Metabolomic Signatures of Brainstem in Mice following Acute and Subchronic Hydrogen Sulfide Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
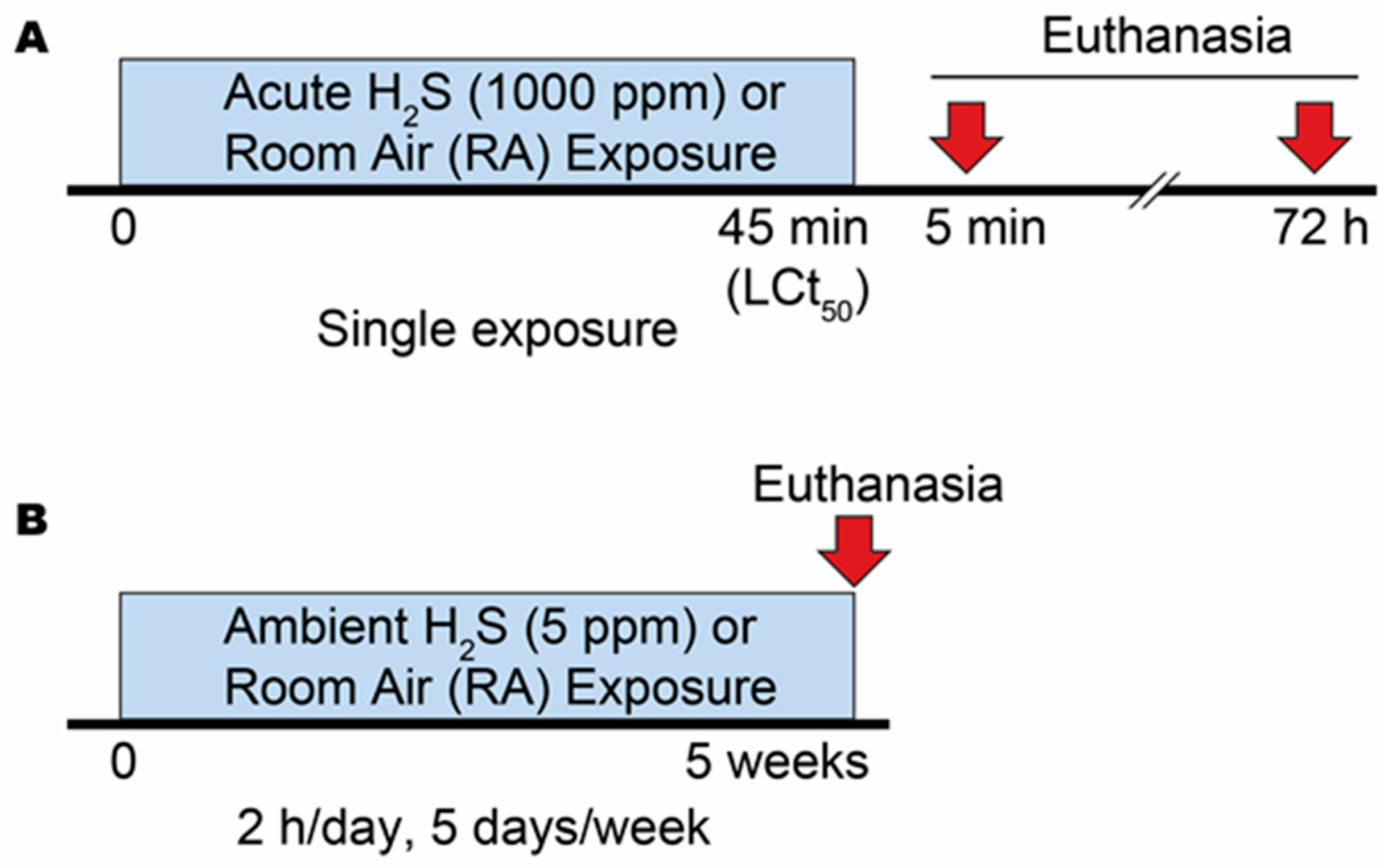
2.2. Gas Exposure
2.2.1. Acute Exposure Experiment
2.2.2. Ambient Exposure Experiment
2.3. Metabolomic Analysis
2.4. Data Analysis
2.5. Enrichment Analysis
2.6. Heatmap
2.7. Statistical Analysis
3. Results
3.1. Exposure to Acute and Ambient Hydrogen Sulfide
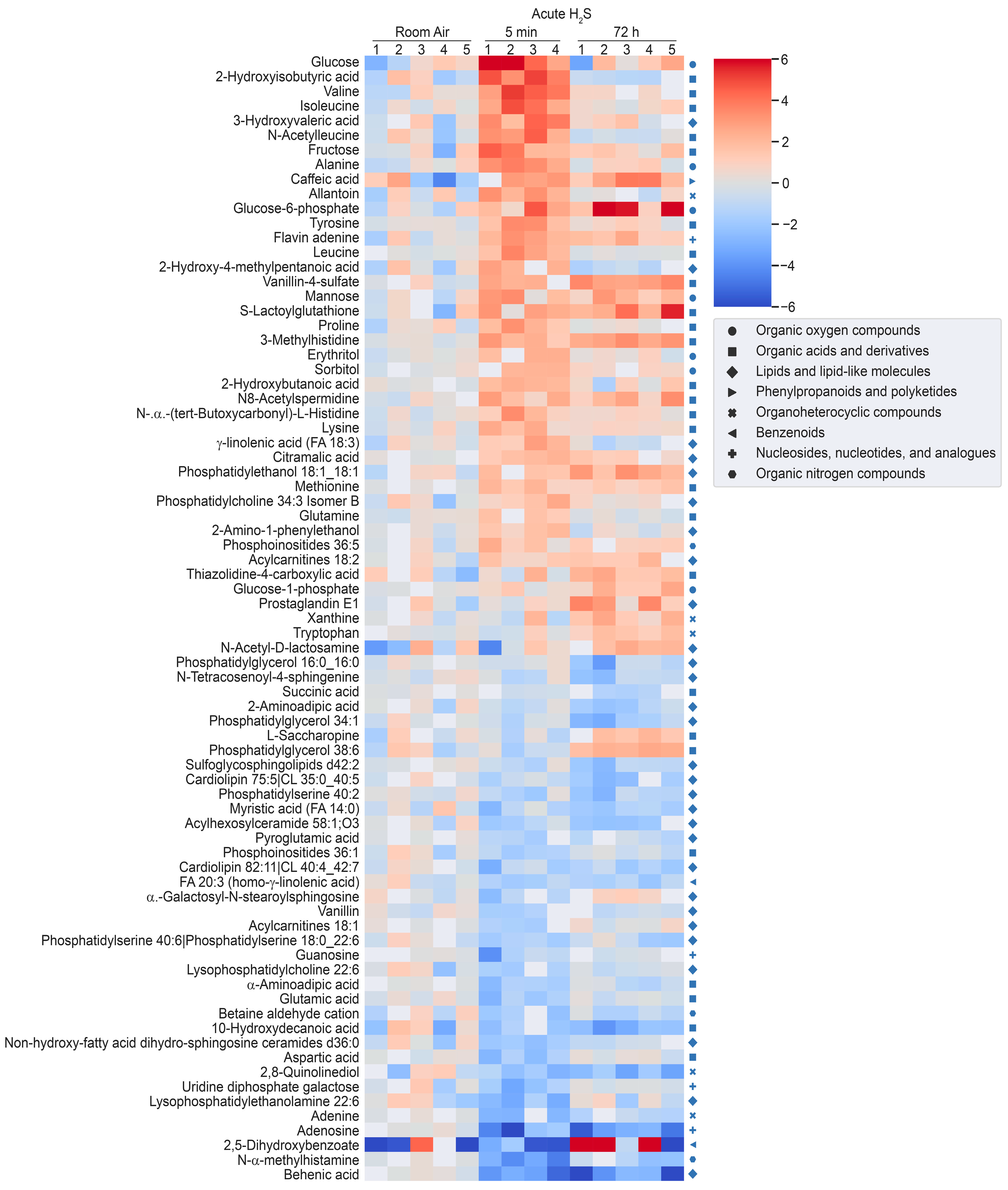
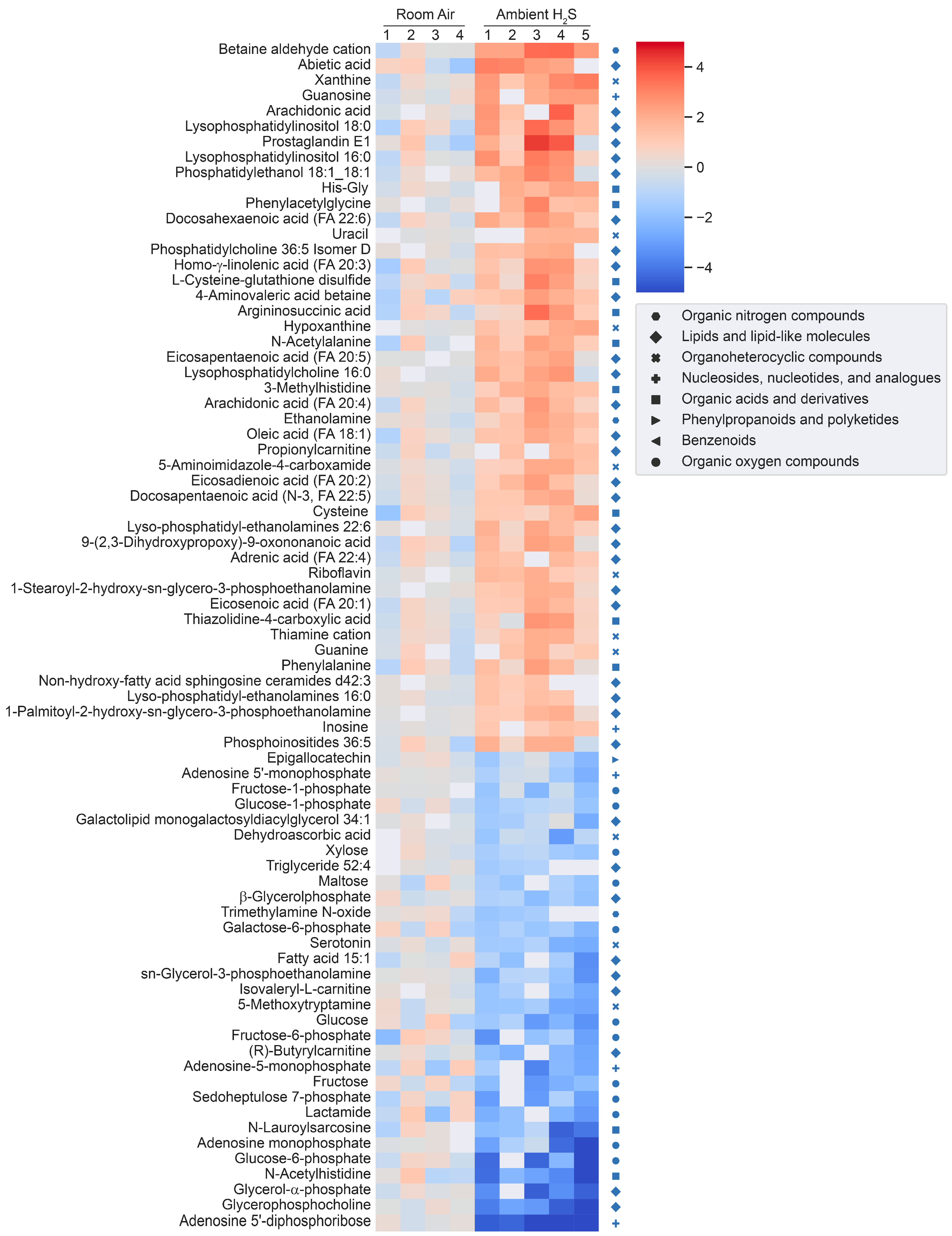
3.2. Metabolomic Changes in the Brainstem following Acute and Ambient Hydrogen Sulfide
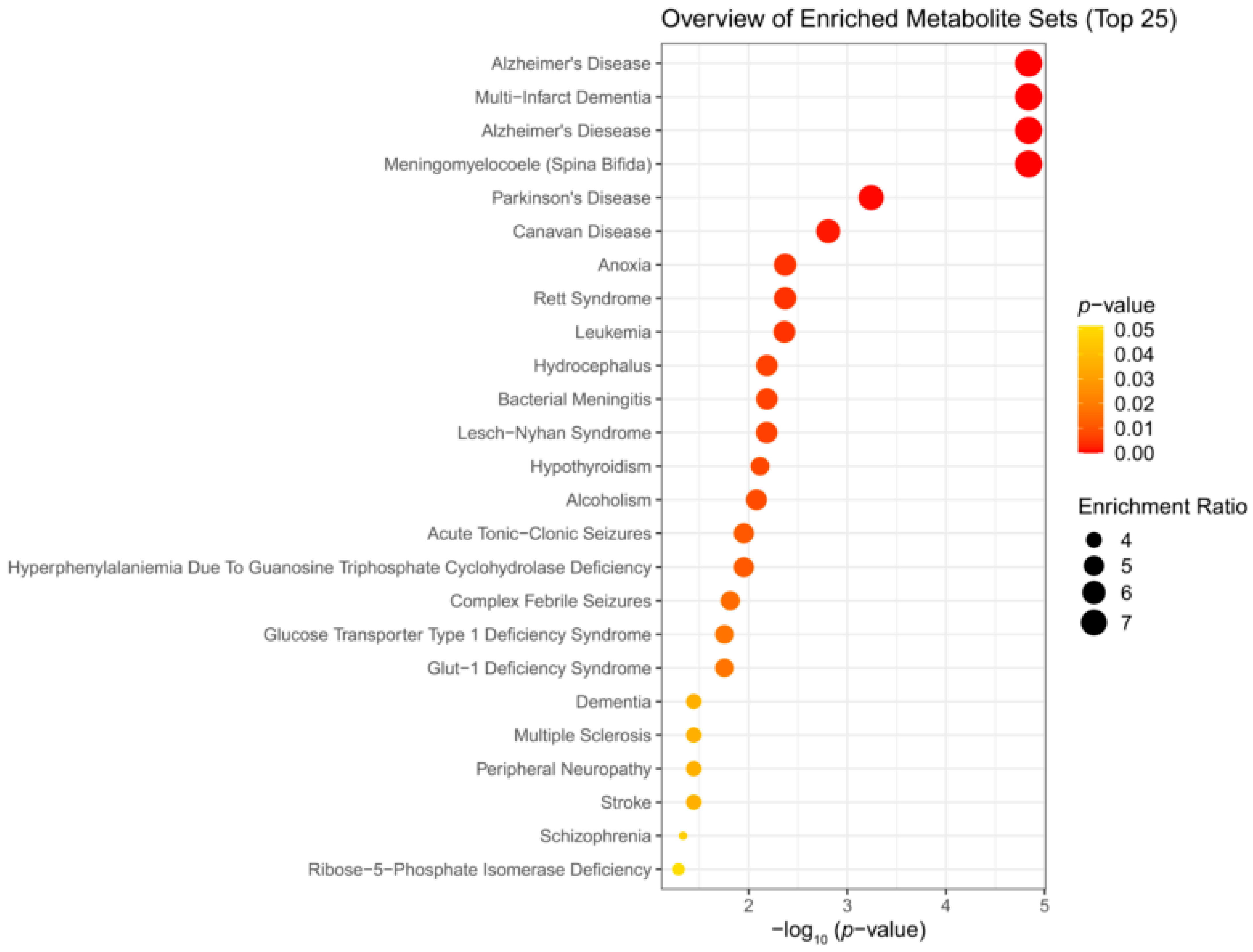
3.3. Enrichment Analysis
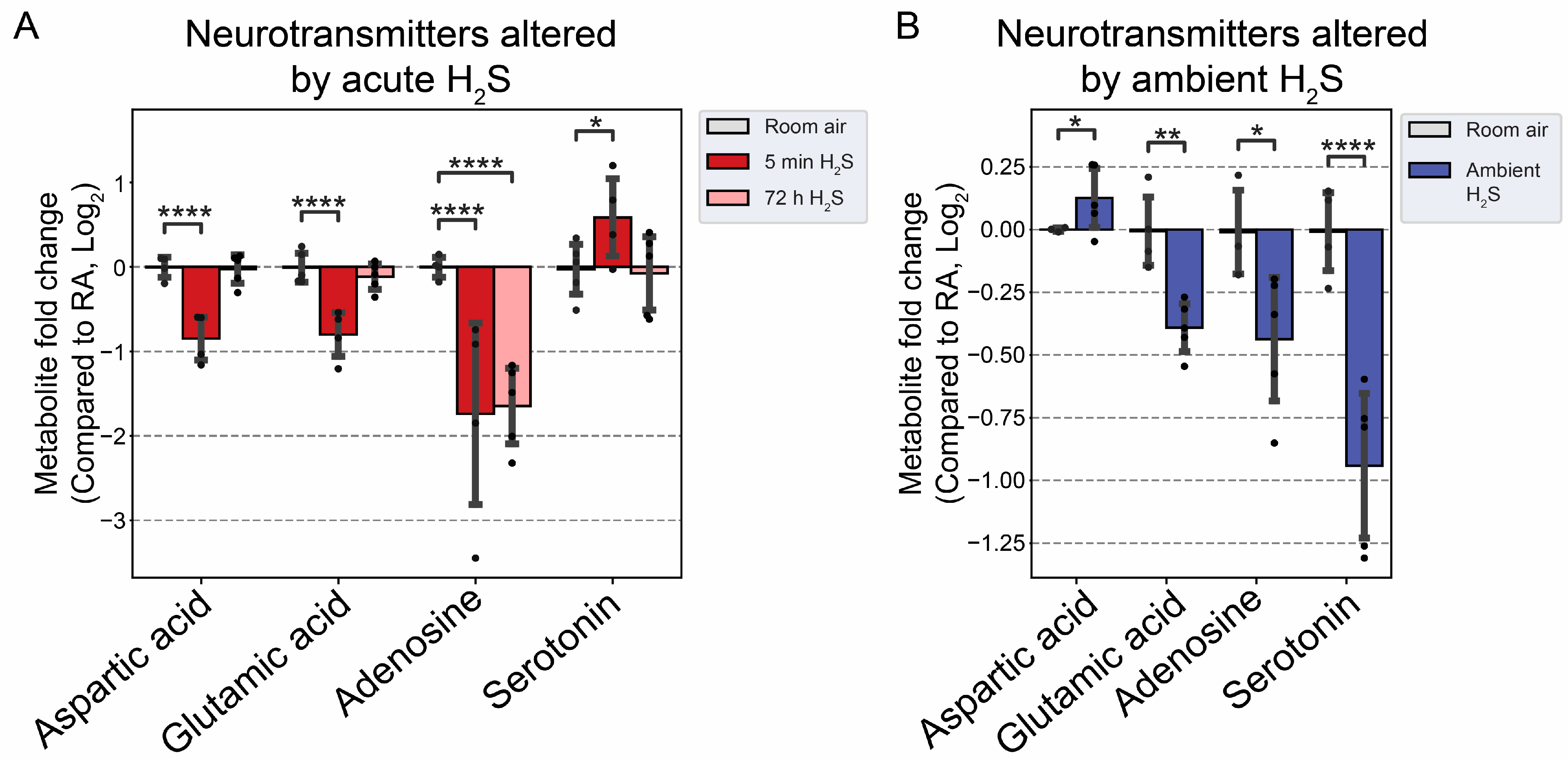
3.4. H2S Dysregulated Brainstem Neurotransmitters
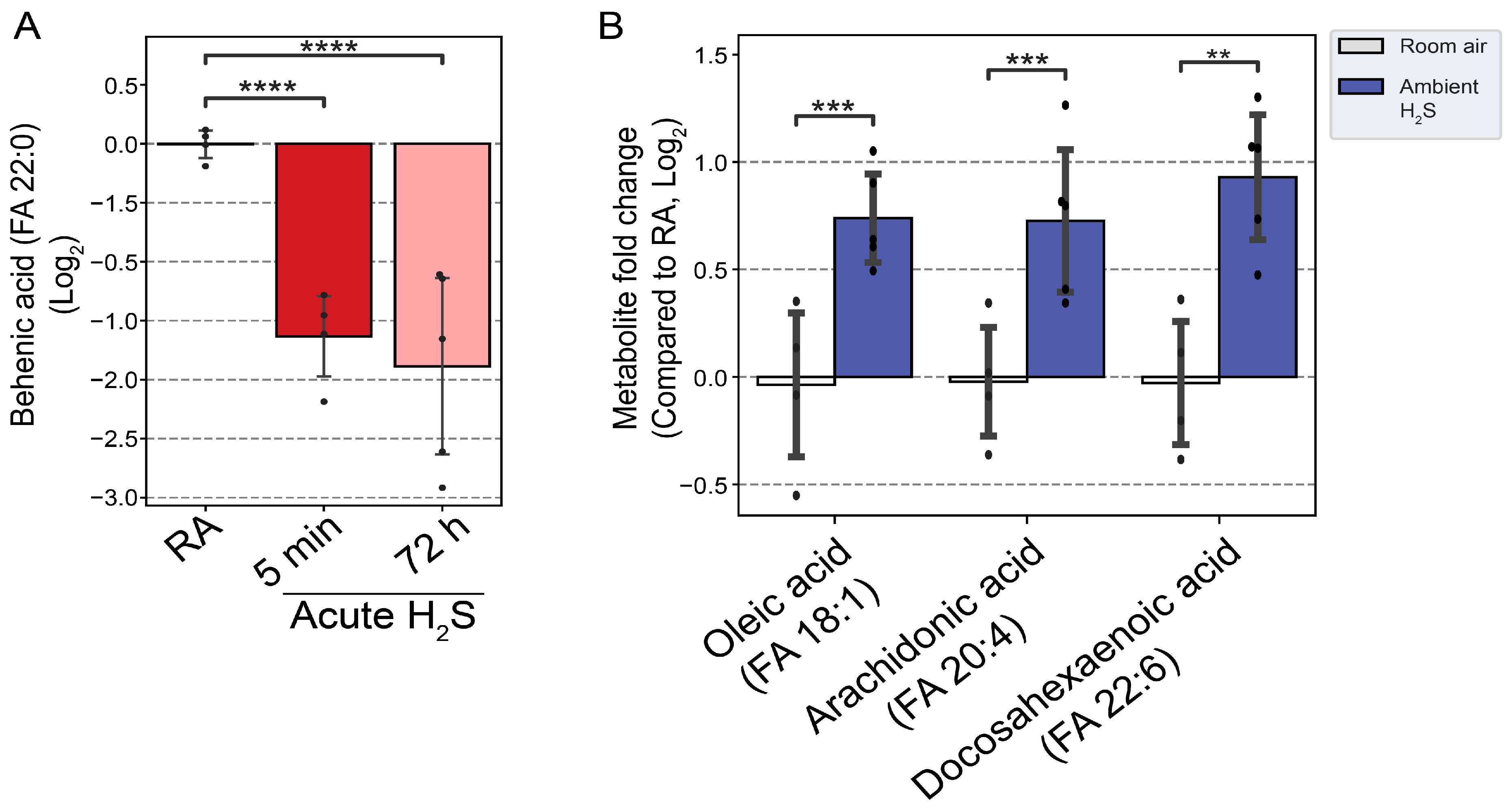
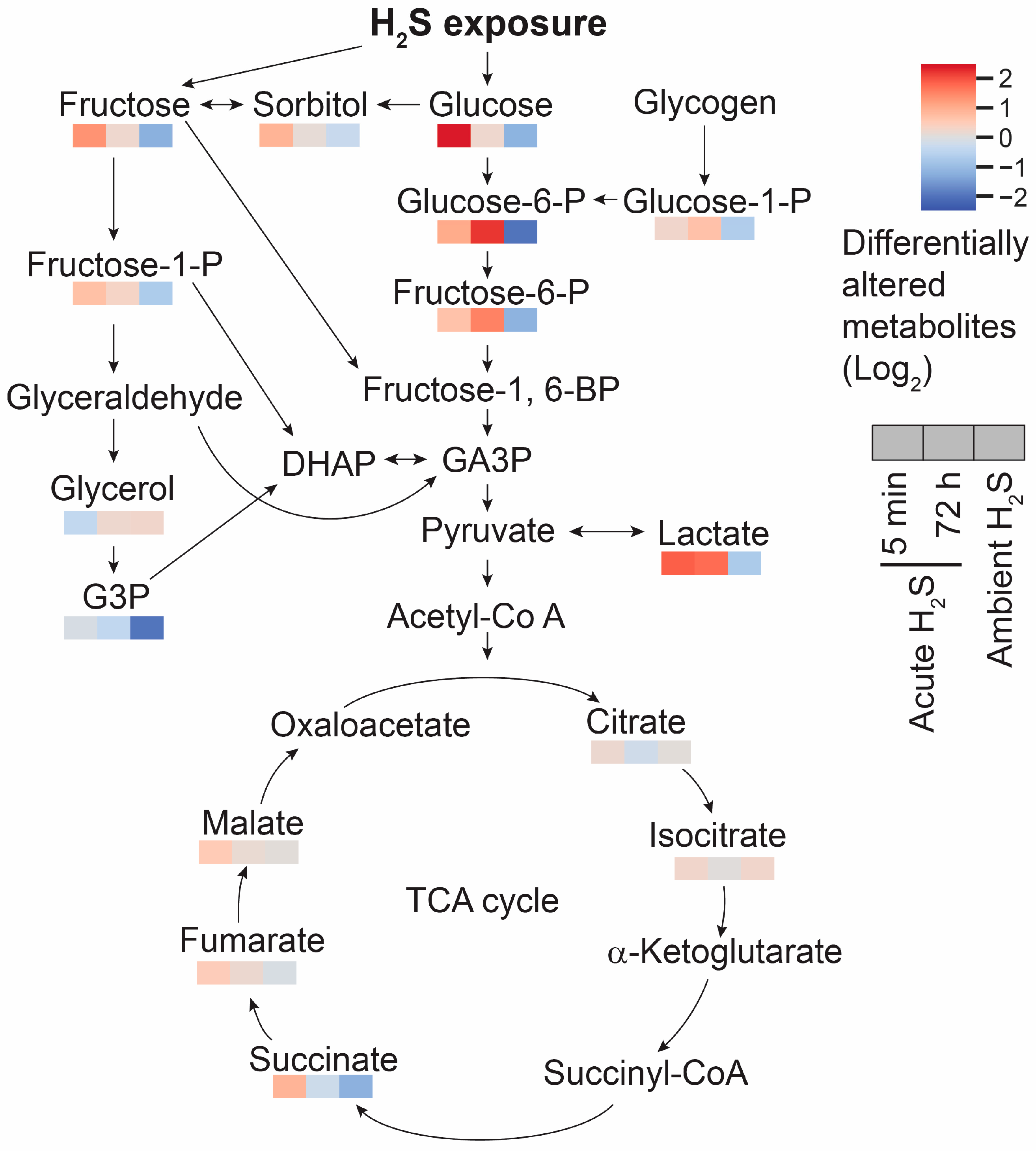
3.5. H2S Dysregulated Energy Homeostasis
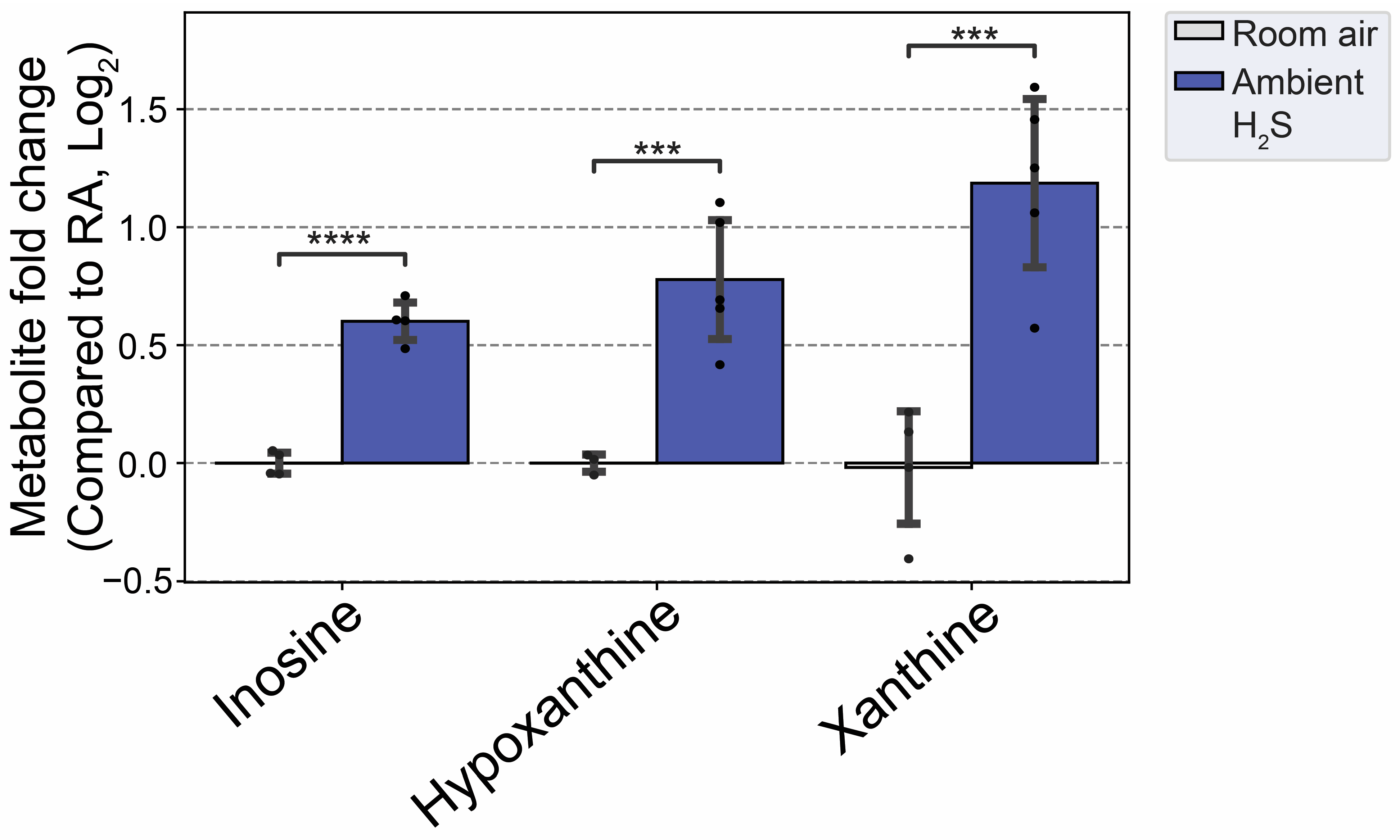
3.6. H2S Dysregulated Inosine Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Santana Maldonado, C.M.; Kim, D.S.; Purnell, B.; Li, R.; Buchanan, G.F.; Smith, J.; Thedens, D.R.; Gauger, P.; Rumbeiha, W.K. Acute hydrogen sulfide-induced neurochemical and morphological changes in the brainstem. Toxicology 2023, 485, 153424. [Google Scholar] [CrossRef] [PubMed]
