Improving the Characteristics of Fruiting Bodies in Lentinus edodes: The Impact of Rolipram-Induced cAMP Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mycelium Culture
2.2. Cultivation Conditions
2.3. Metabolite Extraction
2.4. LC-MS/MS Analysis
2.5. Bioinformatic Analysis
2.6. Exogenous Substances Treatment
2.7. Determination of cAMP
3. Results
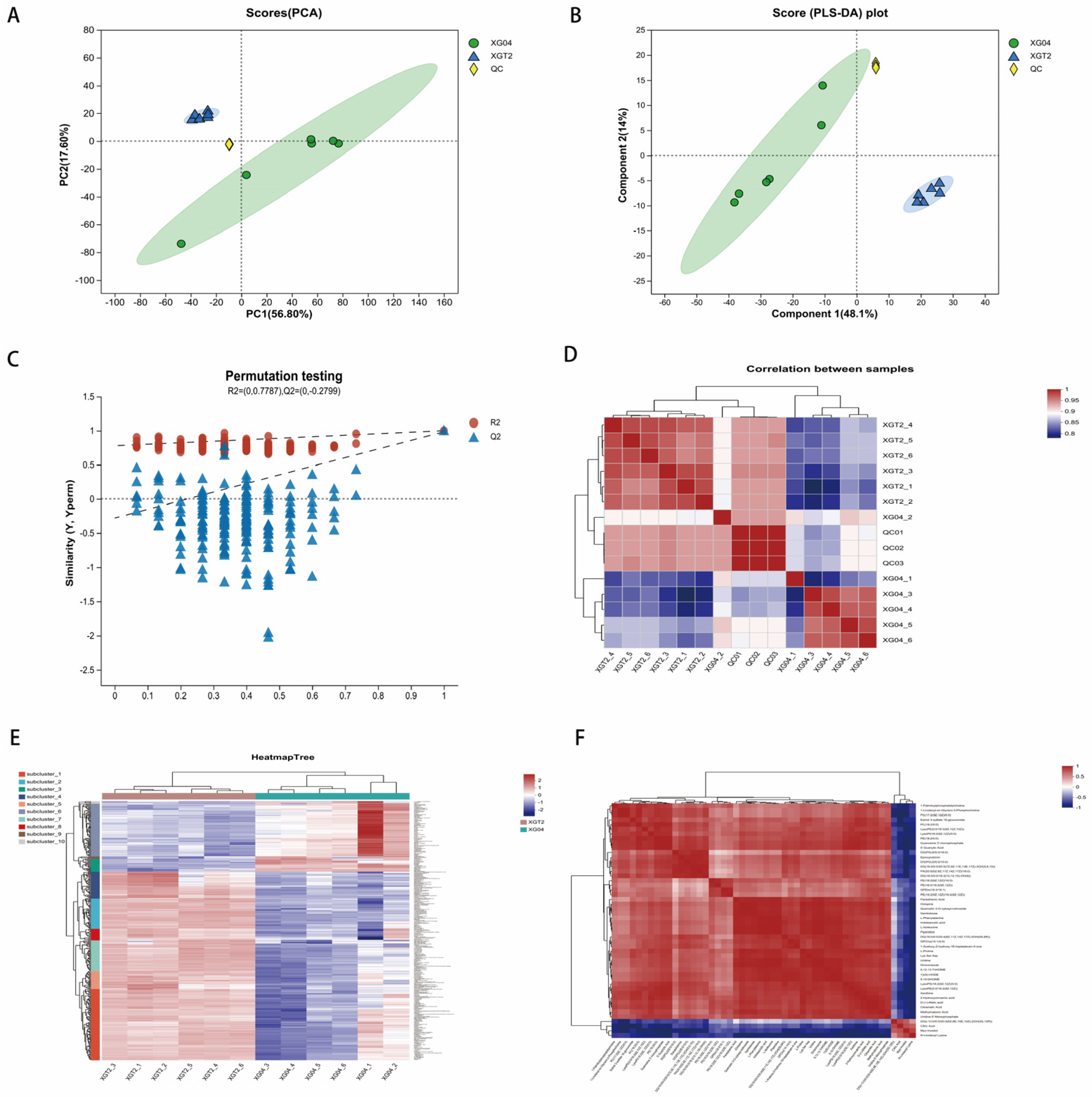
3.1. Sample Confidence and Metabolite Correlation Analysis
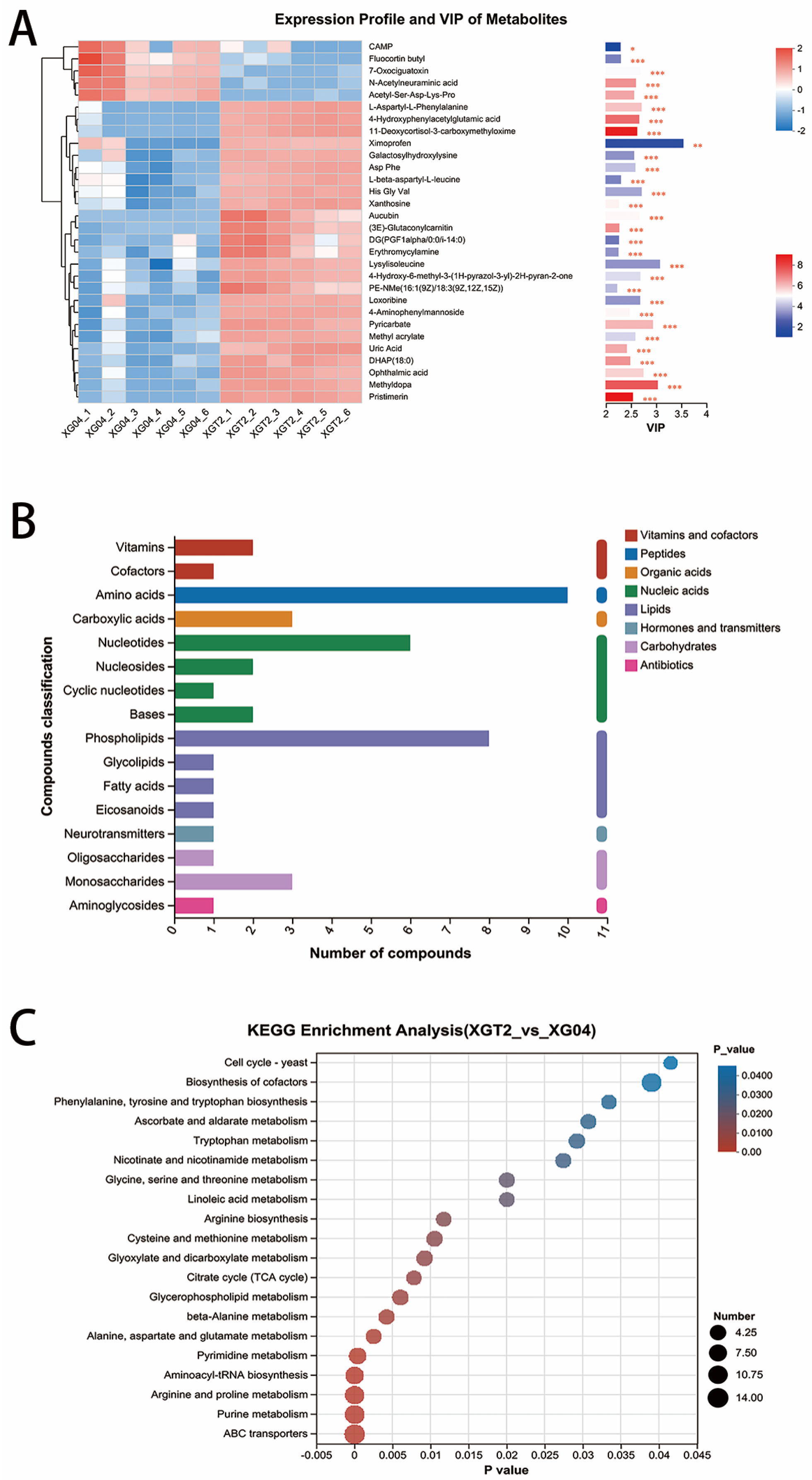
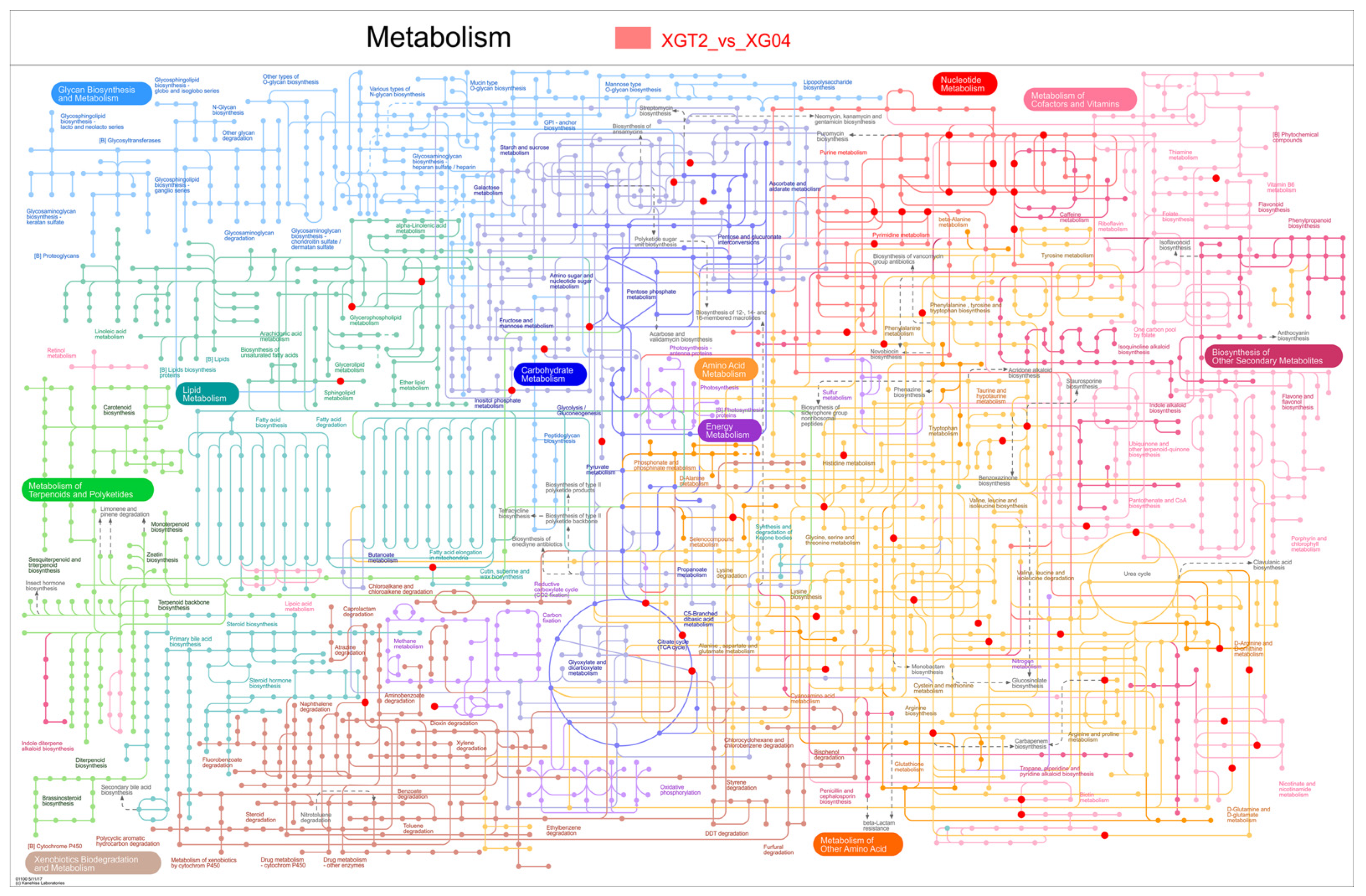
3.2. Metabolome and KEGG Analysis
3.3. Phenotypic Modification by Exogenous Substances
4. Discussion
4.1. cAMP Signaling Pathway and Meiosis Process
4.2. Analyzing Metabolic Pathways and cAMP-Associated Processes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, D.S. An Analysis on the Olomestic and Abroad Markets Prospect of Wild Edible Fungi and the Position of Yunnan Edible Fungi. J. Southwest For. Coll. 2002, 22, 33–38. [Google Scholar]
- Xu, A. Study on Nutritional Value and Characteristics of 18 Commercially Cultivated Edible Fungi. Master’s Thesis, Yangzhou University, Yangzhou, China, 2022. [Google Scholar]
- Miles, P.G.; Chang, S.T. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact; CRC Press: Boca Raton, FL, USA, 2004; p. 451. [Google Scholar]
- China Edible Fungi Association. Analysis of the results of the national Edible fungi Statistical Survey in 2020. China’s Edible Fungus 2022, 41, 85–91. [Google Scholar]
- Xue, J.C.; Zhang, Z.W.; Wei, L.M.; Yu, W.H. Cultivation techniques of a new variety of high-quality Shiitake mushroom, Liaofu No. 4 (0912). Edible Fungi 2015, 37, 44–45. [Google Scholar]
- Zhao, X. Study on High Temperature Stress of Mushroom Based on Multi-Omics Analysis. Ph.D. Thesis, University of Science and Technology of China, Anhui, China, 2019. [Google Scholar]
