Oreo Cookie Treatment Lowers LDL Cholesterol More Than High-Intensity Statin therapy in a Lean Mass Hyper-Responder on a Ketogenic Diet: A Curious Crossover Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Description
2.2. Baseline Diet and Lifestyle
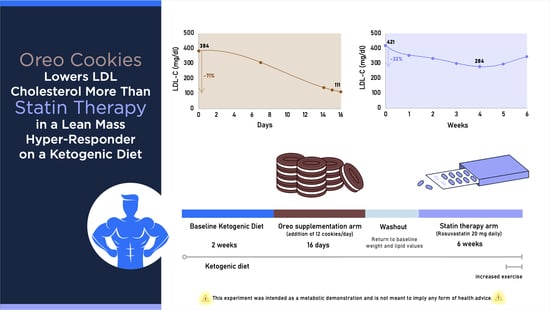
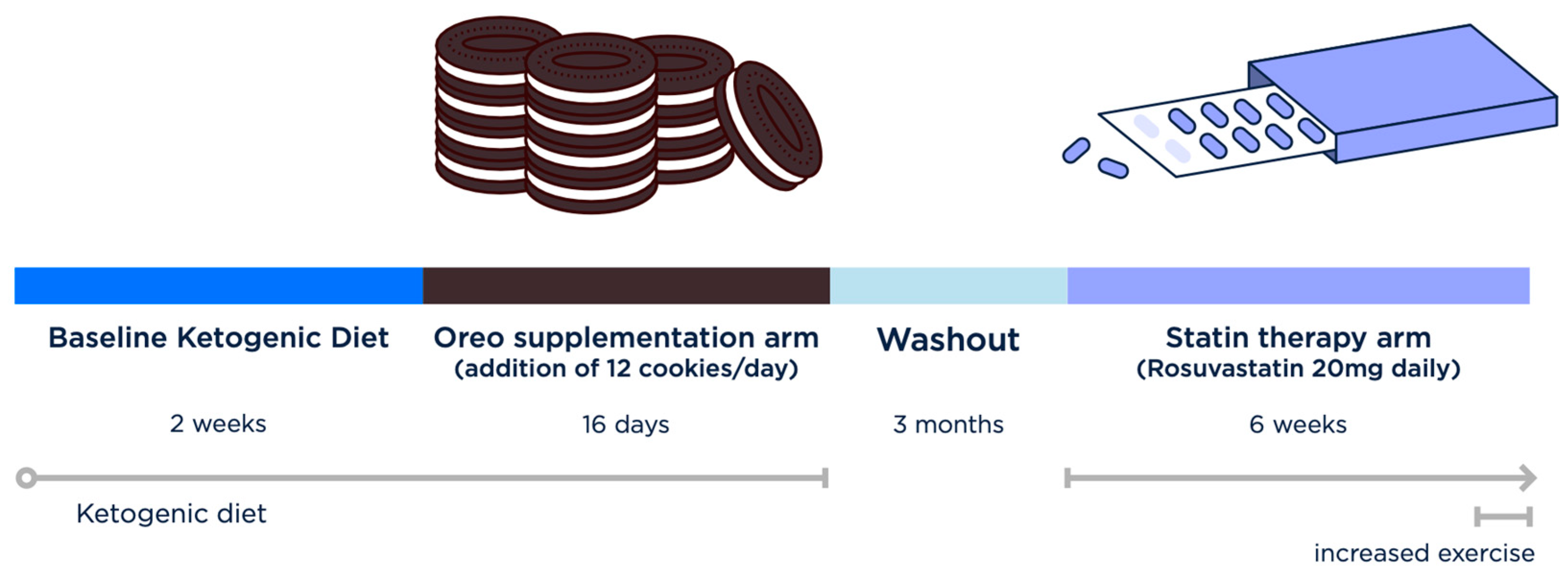
2.3. Interventions
2.4. Blood Work
2.5. Ethics and Safety
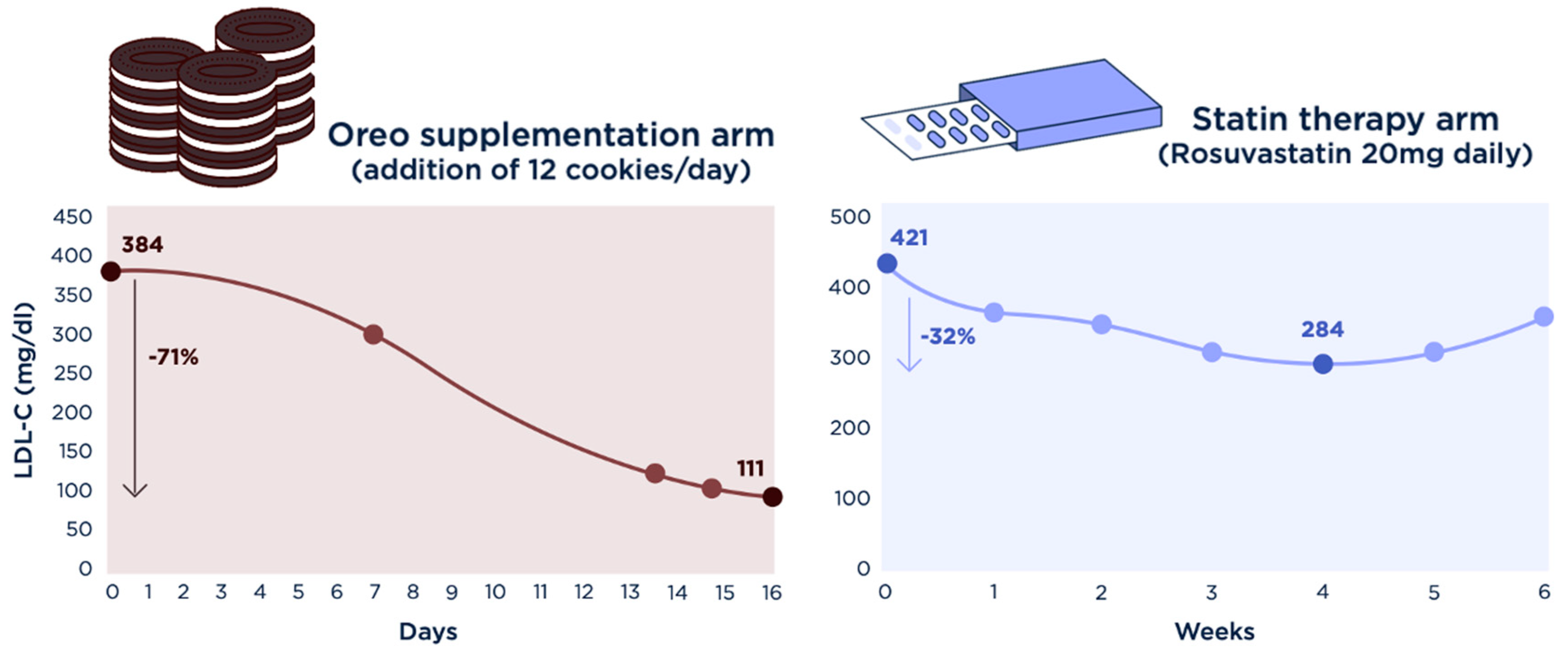
3. Results
4. Discussion
4.1. The Lipid Energy Model Predicts Oreo Cookie Treatment Will Lower LDL Cholesterol
4.2. Evidence Is Lacking to Suggest Saturated Fat Is a Driver of the LMHR Phenotype
4.3. Increased Physical Activity in Week 6 of Statin Arm Increases LDL-C
4.4. Next Step: A Crossover Trial for LDL Cholesterol Lowering Approaches among LMHR
4.5. Additional Learnings and Limitations
4.5.1. Impact of Oreos on Ketosis
4.5.2. Next Step Study Considerations
4.6. Ethical Considerations of Self-Experimentation and the Citizen Scientist Movement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Emergencias 2020, 6, CD001903. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Danan, A.; Westman, E.C.; Saslow, L.R.; Ede, G. The Ketogenic Diet for Refractory Mental Illness: A Retrospective Analysis of 31 Inpatients. Front. Psychiatry 2022, 13, 951376. [Google Scholar] [CrossRef] [PubMed]
- Cukoski, S.; Lindemann, C.H.; Arjune, S.; Todorova, P.; Brecht, T.; Kühn, A.; Oehm, S.; Strubl, S.; Becker, I.; Kämmerer, U.; et al. Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease: KETO-ADPKD—A randomized controlled trial. Cell Rep. Med. 2023, 4, 101283. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Feldman, D.; Soto-Mota, A.; Kalayjian, T.; Ludwig, D.S. Elevated LDL Cholesterol with a Carbohydrate-Restricted Diet: Evidence for a “Lean Mass Hyper-Responder” Phenotype. Curr. Dev. Nutr. 2022, 6, nzab144. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mota, A.; Flores-Jurado, Y.; Norwitz, N.G.; Feldman, D.; A Pereira, M.; Danaei, G.; Ludwig, D.S. Increased LDL-cholesterol on a low-carbohydrate diet in adults with normal but not high body weight: A meta-analysis. Am. J. Clin. Nutr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Soto-Mota, A.; Kaplan, B.; Ludwig, D.S.; Budoff, M.; Kontush, A.; Feldman, D. The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets. Metabolites 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, J.; Liu, H.; Huang, R.; Yan, X.; Song, M.; Tan, G.; Zhi, F. Ketone body β-hydroxybutyrate ameliorates colitis by promoting M2 macrophage polarization through the STAT6-dependent signaling pathway. BMC Med. 2022, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A. HDL and Reverse Remnant-Cholesterol Transport (RRT): Relevance to Cardiovascular Disease. Trends Mol. Med. 2020, 26, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, G.; Arjuman, A.; Suneja, S.; Das, C.; Chandra, N.C. The association between insulin and low-density lipoprotein receptors. Diabetes Vasc. Dis. Res. 2012, 9, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.T.; Yang, N.; Wong, J.M.; Mehta, T.; Allison, D.B.; Ludwig, D.S.; Ebbeling, C.B. Prolonged Glycemic Adaptation Following Transition From a Low- to High-Carbohydrate Diet: A Randomized Controlled Feeding Trial. Diabetes Care 2022, 45, 576–584. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Oreo (12 Cookies) | Statin | |
|---|---|---|---|
| Calories | 2616 | 3350 | 2616 |
| Total Fat (g) | 235 | 263 | 235 |
| SAT (g) | 55 | 63 | 55 |
| MUFA (g) | 154 | 170 | 154 |
| PUFA (g) | 25 | 29 | 25 |
| Protein (g) | 118 | 122 | 118 |