- Santana Maldonado, C.; Weir, A.; Rumbeiha, W.K. A comprehensive review of treatments for hydrogen sulfide poisoning: Past, present, and future. Toxicol. Mech. Methods 2023, 33, 183–196. [Google Scholar] [CrossRef]
- Rumbeiha, W.K.; Kim, D.S.; Min, A.; Nair, M.; Giulivi, C. Disrupted brain mitochondrial morphology after in vivo hydrogen sulfide exposure. Sci. Rep. 2023, 13, 18129. [Google Scholar] [CrossRef]
- Kim, D.S.; Pessah, I.N.; Santana, C.M.; Purnell, B.; Li, R.; Buchanan, G.F.; Rumbeiha, W.K. Investigations into hydrogen sulfide-induced suppression of neuronal activity in vivo and calcium dysregulation in vitro. Toxicol. Sci. 2023, 192, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Anantharam, P.; Padhi, P.; Thedens, D.R.; Li, G.; Gilbreath, E.; Rumbeiha, W.K. Transcriptomic profile analysis of brain inferior colliculus following acute hydrogen sulfide exposure. Toxicology 2020, 430, 152345. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Anantharam, P.; Hoffmann, A.; Meade, M.L.; Grobe, N.; Gearhart, J.M.; Whitley, E.M.; Mahama, B.; Rumbeiha, W.K. Broad spectrum proteomics analysis of the inferior colliculus following acute hydrogen sulfide exposure. Toxicol. Appl. Pharmacol. 2018, 355, 28–42. [Google Scholar] [CrossRef]
- Anantharam, P.; Whitley, E.M.; Mahama, B.; Kim, D.S.; Imerman, P.M.; Shao, D.; Langley, M.R.; Kanthasamy, A.; Rumbeiha, W.K. Characterizing a mouse model for evaluation of countermeasures against hydrogen sulfide-induced neurotoxicity and neurological sequelae. Ann. N. Y. Acad. Sci. 2017, 1400, 46–64. [Google Scholar] [CrossRef]
