Maternal Dietary Deficiencies in Folic Acid and Choline Change Metabolites Levels in Offspring after Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
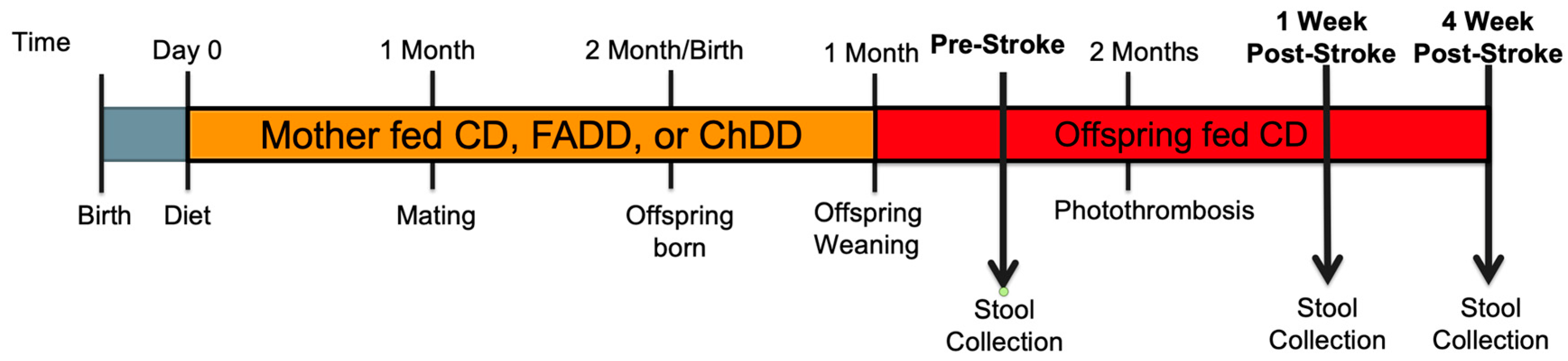
2.1. Animals and Experimental Design
2.2. Photothrombosis Model for Ischemic Stroke
2.3. Fecal Preparation
2.4. Solutions for Experiments
2.5. Untargeted LC-MS Metabolomics
2.6. Data Preprocessing
2.7. Multivariate Analysis
2.8. Pathway and Enrichment Analysis
2.9. Visualization
3. Results
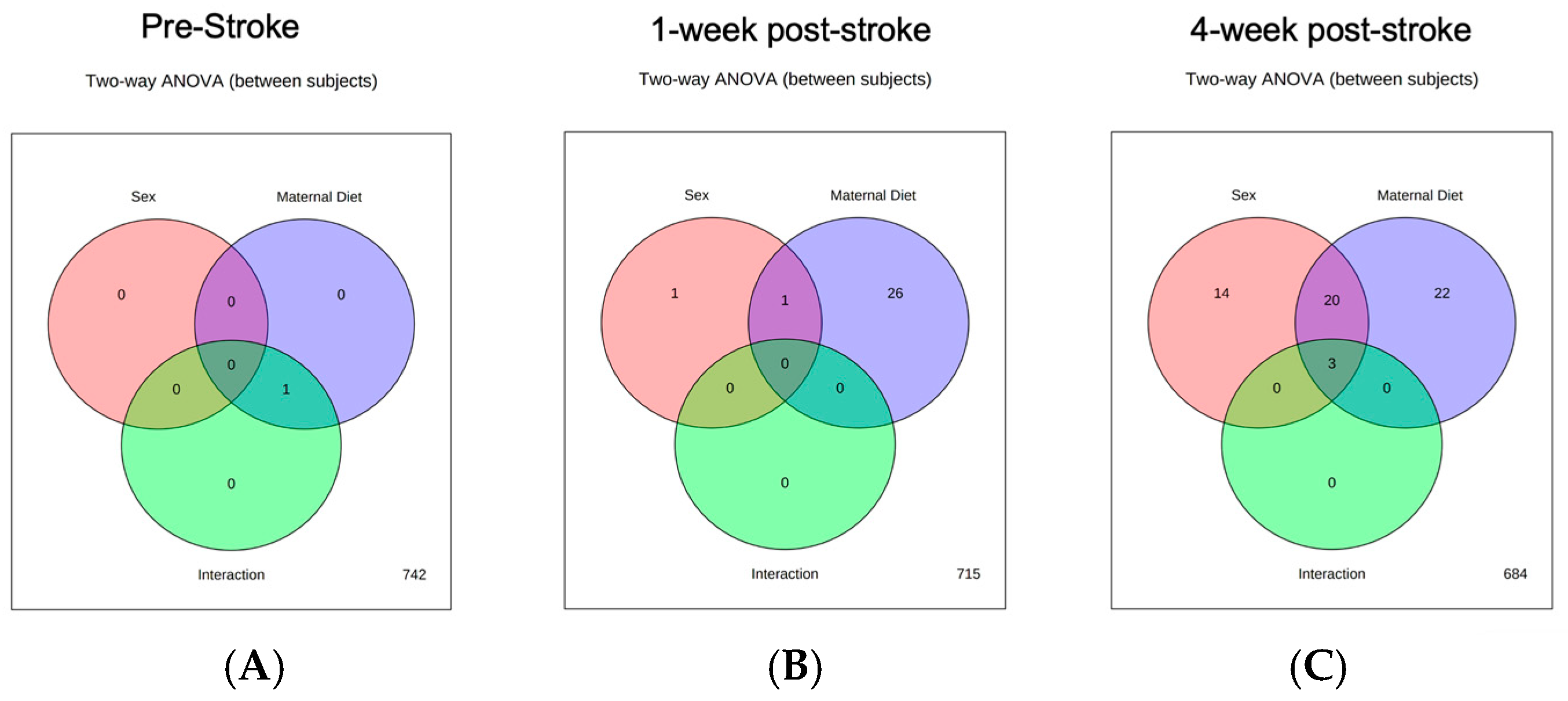
3.1. Pre-Stroke
3.2. One-Week Post-Stroke
3.2.1. Interactions between Maternal Diet and Sex
3.2.2. Sex Differences
3.2.3. Impact of Maternal Diet
3.3. Four-Week Post-Stroke
3.3.1. Interactions between Maternal Diet and Sex
3.3.2. Sex Differences
3.3.3. Impact of Maternal Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2015, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtman, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the Future of Stroke in the United States: A Policy Statement from the American Heart Association and American Stroke Association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and Regional Burden of Stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; George, M.G.; Gillespie, C.; Merritt, R. Trends in Hospitalizations and Cost Associated with Stroke by Age, United States 2003–2012. Int. J. Stroke 2016, 11, 874–881. [Google Scholar] [CrossRef]
- Smajlović, D. Strokes in Young Adults: Epidemiology and Prevention. Vasc. Health Risk Manag. 2015, 11, 157–164. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; de Ferranti, S.D.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Spence, J.D.; Yi, Q.; Hankey, G.J. B Vitamins in Stroke Prevention: Time to Reconsider. Lancet Neurol. 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Hankey, G.J. Nutrition and the Risk of Stroke. Lancet Neurol. 2012, 11, 66–81. [Google Scholar] [CrossRef]
- Kolb, B.; Whishaw, I.Q.; Teskey, G.C. An Introduction to Brain and Behavior, 5th ed.; Worth Publishers: New York, NY, USA, 2016; ISBN 9780716776918. [Google Scholar]
- Pannia, E.; Cho, C.E.; Kubant, R.; Sánchez-Hernández, D.; Huot, P.S.P.; Harvey Anderson, G. Role of Maternal Vitamins in Programming Health and Chronic Disease. Nutr. Rev. 2016, 74, 166–180. [Google Scholar] [CrossRef]
- Carolan-Olah, M.; Duarte-Gardea, M.; Lechuga, J. A Critical Review: Early Life Nutrition and Prenatal Programming for Adult Disease. J. Clin. Nurs. 2015, 24, 3716–3729. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; McDowell, M.A.; Yetley, E.A.; Sempos, C.A.; Burt, V.L.; Radimer, K.L.; Picciano, M.F. Total Folate and Folic Acid Intake from Foods and Dietary Supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 2010, 91, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hamner, H.C.; Cogswell, M.E.; Johnson, M.A. Acculturation Factors Are Associated with Folate Intakes among Mexican American Women. J. Nutr. 2011, 141, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Batres-Marquez, S.; Carriquiry, A.; Schalinske, K. Choline in the Diets of the US Population: NHANES, 2003–2004. In Experimental Biology 2007 (Part II); FASEB: Rockville, MD, USA, 2007. [Google Scholar]
- Zeisel, S.H. Nutrition in Pregnancy: The Argument for Including a Source of Choline. Int. J. Women’s Health 2013, 5, 193–199. [Google Scholar] [CrossRef]
- Gernand, A.D.; Schulze, K.J.; Stewart, C.P.; West, K.P.; Christian, P.; Christian, P. Micronutrient Deficiencies in Pregnancy Worldwide: Health Effects and Prevention. Nat. Rev. Endocrinol. 2016, 12, 274–289. [Google Scholar] [CrossRef]
