Nontargeted Metabolomics to Understand the Impact of Modified Atmospheric Packaging on Metabolite Profiles of Cooked Normal-pH and Atypical Dark-Cutting Beef
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Packaging
2.2. Cooking and Rest Period
2.3. Raw and Cooked Color
2.4. Metmyoglobin Reducing Activity
2.5. Nontargeted Metabolomics Analysis
2.6. Statistical Analysis
3. Results
3.1. Raw Color Attributes
3.2. Cooked Color Attributes
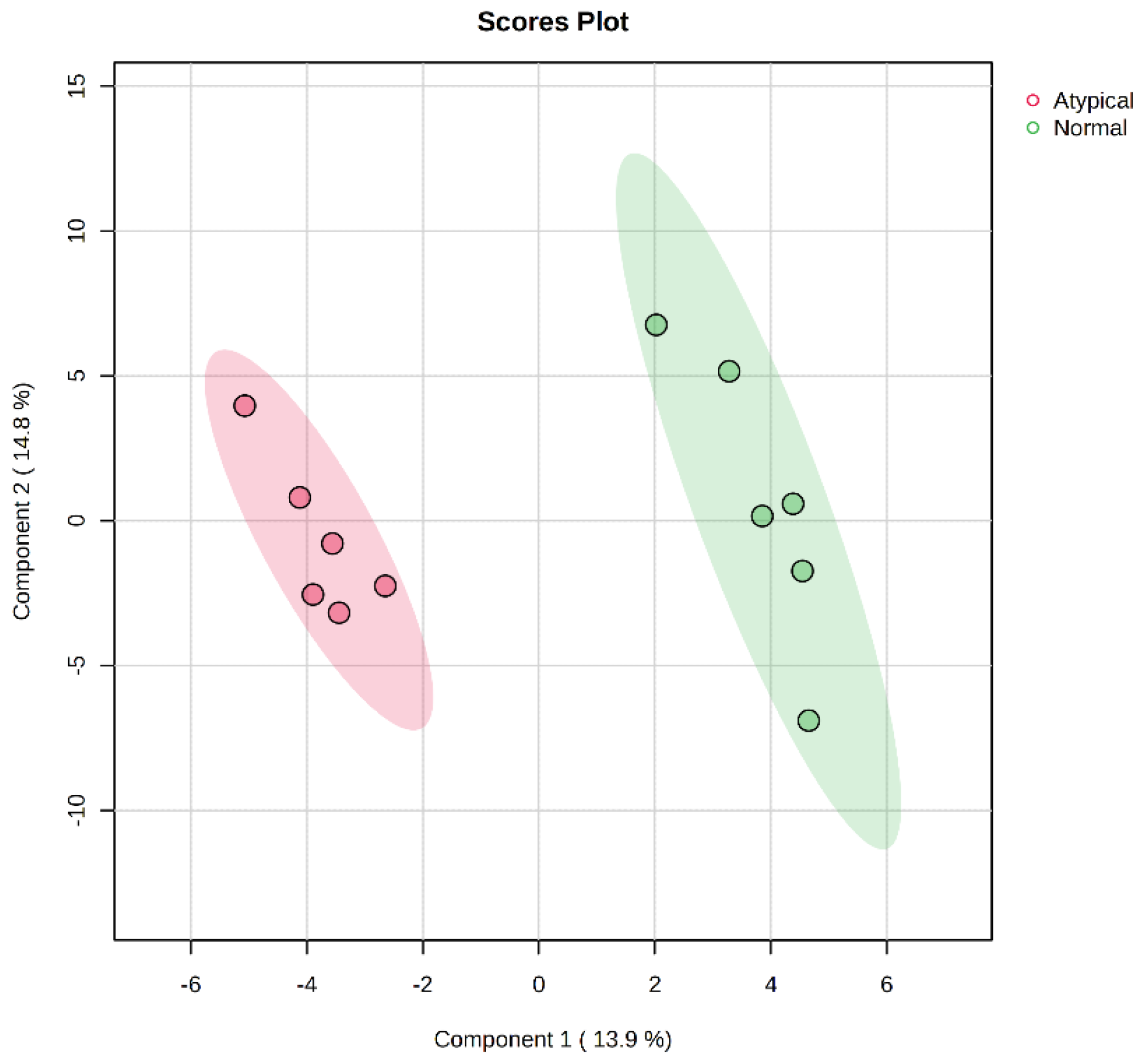
3.3. Effects of Muscle Type on Metabolite Profile
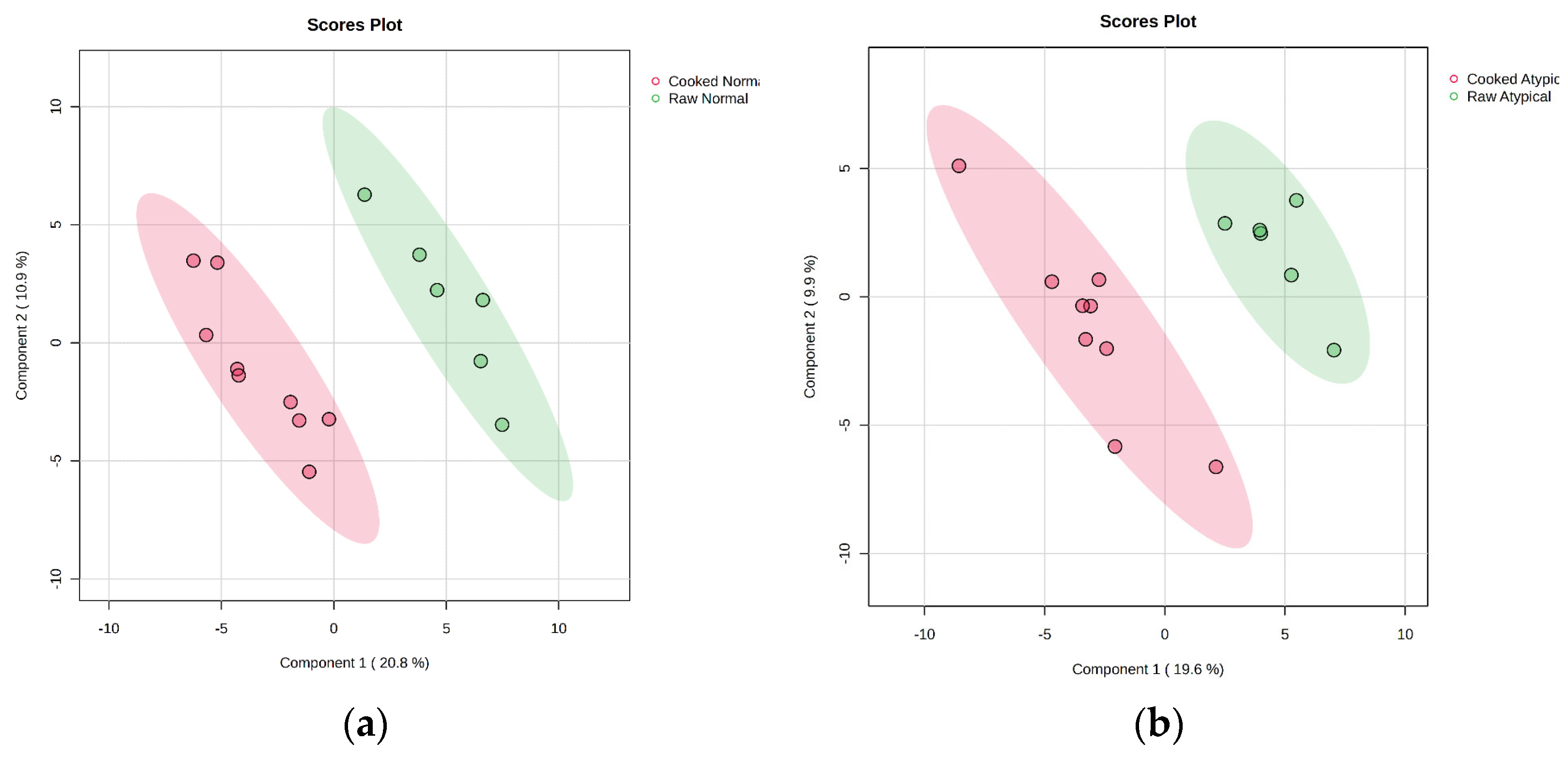
3.4. Effects of Muscle Type and Packaging on Cooked Metabolite Profiles
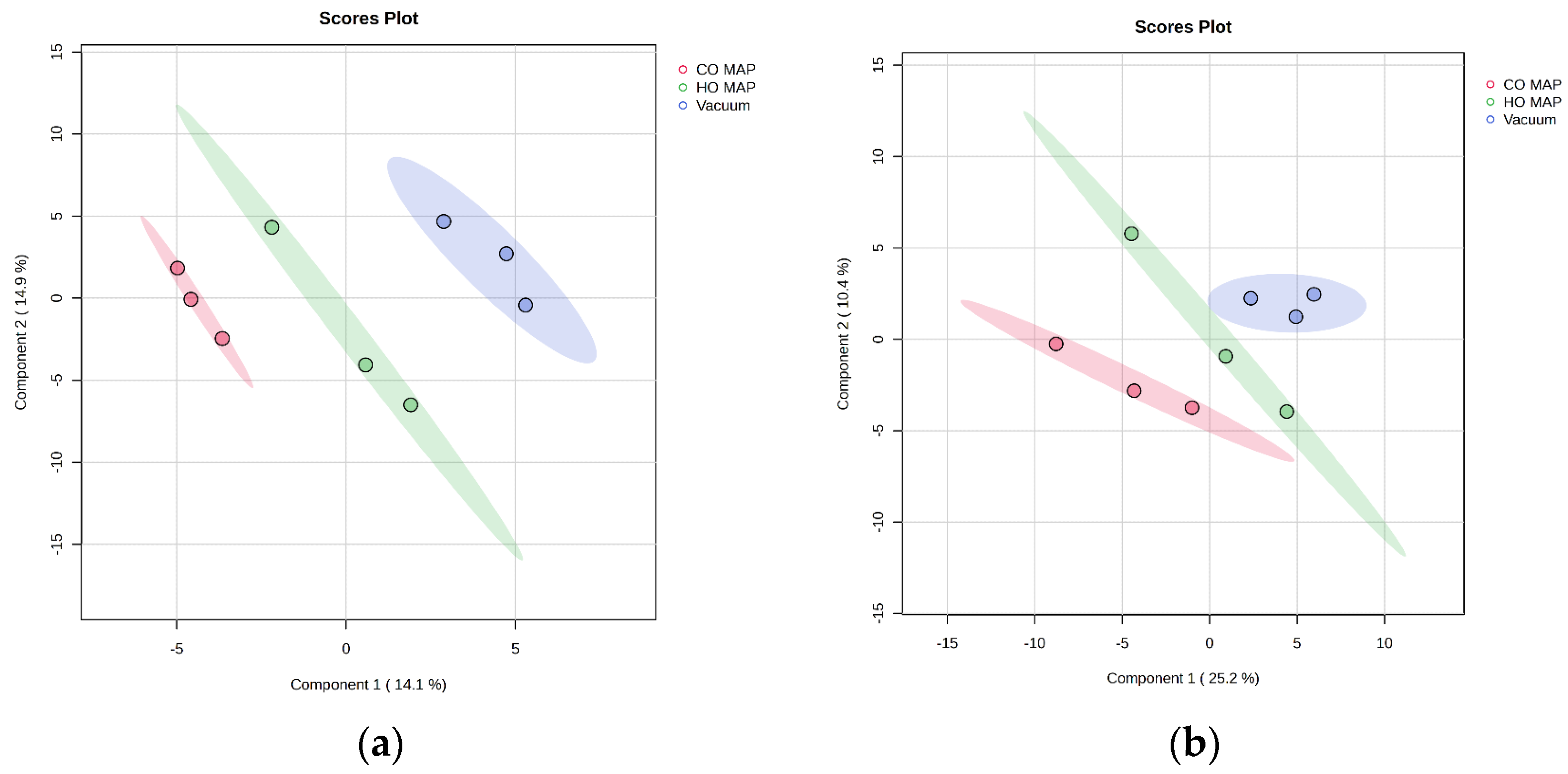
3.5. Packaging Comparison of Atypical Dark-Cutting Beef
3.6. Packaging Comparison of Cooked Normal-pH Beef
4. Discussion
4.1. Impact of Packaging Type and Cooking on Metabolites
4.2. Impact of Packaging and Metabolites on Other Meat Quality Attributes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carpenter, C.E.; Cornforth, D.P.; Whittier, D. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 2001, 57, 359–363. [Google Scholar] [CrossRef]
- Lybarger, K.R.; Beyer, E.S.; Farmer, K.J.; Egger, L.A.; Drey, L.N.; Hunt, M.C.; Vipham, J.L.; Zumbaugh, M.D.; Chao, M.D.; O’Quinn, T.G. Determination of consumer color and discoloration thresholds for purchase of representative retail ground beef. Meat Muscle Biol. 2023, 7, 16757. [Google Scholar] [CrossRef]
- Kiyimba, F.; Cassens, D.; Hartson, S.D.; Rogers, J.; Habiger, J.; Mafi, G.G.; Ramanathan, R. Integrative proteomics and metabolomics profiling to understand the biochemical basis of beef muscle darkening at a slightly elevated pH. J. Anim. Sci. 2023, 101, skac376. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Roy, B.C.; Larsen, I.L.; Aalhus, J.L.; Dixon, W.T.; Bruce, H.L. Understanding the quality of typical and atypical dark cutting beef from heifers and steers. Meat Sci. 2017, 133, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Harr, K.M.; Jewell, N.; Edwards, J.; More, S.; Mafi, G.G.; Pfeiffer, M.; Ramanathan, R. Comparing the effects of packaging normal-pH and atypical dark-cutting beef in modified atmosphere conditions on surface color. Meat Sci. 2024, 213, 109466. