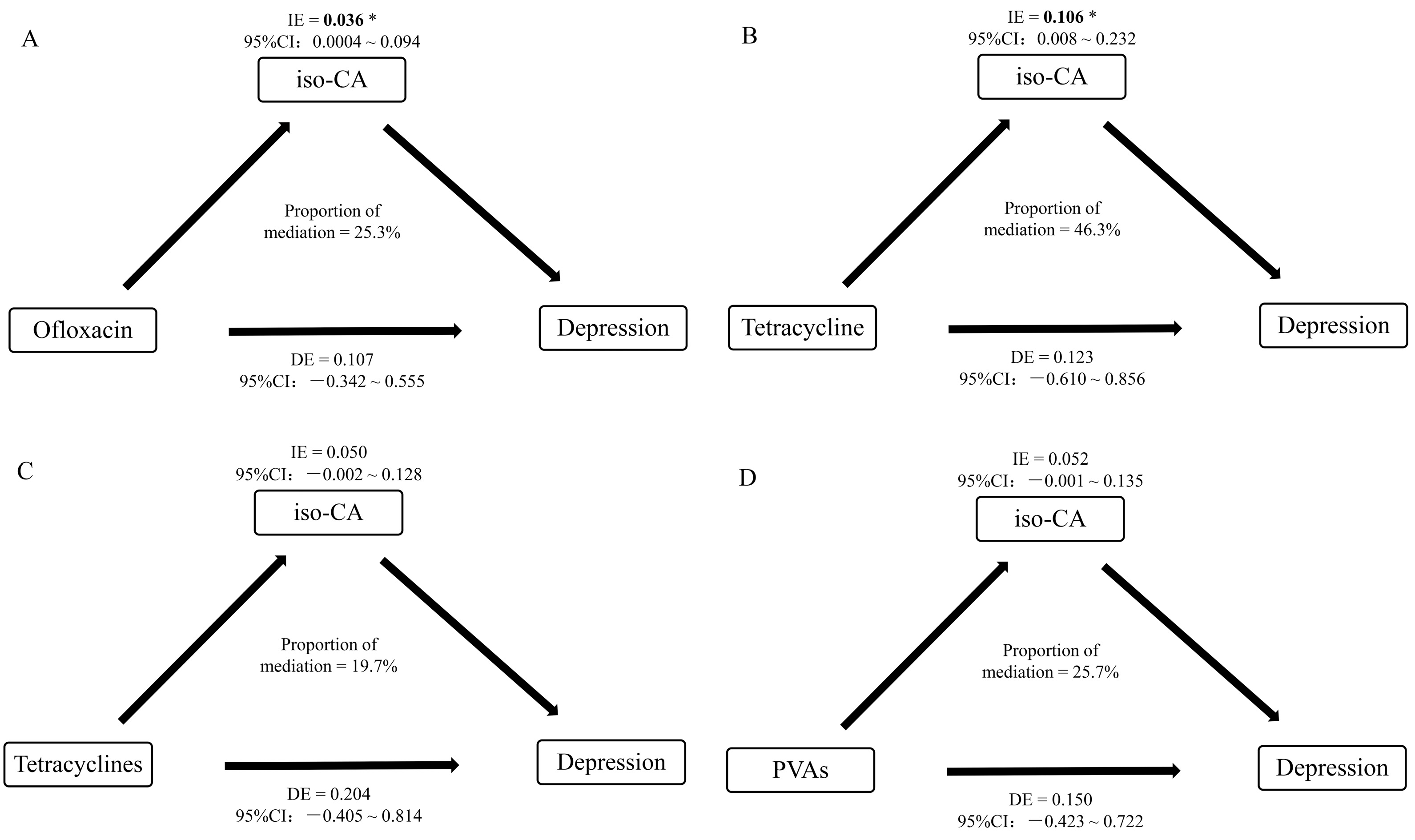
3.3.3. Mediating Effect of Short-Chain Organic Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, A.; Duflo, E.; Grela, E.; McKelway, M.; Schilbach, F.; Sharma, G.; Vaidyanathan, G. Depression and Loneliness among the Elderly in Low- and Middle-Income Countries. J. Econ. Perspect. 2023, 37, 179–202. [Google Scholar] [CrossRef]
- Yan, Y.; Du, Y.; Li, X.; Ping, W.; Chang, Y. Physical function, ADL, and depressive symptoms in Chinese elderly: Evidence from the CHARLS. Front. Public Health 2023, 11, 1017689. [Google Scholar] [CrossRef] [PubMed]
- Alshaya, D.S. Genetic and epigenetic factors associated with depression: An updated overview. Saudi J. Biol. Sci. 2022, 29, 103311. [Google Scholar] [CrossRef] [PubMed]
- Remes, O.; Mendes, J.F.; Templeton, P. Biological, Psychological, and Social Determinants of Depression: A Review of Recent Literature. Brain Sci. 2021, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Luqman, A.; He, M.; Hassan, A.; Ullah, M.; Zhang, L.; Rashid Khan, M.; Din, A.U.; Ullah, K.; Wang, W.; Wang, G. Mood and microbes: A comprehensive review of intestinal microbiota’s impact on depression. Front. Psychiatry 2024, 15, 1295766. [Google Scholar] [CrossRef]
- Claus, S.P.; Guillou, H.; Ellero-Simatos, S. The gut microbiota: A major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2016, 2, 16003. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Wang, Y.; Liao, C.; Jiang, G. A short review of human exposure to antibiotics based on urinary biomonitoring. Sci. Total Environ. 2022, 830, 154775. [Google Scholar] [CrossRef]
- Nel Van Zyl, K.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of antibiotics on the human microbiome: A systematic review. Int. J. Antimicrob. Agents 2022, 59, 106502. [Google Scholar] [CrossRef]
- Pouranayatihosseinabad, M.; Bezabih, Y.; Hawrelak, J.; Peterson, G.M.; Veal, F.; Mirkazemi, C. Antibiotic use and the development of depression: A systematic review. J. Psychosom. Res. 2023, 164, 111113. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Sang, Y.; Liu, K.; Zhu, Y.; Yang, L.; Wang, S.; Sheng, J.; Wang, Q.; Zhang, D.; et al. Antibiotic exposure and potential risk of depression in the Chinese elderly: A biomonitoring-based population study. Environ. Sci. Pollut. Res. 2021, 28, 26794–26806. [Google Scholar] [CrossRef]
- Deng, H.; Yu, Y.; Sha, Q.; Sun, W.; Liang, L.; Ren, F.; Ji, H.; Shen, X.; Fan, X. Construction of antibiotic-induced depression mice model and the function of intestinal microbiota. Front. Microbiol. 2023, 14, 1093486. [Google Scholar] [CrossRef] [PubMed]
- Dinan, K.; Dinan, T. Antibiotics and mental health: The good, the bad and the ugly. J. Intern. Med. 2022, 292, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Mian, F.M.; Stanisz, A.M.; Bindels, L.B.; Cambier, E.; Ben-Amram, H.; Koren, O.; Forsythe, P.; Bienenstock, J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017, 8, 15062. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, H.; Shi, Q.; Wang, N.; Zhang, Z.; Xiong, C.; Liu, J.; Chen, Y.; Jiang, L.; Jiang, Q. Effects of oral florfenicol and azithromycin on gut microbiota and adipogenesis in mice. PLoS ONE 2017, 12, e0181690. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Wang, Y.; Wang, P.; Song, H.; Wang, F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 2019, 14, e0218384. [Google Scholar] [CrossRef]