| Carb, total (g) | 20 | 120 | 20 |
| Fiber (g) | 9 | 11 | 9 |
| Baseline | Oreo (12 Cookies) | Washout | Rosuvastatin, 20 mg | ||||||||
| Day 0 | Day 7 | Day 16 | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | ||
| TC | 494 | 421 | 232 | 539 | 478 | 458 | 406 | 393 | 407 | 468 | |
| LDL-C | 384 | 308 | 111 | 421 | 354 | 340 | 294 | 284 * | 295 | 346 | |
| HDL-C | 99 | 102 | 113 | 107 | 116 | 107 | 102 | 102 | 102 | 112 | |
| TG | 57 | 56 | 39 | 54 | 38 | 54 | 50 | 34 | 49 | 48 | |
| LDL-C reduction, relative (absolute): −71% (−273 mg/dL) | LDL-C reduction *, relative (absolute): −32.5% (−137 mg/dL) | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norwitz, N.G.; Cromwell, W.C. Oreo Cookie Treatment Lowers LDL Cholesterol More Than High-Intensity Statin therapy in a Lean Mass Hyper-Responder on a Ketogenic Diet: A Curious Crossover Experiment. Metabolites 2024, 14, 73. https://doi.org/10.3390/metabo14010073
Norwitz NG, Cromwell WC. Oreo Cookie Treatment Lowers LDL Cholesterol More Than High-Intensity Statin therapy in a Lean Mass Hyper-Responder on a Ketogenic Diet: A Curious Crossover Experiment. Metabolites. 2024; 14(1):73. https://doi.org/10.3390/metabo14010073
Chicago/Turabian StyleNorwitz, Nicholas G., and William C. Cromwell. 2024. "Oreo Cookie Treatment Lowers LDL Cholesterol More Than High-Intensity Statin therapy in a Lean Mass Hyper-Responder on a Ketogenic Diet: A Curious Crossover Experiment" Metabolites 14, no. 1: 73. https://doi.org/10.3390/metabo14010073
APA StyleNorwitz, N. G., & Cromwell, W. C. (2024). Oreo Cookie Treatment Lowers LDL Cholesterol More Than High-Intensity Statin therapy in a Lean Mass Hyper-Responder on a Ketogenic Diet: A Curious Crossover Experiment. Metabolites, 14(1), 73. https://doi.org/10.3390/metabo14010073