- Di Meco, A.; Praticò, D. Early-life Exposure to High-fat Diet Influences Brain Health in Aging Mice. Aging Cell 2019, 18, e13040. [Google Scholar] [CrossRef]
- Gaillard, R. Maternal Obesity during Pregnancy and Cardiovascular Development and Disease in the Offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef]
- Yessoufou, A.; Moutairou, K. Maternal Diabetes in Pregnancy: Early and Long-Term Outcomes on the Offspring and the Concept of “ Metabolic Memory”. J. Diabetes Res. 2011, 2011, 218598. [Google Scholar] [CrossRef]
- Palinski, W. Effect of Maternal Cardiovascular Conditions and Risk Factors on Offspring Cardiovascular Disease. Circulation 2014, 129, 2066–2077. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, X.Y.; Zhou, Y.L.; Shao, B.; Niu, X.T.; Zhang, W.L.; Lin, Y.S. Maternal High-Fat Diet Programs Cerebrovascular Remodeling in Adult Rat Offspring. J. Cereb. Blood Flow. Metab. 2018, 38, 1954–1967. [Google Scholar] [CrossRef]
- Durrant, L.M.; Khorram, O.; Buchholz, J.N.; Pearce, W.J. Maternal Food Restriction Modulates Cerebrovascular Structure and Contractility in Adult Rat Offspring: Effects of Metyrapone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R401–R410. [Google Scholar] [CrossRef][Green Version]
- Campbell, M.E.; Williams, S.J.; Veerareddy, S.; Davidge, S.T. Maternal Nutrient Restriction Reduces Carotid Artery Constriction without Increasing Nitric Oxide Synthesis in the Late Gestation Rat Fetus. Pediatr. Res. 2005, 58, 840–844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clementson, M.; Hurley, L.; Coonrod, S.; Bennett, C.; Marella, P.; Pascual, A.S.; Pull, K.; Wasek, B.; Bottiglieri, T.; Malysheva, O.; et al. Maternal Dietary Deficiencies in Folic Acid or Choline Worsen Stroke Outcomes in Adult Male and Female Mouse Offspring. Neural Regen. Res. 2023, 18, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.; Jauhal, J.; Ille, S.; Pull, K.; Malysheva, O.V.; Jadavji, N.M. Maternal Dietary Deficiencies in Folic Acid and Choline Result in Larger Damage Volume, Reduced Neuro-Degeneration and -Inflammation and Changes in Choline Metabolites after Ischemic Stroke in Middle-Aged Offspring. Nutrients 2023, 15, 1556. [Google Scholar] [CrossRef]
- Pull, K.; Folk, R.; Kang, J.; Jackson, S.; Gusek, B.; Esfandiarei, M.; Jadavji, N.M. Impact of Maternal Dietary Folic Acid or Choline Dietary Deficiencies on Vascular Function in Young and Middle-Aged Female Mouse Offspring after Ischemic Stroke. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H1354–H1359. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, C.; Peng, S.; Zhu, X.; Zhang, Z.; Zhao, Y.; Zhang, J.; Zhao, G.; Zhang, T.; Heng, X.; et al. Pivotal Interplays between Fecal Metabolome and Gut Microbiome Reveal Functional Signatures in Cerebral Ischemic Stroke. J. Transl. Med. 2022, 20, 459. [Google Scholar] [CrossRef]
- Chi, N.-F.; Chang, T.-H.; Lee, C.-Y.; Wu, Y.-W.; Shen, T.-A.; Chan, L.; Chen, Y.-R.; Chiou, H.-Y.; Hsu, C.Y.; Hu, C.-J. Untargeted Metabolomics Predicts the Functional Outcome of Ischemic Stroke. J. Formos. Med. Assoc. 2021, 120, 234–241. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Z.; Cajka, T.; Buffa, J.A.; Nemet, I.; Hurd, A.G.; Gu, X.; Skye, S.M.; Roberts, A.B.; Wu, Y.; et al. Untargeted Metabolomics Identifies Trimethyllysine, a TMAO-Producing Nutrient Precursor, as a Predictor of Incident Cardiovascular Disease Risk. JCI Insight 2018, 3, e99096. [Google Scholar] [CrossRef]
