Abstract
Background/Objectives: The mechanisms of action of phosphine are diverse and include neurotoxicity, metabolic inhibition, and oxidative stress; however, its efficacy at low temperatures is unclear. Methods: Comparative metabolomics is suitable for investigating the response of the spotted-wing fly Drosophila suzukii to exposure toward a combination of cold stimuli and fumigant PH3. Results: Under this combined exposure, 52 metabolites exhibiting significant differences in stress were identified and their physiological roles were analyzed in the Drosophila metabolic pathway. Most metabolites were involved in amino acids, TCA cycle, and nucleic acids. In addition, the alteration levels of cell membrane lipids, such as glycerophospholipids, sphingolipids, and glycerolipids, clearly showed changes in the combined treatment compared to PH3 and low temperatures alone. Aconitic acid, a component of the TCA cycle, was completely inhibited by the combined treatment. Conclusions: These results suggest that treatment-specific indicators could be useful biomarkers to indicate the synergistic effects of PH3 and low temperature on energy metabolism.
1. Introduction
The spotted-wing fly Drosophila suzukii (Matsumara) is widely distributed in Asia, the Americas, and Europe, and is characterized by laying eggs inside fresh fruits using serrated ovipositors [1,2,3,4,5]. Since hatched larvae burrow into the fruit, they are difficult to detect during the early stages of infection [6,7,8,9]. Wounds caused by female ovipositing organs are entry points for secondary pathogens, such as fungi and bacteria, which further aggravate fruit damage [6,7,8]. This invasive behavior has made this species a serious pest worldwide, and its control is crucial at the quarantine stage [10,11,12,13].
Phosphine (PH3) as a fumigant has been widely used to control pests in stored grains and many other stored commodities [14], but its mode of action is not well understood. PH3, which is broken down into harmless phosphates, is very effective for controlling pests in grain storage when used as a combined treatment with carbon dioxide [15,16,17]. Although effective penetration into target pests, lack of residue, and low cost are the major advantages of PH3 [18,19,20], long-term exposure is considered a weak point [21].
Low temperatures are primarily used to disinfect stored agricultural products from pests or for quarantine purposes [22,23,24]. Cold treatment has been used as a control measure against pests such as the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) [25] and Caribbean fruit fly Anastrepha suspensa (Loew) (Diptera: Tephritidae) [26]. In addition, cold treatment significantly reduced adult emergence in both blueberries and strawberries, and extended the shelf life of infested fruits compared to untreated controls [27]. Therefore, low temperatures can be a good option not only for pest control, but also for maintaining the marketability of fruits [27,28].
Recent studies on fumigants have suggested the possibility of controlling pests by using combined treatments at low temperatures. Cold treatment increases the insecticidal activity of phosphine and ethyl formate against pests such as D. suzukii [2,5,29], the oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) [30], and the peach fruit moth Carposina niponensis (Lepidoptera: Carposinadae) [31]. As cold treatment is commonly performed to maintain the marketability of fruits and vegetables, combined treatment with fumigants has the advantages of time efficiency, reduced product damage, and easy control of processing conditions. Although cold fumigation has a synergistic effect on insecticidal activity, the factors that lead to this synergistic effect are not well understood. To obtain evidence of the synergistic effects in pest management, the molecular changes induced by cold conditions and PH3 were investigated using comparative metabolic profiling.
2. Materials and Methods
2.1. Insect Rearing
The spotted-wing fly, D. suzukii (Matsumura) (Diptera: Drosophilidae), was provided by Dr. Bong-Su Kim (Plant Quarantine Technology Center, Animal and Plant Quarantine Agency, Gimcheon, Republic of Korea). D. suzukii was reared in the insect chamber at 20 ± 1 °C and 60 ± 10% relative humidity under a photo-period of 16 h light and 8 h dark [2,5,32]. The insects were maintained in a clean breeding dish (ø 100 mm × h 40 mm) supplied with artificial food and distilled water containing 20% sugar.
2.2. Phosphine and Thermal Treatment
PH3 (Vivakill®, 2% PH3 + 98% CO2) was purchased from Dongbu Farm Hannong Co., Ltd. (Daejeon, Republic of Korea) and supplied by Safefume Co., Ltd. (Fumate™, 99%; Hoengseong, Republic of Korea). One hundred pupae were placed on filter paper soaked in water in a Petri dish. The experimental methods for (1) cold alone, (2) fumigation alone, and (3) combined treatments were as follows [5,32]. Cold treatment was performed at 1 °C for 24 h. PH3 (lethal concentration time; LCT50, 1.1 mg/L) was introduced at 20 °C for 4 h in a 12 L desiccator (Bibby Scientific, Staffordshire, UK) sealed with a glass stopper. The pupae were fumigated for 4 h and then immediately exposed to cold air at 1 °C for 24 h. Pupae from each group were transferred to glass vials and rapidly cooled in liquid nitro-gen to prevent metabolic changes. All treatments and controls were triplicated.
2.3. Metabolite Extraction
Whole metabolites were extracted from D. suzukii pupae in triplicate (100 insects/replicate). Briefly, each sample was suspended in 1 mL of the extracted solution (3:3:2, acetonitrile/isopropyl alcohol/water, v/v/v) and homogenized using a Taco Prep bead beater (Taco, Taichung, Taiwan) while turning it on and off at 30 s intervals for 5 min. Samples were incubated at room temperature for 20 min and centrifuged at 2500× g for 5 min at 4 °C. The supernatant was transferred to a new tube and dried under pure N2 gas. All dried samples were suspended in 200 μL of 50% acetonitrile and sonicated for 5 min. The supernatant was filtered with 0.22 μm pore (Ultrafree-MC, Millipore, Bedford, MA, USA) and immediately loaded into the LC–QTOF/MS for metabolome analysis. The metabolite recovery rate of the sample was investigated with internal standards (L-alanine, Sigma–Aldrich, Oakville, ON, Canada), and the extraction process showed a recovery rate of 50% or greater.