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, L.; Mao, Y.; Hopkins, D.L.; Han, G.; Zhu, L.; Luo, X. Carbon monoxide packaging shows the same color improvement for dark cutting beef as high oxygen packaging. Meat Sci. 2018, 137, 153–159. [Google Scholar] [CrossRef]
- Lu, X.; Cornforth, D.P.; Carpenter, C.E.; Zhu, L.; Luo, X. Effect of oxygen concentration in modified atmosphere packaging on color changes of the M. longissimus thoraces et lumborum from dark cutting beef carcasses. Meat Sci. 2020, 161, 107999. [Google Scholar] [CrossRef]
- Ijaz, M.; Li, X.; Zhang, D.; Hussain, Z.; Ren, C.; Bai, Y.; Zheng, X. Association between meat color of DFD beef and other quality attributes. Meat Sci. 2020, 161, 107954. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef]
- Grobbel, J.P.; Dikeman, M.E.; Hunt, M.C.; Milliken, G.A. Effects of packaging atmospheres on beef instrumental tenderness, fresh color stability, and internal cooked color. J. Anim. Sci. 2008, 86, 1191–1199. [Google Scholar] [CrossRef]
- Xu, B.; Luo, X.; Yang, X.; Zhang, Y.; Sebranek, J.G.; Liang, R. Comparative proteomic analyses to investigate premature browning in high-oxygen modified atmosphere packaged beef patties. Food Chem. 2024, 456, 140022. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.P.; Nair, M.N.; Joseph, P.; Hunt, M.C. Factors influencing internal color of cooked meats. Meat Sci. 2016, 120, 133–144. [Google Scholar] [CrossRef]
- Bao, Y.; Puolanne, E.; Ertbjerg, P. Effect of oxygen concentration in modified atmosphere packaging on color and texture of beef patties cooked to different temperatures. Meat Sci. 2016, 121, 189–195. [Google Scholar] [CrossRef]