- Merenstein, D.; Fraser, C.M.; Roberts, R.F.; Liu, T.; Grant-Beurmann, S.; Tan, T.P.; Smith, K.H.; Cronin, T.; Martin, O.A.; Sanders, M.E.; et al. Bifidobacterium animalis subsp. lactis BB-12 Protects against Antibiotic-Induced Functional and Compositional Changes in Human Fecal Microbiome. Nutrients 2021, 13, 2814. [Google Scholar] [CrossRef]
- Shen, B.; Hu, J.; Song, H.; Wang, Z.; Fan, J.; Sun, Y.; Wang, Q. Antibiotics exacerbated colitis by affecting the microbiota, Treg cells and SCFAs in IL10-deficient mice. Biomed. Pharmacother. 2019, 114, 108849. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, X.; Ouyang, Y.B.; He, C.; Li, N.S.; Xie, C.; Peng, C.; Zhu, Z.H.; Shu, X.; Xie, Y.; et al. Altered Gut Microbiota and Short-Chain Fatty Acids After Vonoprazan-Amoxicillin Dual Therapy for Helicobacter pylori Eradication. Front. Cell Infect. Microbiol. 2022, 12, 881968. [Google Scholar] [CrossRef]
- Hao, W.Z.; Li, X.J.; Zhang, P.W.; Chen, J.X. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res. 2020, 284, 112691. [Google Scholar] [CrossRef]
- Liu, S.; Guo, R.; Liu, F.; Yuan, Q.; Yu, Y.; Ren, F. Gut Microbiota Regulates Depression-Like Behavior in Rats Through the Neuroendocrine-Immune-Mitochondrial Pathway. Neuropsychiatr. Dis. Treat. 2020, 16, 859–869. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Zhang, Q.; Song, Y.; Wang, L.; Zhu, Z. Interactions Between Intestinal Microbiota and Neural Mitochondria: A New Perspective on Communicating Pathway From Gut to Brain. Front. Microbiol. 2022, 13, 798917. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Rendueles, M.; Díaz, M. Microbial production of specialty organic acids from renewable and waste materials. Crit. Rev. Biotechnol. 2015, 35, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Hishiya, N.; Uno, K.; Nakano, A.; Konishi, M.; Higashi, S.; Eguchi, S.; Ariyoshi, T.; Matsumoto, A.; Oka, K.; Takahashi, M.; et al. Association between the gut microbiome and organic acid profiles in a Japanese population with HIV infection. J. Infect. Chemother. 2024, 30, 58–66. [Google Scholar] [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Shin, H.D.; Liu, L.; Du, G.; Chen, J. Protein and metabolic engineering for the production of organic acids. Bioresour. Technol. 2017, 239, 412–421. [Google Scholar] [CrossRef]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Kajitani, N.; Iwata, M.; Miura, A.; Tsunetomi, K.; Yamanashi, T.; Matsuo, R.; Nishiguchi, T.; Fukuda, S.; Nagata, M.; Shibushita, M.; et al. Prefrontal cortex infusion of beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, produces antidepressant-like effects in a rodent model of depression. Neuropsychopharmacol. Rep. 2020, 40, 157–165. [Google Scholar] [CrossRef]