- Rumbeiha, W.; Whitley, E.; Anantharam, P.; Kim, D.S.; Kanthasamy, A. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: Challenges for translational neuroprotective research. Ann. N. Y. Acad. Sci. 2016, 1378, 5–16. [Google Scholar] [CrossRef]
- Tiranti, V.; Viscomi, C.; Hildebrandt, T.; Di Meo, I.; Mineri, R.; Tiveron, C.; Levitt, M.D.; Prelle, A.; Fagiolari, G.; Rimoldi, M.; et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009, 15, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Anantharam, P.; Whitley, E.M.; Mahama, B.; Kim, D.S.; Sarkar, S.; Santana, C.; Chan, A.; Kanthasamy, A.G.; Kanthasamy, A.; Boss, G.R.; et al. Cobinamide is effective for treatment of hydrogen sulfide-induced neurological sequelae in a mouse model. Ann. N. Y. Acad. Sci. 2017, 1408, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Anantharam, P.; Kim, D.S.; Whitley, E.M.; Mahama, B.; Imerman, P.; Padhi, P.; Rumbeiha, W.K. Midazolam Efficacy Against Acute Hydrogen Sulfide-Induced Mortality and Neurotoxicity. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2018, 14, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, T.L. Hydrogen sulfide: Advances in understanding human toxicity. Int. J. Toxicol. 