2.4. Lipid Extraction
Total lipidomes were extracted from whole bodies of D. suzukii pupae in triplicate (100 insects/replicate) using the modified Bligh and Dyer method, as described previously [33]. Briefly, each sample was suspended in 3 mL of extracted solution (2:1, methanol/chloroform, v/v) and homogenized using glass beads by turning the beater on and off at 30 s intervals for 5 min. Samples were incubated at room temperature for 20 min and centrifuged at 1750× g for 10 min at 4 °C. Supernatants were transferred to new tubes to remove tissue debris. One milliliter of chloroform and 1.8 mL of water were added to each sample, and the mixture was vortexed for 1 min. The lower layer was separated by centrifugation at 1750× g for 10 min at 4 °C, followed by transferring to a new tube and drying under pure N2 gas. Dried samples were then suspended in 200 μL of loading solution (1:1, methanol/chloroform, v/v) and sonicated for 5 min. Resulting supernatants were filtered with 0.22 μm pore filters and immediately loaded into the LC–QTOF/MS equipment for lipidomics. Lipid recovery rates for samples were investigated using lipid standards (SPLASH® LIPIDOMIX® Mass Spec Standard, Avanti Polar Lipids, Alabaster, AL, USA), and the extraction process showed recovery rates of 50% or greater [34].
2.5. LC-QTOF/MS
LC-QTOF/MS was performed using a liquid chromatograph triple quadrupole mass spectrometer (Agilent Technologies 1260 and 6530 System, Agilent Technologies, Santa Clara, CA, USA; Metabolomics Research Center for Functional Materials, Kyungsung University) with an electrospray ionization (ESI) source. For metabolome analysis, 5 μL of each sample was injected onto a ZORBAX Eclipse XDB-C18 column (4.6 mm × 50 mm, 1.8 μm; Agilent Technologies, Santa Clara, CA, USA) with a temperature of 55 °C. In the binary mobile phase system, phase A was water with 0.1% formic acid and phase B was acetonitrile with 0.1% formic acid. The mobile phase with a flow rate of 0.5 mL/min had the following composition conditions: initiation at 2% B, followed by a linear gradient to 2% B over 1 min, 100% B at 8 min, 100% B at 10 min, 2% B at 11 min, and 2% B at 20 min. Mass spectrometry was performed in both positive and negative modes. The capillary voltage was set to 2.0 kV in the positive mode and 1.0 kV in the negative mode. Metabolites with a mass range of m/z 100 to 1000 were detected using a quadrupole time-of-flight instrument.
2.6. Data Processing and Statistical Analysis
The data were analyzed in one batch to ensure that the parameters were applied equally to all samples and normalized to the total ion intensity. All entities were extracted from the LC peaks of each sample and analyzed using the Mass Hunter Qualitative soft-ware (Ver. 10.0, Agilent Technologies). All compounds were annotated using the METLIN metabolite database, filtered, scaled, and integrated using Mass Profiler Professional software (Ver. 14.0; Agilent Technologies), principal component analysis (PCA) and Pearson’s correlation analysis were performed. Differentially regulated metabolites were defined as changes in compounds with values of [raw fold change (FC)] > 2 and p < 0.01, compared to the mock control group. Metabolites were evaluated using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca) (accessed on 1 August 2024) and LIPEA (https://hyperlipea.org/home) (accessed on 1 August 2024), and relevant pathways were visualized using the Kyoto Encyclopedia of Genes and Genomes (KEGG).
3. Results and Discussion
3.1. Metabolite Changes according to Stress Conditions
Comparative metabolomics was performed to investigate the physiological effects of low temperature, PH3, and combined treatments (low temperature and PH3) on D. suzukii. When analyzing the total ion chromatogram, peaks that specifically increased or decreased compared to the control were found in each treatment group (Supplementary Figure S1A). By analysis of the mass pattern at 1.57 min, the peak was identified as L-isoleucine, which was only found in the control (Supplementary Figure S1B). A recent study found that transient isoleucine deprivation enhanced nicotine resistance and extended the lifespan of Drosophila melanogaster [35]. These results suggest that stresses such as low temperature and PH3 exactly affected amino acid synthesis in the Drosophila metabolic network. PCA was performed using raw FC data to investigate the reliability of the metabolic analysis (Supplementary Figure S2A). The PCA revealed an aligned cluster of metabolic data for each group and showed a significant distribution pattern in the positive (Supplementary Figure S2A-i) and negative ion modes (Supplementary Figure S2A-ii). Since the correlation analysis showed an association between each experimental group, it can be used to determine treatment-specific indicators based on altered metabolites. Low temperature, PH3, and the combined treatment were correlated with each other and revealed the same pattern in the positive (Supplementary Figure S2B-i) and negative ion modes (Supplementary Figure S2B-ii). These results suggest that the metabolome of D. suzukii is clearly differentiated by low temperature, PH3, and combined treatment.
