Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Preparation
2.3. LC-MS Analysis
2.4. Multivariate Data Analysis
2.4.1. Untargeted Approach
2.4.2. Pseudo-Targeted Approach
2.5. Statistical Analysis
3. Results
3.1. Descriptive Data and Outcome Data
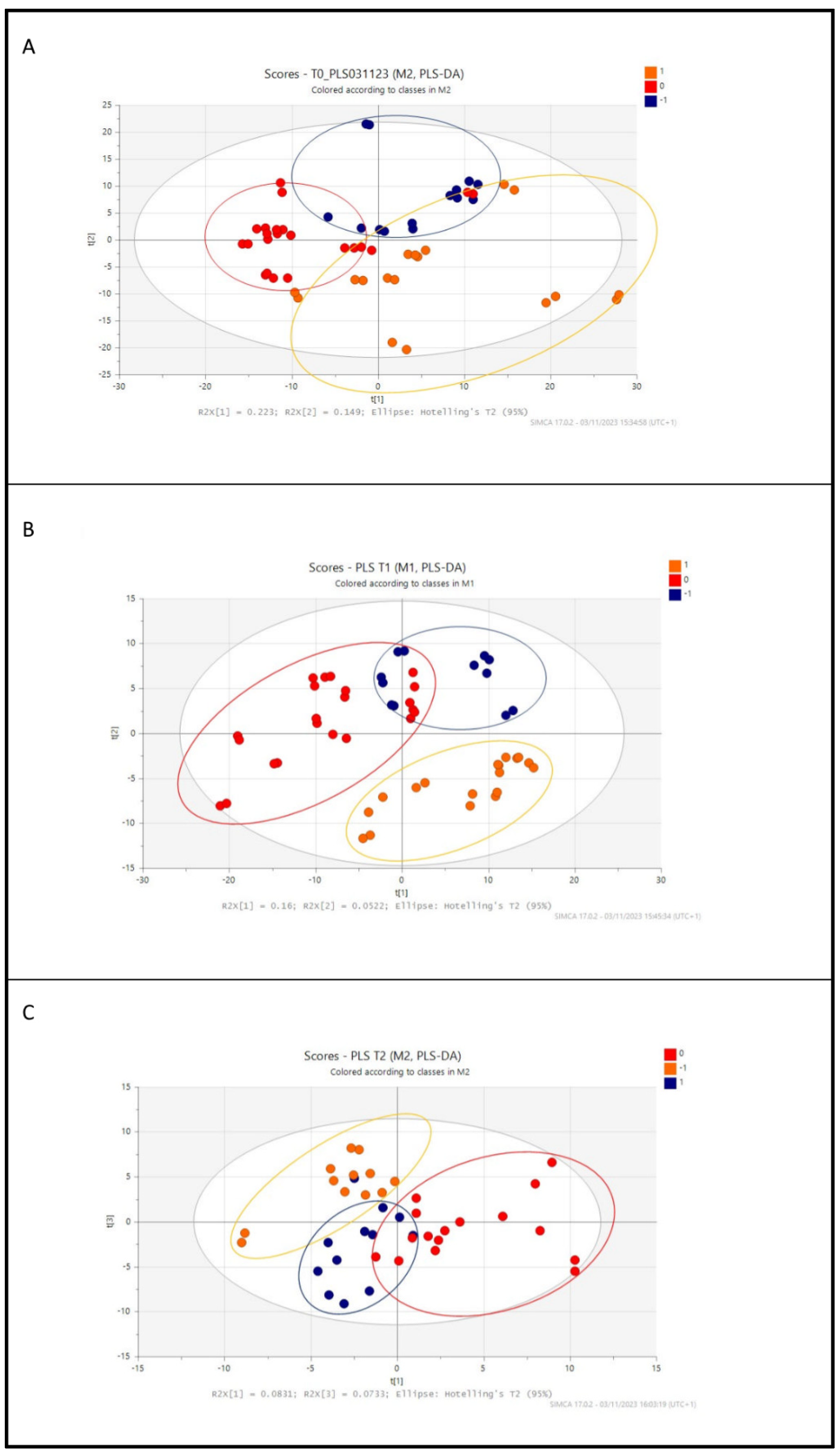
3.2. Metabolic Profile of Preterm Infants According to Diet: Untargeted and Targeted LC-MS Analysis
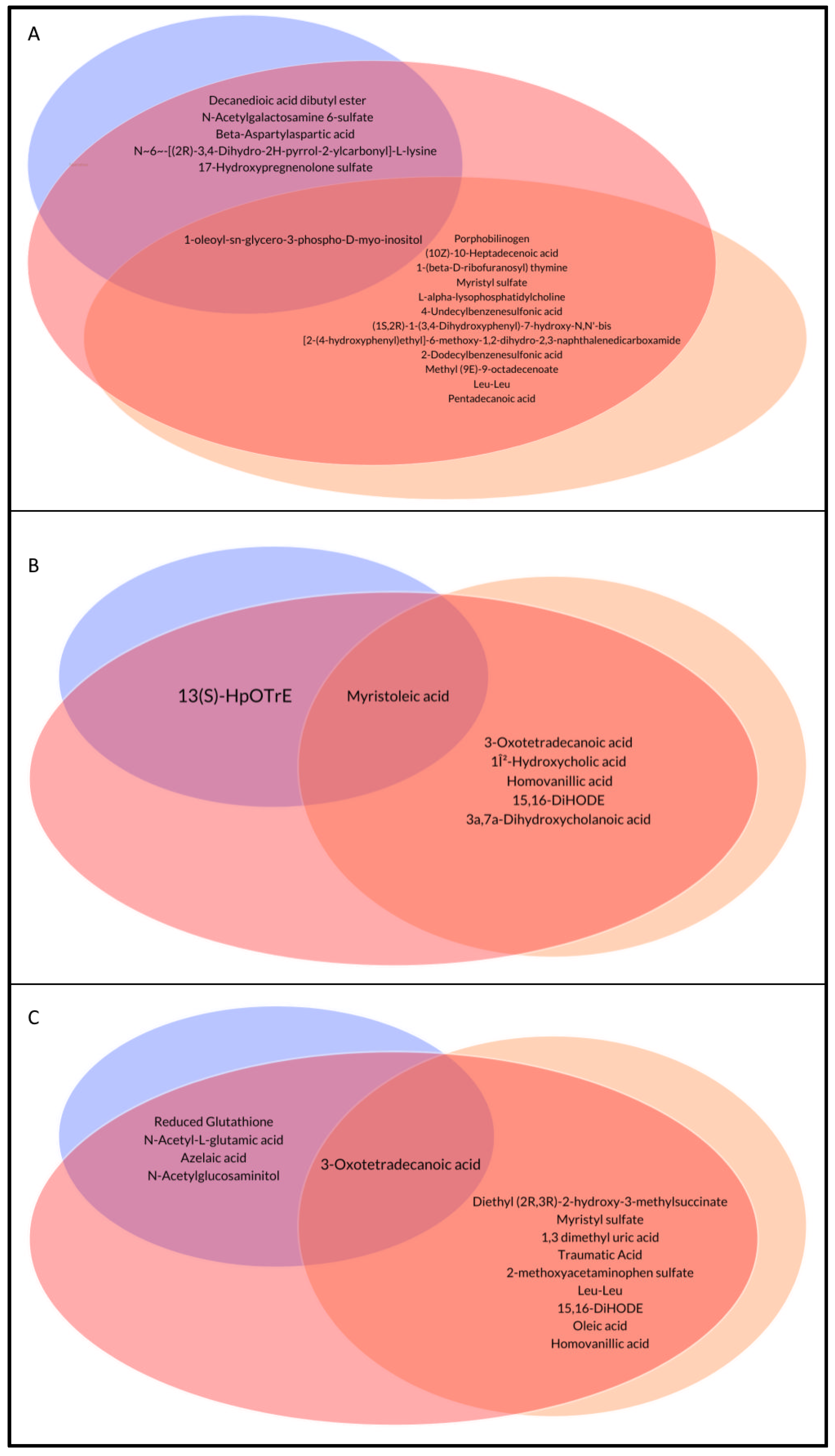
3.3. Analysis of the Identified Metabolites among Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Giannì, M.L.; Canani, R.B.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703. [Google Scholar] [CrossRef] [PubMed]
- Vendt, N.; Grunberg, H.; Tuure, T.; Malminiemi, O.; Wuolijoki, E.; Tillmann, V.; Sepp, E.; Korpela, R. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: Double-blind, randomized trial. J. Hum. Nutr. Diet. 2006, 19, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jakaitis, B.M.; Denning, P.W. Human breast milk and the gastrointestinal innate immune system. Clin. Perinatol. 2014, 41, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Perrone Serafina, L.E.; Giuseppe, D.B.; Maurizio, G.; Francesca, V.; Maria, T.; Marco, C.; Laura, T.A.; Giuseppe, B.; Mariangela, L. Newborn metabolomic profile mirrors that of mother in pregnancy. Med. Hypotheses 2020, 137, 109543. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host. Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Bai, Y.; Peng, W.; Su, W.W. Identification of Naringin Metabolites in Human Urine and Feces. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 647–656. [Google Scholar] [CrossRef]
- D’Urso, G.; Montoro, P.; Lai, C.; Piacente, S.; Sarais, G. LC-ESI/LTQOrbitrap/MS based metabolomics in analysis of leaves from Sardinia (Italy). Ind. Crop Prod. 2019, 128, 354–362. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C. Statistical Power Analysis for the Behavioural Sciences; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Gregory, K.E.; Samuel, B.S.; Houghteling, P.; Shan, G.; Ausubel, F.M.; Sadreyev, R.I.; Walker, W.A. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome 2016, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Larke, J.A.; Kuhn-Riordon, K.; Taft, D.H.; Sohn, K.; Iqbal, S.; Underwood, M.A.; Mills, D.A.; Slupsky, C.M. Preterm Infant Fecal Microbiota and Metabolite Profiles Are Modulated in a Probiotic Specific Manner. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; de Halleux, V.; Arciszewska, M.; Lach, P.; Pokhylko, V.; Klymenko, V.; Schoen, S.; Abrahamse-Berkeveld, M.; Mulder, K.A.; Porcel Rubio, R.; et al. A Partly Fermented Infant Formula with Postbiotics Including 3’-GL, Specific Oligosaccharides, 2’-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial. Nutrients 2020, 12, 3560. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wu, X.; Luo, W.; Chen, J.; Huang, Y.; Ma, K.; Zhang, C.; Wang, J.; Yang, Y.; Deng, M.; et al. Azelaic Acid Regulates the Renin-Angiotensin System and Improves Colitis Based on Network Pharmacology and Experimentation. ACS Omega 2023, 8, 15217–15228. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.; Jiang, H.; Motherway, M.O.; Oscarson, S.; van Sinderen, D. Glycosulfatase-Encoding Gene Cluster in UCC2003. Appl. Environ. Microb. 2016, 82, 6611–6623. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase metabolites of alpha-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016, 6, 31649. [Google Scholar] [CrossRef] [PubMed]
- Cavia-Saiz, M.; Arnaez, J.; Cilla, A.; Puente, L.; Garcia-Miralles, L.C.; Muniz, P. Biomarkers of Oxidative Stress in Healthy Infants within the First Three Days after Birth. Antioxidants 2023, 12, 1249. [Google Scholar] [CrossRef] [PubMed]
- Visca, A.; Bishop, C.T.; Hilton, S.; Hudson, V.M. Oral reduced L-glutathione improves growth in pediatric cystic fibrosis patients. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 802–810. [Google Scholar] [CrossRef] [PubMed]