- Qin, F.; Li, B.; Wang, H.; Ma, S.; Li, J.; Liu, S.; Kong, L.; Zheng, H.; Zhu, R.; Han, Y.; et al. Linking chromatin acylation mark-defined proteome and genome in living cells. Cell 2023, 186, 1066–1085.e36. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C. Clinical validation of the Geriatric Depression Scale (GDS): Chinese version. J. Aging Health 1996, 8, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, K.; Zhang, J.; Liu, X.; Yang, L.; Wei, R.; Wang, S.; Zhang, D.; Xie, S.; Tao, F. Antibiotic body burden of elderly Chinese population and health risk assessment: A human biomonitoring-based study. Envrion. Pollut. 2020, 256, 113311. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-Y.; Zhang, J.-J.; Geng, M.-L.; Zhu, Y.-T.; Liu, X.-J.; Ding, P.; Wang, B.-L.; Liu, W.-W.; Liu, Y.-H.; Tao, F.-B. A Stable Isotope Dilution Assay for Multi-class Antibiotics in Pregnant Urines by LC–MS/MS. Chromatographia 2020, 83, 507–521. [Google Scholar] [CrossRef]
- Schauer, S.; Othman, A. High-Throughput RPLC-MS/MS Quantification of Short- and Medium-Chain Fatty Acids. In Clinical Metabolomics: Methods and Protocols; Giera, M., Sánchez-López, E., Eds.; Springer: New York, NY, USA, 2025; pp. 195–207. [Google Scholar] [CrossRef]
- Wang, S.; Feng, R.; Kong, L.; Zhou, R.; Hu, F.-t.; Sun, S.-J.; Chen, G.-J.; Tao, F.-B.; Liu, K.-Y. An UHPLC-QE-Orbitrap-MS Method for Accurate Quantification of Short-Chain Fatty Acids in Serum From an Older Chinese Population. Chromatographia 2023, 87, 125–136. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Wang, B.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; He, G.; Zhao, Q.; et al. Antibiotics detected in urines and adipogenesis in school children. Environ. Int. 2016, 89–90, 204–211. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, G.; Zhao, H.; Zhai, G.; Chen, J.; Zhao, H. Antibiotics in a general population: Relations with gender, body mass index (BMI) and age and their human health risks. Sci. Total Environ. 2017, 599–600, 298–304. [Google Scholar] [CrossRef]
- Hu, K.; Smedby, K.E.; Sjölander, A.; Montgomery, S.; Valdimarsdóttir, U.; Engstrand, L.; Fang, F.; Fall, K. Use of Antibiotics and Risk of Psychiatric Disorders in Newly Diagnosed Cancer Patients: A Population-Based Cohort Study in Sweden. Cancer Epidemiol. Biomark. Prev. 2022, 31, 528–535. [Google Scholar] [CrossRef]
- Lurie, I.; Yang, Y.X.; Haynes, K.; Mamtani, R.; Boursi, B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: A nested case-control study. J. Clin. Psychiatry 2015, 76, 1522–1528. [Google Scholar] [CrossRef]
- Kaur, K.; Fayad, R.; Saxena, A.; Frizzell, N.; Chanda, A.; Das, S.; Chatterjee, S.; Hegde, S.; Baliga, M.S.; Ponemone, V.; et al. Fluoroquinolone-related neuropsychiatric and mitochondrial toxicity: A collaborative investigation by scientists and members of a social network. J. Community Support. Oncol. 2016, 14, 54–65. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, Y.; Wu, P.; Zhou, H.; Lasanajak, Y.; Fang, Y.; Tang, L.; Ye, L.; Li, X.; Cai, Z.; et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 2019, 148, 104403. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R. Histone deacetylases 1, 2 and 3 in nervous system development. Curr. Opin. Pharmacol. 2020, 50, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, K.A.; Berchtold, N.C.; Malvaez, M.; Carlos, A.J.; McQuown, S.C.; Cunningham, M.J.; Wood, M.A.; Cotman, C.W. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology 2013, 38, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Chen, L.; Miao, Z.; Xu, X. β-hydroxybutyrate alleviates depressive behaviors in mice possibly by increasing the histone3-lysine9-β-hydroxybutyrylation. Biochem. Biophys. Res. Commun. 2017, 490, 117–122. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Iwata, M.; Kajitani, N.; Miura, A.; Matsuo, R.; Murakami, S.; Nakada, Y.; Pu, S.; Shimizu, Y.; Tsubakino, T.; et al. Stress increases blood beta-hydroxybutyrate levels and prefrontal cortex NLRP3 activity jointly in a rodent model. Neuropsychopharmacol. Rep. 2021, 41, 159–167. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef]
- Mu, C.; Zhu, W. Antibiotic effects on gut microbiota, metabolism, and beyond. Appl. Microbiol. Biotechnol. 2019, 103, 9277–9285. [Google Scholar] [CrossRef] [PubMed]
- Pruss, K.M.; Chen, H.; Liu, Y.; Van Treuren, W.; Higginbottom, S.K.; Jarman, J.B.; Fischer, C.R.; Mak, J.; Wong, B.; Cowan, T.M.; et al. Host-microbe co-metabolism via MCAD generates circulating metabolites including hippuric acid. Nat. Commun. 2023, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zou, Y.; Gong, J.; Ge, Z.; Lin, X.; Zhang, W.; Huang, H.; Zhao, J.; Saw, P.E.; et al. A high-fat diet promotes cancer progression by inducing gut microbiota-mediated leucine production and PMN-MDSC differentiation. Proc. Natl. Acad. Sci. USA 2024, 121, e2306776121. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Doubreh, B.; Appaw, W.O.; Abe-Inge, V. Antibiotic residues in poultry eggs and its implications on public health: A review. Sci. Afr. 2023, 19, e01456. [Google Scholar] [CrossRef]
- Samanidou, V.; Nisyriou, S. Multi-residue methods for confirmatory determination of antibiotics in milk. J. Sep. Sci. 2008, 31, 2068–2090. [Google Scholar] [CrossRef]
- Bayou, K.; Haile, N. Review on Antibiotic Residues in Food of Animal Origin: Economic and Public Health Impacts. Appl. J. Hyg. 2017, 6, 1–8. [Google Scholar]
| Characteristics | n (%) | Depression | p-Value a |
|---|---|---|---|
| n (%) | |||
| Gender | |||
| Male | 447 (45.4) | 104 (23.3) | 0.004 |
| Female | 537 (54.6) | 169 (31.5) | |
| Age | |||
| 60~70 | 484 (49.1) | 133 (27.5) | 0.855 |
| >70 | 500 (50.9) | 140 (28.0) | |
| Marital status | |||
| Non-widowed | 256 (26.0) | 100 (39.1) | <0.001 |
| Widowed | 728 (74.0) | 173 (23.8) | |
| Physical activity | |||
| Yes | 292 (29.7) | 43 (14.7) | <0.001 |
| No | 692 (70.3) | 230 (33.2) | |
| Educational level | |||
| Illiteracy | 450 (45.7) | 179 (39.8) | <0.001 |
| Primary school | 236 (23.9) | 55 (23.3) | |
| Middle school | 169 (17.2) | 27 (16.0) | |
| High school | 129 (13.1) | 12 (9.3) | |
| Living alone | |||
| Yes | 134 (13.6) | 62 (46.3) | <0.001 |
| No | 850 (86.4) | 211 (24.9) | |
| Smoke | |||