3.2. Pathway Impact of Altered Metabolites
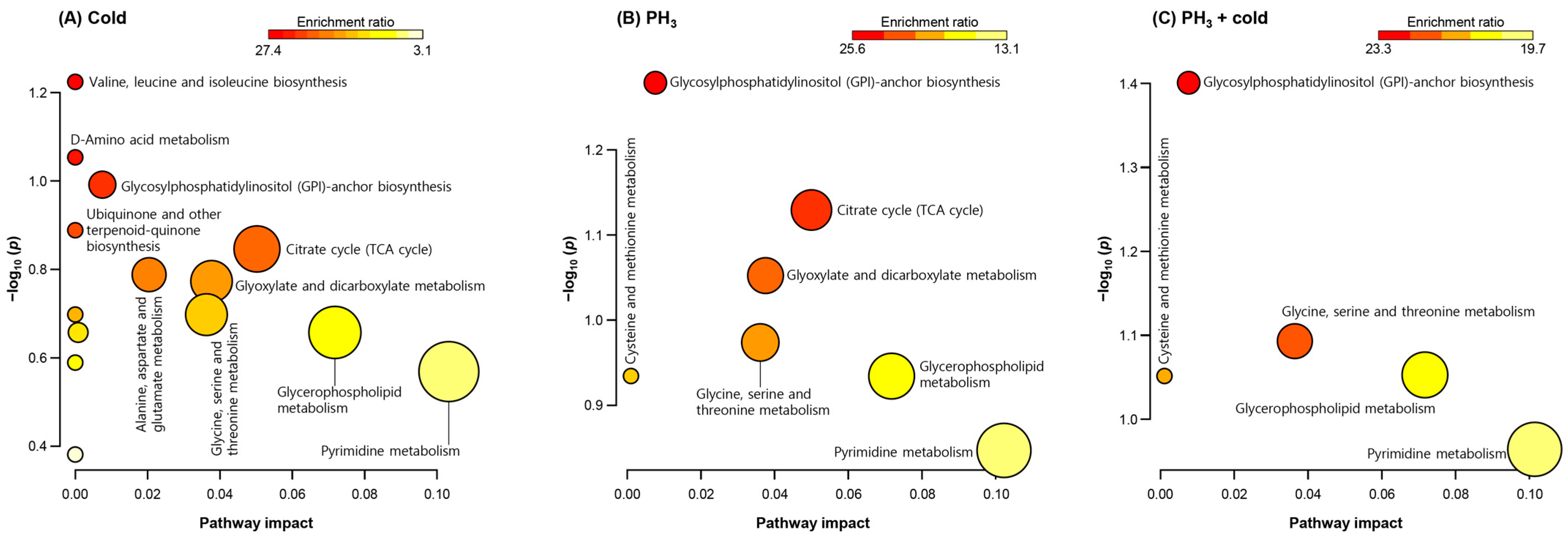
In total, 164 and 98 metabolites were detected in the positive and negative ion modes, respectively, and 80 indicators were filtered using an annotation process based on the metabolite database. Among them, 52 metabolites with significant differences in expression were selected for analysis of their metabolic pathways and were listed as treatment-specific indicators. Metabolites were analyzed using the enrichment ratio and pathway impact scores based on the KEGG database to examine the importance of altered metabolites in the Drosophila metabolic network (Figure 1). GPI-anchor and amino acid biosynthetic pathways were significantly regulated under each stress condition. In addition, metabolites related to purine and pyrimidine metabolism were modulated. The tricarboxylic acid (TCA) cycle was found to be the major metabolic network at low temperatures and PH3 alone (Figure 1A,B), but could not be identified in the combined treatment (Figure 1C). Interestingly, fewer metabolic pathways were altered in the combined treatment than in the low temperature or PH3. The reasons for these results are as follows: (1) only a limited number of metabolites were synergistically affected by the combined treatments; and (2) the combined treatment resulted in significant metabolic changes upon PH3 treatment prior to cold exposure.
Figure 1.
Enrichment ratio and pathway impact scores. Metabolite set enrichment analysis in altered metabolites. (A) Low temperature, (B) PH3, and (C) combined treatment. Analysis was performed using the Kyoto Encyclopedia of Genes and Genomes database.
3.3. Treatment-Specific Metabolites as Biomarkers
Since enrichment and pathway impact scores were evaluated for the metabolites found under each stress condition, these results only showed overall tendencies. Therefore, alignment was performed to confirm which metabolites changed quantitatively in response to stress.
Metabolites detected in all treatments, but not in the mock control, were extracted as candidate treatment-specific indicators (Table 1). Metabolites involved in arachidonic acid metabolism and the immune system were identified in the PH3 treatment. Interestingly, 3-phosphohydroxypyruvate, an intermediate between 3-phosphoglycerate and pyruvate, was detected in all stresses. 3-phosphohydroxypyruvate generates α-ketoglutarate, a major TCA cycle intermediate, during its conversion to 3-phosphoserine [36]. 3-phosphoserine generates the intermediate serine and the final product glycine. Glycine then binds to the TCA cycle intermediate succinyl-CoA. The interconversion of glutamate to α-ketoglutarate produces various amino acids, including alanine, aspartate, and arginine. Recent studies showed that low temperature and PH3 are closely related to energy metabolism [37,38]. PH3 induces nerve excitement by acting on acetylcholine, resulting in excessive energy consumption. Therefore, stress-inducing conditions may stimulate the production of intermediate metabolites of pyruvate, and their overproduction has a clear impact on energy metabolic pathways.

Table 1.
Metabolites specifically found in each stress.
In addition, insects respond to stress by inhibiting or inactivating metabolic pathways. Therefore, the up- or downregulation of metabolites compared to the control was sorted because they can be used as indicators for each stress. The amino acids D-proline (Pro) and L-isoleucine (Ile) were detected in quantitative amounts in the mock control but not in the stressed groups. Suppression of the cryoprotectants Pro and Ile, which are known to accumulate in response to the cold in D. melanogaster, was contrary to previous results [39,40,41]. Interestingly, the downregulation of aconitic acid, an intermediate product of the TCA cycle, by low temperature and PH3, respectively, revealed an improved inhibitory effect.