| FM | FMPB | BM | |
|---|---|---|---|
| Gestational Age | 35 (34.5–35.4) | 35.6 (34.3–36.6) | 35.6 (34.6–36.1) |
| Gender (%) | F (55.6) M (44.4) | F (57.1) M (42.9) | F (63.6) M (36.4) |
| Delivery mode (%) | VD (11.1) CS (88.9) | VD (28.6) CS (71.4) | VD (36.4) CS (63.6) |
| Birth body weight (g) | 2444 ± 318 | 2417 ± 492 | 2367 ± 389 |
| Length (cm) | 47 ± 2.5 | 45 ± 2.3 | 46 ± 2.26 |
| Head circumference (cm) | 33 ± 1.22 | 32 ± 1.49 | 32 ± 1.27 |
| Apgar score 1 min | 8 (7–8) | 8 (8–8) | 8 (8–9) |
| Apgar score 5 min | 9 (8–9) | 9 (9–9) | 9 (9–10) |
| FMPB | BM | FM | FM vs. BM | FMPB vs. BM | |
|---|---|---|---|---|---|
| T0 | p_Value | p_Value | |||
| Porphobilinogen | 3.95 (0.5–5.13) | 0.3 (0.3–0.3) | 1,55 (0.3–3.98) | 0.061 | 0.001 * |
| (10Z)-10-Heptadecenoic acid | 5.9 (5.5–6.13) | 0.3 (0.3–5.3) | 2.65 (0.3–5.5) | 1 | 0.001 * |
| Decanedioic acid dibutyl ester | 5 (2.7–5) | 4.8 (3.25–5.5) | 2.2 (0.3–4.1) | 0.001 * | 1 |
| 1-(beta-D-ribofuranosyl) thymine | 5.55 (2.4–5.95) | 0.3 (0.3–0.3) | 0.3 (0.3–5.88) | 0.206 | 0.001 * |
| Myristyl sulfate | 4.3 (3.8–5.8) | 0.3 (0.3–3.8) | 2.2 (0.3–4.3) | 0.09 | 0.002 * |
| L-alpha-lysophosphatidylcholine | 3.25 (0.3–5.25) | 0.3 (0.3–0.3) | 0.3 (0.3–3.4) | 0.272 | 0.002 * |
| N-Acetylgalactosamine 6-sulfate | 3.95 (0.3–5.25) | 5.5 (0.3–6.15) | 0.3 (0.3–0.3) | 0.003 * | 0.414 |
| Beta-Aspartylaspartic acid | 0.3 (0.3–1.25) | 0.3 (0.3–0.3) | 5.4 (0.3–5.85) | 0.005 * | 1 |
| 4-Undecylbenzenesulfonic acid | 0.3 (0.3–0.3) | 0.3 (0.3–3.75) | 0.3 (0.3–0.3) | 0.156 | 0.007 * |
| (1S,2R)-1-(3,4-Dihydroxyphenyl)-7-hydroxy-N,N’-bis [2-(4-hydroxyphenyl)ethyl]-6-methoxy-1,2-dihydro-2,3-naphthalenedicarboxamide | 6.2 (5.725–6.7) | 5.6 (0.3–6.15) | 4.9 (0.3–6.05) | 1 | 0.026 * |
| 2-Dodecylbenzenesulfonic acid | 0.3 (0.3–0.3) | 0.9 (0.3–4.5) | 0.3 (0.3–4.6) | 1 | 0.011 * |
