Calcitriol Concentration in the Early Phase of Myocardial Infarction and Its Relation to Left Ventricular Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MI Definition
2.3. Biochemical Analysis
2.4. Post-Myocardial Infarction Ejection Fraction
2.5. Statistical Analysis
3. Results
3.1. Vitamin D Status Among All Patients
3.2. Patient Subgroup Analysis
3.3. Vitamin D Status According to LVEF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chanchlani, R.; Ackerman, S.; Piva, E.; Harvey, E. Intraperitoneal Calcitriol for Treatment of Severe Hyperparathyroidism in Children with Chronic Kidney Disease: A Therapy Forgotten. Perit. Dial. Int. 2016, 36, 688–690. [Google Scholar] [CrossRef]
- 25-Hydroxyvitamin D Levels and the Risk of Mortality in the General Population–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18695076/ (accessed on 28 June 2024).
- Meems, L.M.; Brouwers, F.P.; Joosten, M.M.; Lambers Heerspink, H.J.; de Zeeuw, D.; Bakker, S.J.; Gansevoort, R.T.; van Gilst, W.H.; van der Harst, P.; de Boer, R.A. Plasma calcidiol, calcitriol, and parathyroid hormone and risk of new onset heart failure in a population-based cohort study. ESC Heart Fail. 2016, 3, 189–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddy, Y.N.V.; Lewis, G.D.; Shah, S.J.; LeWinter, M.; Semigran, M.; Davila-Roman, V.G.; Anstrom, K.; Hernandez, A.; Braunwald, E.; Redfield, M.M.; et al. INDIE-HFpEF (Inorganic Nitrite Delivery to Improve Exercise Capacity in Heart Failure With Preserved Ejection Fraction): Rationale and Design. Circ Heart Fail. 2017, 10, e003862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barsan, M.; Brata, A.M.; Ismaiel, A.; Dumitrascu, D.I.; Badulescu, A.V.; Duse, T.A.; Dascalescu, S.; Popa, S.L.; Grad, S.; Muresan, L.; et al. The Pathogenesis of Cardiac Arrhythmias in Vitamin D Deficiency. Biomedicines 2022, 10, 1239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Graczyk, S.; Grzeczka, A.; Pasławska, U.; Kordowitzki, P. The Possible Influence of Vitamin D Levels on the Development of Atrial Fibrillation-An Update. Nutrients 2023, 15, 2725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giovannucci, E.; Liu, Y.; Hollis, B.W.; Rimm, E.B. 25-Hydroxyvitamin D and Risk of Myocardial Infarction in Men: A Prospective Study. Arch. Intern. Med. 2008, 168, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, C.; Ruan, C.; Chen, M.; Cao, R.; Sheng, L.; Chang, N.; Xu, T.; Zhao, P.; Liu, X.; et al. Novel Insights into the Cardioprotective Effects of Calcitriol in Myocardial Infarction. Cells 2022, 11, 1676. Available online: https://www.mdpi.com/2073-4409/11/10/1676 (accessed on 28 June 2024). [CrossRef]
- Tamayo, M.; Martín-Nunes, L.; Val-Blasco, A.; G.M-Piedras, M.J.; Navarro-García, J.A.; Lage, E.; Prieto, P.; Ruiz-Hurtado, G.; Fernández-Velasco, M.; Delgado, C. Beneficial Effects of Paricalcitol on Cardiac Dysfunction and Remodelling in a Model of Established Heart Failure. Br. J. Pharmacol. 2020, 177, 3273–3290. [Google Scholar] [CrossRef] [PubMed]
- Supplementation With Vitamin D and Omega-3 Fatty Acids and Incidence of Heart Failure Hospitalization: VITAL-Heart Failure.—Abstract—Europe PMC. Available online: https://europepmc.org/article/pmc/pmc7054158 (accessed on 28 June 2024).