- Killinger, K.M.; Hunt, M.C.; Campbell, R.E.; Kropf, D.H. Factors affecting premature browning during cooking of store-purchased ground beef. J. Food Sci. 2000, 65, 585–588. [Google Scholar] [CrossRef]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.B.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. American Meat Science Association guidlines for meat color measurement. Meat Muscle Biol. 2023, 6, 81. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Claus, J.R. Color stability and reversion in carbon monoxide packaged ground beef. Meat Sci. 2010, 85, 525–530. [Google Scholar] [CrossRef]
- Djimsa, B.A.; Abraham, A.; Mafi, G.G.; VanOverbeke, D.L.; Ramanathan, R. Effects of metmyoglobin reducing activity and thermal stability of NADH-dependent reductase and lactate dehydrogenase on premature browning in ground beef. J. Food Sci. 2017, 82, 304–313. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Kiyimba, F.; Hartson, S.D.; Rogers, J.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Changes in glycolytic and mitochondrial protein profiles regulates postmortem muscle acidification and oxygen consumption in dark-cutting beef. J. Proteom. 2021, 232, 104016. [Google Scholar] [CrossRef]
- Ramanathan, R.; Kiyimba, F.; Gonzalez, J.; Mafi, G.; Desilva, U. Impact of up- and downregulation of metabolites and mitochondrial content on pH and color of the longissimus muscle from normal-pH and dark-cutting beef. J. Agric. Food Chem. 2020, 68, 7194–7203. [Google Scholar] [CrossRef]
- Cônsolo, N.R.B.; Rosa, A.F.; Barbosa, L.C.G.S.; Maclean, P.H.; Higuera-Padilla, A.; Colnago, L.A.; Titto, E.A.L. Preliminary study on the characterization of Longissimus lumborum dark cutting meat in Angus × Nellore crossbreed cattle using NMR-based metabolomics. Meat Sci. 2021, 172, 108350. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kong, X.; Yang, X.; Zhu, L.; Liang, R.; Luo, X.; Zhang, L.; Hopkins, D.L.; Mao, Y.; Zhang, Y. Effect of energy metabolism and proteolysis on the toughness of intermediate ultimate pH beef. Meat Sci. 2022, 188, 108798. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Kim, H.R.; Lee, C.Y.; Hyun, S.-A.; Ko, M.Y.; Lee, B.-S.; Hwang, D.Y.; Ka, M. 2-Phenylethylamine (PEA) Ameliorates Corticosterone-Induced Depression-Like Phenotype via the BDNF/TrkB/CREB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 9103. [Google Scholar] [CrossRef]