In invertebrates, PH3 increases the signaling of the excitatory neurotransmitter acetylcholine by inhibiting acetylcholine esterase [15]. Persistent synaptic signaling by acetylcholine leads to hyperactivity, convulsions, and ultimately, excitotoxicity. PH3 directly interferes with mitochondrial respiration and causes a lack of energy metabolism, which can be confirmed by a decrease in oxygen consumption after 4 h of exposure to PH3 [42,43]. In addition, PH3 acts as a reducing agent, inhibiting cytochrome c oxidase and inducing the production of hydrogen peroxide, which is a reactive oxygen species (ROS) [44,45]. These PH3 responses ultimately resulted in metabolic inhibition, thereby supporting our finding that many metabolites were reduced or suppressed by PH3. Collectively, these results suggest that low temperature and PH3 share similar metabolic mechanisms that inhibit mitochondrial function and downregulate cellular metabolism.
3.4. Comparative Lipidomic Profiling by Stress
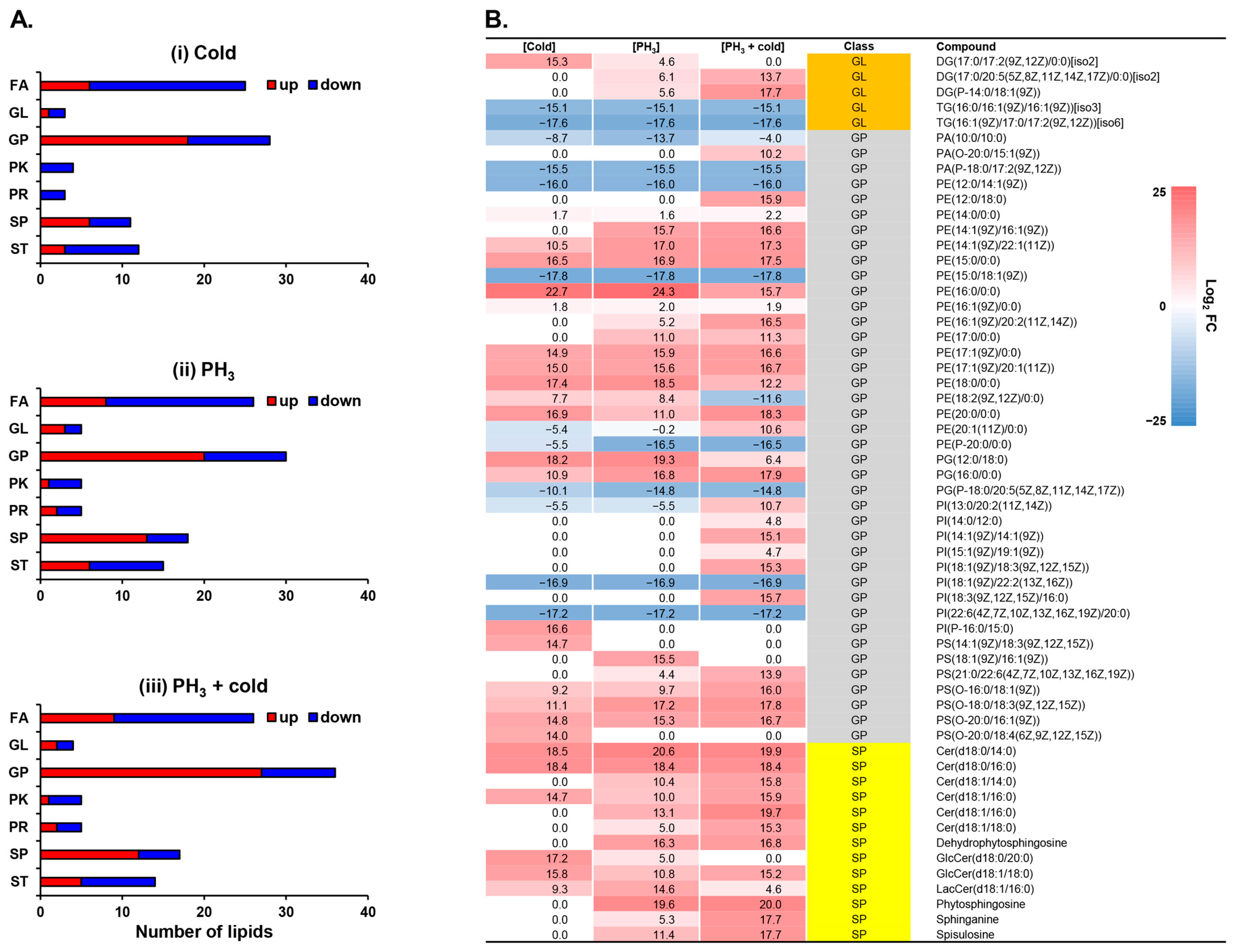
Pathway analysis revealed that the metabolites involved in glycosylphosphatidylinositol (GPI)-anchor biosynthesis were significantly regulated by low temperatures, PH3, and combined stress (Figure 1). GPI-anchors are covalently linked to the carboxyl terminus of proteins and mediate protein attachment to lipid bilayers [46,47]. GPI, a lipid anchor for cell surface proteins, is associated with lipid rafts enriched in sphingolipids and cholesterol. Therefore, to investigate the changes in lipid profiles in response to stress, 116 lipids were identified through multivariate statistical analysis and annotation (Figure 2). PCA and correlation analyses showed that the clusters of each stress were well aligned and clearly distinguished from the mock control (Supplementary Figure S3). Most lipid classes were quantitatively altered, including fatty acids (FAs), glycerophospholipids (GPs), sphingolipids (SPs), and sterol lipids (STs), but not glycerolipids (GLs), polyketides (PKs), or prenols (PRs) (Figure 2A).