- Wu, M.-H.; Chang, C.-T.; Lin, Y.-N.; Chen, C.-J. Identification of a Potential Prognostic Plasma Biomarker of Acute Ischemic Stroke via Untargeted LC-MS Metabolomics. Proteomics Clin. Appl. 2023, 17, 2200081. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Deng, L.; Malysheva, O.V.; Caudill, M.A.; Rozen, R. MTHFR Deficiency or Reduced Intake of Folate or Choline in Pregnant Mice Results in Impaired Short-Term Memory and Increased Apoptosis in the Hippocampus of Wild-Type Offspring. Neuroscience 2015, 300, 1–9. [Google Scholar] [CrossRef]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of Reproducible Brain Infarction by Photochemically Initiated Thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef]
- Carmichael, S.T. Rodent Models of Focal Stroke: Size, Mechanism, and Purpose. NeuroRx 2005, 2, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Jadavji, N.M.; Emmerson, J.; Willmore, W.G.; MacFarlane, A.J.; Smith, P. B-Vitamin and Choline Supplementation Increases Neuroplasticity and Recovery after Stroke. Neurobiol. Dis. 2017, 103, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Kim, J.-E.; Sivula, M.; Strittmatter, S.M. Nogo Receptor Antagonism Promotes Stroke Recovery by Enhancing Axonal Plasticity. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6209–6217. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.M.; Romero, A.S.; Merkley, S.D.; Meyer-Hagen, J.L.; Forbes, C.; Hayek, E.E.; Sciezka, D.P.; Templeton, R.; Gonzalez-Estrella, J.; Jin, Y.; et al. In Vivo Tissue Distribution of Polystyrene or Mixed Polymer Microspheres and Metabolomic Analysis after Oral Exposure in Mice. Environ. Health Perspect. 2024, 132, 47005. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhang, P.; Zhu, J.; Raftery, D. Globally Optimized Targeted Mass Spectrometry: Reliable Metabolomics Analysis with Broad Coverage. Anal. Chem. 2015, 87, 12355–12362. [Google Scholar] [CrossRef] [PubMed]
- Scieszka, D.; Jin, Y.; Noor, S.; Barr, E.; Garcia, M.; Begay, J.; Herbert, G.; Hunter, R.P.; Bhaskar, K.; Kumar, R.; et al. Biomass Smoke Inhalation Promotes Neuroinflammatory and Metabolomic Temporal Changes in the Hippocampus of Female Mice. J. Neuroinflamm. 2023, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Scieszka, D.P.; Garland, D.; Hunter, R.; Herbert, G.; Lucas, S.; Jin, Y.; Gu, H.; Campen, M.J.; Cannon, J.L. Multi-Omic Assessment Shows Dysregulation of Pulmonary and Systemic Immunity to e-Cigarette Exposure. Respir. Res. 2023, 24, 138. [Google Scholar] [CrossRef]
- Wei, Y.; Jasbi, P.; Shi, X.; Turner, C.; Hrovat, J.; Liu, L.; Rabena, Y.; Porter, P.; Gu, H. Early Breast Cancer Detection Using Untargeted and Targeted Metabolomics. J. Proteome Res. 2021, 20, 3124–3133. [Google Scholar] [CrossRef]
- Clementson, M.; Hurley, L.; Coonrod, S.; Bennett, C.; Marella, P.; Pascual, A.S.; Pull, K.; Wasek, B.; Bottiglieri, T.; Malysheva, O.; et al. Maternal Dietary Deficiencies in Folates or Choline during Pregnancy and Lactation Worsen Stroke Outcome in 3-Month-Old Male and Female Mouse Offspring. bioRxiv 2022, 2022-09. [Google Scholar] [CrossRef]
- Wetzel, C.H.; Vedder, H.; Holsboer, F.; Zieglgänsberger, W.; Deisz, R.A. Bidirectional Effects of the Neuroactive Steroid Tetrahydrodeoxycorticosterone on GABA-Activated Cl-Currents in Cultured Rat Hypothalamic Neurons. Br. J. Pharmacol. 1999, 127, 863–868. [Google Scholar] [CrossRef]
- Mediratta, P.K.; Gambhir, M.; Sharma, K.K.; Ray, M. Antinociceptive Activity of a Neurosteroid Tetrahydrodeoxycorticosterone (5alpha-Pregnan-3alpha-21-Diol-20-One) and Its Possible Mechanism(s) of Action. Indian. J. Exp. Biol. 2001, 39, 1299–1301. [Google Scholar] [PubMed]