- Ramanathan, R.; Lusk, J.L.; Reuter, R.R.; Mafi, G.G.; VanOverbeke, D.L. Consumer practices and risk factors that predispose to premature browning in cooked ground beef. Meat Muscle Biol. 2019, 3, 526–531. [Google Scholar] [CrossRef]
- Hunt, M.C.; Sørheim, O.; Slinde, E. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 1999, 64, 847–851. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Luo, X.; Zhang, Y.; Zhu, L.; Xu, B.; Hopkins, D.L.; Liang, R. Influence of oxygen concentration on the fresh and internal cooked color of modified atmosphere packaged dark-cutting beef stored under chilled and superchilled conditions. Meat Sci. 2022, 188, 108773. [Google Scholar] [CrossRef]
- Moiseev, I.V.; Cornforth, D.P. Treatments for prevention of persistent pinking in dark-cutting beef patties. J. Food Sci. 1999, 64, 738–743. [Google Scholar] [CrossRef]
- Apple, J.K.; Sawyer, J.T.; Meullenet, J.-F.; Yancey, J.W.S.; Wharton, M.D. Lactic acid enhancement can improve the fresh and cooked color of dark-cutting beef1,2. J. Anim. Sci. 2011, 89, 4207–4220. [Google Scholar] [CrossRef] [PubMed]
- Stackhouse, R.J.; Apple, J.K.; Yancey, J.W.S.; Keys, C.A.; Johnson, T.M.; Mehall, L.N. Postrigor citric acid enhancement can alter cooked color but not fresh color of dark-cutting beef1. J. Anim. Sci. 2016, 94, 1738–1754. [Google Scholar] [CrossRef][Green Version]
- dos Santos-Donado, P.R.; Donado-Pestana, C.M.; Ossamu Tanaka, F.A.; Venturini, A.C.; Francisquine Delgado, E.; Contreras-Castillo, C.J. Effects of high-oxygen, carbon monoxide modified atmospheres and vacuum packaging on quality of Longissimus thoracis et lumborum steaks from Nellore cows during ageing. Food Res. Int. 2021, 143, 110226. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.; Faustman, C. Metmyoglobin reducing activity. Meat Sci. 2005, 71, 407–439. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Zhang, D.; Hou, C.; Mahmood, M.; Hussain, Z.; Zheng, X.; Li, X. Changes in postmortem metabolites profile of atypical and typical DFD beef. Meat Sci. 2022, 193, 108922. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Escudero, A.; Beltrán, J.A.; Olleta, J.L.; Sañudo, C.; Mar Campo, M.d. Color, lipid oxidation, sensory quality, and aroma compounds of beef steaks displayed under different levels of oxygen in a modified atmosphere package. J. Food Sci. 2012, 77, S10–S18. [Google Scholar] [CrossRef]
- Dashdorj, D.; Amna, T.; Hwang, I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: An overview. Eur. Food Res. Technol. 2015, 241, 157–171. [Google Scholar] [CrossRef]
- Adhikari, K.; Chambers Iv, E.; Miller, R.; Vazquez-Araujo, L.; Bhumiratana, N.; Philip, C. Development of a lexicon for beef flavor in intact muscle. J. Sens. Stud. 2011, 26, 413–420. [Google Scholar] [CrossRef]
- O’Quinn, T.G.; Legako, J.F.; Woerner, D.R.; Kerth, C.R.; Nair, M.N.; Brooks, J.C.; Lancaster, J.M.; Miller, R.K. A current review of U.S. beef flavor II: Managing beef flavor. Meat Sci. 2024, 209, 109403. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Liu, W.; Zhao, L. Novel Umami Ingredients: Umami Peptides and Their Taste. J. Food Sci. 2017, 82, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Setyabrata, D.; Cooper, B.R.; Sobreira, T.J.P.; Legako, J.F.; Martini, S.; Kim, Y.H.B. Elucidating mechanisms involved in flavor generation of dry-aged beef loins using metabolomics approach. Food Res. Int. 2021, 139, 109969. [Google Scholar] [CrossRef]