| Yes | 189 (19.2) | 46 (24.3) | 0.278 |
| No | 795 (80.8) | 227 (28.6) | |
| Drinking | |||
| Yes | 370 (37.6) | 83 (22.4) | 0.004 |
| No | 614 (62.4) | 190 (30.9) | |
| Dietary structure | |||
| Vegetable-based | 551 (56.0) | 172 (31.2) | 0.009 |
| Balanced | 376 (38.2) | 92 (24.7) | |
| Meat-based | 57 (5.8) | 9 (15.8) | |
| Salt | |||
| Low | 465 (47.3) | 134 (28.8) | 0.763 |
| General | 228 (23.1) | 62 (27.2) | |
| High | 291 (29.6) | 77 (26.5) | |
| Oil | |||
| Low | 450 (45.7) | 133 (29.6) | 0.227 |
| General | 322 (32.8) | 78 (24.2) | |
| High | 212 (21.5) | 62 (29.2) | |
| Sugar | |||
| Low | 617 (62.6) | 178 (28.8) | 0.428 |
| General | 256 (26.2) | 64 (24.8) | |
| High | 111 (11.3) | 32 (28.8) | |
| Chronic diseases | |||
| Yes | 362 (36.8) | 101 (27.9) | 0.933 |
| No | 622 (40.0) | 172 (27.7) | |
| Cognitive impairment | |||
| Yes | 407 (41.4) | 62 (15.2) | <0.001 |
| No | 577 (58.6) | 211 (36.6) | |
| Depression | |||
| Yes | 273 (27.7) | - | - |
| No | 711 (72.3) | - |
| Metabolites | Model 1 a | Model 2 a | ||
|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | |
| AA | 0.230 (−0.624, 1.083) | 0.598 | 0.395 (−0.394, 1.184) | 0.326 |
| PA | 1.013 (−0.933, 2.959) | 0.307 | 0.581 (−1.228, 2.390) | 0.529 |
| Crotonic acid | 0.380 (−0.845, 1.606) | 0.543 | 0.056 (−1.074, 1.185) | 0.923 |
| iso-BA | −0.058 (−0.952, 0.836) | 0.899 | −0.150 (−0.974, 0.674) | 0.721 |
| BA | 1.042 (−0.986, 3.070) | 0.314 | 0.491 (−1.389, 2.370) | 0.609 |
| iso-VA | −0.370 (−1.187, 0.447) | 0.374 | −0.430 (−1.181, 0.321) | 0.262 |
| VA | 0.529 (−0.333, 1.391) | 0.229 | 0.266 (−0.539, 1.071) | 0.516 |
| iso-CA | −4.075 (−5.649, −2.501) | <0.001 | −1.758 (−3.266, −0.250) | 0.022 |
| CA | 2.470 (1.184, 3.756) | <0.001 | 1.024 (−0.200, 2.249) | 0.101 |
| LA | −0.916 (−2.944, 1.112) | 0.376 | 0.316 (−1.562, 2.194) | 0.742 |
| BHB | 0.769 (−0.986, 2.525) | 0.390 | 1.812 (0.190, 3.434) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Kong, L.; Hu, F.; Wang, S.; Geng, M.; Cao, H.; Tao, X.; Tao, F.; Liu, K. Metabolic Alterations of Short-Chain Organic Acids in the Elderly Link Antibiotic Exposure with the Risk for Depression. Metabolites 2024, 14, 689. https://doi.org/10.3390/metabo14120689
Sun S, Kong L, Hu F, Wang S, Geng M, Cao H, Tao X, Tao F, Liu K. Metabolic Alterations of Short-Chain Organic Acids in the Elderly Link Antibiotic Exposure with the Risk for Depression. Metabolites. 2024; 14(12):689. https://doi.org/10.3390/metabo14120689
Chicago/Turabian StyleSun, Shujing, Li Kong, Fangting Hu, Sheng Wang, Menglong Geng, Hongjuan Cao, Xingyong Tao, Fangbiao Tao, and Kaiyong Liu. 2024. "Metabolic Alterations of Short-Chain Organic Acids in the Elderly Link Antibiotic Exposure with the Risk for Depression" Metabolites 14, no. 12: 689. https://doi.org/10.3390/metabo14120689
APA StyleSun, S., Kong, L., Hu, F., Wang, S., Geng, M., Cao, H., Tao, X., Tao, F., & Liu, K. (2024). Metabolic Alterations of Short-Chain Organic Acids in the Elderly Link Antibiotic Exposure with the Risk for Depression. Metabolites, 14(12), 689. https://doi.org/10.3390/metabo14120689