2010, 29, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, T.L. Hydrogen Sulfide intoxication. In Handbook of Clinical Neurology, 3rd ed.; Lotti, M., Bleecker, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131. [Google Scholar]
- Reiffenstein, R.J. Neurochemical and neurophysiological effects of high dose hydrogen sulphide. In Proceedings of the International Conference on Hydrogen Sulphide Toxicity, Banff, AB, Canada, 18–21 June 1989; Prior, M.G., Roth, S.H., Green, F.H.Y., Hulbert, W.C., Reiffenstein, R., Eds.; The Sulphide Research Network: Banff, AB, Canada, 1989. [Google Scholar]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Vicas, I.M.O. Therapeutics and Management of H2S Poisoning. In Proceedings of the International Conference on Hydrogen Sulphide Toxicity, Banff, AB, Canada, 18–21 June 1989; Prior, M.G., Roth, S.H., Green, F.H.Y., Hulbert, W.C., Reiffenstein, R., Eds.; Sulphide Research Network. Department of Pharmacology, University of Alberta: Banff, AB, Canada, 1989; pp. 217–219. [Google Scholar]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.J.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute Hydrogen sulfide poisoning. Biochem. Pharmacol. 1989, 38, 973–981. [Google Scholar] [CrossRef]
- Sprague, W. Arrive Guidelines 2.0. Vet. Clin. Pathol. 2020, 49, 378–379. [Google Scholar] [CrossRef]