- Zhan, X.K. Metabolomics of Pure Culture of Boletus Lanmao Based on LC-MS Method. Master’s Thesis, Yunnan University, Kunming, China, 2020. [Google Scholar]
- Tong, M.; Deng, Z.; Yang, M.; Xu, C.; Zhang, X.; Zhang, Q.; Liu, Q. Transcriptomic but not genomic variability confers phenotype of breast cancer stem cells. Cancer Commun. 2018, 38, 56. [Google Scholar] [CrossRef]
- Lv, Z.P.; Peng, Y.Z.; Zhang, B.B.; Fan, H.; Liu, D.; Guo, Y.M. Glucose and lipid metabolism disorders in the chickens with dexamethasone-induced oxidative stress. J. Anim. Physiol. Anim. Nutr. 2018, 102, e706–e717. [Google Scholar] [CrossRef]
- Zhang, X. Identification of Growth and Development and Drought Stress Tolerance of Sesame by cAMP Signaling. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2022. [Google Scholar]
- Perrin, C.M. Growth and Adhesion Properties of Monosodium Urate Monohydrate (MSU) Crystals. Ph.D. Thesis, Georgetown University, Washington, DC, USA, 2011. [Google Scholar]
- Liang, Q.; Wang, C.; Li, B. Metabolomic analysis using liquid chromatography/mass spectrometry for gastric cancer. Appl. Biochem. Biotechnol. 2015, 176, 2170–2184. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, S.; Xie, J.; Li, R. Identification of multiple dysregulated metabolic pathways by GC-MS-based profiling of lung tissue in mice with PM2.5-induced asthma. Chemosphere 2019, 220, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Kouzuma, A.; Watanabe, K. CpdA is involved in amino acid metabolism in Shewanella oneidensis MR-1. Biosci. Biotechnol. Biochem. 2018, 82, 166–172. [Google Scholar] [CrossRef]
- Mitrokhin, V.M.; Kamkina, O.V.; Kamkin, A.G.; Rodina, A.S.; Zolotareva, A.D.; Zolotarev, V.I.; Kazansky, V.E.; Gorbacheva, L.R.; Bilichenko, A.S.; Shileiko, S.A.; et al. Simulated Microgravity and Hypergravity Affect the Expression Level of Soluble Guanylate Cyclase, Adenylate Cyclase, and Phosphodiesterase Genesin Rat Ventricular Cardiomyocytes. Bull. Exp. Biol. Med. 2024, 176, 359–362. [Google Scholar] [CrossRef]