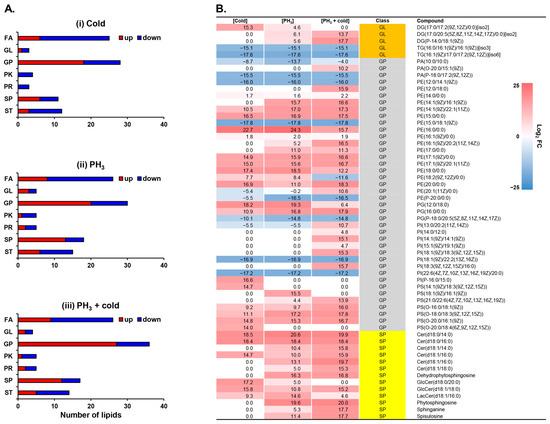
Figure 2.
Lipidomic profiling altered by exposure to low temperature and PH3. (A) The number of lipids showing relative increases and decreases in D. suzukii after stress. (B) Heatmap of membrane-associated lipids. FA: fatty acid; GL: glycerolipid; GP: glycerophospholipid; PK: polyketide; PR: prenol lipid; SP: sphingolipid; ST: sterol lipid.
In this study, each stress condition revealed significant regulation of cell surface-related lipids, such as GPs and SPs (Table 1 and Figure 2A). A recent study has shown that lipids provide an energy source for PH3-resistant insects to survive and an environment suitable for protecting mitochondria from PH3 [48]. In D. suzukii, phospholipids in the cell membrane are mainly composed of phosphatidylethanolamine (PE) and a GP class, and low temperatures cause quantitative differences [49]. SPs, components of lipid rafts, are involved in cell membrane receptors and signal transduction, and low temperatures cause changes in the structure and profile of lipid rafts [50,51,52]. Low temperatures can induce changes in the phospholipid bilayer properties of cell membranes, thereby damaging their integrity [53]. These changes in membrane fluidity can lead to neuromuscular dysfunction, chills coma, and ultimately death [54,55,56,57]. Therefore, the altered levels of cell surface-related lipid GLs, GPs, and SPs are presented for each type of stress (Figure 2B). Overall, many metabolites were upregulated compared to the mock control. Heatmap analysis showed that sphingolipids were upregulated by stress and were synergistically affected by the combined treatment. Interestingly, in the GP class, PE and PS were upregulated by the combination treatment, whereas PA, PG, and PI were downregulated. Lipids are the main components of the fat body in insects and most lipids are stored in the form of triglycerides (TGs) [58,59]. In contrast, the major lipid diglyceride (DG) in insect hemolymph increases rapidly during energy requirements such as flight [59,60]. Considering the mechanism of action of PH3 in relation to energy depletion, the increase in DG and decrease in TG in response to stress suggests that PH3 affects the energy metabolic pathways of D. suzukii.
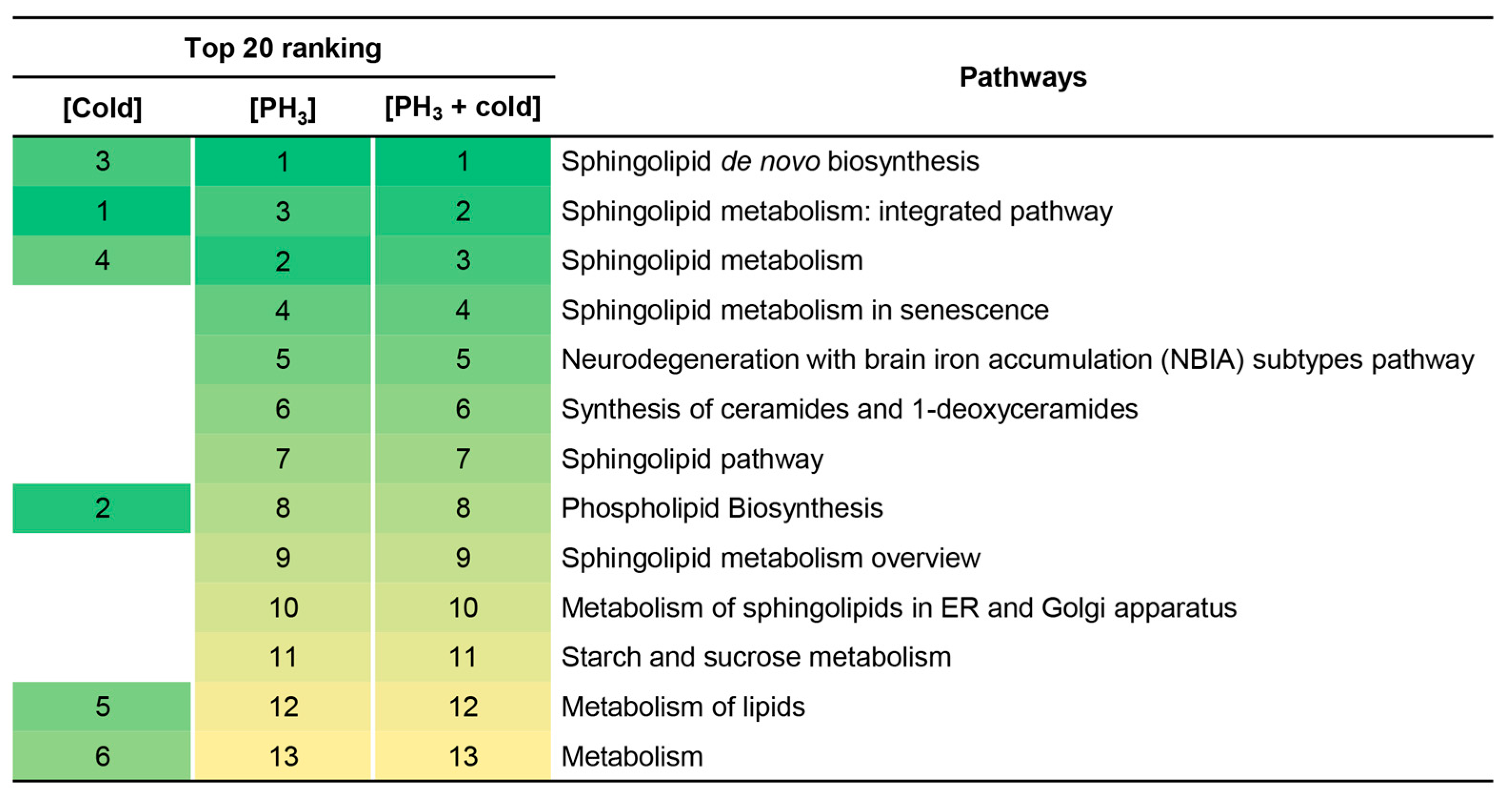
In addition, the metabolome set enrichment analysis revealed that sphingolipid-related metabolic pathways were primarily affected by stress (Figure 3). There was no difference between the fumigant alone and the combined treatment, but this result may be due to the effect of PH3 already prior to the mechanism of action of low temperature on sphingolipids. These results support the reason why fewer metabolic pathways were changed in combined treatment (Figure 2).