- Occupational Safety and Health Administration. Hydrogen Sulfide. Available online: https://www.osha.gov/hydrogen-sulfide/hazards (accessed on 21 December 2023).
- Occupational Safety and Health Administration. Hydrogen Sulfide. Available online: https://www.osha.gov/SLTC/hydrogensulfide/standards.html (accessed on 10 December 2023).
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Smilowitz, J.T.; Fiehn, O. Validating Quantitative Untargeted Lipidomics Across Nine Liquid Chromatography-High-Resolution Mass Spectrometry Platforms. Anal. Chem. 2017, 89, 12360–12368. [Google Scholar] [CrossRef] [PubMed]
- Tvedt, B.; Edland, A.; Skyberg, K.; Forberg, O. Delayed neuropsychiatric sequelae after acute hydrogen sulfide poisoning: Affection of motor function, memory, vision and hearing. Acta Neurol. Scand. 1991, 84, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.; Kim, H.; Choi, Y.; Lee, H.; Hong, E.S.; Park, J.K.; Lee, K.M.; Kim, Y. Neurologic sequela of hydrogen sulfide poisoning. Ind. Health 2004, 42, 83–87. [Google Scholar] [CrossRef]
- Matsuo, F.; Cummins, J.W.; Anderson, R.E. Neurological sequelae of massive hydrogen sulfide inhalation. Arch. Neurol. 1979, 36, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.W.; Safir, E.F.; Summerville, G.P.; Middleberg, R.A. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am. J. Emerg. Med. 1995, 13, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Basinger, H.; Hogg, J.P. Neuroanatomy, Brainstem; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK544297/ (accessed on 10 December 2023).