- Márk, L.; Paragh, G. Change in the cholesterol metabolism associated with the combined inhibition of synthesis and absorption. Orv. Hetil. 2007, 148, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Womack, M.D.; Pyner, S.; Barrett-Jolley, R. Inhibition by Alpha-Tetrahydrodeoxycorticosterone (THDOC) of Pre-Sympathetic Parvocellular Neurones in the Paraventricular Nucleus of Rat Hypothalamus. Br. J. Pharmacol. 2006, 149, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Patchev, V.K.; Montkowski, A.; Rouskova, D.; Koranyi, L.; Holsboer, F.; Almeida, O.F. Neonatal Treatment of Rats with the Neuroactive Steroid Tetrahydrodeoxycorticosterone (THDOC) Abolishes the Behavioral and Neuroendocrine Consequences of Adverse Early Life Events. J. Clin. Invest. 1997, 99, 962–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rupprecht, R. The Neuropsychopharmacological Potential of Neuroactive Steroids. J. Psychiatr. Res. 1997, 31, 297–314. [Google Scholar] [CrossRef]
- Kang, X.-C.; Chen, T.; Zhou, J.-L.; Shen, P.-Y.; Dai, S.-H.; Gao, C.-Q.; Zhang, J.-Y.; Xiong, X.-Y.; Liu, D.-B. Phytosterols in Hull-Less Pumpkin Seed Oil, Rich in ∆7-Phytosterols, Ameliorate Benign Prostatic Hyperplasia by Lowing 5α-Reductase and Regulating Balance between Cell Proliferation and Apoptosis in Rats. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Piette, P.C.M. The Pharmacodynamics and Safety of Progesterone. Best. Pract. Res. Clin. Obs. Gynaecol. 2020, 69, 13–29. [Google Scholar] [CrossRef]
- Huang, S.S.; Liu, I.-H.; Chen, C.-L.; Chang, J.-M.; Johnson, F.E.; Huang, J.S. 7-Dehydrocholesterol (7-DHC), But Not Cholesterol, Causes Suppression of Canonical TGF-β Signaling and Is Likely Involved in the Development of Atherosclerotic Cardiovascular Disease (ASCVD). J. Cell Biochem. 2017, 118, 1387–1400. [Google Scholar] [CrossRef]
- Waterham, H.R.; Wanders, R.J. Biochemical and Genetic Aspects of 7-Dehydrocholesterol Reductase and Smith-Lemli-Opitz Syndrome. Biochim. Biophys. Acta 2000, 1529, 340–356. [Google Scholar] [CrossRef]
- Xiao, J.; Li, W.; Zheng, X.; Qi, L.; Wang, H.; Zhang, C.; Wan, X.; Zheng, Y.; Zhong, R.; Zhou, X.; et al. Targeting 7-Dehydrocholesterol Reductase Integrates Cholesterol Metabolism and IRF3 Activation to Eliminate Infection. Immunity 2020, 52, 109–122.e6. [Google Scholar] [CrossRef]
- Honda, M.; Tint, G.S.; Honda, A.; Nguyen, L.B.; Chen, T.S.; Shefer, S. 7-Dehydrocholesterol down-Regulates Cholesterol Biosynthesis in Cultured Smith-Lemli-Opitz Syndrome Skin Fibroblasts. J. Lipid Res. 1998, 39, 647–657. [Google Scholar] [CrossRef] [PubMed]
- López-Dyck, E.; Andrade-Urzúa, F.; Elizalde, A.; Ferrer-Villada, T.; Dagnino-Acosta, A.; Huerta, M.; Osuna-Calleros, Z.; Rangel-Sandoval, C.; Sánchez-Pastor, E. ACPA and JWH-133 Modulate the Vascular Tone of Superior Mesenteric Arteries through Cannabinoid Receptors, BKCa Channels, and Nitric Oxide Dependent Mechanisms. Pharmacol. Rep. 2017, 69, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Onat, T.; Demir Caltekin, M.; Doğanyigit, Z.; Turkler, C.; Kaymak, E.; Kara, M.; Serdar Yalvac, E. Activation of Cannabinoid 2 Receptors by JWH-133 Protects against Ovarian Ischemia-Reperfusion Injury in Rats. Biotech. Histochem. 2021, 96, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, J.; Krzemień, W.; Zawilska, J.B. JWH-133, a Selective Cannabinoid CB₂ Receptor Agonist, Exerts Toxic Effects on Neuroblastoma SH-SY5Y Cells. J. Mol. Neurosci. 2016, 58, 441–445. [Google Scholar] [CrossRef]