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.-T.; Camargo, C.A. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Glob. Heart 2018, 13, 305–338. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Arabi, A. Association between Vitamin D and Cardiovascular Health: Myth or Fact? A Narrative Review of the Evidence. Womens Health 2023, 19, 17455057231158222. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.C.; Martini, L.A. Vitamin D and Cardiovascular Disease. Nutrients 2010, 2, 426–437. [Google Scholar] [CrossRef]
- Zittermann, A.; Zelzer, S.; Herrmann, M.; Gummert, J.F.; Kleber, M.; Trummer, C.; Theiler-Schwetz, V.; Keppel, M.H.; Maerz, W.; Pilz, S. Determinants of Circulating Calcitriol in Cardiovascular Disease. J. Steroid Biochem. Mol. Biol. 2024, 241, 106528. [Google Scholar] [CrossRef] [PubMed]
- Le, T.Y.L.; Ogawa, M.; Kizana, E.; Gunton, J.E.; Chong, J.J.H. Vitamin D Improves Cardiac Function After Myocardial Infarction Through Modulation of Resident Cardiac Progenitor Cells. Heart Lung Circ. 2018, 27, 967–975. [Google Scholar] [CrossRef]
- Rahman, A.; Hershey, S.; Ahmed, S.; Nibbelink, K.; Simpson, R.U. Heart Extracellular Matrix Gene Expression Profile in the Vitamin D Receptor Knockout Mice. J. Steroid Biochem. Mol. Biol. 2007, 103, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Ferder, M.; Inserra, F.; Manucha, W.; Ferder, L. The World Pandemic of Vitamin D Deficiency Could Possibly Be Explained by Cellular Inflammatory Response Activity Induced by the Renin-Angiotensin System. Am. J. Physiol. Cell Physiol. 2013, 304, C1027–C1039. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, M.; Li, Y.C.; Quiroz, Y.; Bravo, Y.; Seeherunvong, W.; Faul, C.; Weisinger, J.R.; Rodriguez-Iturbe, B. Paricalcitol Downregulates Myocardial Renin-Angiotensin and Fibroblast Growth Factor Expression and Attenuates Cardiac Hypertrophy in Uremic Rats. Am. J. Hypertens. 2014, 27, 720–726. [Google Scholar] [CrossRef][Green Version]
- Luo, Q.; Yan, W.; Nie, Q.; Han, W. Vitamin D and Heart Failure: A Two-Sample Mendelian Randomization Study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2612–2620. [Google Scholar] [CrossRef]
- Tishkoff, D.X.; Nibbelink, K.A.; Holmberg, K.H.; Dandu, L.; Simpson, R.U. Functional Vitamin D Receptor (VDR) in the t-Tubules of Cardiac Myocytes: VDR Knockout Cardiomyocyte Contractility. Endocrinology 2008, 149, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.M.; Hanwell, H.E. “Calcitriol” Is Not Synonymous with “Vitamin D”. Mult. Scler. Int. 2012, 2012, 650462. [Google Scholar] [CrossRef][Green Version]
- Simpson, R.U.; Thomas, G.A.; Arnold, A.J. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985, 260, 8882–8889. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, R.E.; Kim, S.N.; Saunders, D.E.; Simpson, R.U. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am. J. Physiol. 1990, 258, E134–E142. [Google Scholar] [CrossRef]
- Green, J.J.; Robinson, D.A.; Wilson, G.E.; Simpson, R.U.; Westfall, M.V. Calcitriol modulation of cardiac contractile performance via protein kinase C. J. Mol. Cell. Cardiol. 2006, 41, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, R.E.; Simpson, R.U. Vitamin D3 and cardiovascular function in rats. J. Clin. Investig. 1987, 79, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Kong, J.; Chen, S. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E125–E132. [Google Scholar] [CrossRef]