- Mudumba, S.; Menezes, A.; Fries, D.; Blankenship, J. Differentiation of PC12 cells induced by N8-acetylspermidine and by N8-acetylspermidine deacetylase inhibition. Biochem. Pharmacol. 2002, 63, 2011–2018. [Google Scholar] [CrossRef]
- Nayak, A.; Liu, C.; Mehta, A.; Ko, Y.A.; Tahhan, A.S.; Dhindsa, D.S.; Uppal, K.; Jones, D.P.; Butler, J.; Morris, A.A.; et al. N8-Acetylspermidine: A Polyamine Biomarker in Ischemic Cardiomyopathy With Reduced Ejection Fraction. J. Am. Heart Assoc. 2020, 9, e016055. [Google Scholar] [CrossRef]
- Baldelli, R.J.; Green, F.H.; Auer, R.N. Sulfide toxicity: Mechanical ventilation and hypotension determine survival rate and brain necrosis. J. Appl. Physiol. 1993, 75, 1348–1353. [Google Scholar] [CrossRef]
- Boison, D. Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol. 2008, 8, 2–7. [Google Scholar] [CrossRef]
- Faraguna, U.; Ferrucci, M.; Giorgi, F.S.; Fornai, F. Editorial: The Functional Anatomy of the Reticular Formation. Front. Neuroanat. 2019, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Mangold, S.A.; Das, J.M. Neuroanatomy, Reticular Formation; StatPearls: St. Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556102/ (accessed on 10 December 2023).