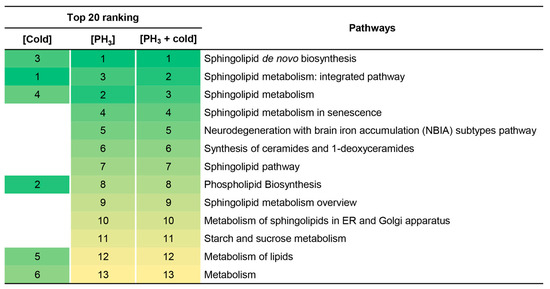
Figure 3.
Top 20 signaling pathways enriched at low temperature, PH3, and combined treatment. Numbers and colors indicate the ranking (high: green; low: yellow) of the respective signaling pathways.
4. Conclusions
Since studies on fumigants or low temperatures in the D. suzukii model are individual, research on metabolic mechanisms is required to understand the synergistic effect of PH3, which inhibits cytochrome oxidase activity, induces ROS production, and regulates metabolism at low temperatures. Most metabolites acted on D. suzukii metabolic pathways related to amino acid, lipid, and energy biosynthesis. In particular, the synergistic alteration of aconitic acid metabolites involved in the TCA cycle may be an important indicator of physiological changes in D. suzukii. In addition, the altered levels of the cell membrane lipids GP and SP revealed the synergistic effect of PH3 and low temperatures. Since these metabolites were specifically detected in each stress condition, they can be used as indicators to determine whether the treatment was successfully performed. Therefore, this study provides useful information on treatment-specific biomarkers for low temperature or fumigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo14100526/s1, Figure S1: Total ion current profile and distribution of altered metabolites; Figure S2: Comparative analysis of expression patterns between stresses according to metabolic changes; Figure S3: Comparative analysis of expression patterns between stresses according to lipidomic changes.
Author Contributions
Conceptualization, J.L., H.-K.K., J.-C.J., S.-J.S., G.-H.K., H.-N.K. and D.-W.L.; Data curation, J.L., H.-K.K., G.-H.K., H.-N.K. and D.-W.L.; Formal analysis, J.L., J.-C.J., S.-J.S., H.-N.K. and D.-W.L.; Funding acquisition, J.L., G.-H.K. and D.-W.L.; Investigation, J.L., J.-C.J., S.-J.S., H.-N.K. and D.-W.L.; Methodology, J.L., H.-N.K. and D.-W.L.; Project administration, G.-H.K. and H.-N.K.; Resources, J.-C.J., S.-J.S. and H.-N.K.; Software, J.L., H.-N.K. and D.-W.L.; Supervision, G.-H.K., H.-N.K. and D.-W.L.; Validation, J.L., H.-N.K. and D.-W.L.; Visualization, J.L., H.-K.K., H.-N.K. and D.-W.L.; Writing—original draft, J.L., H.-K.K., G.-H.K., H.-N.K. and D.-W.L.; Writing—review and editing, J.L., H.-K.K., G.-H.K., H.-N.K. and D.-W.L. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc., via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Animal and Plant Quarantine Agency, Republic of Korea [grant no. Z-1543086-2021-23-05]. This research was funded by the Korea Basic Science Institute (National Research Facilities and Equipment Center) and supported by the Ministry of Education [grant no. 2019R1A6C1010044]. This research was funded by the NRF Basic Science Research Program grant and supported by the Ministry of Education [grant no. RS-2023-00245952].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in insert article or supplementary material here.