- Takagi, Y.; Katayose, Y.; Shishido, K. Intracellular levels of cyclic AMP and adenylate cyclase activity during mycelial development in fruiting body formation in Lentinus edodes. FEMS Microbiol. Lett. 2010, 55, 275–278. [Google Scholar] [CrossRef][Green Version]
- Murry, R.; Traxler, L.; Pötschner, J.; Krüger, T.; Kniemeyer, O.; Krause, K.; Kothe, E. Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune. J. Fungi 2021, 7, 470. [Google Scholar] [CrossRef]
- Wirth, S.; Kunert, M.; Ahrens, L.M.; Krause, K.; Broska, S.; Paetz, C.; Kniemeyer, O.; Jung, E.M.; Boland, W.; Kothe, E. The regulator of G-protein signalling Thn1 links pheromone response to volatile production in Schizophyllum commune. Environ. Microbiol. 2018, 20, 3684–3699. [Google Scholar] [CrossRef]
- Knabe, N.; Jung, E.M.; Freihorst, D.; Hennicke, F.; Horton, J.S.; Kothe, E. A central role for Ras1 in morphogenesis of the basidiomycete Schizophyllum commune. Eukaryot. Cell 2013, 12, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Chaveroche, M.K.; Shimizu, K.; Keller, N.; d’Enfert, C. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 2002, 44, 1001–1016. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.E.; Horton, J.S. Mushrooms by magic: Making connections between signal transduction and fruiting body development in the basidiomycete fungus Schizophyllum commune. FEMS Microbiol. Lett. 2006, 262, 1–8. [Google Scholar] [CrossRef]
- Kinoshita, H.; Sen, K.; Iwama, H.; Samadder, P.P.; Kurosawa, S.; Shibai, H. Effects of indole and caffeine on cAMP in the ind1 and cfn1 mutant strains of Schizophyllum commune during sexual development. FEMS Microbiol. Lett. 2002, 206, 247–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, J.; Chen, H.; Ma, N.; Chen, L.; Zhou, X.; Zhou, Y.J.; Zhou, Z. cAMP/PKA-induced meiosis arrest in oocytes. Genom. Appl. Biol. 2020, 39, 775–780. [Google Scholar] [CrossRef]