| N~6~-[(2R)-3,4-Dihydro-2H-pyrrol-2-ylcarbonyl]-L-lysine | 0.3 (0.3–4.6) | 0.3 (0.3–0.3) | 2.1 (0.3–5.9) | 0.011 * | 0.62 |
| 17-Hydroxypregnenolone sulfate | 0.3 (0.3–1.675) | 0.3 (0.3–0.3) | 0.3 (0.3–5.5) | 0.015 * | 0.912 |
| Methyl (9E)-9-octadecenoate | 3.8 (0.3–5.8) | 0.3 (0.3–0.3) | 0.3 (0.3–5.5) | 0.127 | 0.033 * |
| 1-oleoyl-sn-glycero-3-phospho-D-myo-inositol | 0.3 (0.3–0.3) | 0.3 (0.3–3.45) | 0.3 (0.3–4.575) | 1 | 0.101 |
| Leu-Leu | 3.9 (2–4.7) | 0.3 (0.3–3.3) | 2.3 (0.5–5.05) | 0.059 | 0.02 * |
| Pentadecanoic acid | 0.3 (0.3–4.9) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) | 0.413 | 0.043 * |
| FM vs. BM | FMPB vs. BM | ||||
| T1 | p_value | p_value | |||
| 3-Oxotetradecanoic acid | 5.8 (0.3–6.2) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) | 1 | 0.001 * |
| Taurohyocholic acid | 0.3 (0.3–0.5) | 5.7 (3.2–5.8) | 2.7 (0.3–4.8) | 0.023 * | 0.001 * |
| Cholic acid | 0.3 (0.3–0.3) | 0.3 (0.3–5.8) | 0.3 (0.3–0.3) | 0.017 * | 0.007 * |
| 1β-Hydroxycholic acid | 0.3 (0.3–0.3) | 2.55 (0.3–6.05) | 0.3 (0.3–0.3) | 0.113 | 0.02 * |
| Myristoleic acid | 5.5 (0.3–6.1) | 0.3 (0.3–3.2) | 0.3 (0.3–0.3) | 1.000 | 0.067 |
| 13(S)-HpOTrE | 2.6 (1.7–5.2) | 4.75 (3.7–5.35) | 2.6 (0.3–4.1) | 0.028 * | 0.388 |
| Homovanillic acid | 5.6 (0.3–6) | 0.3 (0.3–0.3) | 0.3 (0.3–0.3) | 1 | 0.037 * |
| 15,16-DiHODE | 3.7 (0.3–5.1) | 5 (4–6.2) | 4.3 (2.2–5.1) | 0.313 | 0.039 * |
| 3a,7a-Dihydroxycholanoic acid | 0.3 (0.3–0.3) | 0.3 (0.3–6.1) | 0.3 (0.3–0.3) | 0.296 | 0.043 * |
| FM vs. BM | FMPB vs. BM | ||||
| T2 | p_value | p_value | |||
| Diethyl (2R,3R)-2-hydroxy-3-methylsuccinate | 7.3 (7.1–7.5) | 6.4 (5.9–6.5) | 6 (5.7–6.6) | 1 | 0.001 * |
| Reduced Glutathione | 3.1 (0.3–6.1) | 5.1 (0.3–5.7) | 6.5 (6.4–6.5) | 0.001 * | 1 |
| N-Acetyl-L-glutamic acid | 5.4 (3.1–6.2) | 4.3 (2.3–5.9) | 6.8 (6.2–7) | 0.001 * | 1 |
| Myristyl sulfate | 4.3 (4.3–5) | 5.3 (4.8–6.3) | 5.8 (5.8–5.8) | 1.000 | 0.007 * |