Acknowledgments
We would like to thank Bong-Su Kim (Plant Quarantine Technology Center, Animal and Plant Quarantine Agency, Gimcheon, Republic of Korea) for providing D. suzukii.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atallah, J.; Teixeira, L.; Salazar, R.; Zaragoza, G.; Kopp, A. The making of a pest: The evolution of a fruit-enetrating ovipositor in Drosophila suzukii and related species. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20132840. [Google Scholar]
- Jeon, J.-C.; Kim, H.-K.; Koo, H.-N.; Kim, B.-S.; Yang, J.-O.; Kim, G.-H. Synergistic effect of cold treatment combined with ethyl formate fumigation against Drosophila suzukii (Diptera: Drosophilidae). Insects 2022, 13, 664. [Google Scholar] [CrossRef] [PubMed]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.; Grunwald Kadow, T.C.; Gompel, C.; Prud’homme, B. Evolution of multiple sensory systems drives novel egg-laying behavior in the fruit pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef]
- Muto, L.; Kamimura, Y.; Tanaka, K.M.; Takahashi, A. An innovative ovipositor for niche exploitation impacts genital coevolution between sexes in a fruit-damaging Drosophila. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20181635. [Google Scholar] [CrossRef]
- Seok, S.-J.; Kim, H.-K.; Koo, H.-N.; Kim, G.-H. Combined Effects of Cold Treatment and Phosphine in Drosophila suzukii (Diptera: Drosophilidae). Appl. Sci. 2022, 12, 12531. [Google Scholar] [CrossRef]
- Biondi, A.; Traugott, M.; Desneux, N. Special issue on Drosophila suzukii: From global invasion to sustainable control. J. Pest Sci. 2016, 89, 603–604. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Woltz, J.M.; Lee, J.C. Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol. Control. 2017, 110, 62–69. [Google Scholar] [CrossRef]
- Adrion, J.R.; Kousathanas, A.; Pascual, M.; Burrack, H.J.; Haddad, N.M.; Bergland, A.O.; Machado, H.; Sackton, T.B.; Schlenke, T.A.; Watada, M.; et al. Drosophila suzukii: The genetic footprint of a recent, worldwide invasion. Mol. Biol. Evol. 2014, 31, 3148–3163. [Google Scholar] [CrossRef]
- Berry, J.A. Drosophila suzukii: Pathways and pathway management by regulation. In Drosophila suzukii Management; Springer: Cham, Switzerland, 2020; pp. 29–39. [Google Scholar]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Hauser, M. A historic account of the invasion of Drosophila suzukii (Matsumura)(Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag. Sci. 2011, 67, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Ebert, P.R. Pesticidal toxicity of phosphine and its interaction with other pest control treatments. Curr. Issues Mol. Biol. 2023, 45, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Athié, I.; Gomes, R.A.; Bolonhezi, S.; Valentini, S.R.; De Castro, M.F.P.M. Effects of carbon dioxide and phosphine mixtures on resistant populations of stored-grain insects. J. Stored Prod. Res. 1998, 34, 27–32. [Google Scholar] [CrossRef]
- Constantin, M.; Jagadeesan, R.; Chandra, K.; Ebert, P.; Nayak, M.K. Synergism between phosphine (PH3) and carbon dioxide (CO2): Implications for managing PH3 resistance in rusty grain beetle (Laemophloeidae: Coleoptera). J. Econ. Entomol. 2020, 113, 1999–2006. [Google Scholar] [CrossRef]
- Manivannan, S.; Koshy, G.E.; Patil, S.A. Response of phosphine-resistant mixed-age cultures of lesser grain borer, Rhyzopertha dominica (F.) to different phosphine-carbon dioxide mixtures. J. Stored Prod. Res. 2016, 69, 175–178. [Google Scholar] [CrossRef]
- Nath, N.S.; Bhattacharya, I.; Tuck, A.G.; Schlipalius, D.I.; Ebert, P.R. Mechanisms of phosphine toxicity. J. Toxicol. 2011, 2011, 494168. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Athanassiou, C.G. A review of insect cold hardiness and its potential in stored product insect control. Crop Prot. 2017, 91, 93–99. [Google Scholar] [CrossRef]
- Chaudhry, M. Review a review of the mechanisms involved in the action of phosphine as an insecticide and phosphine resistance in stored-product insects. Pestic. Sci. 1997, 49, 213–228. [Google Scholar] [CrossRef]
- Chaudhry, M. Phosphine resistance. Pestic. Outlook 2000, 11, 88–91. [Google Scholar] [CrossRef]
- Dohino, T.; Masaki, S.; Matsuoka, I.; Tanno, M.; Takano, T. Low temperature as an alternative to fumigation for disinfesting stored products. Res. Bull. Plant Prot. Serv. 1999, 35, 5–14. [Google Scholar]
- Fields, P.G. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. Res. 1992, 28, 89–118. [Google Scholar] [CrossRef]
- Mason, L.J.; Strait, C.A. Stored product integrated pest management with extreme temperatures. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2019; pp. 141–177. [Google Scholar]
- De Lima, C.P.; Jessup, A.; Mansfield, E.; Daniels, D. Cold treatment of table grapes infested with Mediterranean fruit fly Ceratitis capitata (Wiedemann) and Queensland fruit fly Bactrocera tryoni (Froggatt) Diptera: Tephritidae. N. Z. J. Crop Hortic. Sci. 2011, 39, 95–105. [Google Scholar] [CrossRef]
- Benschoter, C. Low-temperature storage as a quarantine treatment for the Caribbean fruit fly (Diptera: Tephritidae) in Florida citrus. J. Econ. Entomol. 1984, 77, 1233–1235. [Google Scholar] [CrossRef]
- Saeed, N.; Tonina, L.; Battisti, A.; Mori, N. Postharvest short cold temperature treatment to preserve fruit quality after Drosophila suzukii damage. Int. J. Pest Manag. 2020, 66, 23–30. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Abdalla, N.A.; Taha, H.S.; Fári, M. Postharvest management of fruits and vegetables storage. Sustain. Agric. Rev. 2015, 15, 65–152. [Google Scholar]