- Liu, L.; Kong, N.; Xia, G.; Zhang, M. Molecular control of oocyte meiotic arrest and resumption. Reprod. Fertil. Dev. 2013, 25, 463–471. [Google Scholar] [CrossRef]
- Rimal, A.; Swayne, T.M.; Kamdar, Z.P.; Tewey, M.A.; Winter, E. Isc10, an inhibitor of the Smk1 MAPK, prevents activation loop autophosphorylation and substrate phosphorylation through separate mechanisms. J. Biol. Chem. 2022, 298, 102450. [Google Scholar] [CrossRef]
- Yamashita, A.; Sakuno, T.; Watanabe, Y.; Yamamoto, M. Synchronous induction of meiosis in the fission yeast Schizosaccharomyces pombe. Cold Spring Harbor. Protoc. 2017, 2017, pdb-prot091777. [Google Scholar] [CrossRef]
- Cai, J.; Ge, W.W. Arrest and recovery of oocyte’s first meiosis. Life Sci. 2019, 31, 921–930. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Miao, Y.L.; Schatten, H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle 2009, 8, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Hord, C.L.; Sun, Y.J.; Pillitteri, L.J.; Torii, K.U.; Wang, H.; Zhang, S.; Ma, H. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases. Mol. Plant 2008, 1, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Kajiura-Kobayashi, H.; Yoshida, N.; Sagata, N.; Yamashita, M.; Nagahama, Y. The Mos/MAPK pathway is involved in metaphase II arrest as a cytostatic factor but is neither necessary nor sufficient for initiating oocyte maturation in goldfish. Dev. Genes Evol. 2000, 210, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Yu, S.F.; Wang, C.L.; Wang, L. cAMP Signalling Pathway in Biocontrol Fungi. Curr. Issues Mol. Biol. 2022, 44, 2622–2634. [Google Scholar] [CrossRef]
- Kayikci, Ö.; Magwene, P.M. Divergent Roles for cAMP-PKA Signaling in the Regulation of Filamentous Growth in Saccharomyces cerevisiae and Saccharomyces bayanus. G3 2018, 8, 3529–3538. [Google Scholar] [CrossRef]
- Kües, U. Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol. Mol. Biol. Rev. 2000, 64, 316–353. [Google Scholar] [CrossRef]