- Kerth, C.R.; Legako, J.F.; Woerner, D.R.; Brooks, J.C.; Lancaster, J.M.; O’Quinn, T.G.; Nair, M.; Miller, R.K. A current review of U.S. beef flavor I: Measuring beef flavor. Meat Sci. 2024, 210, 109437. [Google Scholar] [CrossRef]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Mottram, D.S. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992, 31, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kamiya, M.; Higuchi, M. Gas chromatography–mass spectrometry-based metabolomic analysis of Wagyu and Holstein beef. Metabolites 2020, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; Vierck, K.R.; Legako, J.F.; Kim, J.; Johnson, B.J.; Brooks, J.C. Determination of package and muscle-type influence on proteolysis, beef-flavor-contributing free amino acids, final beef flavor, and tenderness. Meat Muscle Biol. 2020, 4, 10933. [Google Scholar] [CrossRef]
| Raw Color | Cooked Internal Color | ||||||
|---|---|---|---|---|---|---|---|
| L* | a* | Chroma | L* | a* | Chroma | 630 nm/580 nm | |
| Loin type | |||||||
| Atypical dark-cutting | 42.76 b | 21.10 | 24.57 | 52.40 | 18.78 | 27.34 | 2.33 |
| Normal | 45.85 a | 21.79 | 25.92 | 50.28 | 18.59 | 28.00 | 2.34 |
| Standard error: | 0.90 | 0.70 | 0.79 | 0.79 | 0.49 | 0.50 | 0.09 |
| p-value: | 0.03 | 0.50 | 0.23 | 0.09 | 0.79 | 0.36 | 0.95 |
| Packaging type | |||||||
| CO-MAP | 42.92 | 23.34 b | 26.87 b | 50.81 | 22.82 a | 31.10 a | 2.94 a |
| HiOx-MAP | 44.90 | 25.89 a | 31.21 a | 52.03 | 11.50 b | 21.33 b | 1.32 b |
| Vacuum package | 45.10 | 15.11 c | 17.65 c | 51.19 | 21.74 a | 30.59 a | 2.73 a |
| Standard error: | 0.95 | 0.82 | 0.97 | 0.70 | 0.56 | 0.61 | 0.11 |
| p-value: | 0.17 | <0.01 | <0.01 | 0.25 | <0.01 | <0.01 | <0.01 |
| Metabolite | p-Value | Abundance (Atypical/Normal) | Role |
|---|---|---|---|
| 2-hydroxygluataric acid | 0.040 | over | Lysine and tryptophan degradation |
| Arachidonic acid | <0.001 | over | Fatty acid metabolism |
| Linoleic acid | 0.003 | over | Fatty acid metabolism |
| Heptadecanoic acid | 0.026 | over | Fatty acid metabolism |
| Threonic acid | 0.025 | over | Sugar acid |
| Phenylethylamine | 0.002 | over | Neurotransmitter |
| Dehydroascorbic acid | 0.026 | over | Antioxidant metabolism |
| Palmitoleic acid | 0.046 | less | Fatty acid metabolism |
| Lactic acid | 0.014 | less | Glycolysis |