| Previous Health Issues |
|---|
| pregnancy |
| age under 18 years |
| chronic inflammation diseases |
| hematologic diseases |
| liver diseases—AST or ALT > 150 UI/L |
| kidney diseases—GFR < 30 mL/min/1.73 m2 |
| PCI complications |
| hypersensitivity reactions to antiplatelet drugs |
| Parameters | All Patients | |
|---|---|---|
| Median | IQR | |
| Age [years] | 66.5 | 18.29 |
| LVEF % | 49 | 13.25 |
| Initial hs-TnT [μg/L] | 0.11 | 0.51 |
| Hba1c [%] | 5.9 | 0.62 |
| LDL–cholesterol [mg/dL] | 124.5 | 62.75 |
| WBCs [g/L] | 9.98 | 3.9 |
| PLTs [g/L] | 222.5 | 72.25 |
| Hgb [mmol/L] | 9.1 | 1.1 |
| RBCs [t/L] | 4.63 | 0.69 |
| GFR [mL/min/1.73 m2] | 82 | 31 |
| BMI [kg/m2] | 28.01 | 5.64 |
| Vitamin D Status | All Patients | |
|---|---|---|
| Median | IQR | |
| 25-OHD3 [ng/mL] | 15.49 | 8.71 |
| 1.25-OH2D3 [pg/mL] | 45.48 | 11.75 |
| VDBP [ng/mL] | 19.31 | 21.34 |
| Parameters | LVEF ≥ 40% | LVEF < 40% | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Age [years] | 67 | 17.5 | 57 | 13 | 0.76 |
| Initial hs-TnT [μg/L] | 0.114 | 0.501 | 0.213 | 0.837 | 0.34 |
| Hba1c [%] | 5.8 | 0.7 | 5.9 | 0.325 | 0.37 |
| LDLs [mg/dL] | 132 | 66 | 123.5 | 45 | 0.38 |
| WBCs [g/L] | 9.94 | 3.79 | 10.46 | 5.69 | 0.25 |
| PLTs [g/L] | 221 | 87 | 221.5 | 37.5 | 0.87 |
| Hgb [mmol/L] | 9 | 1.1 | 9.3 | 0.775 | 0.79 |
| RBCs [t/L] | 4.59 | 0.73 | 4.82 | 0.41 | 0.68 |
| GFR [mL/min/1.73 m2] | 82 | 33 | 81.5 | 26 | 0.83 |
| BMI [kg/m2] | 28.72 | 5.71 | 27.23 | 4.69 | 0.09 |
| Vitamin D Status | LVEF ≥ 40% | LVEF < 40% | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| 25-OHD3 [ng/mL] | 16.46 | 7.98 | 14.61 | 9.04 | 0.013 |
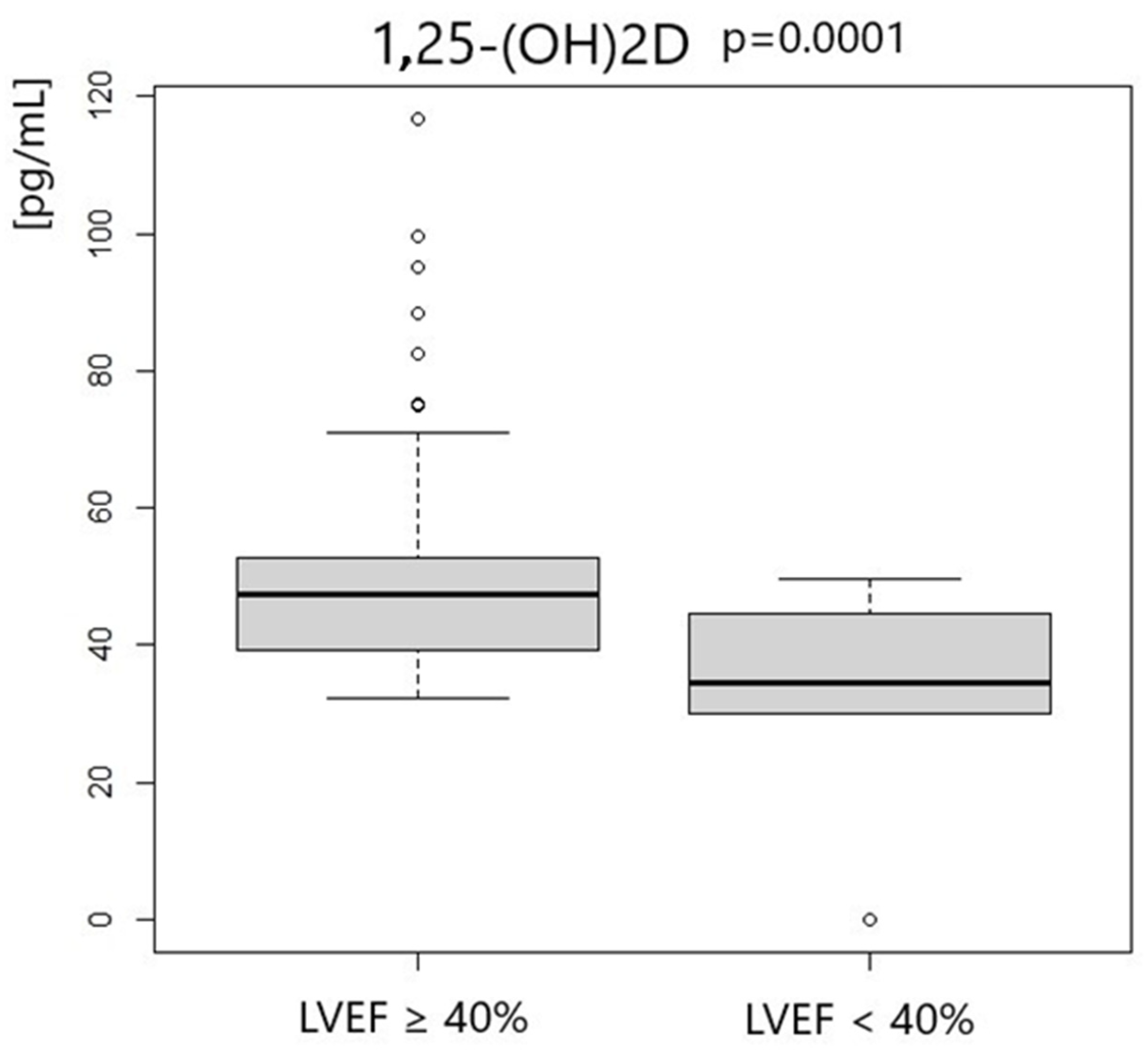