- Aydogan Kirmizi, D.; Baser, E.; Doganyigit, Z. The Activation of Cannabinoid Type-2 Receptor with JWH-133 Protects Uterine Ischemia/Reperfusion-Induced Damage. Pharmacology 2021, 106, 106–113. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R Agonist JWH-133 Attenuates Chronic Inflammation by Restraining M1 Macrophage Polarization via Nrf2/HO-1 Pathway in Diet-Induced Obese Mice. Life Sci. 2020, 260, 118424. [Google Scholar] [CrossRef]
- Zanatta, Â.; Rodrigues, M.D.N.; Amaral, A.U.; Souza, D.G.; Quincozes-Santos, A.; Wajner, M. Ornithine and Homocitrulline Impair Mitochondrial Function, Decrease Antioxidant Defenses and Induce Cell Death in Menadione-Stressed Rat Cortical Astrocytes: Potential Mechanisms of Neurological Dysfunction in HHH Syndrome. Neurochem. Res. 2016, 41, 2190–2198. [Google Scholar] [CrossRef]
- Sumien, N.; Shetty, R.A.; Gonzales, E.B. Creatine, Creatine Kinase, and Aging. Subcell. Biochem. 2018, 90, 145–168. [Google Scholar] [CrossRef]
- Prokopová, I. Noradrenaline and behavior. Cesk Fysiol. 2010, 59, 51–58. [Google Scholar]
- Harper, M.S.; Amanda Shen, Z.; Barnett, J.F.; Krsmanovic, L.; Myhre, A.; Delaney, B. N-Acetyl-Glutamic Acid: Evaluation of Acute and 28-Day Repeated Dose Oral Toxicity and Genotoxicity. Food Chem. Toxicol. 2009, 47, 2723–2729. [Google Scholar] [CrossRef]
- Caldovic, L.; Ah Mew, N.; Shi, D.; Morizono, H.; Yudkoff, M.; Tuchman, M. N-Acetylglutamate Synthase: Structure, Function and Defects. Mol. Genet. Metab. 2010, 100 (Suppl. S1), S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, Z.; Chai, G.; Xiong, Y.; Li, B.; Zhang, H.; Xin, R.; Qian, X.; Tang, Z.; Wu, J.; et al. Dehydrocostus Lactone Suppresses LPS-Induced Acute Lung Injury and Macrophage Activation through NF-κB Signaling Pathway Mediated by P38 MAPK and Akt. Molecules 2019, 24, 1510. [Google Scholar] [CrossRef] [PubMed]
- Tharappel, A.M.; Cheng, Y.; Holmes, E.H.; Ostrander, G.K.; Tang, H. Castanospermine Reduces Zika Virus Infection-Associated Seizure by Inhibiting Both the Viral Load and Inflammation in Mouse Models. Antivir. Res. 2020, 183, 104935. [Google Scholar] [CrossRef] [PubMed]
- Saul, R.; Chambers, J.P.; Molyneux, R.J.; Elbein, A.D. Castanospermine, a Tetrahydroxylated Alkaloid That Inhibits Beta-Glucosidase and Beta-Glucocerebrosidase. Arch. Biochem. Biophys. 1983, 221, 593–597. [Google Scholar] [CrossRef]
- Paik, M.-J.; Lee, S.; Cho, K.-H.; Kim, K.-R. Urinary Polyamines and N-Acetylated Polyamines in Four Patients with Alzheimer’s Disease as Their N-Ethoxycarbonyl-N-Pentafluoropropionyl Derivatives by Gas Chromatography-Mass Spectrometry in Selected Ion Monitoring Mode. Anal. Chim. Acta 2006, 576, 55–60. [Google Scholar] [CrossRef]
- Biagioli, M.; Carino, A.; Cipriani, S.; Francisci, D.; Marchianò, S.; Scarpelli, P.; Sorcini, D.; Zampella, A.; Fiorucci, S. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J. Immunol. 2017, 199, 718–733. [Google Scholar] [CrossRef]
- Shikata, K.; Niiro, H.; Azuma, H.; Ogino, K.; Tachibana, T. Apoptotic Activities of C2-Ceramide and C2-Dihydroceramide Homologues against HL-60 Cells. Bioorg. Med. Chem. 2003, 11, 2723–2728. [Google Scholar] [CrossRef]