| Metabolite | p-Value | Abundance (Cooked/Raw) | Type of Metabolite |
|---|---|---|---|
| Aspartic acid | 0.019 | over | Amino acid |
| Tryptophan | 0.018 | over | Amino acid |
| Glycyl-proline | 0.005 | over | Dipeptide |
| Isoleucine | 0.032 | over | Amino acid |
| Leucine | 0.030 | over | Amino acid |
| Serine | 0.029 | over | Amino acid |
| Phenylalanine | 0.031 | over | Amino acid |
| Isomaltose | 0.010 | over | Carbohydrate |
| Lyxose | 0.003 | over | Carbohydrate |
| Cellobiose | 0.012 | over | Carbohydrate |
| Squalene | 0.005 | less | Lipid |
| Dehydroascorbic acid | 0.013 | less | Vitamin (oxidized) |
| Nicotinamide | 0.005 | less | Vitamin |
| Threonic acid | 0.033 | less | Sugar acid |
| Metabolite | p-Value | Abundance (Cooked/Raw) | Type of Molecule |
|---|---|---|---|
| Aspartic acid | 0.028 | over | Amino acid |
| Cysteine | 0.005 | over | Amino acid |
| Glutamic acid | 0.007 | over | Amino acid |
| Isoleucine | 0.001 | over | Amino acid |
| Leucine | 0.001 | over | Amino acid |
| Methionine | 0.014 | over | Amino acid |
| Phenylalanine | 0.018 | over | Amino acid |
| Threonine | 0.007 | over | Amino acid |
| Tryptophan | 0.047 | over | Amino acid |
| Tyrosine | 0.031 | over | Amino acid |
| Valine | 0.002 | over | Amino acid |
| 3,6-anhydro-D-galactose | 0.008 | over | Carbohydrate |
| Lyxose | <0.001 | over | Carbohydrate |
| N-acetylneuraminic acid | 0.010 | over | Carbohydrate |
| Xylulose | 0.019 | over | Carbohydrate |
| Glycerol | 0.019 | over | Lipid base |
| Glycerol-3-galactoside | 0.037 | over | Glycoside |
| Glycyl-proline | <0.001 | over | Dipeptide |
| Hypoxanthine | 0.046 | over | Purine base |
| Creatinine | 0.011 | less | Metabolite |
| Dehydroascorbic acid | 0.009 | less | Vitamin (oxidized) |
| Guanosine | 0.047 | less | Nucleoside |
| Lactic acid | 0.013 | less | Organic acid |
| Metabolite | CO vs. HO | CO vs. VP | HO vs. VP | ||
|---|---|---|---|---|---|
| p-Value | Abundance (CO/HO) | p-Value | p-Value | Abundance (HO/VP) | |