- Kwon, T.H.; Park, C.G.; Lee, B.-H.; Zarders, D.R.; Roh, G.H.; Kendra, P.E.; Cham, D.H. Ethyl formate fumigation and ethyl formate plus cold treatment combination as potential phytosanitary quarantine treatments of Drosophila suzukii in blueberries. J. Asia-Pac. Entomol. 2021, 24, 129–135. [Google Scholar] [CrossRef]
- Burikam, I.; Sarnthoy, O.; Charernsom, K.; Kanno, T.; Homma, H. Cold temperature treatment for mangosteens infested with the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 1992, 85, 2298–2301. [Google Scholar] [CrossRef]
- Bo, L.; Fanhua, Z.; Yuejin, W. Toxicity of phosphine to Carposina niponensis (Lepidoptera: Carposinadae) at low temperature. J. Econ. Entomol. 2010, 103, 1988–1993. [Google Scholar] [CrossRef]
- Lee, S.; Moon, H.H.; Kim, H.-K.; Koo, H.-N.; Kim, G.-H. Proteomic Analysis of Drosophila suzukii (Diptera: Drosophilidae) by Fumigant and Low-temperature Combination Treatment. Korean J. Appl. Entomol. 2024, 63, 109–117. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Lee, J.; Lee, D.-W.; Jeong, B.-H. Large-scale lipidomic profiling identifies novel potential biomarkers for prion diseases and highlights lipid raft-related pathways. Vet. Res. 2021, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fulton, T.L.; Mirth, C.K.; Piper, M.D. Restricting a single amino acid cross-protects Drosophila melanogaster from nicotine poisoning through mTORC1 and GCN2 signalling. Open Biol. 2022, 12, 220319. [Google Scholar] [CrossRef]
- Chandel, N.S. Amino acid metabolism. Cold Spring Harbor Perspect. Biol. 2021, 13, a040584. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Insect cold hardiness: Metabolic, gene, and protein adaptation. Can. J. Zool. 2012, 90, 456–475. [Google Scholar] [CrossRef]
- Colinet, H.; Larvor, V.; Laparie, M.; Renault, D. Exploring the plastic response to cold acclimation through metabolomics. Funct. Ecol. 2012, 26, 711–722. [Google Scholar] [CrossRef]
- Koštál, V.; Korbelová, J.; Rozsypal, J.; Zahradníčková, H.; Cimlová, J.; Tomčala, A.; Šimek, P. Long-term cold acclimation extends survival time at 0°C and modifies the metabolomic profiles of the larvae of the fruit fly Drosophila melanogaster. PLoS ONE. 2011, 6, e25025. [Google Scholar] [CrossRef] [PubMed]
- Koštál, V.; Zahradníčková, H.; Šimek, P. Hyperprolinemic larvae of the drosophilid fly, Chymomyza costata, survive cryopreservation in liquid nitrogen. Proc. Natl. Acad. Sci. USA 2011, 108, 13041–13046. [Google Scholar] [CrossRef]
- Chefurka, W.; Kashi, K.; Bond, E. The effect of phosphine on electron transport in mitochondria. Pest. Biochem. Physiol. 1976, 6, 65–84. [Google Scholar] [CrossRef]
- Price, N. The effect of phosphine on respiration and mitochondrial oxidation in susceptible and resistant strains of Rhyzopertha dominica. Insect Biochem. 1980, 10, 65–71. [Google Scholar] [CrossRef]
- Bolter, C.J.; Chefurka, W. Extramitochondrial release of hydrogen peroxide from insect and mouse liver mitochondria using the respiratory inhibitors phosphine, myxothiazol, and antimycin and spectral analysis of inhibited cytochromes. Arch. Biochem. Biophys. 1990, 278, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schlipalius, D.I.; Valmas, N.; Tuck, A.G.; Jagadeesan, R.; Ma, L.; Kaur, R.; Goldinger, A.; Anderson, C.; Kuang, J.; Zuryn, S. A core metabolic enzyme mediates resistance to phosphine gas. Science 2012, 338, 807–810. [Google Scholar] [CrossRef]
- Borges, A.R.; Link, F.; Engstler, M.; Jones, N.G. The glycosylphosphatidylinositol anchor: A linchpin for cell surface versatility of trypanosomatids. Front. Cell. Dev. Biol. 2021, 9, 720536. [Google Scholar] [CrossRef]
- Kinoshita, T. Glycosylphosphatidylinositol (GPI) anchors: Biochemistry and cell biology: Introduction to a thematic review series. J. Lipid Res. 2016, 57, 4–5. [Google Scholar] [CrossRef]
- Alnajim, I.; Aldosary, N.; Agarwal, M.; Liu, T.; Du, X.; Ren, Y. Role of lipids in phosphine resistant stored-grain insect pests Tribolium castaneum and Rhyzopertha dominica. Insects 2022, 13, 798. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, T.; Colinet, H. Cold acclimation triggers lipidomic and metabolic adjustments in the spotted wing drosophila Drosophila suzukii (Matsumara). Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 316, R751–R763. [Google Scholar] [CrossRef]
- Bieberich, E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chem. Phys. Lipids. 2018, 216, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Simons, K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005, 118, 1099–1102. [Google Scholar] [CrossRef]
- van Meer, G.; Lisman, Q. Sphingolipid transport: Rafts and translocators. J. Biol. Chem. 2002, 277, 25855–25858. [Google Scholar] [CrossRef]
- Koštál, V. Cell structural modifications in insects at low temperature. In Low Temperature Biology of Insects; Cambridge University Press: Cambridge, MA, USA, 2010; pp. 116–140. [Google Scholar]
- Hosler, J.S.; Burns, J.E.; Esch, H.E. Flight muscle resting potential and species-specific differences in chill-coma. J. Insect Physiol. 2000, 46, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E. A primer on insect cold-tolerance. In Low Temperature Biology of Insects; Cambridge University Press: Cambridge, MA, USA, 2010; pp. 3–34. [Google Scholar]
- MacMillan, H.A.; Sinclair, B.J. Mechanisms underlying insect chill-coma. J. Insect Physiol. 2011, 57, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yocum, G.D.; Žďárek, J.; Joplin, K.H.; Lee, R.E., Jr.; Smith, D.C.; Manter, K.D.; Denlinger, D.L. Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J. Insect Physiol. 1994, 40, 13–21. [Google Scholar] [CrossRef]
- Ad, M.; Van der Horst, D.J.; Van Marrewijk, W.J. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Jutsum, A.; Goldsworthy, G. Fuels for flight in Locusta. J. Insect Physiol. 1976, 22, 243–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).