- Pedroso, J.A.B.; Zampieri, T.T.; Donato, J. Reviewing the Effects of L-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients 2015, 7, 3914–3937. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxid. Med. Cell Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Junaid, M. a Folic Acid Supplementation in Pregnancy and Implications in Health and Disease. J. Biomed. Sci. 2014, 21, 77. [Google Scholar] [CrossRef]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.; Uher, R.; Reichenberg, A.; Sandin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods before and during Pregnancy with the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Julvez, J.; Fortuny, J.; Mendez, M.; Torrent, M.; Ribas-Fitó, N.; Sunyer, J. Maternal Use of Folic Acid Supplements during Pregnancy and Four-Year-Old Neurodevelopment in a Population-Based Birth Cohort. Paediatr. Perinat. Epidemiol. 2009, 23, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an Essential Nutrient for Humans. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1991, 5, 2093–2098. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef]
| Metabolite | Sex of Mice | CD | FADD | ChDD | p-Values Interaction Sex Maternal Diet |
|---|---|---|---|---|---|
| HEPES | Females | 5.56 ± 0.04 | 5.29 ± 0.02 | 6.41 ± 0.22 | 0.01 |
| Males | 5.68 ± 0.20 | 5.39 ± 0.04 | 5.41 ± 0.03 | 0.994 | |
| 0.00052 |
| Metabolite | Sex of Mice | CD | FADD | ChDD | p-Values Interaction Sex Maternal Diet |
|---|---|---|---|---|---|
| PALDA | Female | 5.80 ± 0.193 | 4.32 ± 0.07 | 5.41 ± 0.25 | 0.0213 |
| Male | 5.04 ± 0.27 | 4.65 ± 0.19 | 4.69 ± 0.14 | 0.0307 | |
| 0.0006 | |||||
| N6-(2-1Himidazol-4-yl)ethyl)lysine | Females | 5.05 ± 0.03 | 4.84 ± 0.03 | 5.04 ± 0.03 | n.s. |
| Males | 4.97 ± 0.02 | 4.83 ± 0.02 | 4.97 ± 0.03 | 0.0221 | |
| 0.0001 | |||||
| 3-Oxopalmitic Acid | Female | 4.87 ± 0.01 | 4.79 ± 0.08 | 4.86 ± 0.04 | 0.001 |
| Male | 5.56 ± 0.13 | 5.15 ± 0.11 | 5.01 ± 0.14 | n.s. | |
| 0.017 | |||||
| 7-Dehydrocholesterol | Female | 6.27 ± 0.04 | 4.464 ± 0.01 | 6.087 ± 0.10 | 0.0001 |
| Male | 5.35 ± 0.19 | 5.352 ± 0.19 | 5.459 ± 0.13 | n.n. | |
| 0.0001 | |||||
| Yakkasterone | Female | 6.05 ± 0.16 | 4.89 ± 0.26 | 6.097 ± 0.10 | n.s. |
| Male | 6.21 ± 0.35 | 5.01 ± 0.13 | 6.23 ± 0.10 | n.s. | |
| 0.0001 | |||||
| Tetrahydrodeoxycorticosterone | Female | 6.49 ± 0.38 | 6.58 ± 0.33 | 4.68 ± 0.16 | 0.001 |
| Male | 6.26 ± 0.08 | 5.53 ± 0.08 | 6.45 ± 0.06 | n.s. | |
| 0.013 | |||||
| Avenasterol | Female | 6.36 ± 0.11 | 4.64 ± 0.04 | 6.08 ± 0.10 | 0.011 |
| Male | 5.86 ± 0.28 | 5.50 ± 0.30 | 6.17 ± 0.09 | n.s. | |
| n.s. | |||||
| 4betamethylzymosterol-4-carbaldehyde | Female | 6.52 ± 0.06 | 5.01 ± 0.04 | 6.44 ± 0.12 | n.s. |
| Male | 6.16 ± 0.36 | 5.69 ± 0.26 | 6.59 + 0.12 | n.s. | |
| 0.001 | |||||
| 4a,10-Dihydroxy-2,2,6a,6b,9,9,12a-heptamethyl-2,3,4,4a,6,6a,6b | Female | 6.71 ± 0.09 | 5.12 ± 0.20 | 6.713 ± 0.13 | n.s. |
| Male | 6.38 ± 0.43 | 5.88 ± 0.40 | 6.907 ± 0.07 | n.s. | |
| 0.004 | |||||
| 3-dehydro-6-deoxoteasterone | Female | 6.14 ± 0.16 | 4.87 ± 0.09 | 5.83 ± 0.15 | n.s. |
| Male | 5.96 ± 0.21 | 5.48 ± 0.26 | 6.01 ± 0.03 | n.s. | |
| 0.0001 | |||||
| 2-Aminoethyl(2R)-3-[(1Z)-1-h(14Z)-14-Tricosen-10-one | Female | 6.18 ± 0.19 | 4.89 ± 0.11 | 5.74 ± 0.30 | n.s. |
| Male | 5.71 ± 0.21 | 5.16 ± 0.13 | 5.96 ± 0.081 | n.s. | |
| 0.0001 | |||||
| 1-Piperidinocyclohexanecarbonitrile | Female | 6.41 ± 0.18 | 5.75 ± 0.12 | 6.69 ± 0.14 | n.s. |
| Male | 6.28 ± 0.15 | 5.70 ± 0.06 | 6.52 ± 0.11 | n.s. | |
| 0.0001 | |||||
| 1-(3,5-Dihydroxyphenyl)-2-heptadecanone | Female | 6.13 ± 0.07 | 4.66 ± 0.07 | 6.00 ± 0.13 | n.s. |
| Male | 6.23 ± 0.40 | 4.82 ± 0.07 | 6.30 ± 0.14 | n.s. | |
| 0.0001 | |||||
| (R)-palmiticmonoisopropanolamide | Female | 6.46 ± 0.09 | 3.81 ± 0.03 | 6.10 ± 0.31 | 0.05 |
| Male | 5.78 ± 0.48 | 5.15 ± 0.61 | 6.49 ± 0.12 | n.s. | |
| 0.0002 | |||||