| Citric acid | 0.005 | less | NS | 0.002 | over |
| Fumaric acid | 0.002 | less | NS | NS | - |
| Maltose | 0.017 | less | NS | 0.009 | over |
| Maltotriose | 0.007 | less | NS | 0.044 | over |
| Threonic acid | 0.017 | over | NS | 0.017 | less |
| Trans-4-hydroxyproline | 0.031 | over | NS | NS | - |
| Xanthine | 0.003 | less | NS | 0.002 | over |
| Metabolite | CO vs. HO | CO vs. VP | HO vs. VP | ||
|---|---|---|---|---|---|
| p-Value | Abundance (CO/HO) | Abundance | p-Value | Abundance (HO/VP) | |
| citric acid | <0.001 | less | NS | <0.001 | over |
| fumaric acid | 0.010 | less | NS | <0.001 | over |
| malic acid | 0.002 | over | NS | 0.002 | less |
| threonic acid | 0.003 | over | NS | <0.001 | less |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harr, K.M.; Jewell, N.; Mafi, G.G.; Pfeiffer, M.M.; Ramanathan, R. Nontargeted Metabolomics to Understand the Impact of Modified Atmospheric Packaging on Metabolite Profiles of Cooked Normal-pH and Atypical Dark-Cutting Beef. Metabolites 2024, 14, 532. https://doi.org/10.3390/metabo14100532
Harr KM, Jewell N, Mafi GG, Pfeiffer MM, Ramanathan R. Nontargeted Metabolomics to Understand the Impact of Modified Atmospheric Packaging on Metabolite Profiles of Cooked Normal-pH and Atypical Dark-Cutting Beef. Metabolites. 2024; 14(10):532. https://doi.org/10.3390/metabo14100532
Chicago/Turabian StyleHarr, Keayla M., Noah Jewell, Gretchen G. Mafi, Morgan M. Pfeiffer, and Ranjith Ramanathan. 2024. "Nontargeted Metabolomics to Understand the Impact of Modified Atmospheric Packaging on Metabolite Profiles of Cooked Normal-pH and Atypical Dark-Cutting Beef" Metabolites 14, no. 10: 532. https://doi.org/10.3390/metabo14100532
APA StyleHarr, K. M., Jewell, N., Mafi, G. G., Pfeiffer, M. M., & Ramanathan, R. (2024). Nontargeted Metabolomics to Understand the Impact of Modified Atmospheric Packaging on Metabolite Profiles of Cooked Normal-pH and Atypical Dark-Cutting Beef. Metabolites, 14(10), 532. https://doi.org/10.3390/metabo14100532