| 1,25-OH2D3 [pg/mL] | 47.34 | 13.35 | 34.65 | 12.41 | 0.0001 |
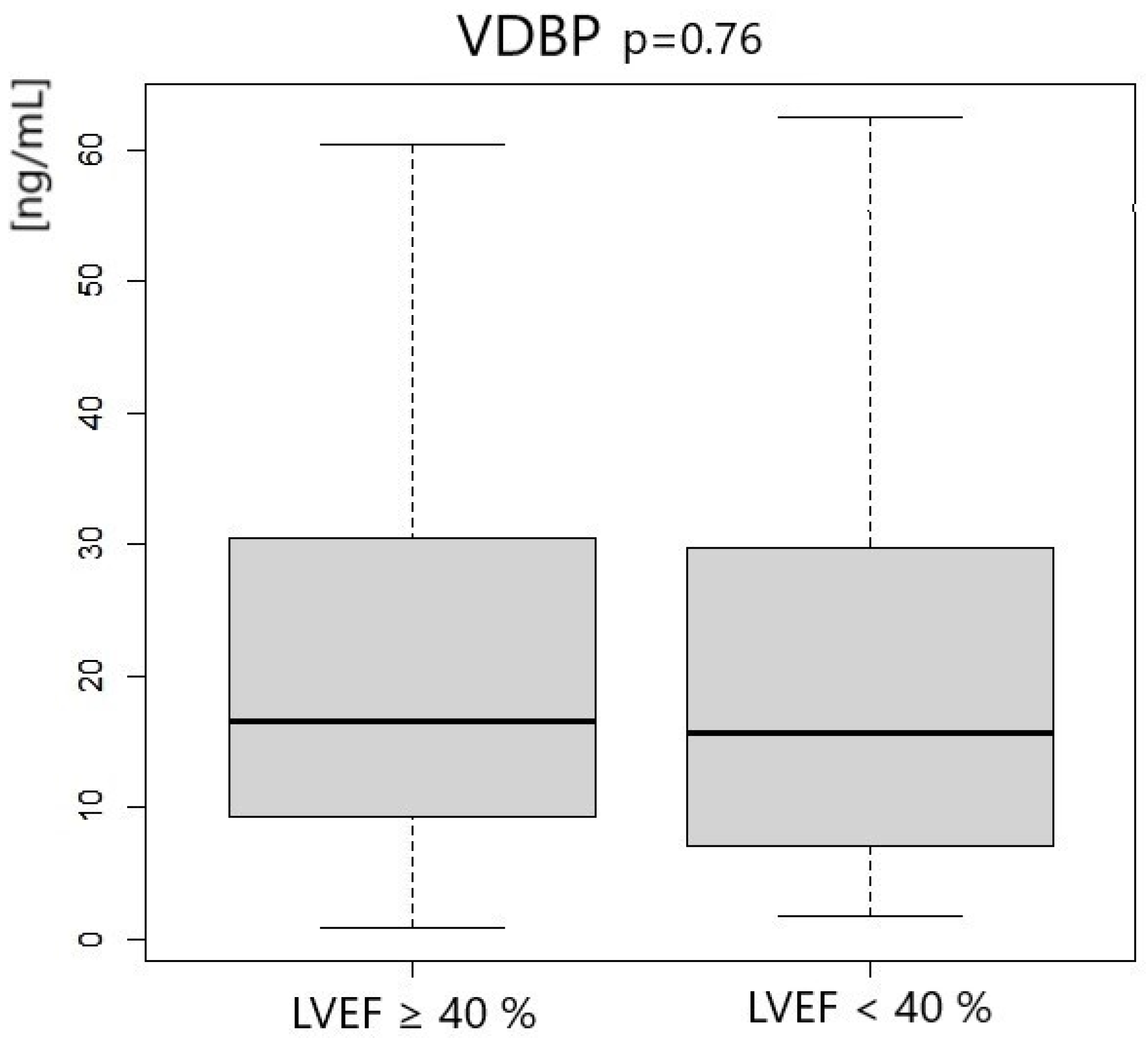
| VDBP [ng/mL] | 16.54 | 21.18 | 15.64 | 22.68 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olędzki, S.; Siennicka, A.; Maciejewska-Markiewicz, D.; Stachowska, E.; Jakubiak, N.; Kiedrowicz, R.; Jakubczyk, K.; Skonieczna-Żydecka, K.; Gutowska, I.; Kaźmierczak, J. Calcitriol Concentration in the Early Phase of Myocardial Infarction and Its Relation to Left Ventricular Ejection Fraction. Metabolites 2024, 14, 686. https://doi.org/10.3390/metabo14120686
Olędzki S, Siennicka A, Maciejewska-Markiewicz D, Stachowska E, Jakubiak N, Kiedrowicz R, Jakubczyk K, Skonieczna-Żydecka K, Gutowska I, Kaźmierczak J. Calcitriol Concentration in the Early Phase of Myocardial Infarction and Its Relation to Left Ventricular Ejection Fraction. Metabolites. 2024; 14(12):686. https://doi.org/10.3390/metabo14120686
Chicago/Turabian StyleOlędzki, Szymon, Aldona Siennicka, Dominika Maciejewska-Markiewicz, Ewa Stachowska, Natalia Jakubiak, Radosław Kiedrowicz, Karolina Jakubczyk, Karolina Skonieczna-Żydecka, Izabela Gutowska, and Jarosław Kaźmierczak. 2024. "Calcitriol Concentration in the Early Phase of Myocardial Infarction and Its Relation to Left Ventricular Ejection Fraction" Metabolites 14, no. 12: 686. https://doi.org/10.3390/metabo14120686
APA StyleOlędzki, S., Siennicka, A., Maciejewska-Markiewicz, D., Stachowska, E., Jakubiak, N., Kiedrowicz, R., Jakubczyk, K., Skonieczna-Żydecka, K., Gutowska, I., & Kaźmierczak, J. (2024). Calcitriol Concentration in the Early Phase of Myocardial Infarction and Its Relation to Left Ventricular Ejection Fraction. Metabolites, 14(12), 686. https://doi.org/10.3390/metabo14120686