| 1,3 dimethyl uric acid | 0.3 (0.3–0.3) | 2.8 (0.3–4.95) | 0.3 (0.3–1.7) | 0.055 | 0.002 * |
| Traumatic Acid | 5.7 (5.1–5.85) | 3.6 (1.7–4.85) | 5 (4.6–5.65) | 0.058 | 0.003 * |
| 2-methoxyacetaminophen sulfate | 2.85 (0.85–5.9) | 6.1 (5.4–6.55) | 5.45 (4.65–5.6) | 0.212 | 0.01 * |
| Leu-Leu | 4.3 (2.4–5.5) | 1.85 (0.85–3.55) | 2.2 (1.3–3.2) | 1 | 0.038 * |
| Azelaic acid | 6.7 (6.5–7.1) | 7.5 (6.7–7.5) | 6.7 (5.75–6.7) | 0.026 * | 0.17 |
| N-Acetylglucosaminitol | 5.5 (2.05–6.15) | 2.45 (0.3–5.4) | 5.8 (2.75–6.5) | 0.043 * | 1 |
| 15,16-DiHODE | 4.7 (4.15–5.3) | 5.5 (4.9–6) | 5.4 (5.15–5.9) | 0.978 | 0.021 * |
| Oleic acid | 5.8 (5.5–5.8) | 6.6 (5.8–6.6) | 5.8 (5.8–6.6) | 1 | 0.035 * |
| 3-Oxotetradecanoic acid | 0.3 (0.3–4.1) | 3.3 (0.3–6.25) | 5.8 (5.3–6) | 1 | 0.24 |
| Homovanillic acid | 5.8 (5.4–6.2) | 2.9 (0.65–5.65) | 5.3 (3.65–5.7) | 0.44 | 0.047 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bernardo, G.; D’Urso, G.; Spadarella, S.; Giordano, M.; Leone, G.; Casapullo, A. Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants. Metabolites 2024, 14, 72. https://doi.org/10.3390/metabo14010072
De Bernardo G, D’Urso G, Spadarella S, Giordano M, Leone G, Casapullo A. Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants. Metabolites. 2024; 14(1):72. https://doi.org/10.3390/metabo14010072
Chicago/Turabian StyleDe Bernardo, Giuseppe, Gilda D’Urso, Simona Spadarella, Maurizio Giordano, Giuseppina Leone, and Agostino Casapullo. 2024. "Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants" Metabolites 14, no. 1: 72. https://doi.org/10.3390/metabo14010072
APA StyleDe Bernardo, G., D’Urso, G., Spadarella, S., Giordano, M., Leone, G., & Casapullo, A. (2024). Analysis of the Fecal Metabolomic Profile in Breast vs. Different Formula Milk Feeding in Late Preterm Infants. Metabolites, 14(1), 72. https://doi.org/10.3390/metabo14010072