- Straatsma, G.; Sonnenberg, A.S.; Van Griensven, L.J. Development and growth of fruit bodies and crops of the button mushroom, Agaricus bisporus. Fungal Biol. 2013, 117, 697–707. [Google Scholar] [CrossRef]
- Knoll, A.; Puchta, H. The role of DNA helicases and their interaction partners in genome stability and meiotic recombination in plants. J. Exp. Bot. 2011, 62, 1565–1579. [Google Scholar] [CrossRef]
- Haag, J.E.; Wouwer, A.V.; Bogaerts, P. Dynamic modeling of complex biological systems: A link between metabolic and macroscopic description. Math. Biosci. 2005, 193, 25–49. [Google Scholar] [CrossRef]
- Takaine, M.; Ueno, M.; Kitamura, K.; Imamura, H.; Yoshida, S. Reliable imaging of ATP in living budding and fission yeast. J. Cell Sci. 2019, 132, jcs230649. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, F.; Xu, C.; Wang, Y.; Deng, C.; Meng, Q.; Zhu, W. Improving the Characteristics of Fruiting Bodies in Lentinus edodes: The Impact of Rolipram-Induced cAMP Modulation. Metabolites 2024, 14, 619. https://doi.org/10.3390/metabo14110619
Li H, Chen F, Xu C, Wang Y, Deng C, Meng Q, Zhu W. Improving the Characteristics of Fruiting Bodies in Lentinus edodes: The Impact of Rolipram-Induced cAMP Modulation. Metabolites. 2024; 14(11):619. https://doi.org/10.3390/metabo14110619
Chicago/Turabian StyleLi, Hongman, Fei Chen, Chong Xu, Yanhua Wang, Chunhai Deng, Qingguo Meng, and Weiwei Zhu. 2024. "Improving the Characteristics of Fruiting Bodies in Lentinus edodes: The Impact of Rolipram-Induced cAMP Modulation" Metabolites 14, no. 11: 619. https://doi.org/10.3390/metabo14110619
APA StyleLi, H., Chen, F., Xu, C., Wang, Y., Deng, C., Meng, Q., & Zhu, W. (2024). Improving the Characteristics of Fruiting Bodies in Lentinus edodes: The Impact of Rolipram-Induced cAMP Modulation. Metabolites, 14(11), 619. https://doi.org/10.3390/metabo14110619