- Cardoso, S.M.; Proenca, M.T.; Santos, S.; Santana, I.; Oliveira, C.R. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol. Aging 2004, 25, 105–110. [Google Scholar] [CrossRef]
- Shivanthan, M.C.; Perera, H.; Jayasinghe, S.; Karunanayake, P.; Chang, T.; Ruwanpathirana, S.; Jayasinghe, N.; De Silva, Y.; Jayaweerabandara, D. Hydrogen sulphide inhalational toxicity at a petroleum refinery in Sri Lanka: A case series of seven survivors following an industrial accident and a brief review of medical literature. J. Occup. Med. Toxicol. 2013, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, P.F.; Zhou, J.; Xiao, W.; He, J.G.; Guan, X.L.; Zhang, J.T.; Hu, Z.L.; Wang, F.; Chen, J.G. Aggravation of seizure-like events by hydrogen sulfide: Involvement of multiple targets that control neuronal excitability. CNS Neurosci. Ther. 2014, 20, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; McBain, C.J. Glutamate and Aspartate Are the Major Excitatory Transmitters in the Brain. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects, 6th ed.; Lippincott: New York, NY, USA, 1999. [Google Scholar]
- Maura, G.; Marcoli, M.; Pepicelli, O.; Rosu, C.; Viola, C.; Raiteri, M. Serotonin inhibition of the NMDA receptor/nitric oxide/cyclic GMP pathway in human neocortex slices: Involvement of 5-HT(2C) and 5-HT(1A) receptors. Br. J. Pharmacol. 2000, 130, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Almallouhi, E.; Rahwan, M.; Dainton, H.; Bonilha, L. Focal seizure as a manifestation of serotonin syndrome: Case report. Avicenna J. Med. 2019, 9, 119–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhu, W.; Yu, J.; Wang, Q.; Zhang, J.; Cui, Y.; Pan, X.; Gao, X.; Sun, H. Succinate accumulation induces mitochondrial reactive oxygen species generation and promotes status epilepticus in the kainic acid rat model. Redox Biol. 2020, 28, 101365. [Google Scholar] [CrossRef]
- Hussain, G.; Schmitt, F.; Loeffler, J.P.; Gonzalez de Aguilar, J.L. Fatting the brain: A brief of recent research. Front. Cell. Neurosci. 2013, 7, 144. [Google Scholar] [CrossRef]
- Thomas, M.H.; Pelleieux, S.; Vitale, N.; Olivier, J.L. Arachidonic acid in Alzheimer’s disease. J. Neurol. Neuromed. 2016, 1, 1–6. [Google Scholar]
- Kerdiles, O.; Laye, S.; Calon, F. Omega-3 polyunsaturated fatty acids and brain health: Preclinical evidence for the prevention of neurodegenerative diseases. Trends Food Sci. Technol. 2017, 69, 203–213. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jinnah, H.A.; Kamatani, N. Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front. Pharmacol. 2019, 10, 98. [Google Scholar] [CrossRef]
- Nascimento, F.P.; Macedo-Junior, S.J.; Lapa-Costa, F.R.; Cezar-Dos-Santos, F.; Santos, A.R.S. Inosine as a Tool to Understand and Treat Central Nervous System Disorders: A Neglected Actor? Front. Neurosci. 2021, 15, 703783. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, N.F.; Ibrahim, S.M.; Moustafa, P.E.; Elbaset, M.A. Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-kappaB/Nrf2 and TGF-beta/PKC/TRPV1 signaling pathways. Biomed. Pharmacother. 2022, 145, 112395. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; Kekesi, K.A.; Bobest, M.; Torok, T.; Szilagyi, N.; Szikra, T.; Szepesi, Z.; Nyilas, R.; Dobolyi, A.; Palkovits, M.; et al. Post mortem degradation of nucleosides in the brain: Comparison of human and rat brains for estimation of in vivo concentration of nucleosides. J. Neurosci. Methods 2005, 148, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.K.; Quigley, J.M.; Tinsley, H.F. Islamic State chemical weapons: A case contained by its context? CTC Sentin. 2018, 11, 27–31. [Google Scholar]
- HAZMAT/SAFETY. TSA Issues Security Awareness Message on Potential Use of H2S in Terrorist Attack. Available online: https://www.bulktransporter.com/hazmatsafety/tsa-issues-security-awareness-message-potential-use-h2s-terrorist-attack (accessed on 10 December 2023).
- Wasch, H.H.; Estrin, W.J.; Yip, P.; Bowler, R.; Cone, J.E. Prolongation of the P-300 latency associated with hydrogen sulfide exposure. Arch. Neurol. 1989, 46, 902–904. [Google Scholar] [CrossRef]
- Fomenko, M.V.; Yanshole, L.V.; Tsentalovich, Y.P. Stability of Metabolomic Content during Sample Preparation: Blood and Brain Tissues. Metabolites 2022, 12, 811. [Google Scholar] [CrossRef]
- Dienel, G.A. Stop the rot. Enzyme inactivation at brain harvest prevents artifacts: A guide for preservation of the in vivo concentrations of brain constituents. J. Neurochem. 2021, 158, 1007–1031. [Google Scholar] [CrossRef]





| Metabolites | m/z | 5 min | 72 h | ||
|---|---|---|---|---|---|
| Fold Change | Significance | Fold Change | Significance | ||
| 10-hydroxydecanoic acid | 187.135 | 1.77 | **** | 0.31 | |
| 2,5-dihydroxybenzoate | 329.025 | 1.52 | **** | 0.21 | |