| (22S)-22-hydroxycampest-4-en-3-one | Female | 6.81 ± 0.16 | 4.73 ± 0.02 | 6.53 ± 0.18 | 0.0099 |
| Male | 6.23 ± 0.37 | 6.00 ± 0.41 | 6.40 ± 0.21 | n.s. | |
| 0.006 | |||||
| (14Z)-14-Tricosen-10-one | Female | 6.81 ± 0.16 | 4.84 ± 0.04 | 6.44 ± 0.39 | 0.0004 |
| Male | 6.24 ± 0.36 | 6.58 ± 0.12 | 6.02 ± 0.21 | n.s. | |
| 0.0208 | |||||
| (3S,5E,7E,24R)-(6,19,19-~2~H_3_)-9,10-Secocholesta-5,7,10-trie | Female | 5.92 ± 0.09 | 4.35 ± 0.02 | 5.98 ± 0.09 | n.s. |
| Male | 5.78 ± 0.32 | 5.01 ± 0.26 | 5.93 ± 1.00 | n.s. | |
| 0.0001 |
| Metabolite | Sex of Mice | CD | FADD | ChDD | p-Values Interaction Sex Maternal Diet |
|---|---|---|---|---|---|
| HEPES | Females | 5.27 ± 0.03 | 5.41 ± 0.02 | 5.50 ± 0.06 | 0.032 |
| Males | 5.39 ± 0.03 | 5.44 ± 0.04 | 5.42 ± 0.03 | n.s. | |
| 0.0072 | |||||
| Fenamole | Female | 5.89 + 0.14 | 6.52 + 0.067 | 6.12 + 0.085 | n.s. |
| Male | 6.46 ± 0.12 | 5.92 ± 0.058 | 6.37 ± 0.042 | 0.03 | |
| 0.02 | |||||
| 2,3-Dihydroxypropylstearate | Female | 6.43 ± 0.21 | 6.41 ± 0.12 | 6.32 ± 0.21 | n.s. |
| Male | 6.24 ± 0.15 | 6.13 ± 0.07 | 6.27 ± 0.11 | n.s. | |
| 0.0004 | |||||
| n-Hexanamide | Female | 5.89 ± 0.29 | 6.69 ± 0.04 | 6.67 ± 0.02 | 0.0073 |
| Male | 7.25 ± 0.13 | 7.25 ± 0.35 | 6.57 ± 0.30 | 0.0019 | |
| n.s. | |||||
| N-Acetyl-Lglutamic Acid | Female | 7.01 ± 0.15 | 7.27 ± 0.12 | 7.24 ± 0.10 | 0.0029 |
| Male | 7.75 ± 0.11 | 7.18 ± 0.11 | 7.20 ± 0.13 | 0.051 | |
| n.s. | |||||
| JWH 133 | Female | 6.48 ± 0.29 | 6.68 ± 0.08 | 6.75 ± 0.07 | n.s. |
| Male | 6.63 ± 0.06 | 6.68 ± 0.07 | 6.74 ± 0.05 | n.s. | |
| <0.0001 | |||||
| Homocitrulline | Female | 5.68 ± 0.03 | 6.27 ± 0.09 | 5.91 ± 0.07 | 0.0001 |
| Male | 6.32 ± 0.08 | 5.72 ± 0.12 | 5.94 ± 0.16 | n.s. | |
| n.s. | |||||
| Sphinganine | Female | 6.73 ± 0.17 | 7.14 ± 0.03 | 7.07 ± 0.03 | 0.018 |
| Male | 7.16 ± 0.03 | 7.11 ± 0.03 | 7.07 ± 0.01 | 0.02 | |
| 0.053 | |||||
| R-(+)-Etiracetam | Female | 5.21 ± 0.22 | 5.99 + 0.04 | 5.71 ± 0.05 | 0.0005 |
| Male | 5.81 ± 0.06 | 5.60 ± 0.10 | 5.57 ± 0.09 | n.s. | |
| n.s. | |||||
| Pyrrolidine | Female | 5.59 ± 0.10 | 6.08 ± 0.12 | 5.75 ± 0.12 | 0.01 |
| Male | 6.14 ± 0.09 | 5.91 ± 0.12 | 5.93 ± 0.10 | 0.04 | |
| n.s. | |||||
| 1-(4-Aminobutyl)urea | Female | 4.61 ± 0.19 | 5.94 ± 0.08 | 5.44 ± 0.17 | 0.0015 |
| Male | 5.40 ± 0.09 | 5.15 ± 0.34 | 5.30 ± 0.24 | n.s. | |
| 0.025 | |||||
| Propoxur | Female | 5.31 ± 0.19 | 6.017 ± 0.07 | 5.93 ± 0.07 | n.s. |
| Male | 5.94 ± 0.14 | 6.072 ± 0.09 | 6.18 ± 0.14 | 0.006 | |
| 0.003 | |||||
| PRIMA-1 | Female | 5.20 ± 0.22 | 6.00 ± 0.04 | 5.71 ± 0.05 | 0.0005 |
| Male | 5.81 ± 0.06 | 5.60 ± 0.10 | 5.57 ± 0.09 | n.s. | |
| n.s. | |||||
| Phytosphingosine | Female | 7.56 ± 0.21 | 8.14 ± 0.19 | 8.02 ± 0.07 | 0.022 |
| Male | 8.13 ± 0.07 | 8.13 ± 0.018 | 8.15 ± 0.02 | 0.012 | |
| 0.02 | |||||
| N-lauroylglycine | Female | 5.96 ± 0.13 | 6.46 ± 0.04 | 6.50 ± 0.09 | 0.0036 |
| Male | 6.34 ± 0.09 | 6.22 ± 0.11 | 6.32 ± 0.04 | n.s. | |
| 0.021 | |||||
| Nicaraven | Female | 5.24 ± 0.09 | 4.88 ± 0.07 | 4.84 ± 0.05 | n.s. |
| Male | 5.06 ± 0.07 | 5.02 ± 0.10 | 4.94 ± 0.03 | n.s. | |
| 0.003 | |||||
| N~6~,N~6~-Dimethyllysine | Female | 5.05 ± 0.14 | 5.78 ± 0.07 | 5.47 ± 0.13 | 0.0088 |
| Male | 5.45 ± 0.12 | 5.31 ± 0.16 | 5.63 ± 0.18 | n.s. | |
| 0.049 | |||||
| Misoprostol | Female | 6.18 ± 0.14 | 5.85 ± 0.08 | 5.69 ± 0.05 | 0.032 |
| Male | 5.88 ± 0.05 | 5.82 ± 0.11 | 5.91 ± 0.10 | n.s. | |
| 0.036 | |||||
| Lovastatin | Female | 6.86 ± 0.13 | 6.43 ± 0.10 | 6.52 ± 0.05 | n.s. |
| Male | 6.61 ± 0.04 | 6.50 ± 0.05 | 6.53 ± 0.08 | n.s. | |
| 0.0081 | |||||
| HMMNI | Female | 4.87 ± 0.19 | 5.48 ± 0.23 | 5.50 ± 0.05 | n.s. |
| Male | 6.08 ± 0.19 | 6.03 ± 0.19 | 5.78 ± 0.44 | 0.0014 | |
| n.s. | |||||
| Gly-Leu | Female | 5.38 ± 0.10 | 6.46 ± 0.13 | 5.96 ± 0.11 | 0.003 |
| Male | 6.21 ± 0.15 | 6.07 ± 0.30 | 5.88 ± 0.20 | n.s. | |
| 0.026 | |||||
| Chamazulene | Female | 5.78 ± 0.35 | 5.01 ± 0.01 | 4.929 ± 0.02 | 0.02 |
| Male | 4.99 ± 0.03 | 4.97 ± 0.03 | 4.99 ± 0.02 | 0.05 | |
| 0.02 | |||||
| Esmolol | Female | 6.00 ± 0.22 | 6.39 ± 0.07 | 6.43 ± 0.04 | 0.013 |
| Male | 6.59 ± 0.03 | 6.51 ± 0.08 | 6.35 ± 0.06 | 0.025 | |
| 0.0364 | |||||
| Dodecylethanolamide | Female | 6.38 ± 0.09 | 6.15 ± 0.03 | 6.16 ± 0.01 | 0.038 |
| Male | 6.24 ± 0.02 | 6.23 ± 0.03 | 6.20 ± 0.02 | n.s. | |
| 0.009 | |||||
| Creatine | Female | 7.21 ± 0.17 | 6.86 ± 0.20 | 7.06 ± 0.14 | 0.01 |
| Male | 6.21 ± 0.22 | 6.98 ± 0.10 | 7.15 ± 0.33 | n.s. | |
| n.s. | |||||
| C2 Dihydroceramide | Female | 5.55 ± 0.09 | 5.87 ± 0.06 | 5.88 ± 0.03 | 0.003 |
| Male | 5.93 ± 0.04 | 5.73 ± 0.13 | 5.84 ± 0.05 | n.s. | |
| n.s. | |||||
| BAR501 | Female | 4.61 ± 0.06 | 5.15 ± 0.04 | 4.96 ± 0.09 | 0.006 |
| Male | 5.06 ± 0.08 | 4.99 ± 0.11 | 4.97 ± 0.15 | n.s. | |
| 0.042 | |||||
| APM | Female | 5.59 ± 0.26 | 6.42 ± 0.07 | 6.21 ± 0.10 | 0.024 |
| Male | 6.16 ± 0.12 | 6.13 ± 0.14 | 6.14 ± 0.13 | n.s. | |
| 0.038 | |||||
| Adenine | Female | 6.51 ± 0.14 | 7.04 ± 0.20 | 6.91 ± 0.21 | 0.012 |
| Male | 7.39 ± 0.16 | 6.76 ± 0.19 | 7.00 ± 0.15 | n.s. | |
| n.s. | |||||
| Acetylcadaverine | Female | 5.31 ± 0.01 | 5.80 ± 0.16 | 5.42 ± 0.05 | 0.0038 |
| Male | 5.58 ± 0.07 | 5.44 ± 0.06 | 5.38 ± 0.03 | n.s. | |
| 0.041 | |||||
| Acetohydroxamic acid | Female | 7.31 ± 0.08 | 7.75 ± 0.07 | 7.48 ± 0.09 | 0.0084 |
| Male | 7.83 ± 0.12 | 7.53 ± 0.11 | 7.73 ± 0.17 | n.s. | |
| n.s. | |||||
| 5-Hydroxytryptophol | Female | 5.32 ± 0.14 | 6.02 ± 0.07 | 5.93 ± 0.07 | n.s. |
| Male | 5.94 ± 0.19 | 6.07 ± 0.09 | 6.18 ± 0.14 | 0.006 | |
| 0.003 | |||||
| 1-PP | Female | 5.61 ± 0.08 | 6.07 ± 0.08 | 5.79 ± 0.10 | 0.011 |
| Male | 6.14 ± 0.13 | 5.85 ± 0.12 | 6.01 ± 0.16 | n.s. | |
| n.s. | |||||
| (4R)-5-Hydroxy-L-leucine | Female | 5.91 ± 0.16 | 6.66 ± 0.10 | 6.20 ± 0.13 | 0.003 |
| Male | 6.64 ± 0.13 | 6.25 ± 0.25 | 6.37 ± 0.08 | n.s. | |
| n.s. | |||||
| (+)-castanospermine | Female | 5.05 ± 0.29 | 5.94 ± 0.08 | 5.59 ± 0.10 | 0.028 |
| Male | 5.60 ± 0.08 | 5.59 ± 0.10 | 5.69 ± 0.14 | n.s. | |
| 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, F.; Mosley, M.-T.; Jasbi, P.; Chi, J.; Gu, H.; Jadavji, N.M. Maternal Dietary Deficiencies in Folic Acid and Choline Change Metabolites Levels in Offspring after Ischemic Stroke. Metabolites 2024, 14, 552. https://doi.org/10.3390/metabo14100552
Anwar F, Mosley M-T, Jasbi P, Chi J, Gu H, Jadavji NM. Maternal Dietary Deficiencies in Folic Acid and Choline Change Metabolites Levels in Offspring after Ischemic Stroke. Metabolites. 2024; 14(10):552. https://doi.org/10.3390/metabo14100552
Chicago/Turabian StyleAnwar, Faizan, Mary-Tyler Mosley, Paniz Jasbi, Jinhua Chi, Haiwei Gu, and Nafisa M. Jadavji. 2024. "Maternal Dietary Deficiencies in Folic Acid and Choline Change Metabolites Levels in Offspring after Ischemic Stroke" Metabolites 14, no. 10: 552. https://doi.org/10.3390/metabo14100552
APA StyleAnwar, F., Mosley, M.-T., Jasbi, P., Chi, J., Gu, H., & Jadavji, N. M. (2024). Maternal Dietary Deficiencies in Folic Acid and Choline Change Metabolites Levels in Offspring after Ischemic Stroke. Metabolites, 14(10), 552. https://doi.org/10.3390/metabo14100552