| 2,8-quinolinediol | 162.057 | 1.46 | *** | 0.32 | |
| 2-amino-1-phenylethanol | 120.08 | 1.41 | **** | −0.2 | |
| 2-aminoadipic acid | 162.076 | 1.36 | *** | 0.48 | |
| 2-hydroxy-4-methylpentanoic acid | 131.071 | 1.35 | **** | 0.23 | |
| 2-hydroxybutanoic acid | 131 | 1.22 | * | 1.18 | ** |
| 2-hydroxyisobutyric acid | 103.041 | 1.15 | *** | 0.02 | |
| 3-hydroxyvaleric acid | 117.056 | 1.15 | 2.88 | * | |
| 3-methylhistidine | 170.091 | 1.11 | *** | 0.25 | |
| Acylcarnitines 18:1 | 426.358 | 0.9 | **** | 0.31 | *** |
| Acylcarnitines 18:2 | 424.344 | 0.87 | *** | 0.19 | |
| Acylhexosylceramide 58:1; O3 | 1048.91 | 1.07 | *** | −0.04 | |
| Adenine | 136.062 | 1.1 | 1.33 | * | |
| Adenosine | 236 | 1.07 | *** | 0.68 | ** |
| Alanine | 116 | 1.01 | *** | −0.44 | |
| Allantoin | 157.038 | 1 | *** | 1.29 | **** |
| α-galactosyl-N-stearoylsphingosine | 728.598 | 1.79 | **** | −0.43 | |
| α-aminoadipic acid | 260 | 0.97 | * | 0.81 | |
| Aspartic acid | 232 | 0.96 | 1.49 | ** | |
| Behenic acid | 117 | 0.93 | *** | 0.08 | |
| Betaine aldehyde cation | 120.1019_ 102.0893 | 0.93 | *** | 1.19 | **** |
| Caffeic acid | 181.057 | 0.91 | **** | 0.13 | |
| Cardiolipin 75:5|Cardiolipin 35:0_40:5 | 1496.04 | 0.79 | *** | 0.29 | |
| Cardiolipin 82:11|Cardiolipin 40:4_42:7 | 1582.09 | 0.78 | ** | −0.24 | |
| Citramalic acid | 247 | 0.83 | ** | 0.26 | |
| Erythritol | 217 | 0.78 | * | 0.76 | ** |
| Flavin adenine | 246 | 0.66 | ** | 0.2 | |
| Fructose | 307 | 0.63 | **** | −0.06 | |
| γ-Linolenic acid (FA 18:3) | 277.216 | 0.69 | * | 1.06 | *** |
| Glucose | 319 | 0.62 | *** | −0.02 | |
| Glucose-1-phosphate | 217 | 0.59 | * | 0.45 | |
| Glucose-6-phosphate | 387 | 0.47 | * | 0.65 | ** |
| Glutamic acid | 246 | 0.27 | 0.8 | * | |
| Glutamine | 156 | 0.26 | 0.7 | ** | |
| Guanosine | 324 | 0.12 | 1.07 | ** | |
| Homo-gamma-linolenic acid (FA 20:3) | 305.249 | 0.68 | *** | 0.48 | ** |
| Isoleucine | 158 | 0.09 | 0.71 | * | |
| Leucine | 158 | −0.06 | 0.87 | * | |
| L-Saccharopine | 277.139 | −0.23 | *** | −0.58 | **** |
| Lysine | 317 | −0.24 | −0.62 | ** | |
| Lysophosphatidylcholine 22:6 | 568.339 | −0.07 | −0.61 | ** | |
| Lysophosphatidylethanolamine 22:6 | 526.288 | −0.18 | −0.58 | ** | |
| Mannose | 205 | −0.24 | −0.8 | *** | |
| Methionine | 150.056 | −0.26 | 0.71 | ** | |
| Myristic acid (FA 14:0) | 227.202 | 0.75 | *** | 0.47 | ** |
| N-(Octadecanoyl)-sphinganine (Cer-NDS d36:0) | 566.547 | 0.86 | * | 0.88 | * |
| N-α-(Tert-Butoxycarbonyl)-L-histidine | 110.069 | −0.27 | 0.93 | **** | |
| N8-Acetylspermidine | 188.175 | −0.28 | −0.6 | ** | |
| N-Acetyl-D-lactosamine | 406.13 | −0.35 | −0.9 | *** | |
| N-Acetylleucine | 172.096 | −0.4 | −0.66 | ** | |
| N-α-Methylhistamine | 126.101 | −0.49 | −0.68 | * | |
| N-tetracosenoyl-4-sphingenine | 648.6272_ 630.6146 | −0.5 | * | −0.89 | ** |
| Phosphatidylcholine 34:3 Isomer B | 756.5538_ 778.5289 | −0.58 | *** | −0.39 | *** |
| Phosphatidylglycerol 16:0_16:0 | 721.502 | −0.6 | ** | −0.6 | ** |
| Phosphatidylserine 40:2 | 844.603 | −0.69 | * | −0.19 | |
| Phosphatidylserine 40:6|PS 18:0_22:6 | 836.547 | −0.74 | *** | −0.29 | * |
| Phosphoinositides 34:1 | 835.536 | −0.61 | ** | −0.59 | ** |
| Phosphoinositides 36:1 | 863.56 | −0.65 | ** | 0.38 | * |
| Phosphoinositides 36:5 | 855.5 | −0.66 | *** | −0.3 | |
| Phosphoinositides 38:6 | 881.512 | −0.66 | *** | 0.07 | |
| Phostatidylethanol 18:1_18:1 | 727.527 | −0.6 | * | −0.19 | |
| Proline | 142 | −0.66 | * | −0.37 | |
| Prostaglandin E1 | 353.232 | −0.67 | ** | −0.16 | |
| Pyroglutamic acid | 130.05 | −0.78 | **** | −0.11 | |
| S-Lactoylglutathione | 380.111 | −0.82 | −1.13 | * | |
| Sorbitol | 217 | −0.83 | ** | −0.3 | |
| Succinic acid | 117.019 | −0.83 | **** | −0.02 | |
| Sulfoglycosphingolipids d42:2 | 890.639 | −0.8 | * | −0.41 | |
| Thiazolidine-4-carboxylic acid | 134.025 | −0.84 | * | −1.05 | ** |
| Tryptophan | 202 | −0.87 | ** | −0.08 | |
| Tyrosine | 218 | −1.06 | * | −0.05 | |
| Uridine diphosphate galactose | 565.044 | −1.18 | **** | −0.49 | **** |
| Valine | 144 | −1.42 | **** | −1.58 | **** |
| Vanillin | 151.04 | −1.53 | 4.94 | * | |
| Vanillin-4-sulfate | 230.996 | −1.54 | **** | −0.64 | *** |
| Xanthine | 153.037 | −1.6 | **** | −1.71 | **** |
| Metabolites | m/z | Fold Change | Significance |
|---|---|---|---|
| (R)-Butyrylcarnitine | 232.1547 | −1.18 | *** |
| 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine | 454.2927 | 0.61 | ** |
| 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine | 482.3181 | 0.68 | ** |
| 3-Methylhistidine | 170.0913 | 0.77 | *** |
| 4-Aminovaleric acid betaine | 160.133 | 0.8 | ** |
| 5-Aminoimidazole-4-carboxamide | 110.0322 | 0.72 | ** |
| 5-Methoxytryptamine | 174 | −1.03 | *** |
| 9-(2,3-Dihydroxypropoxy)-9-oxononanoic acid | 261.1345 | 0.7 | ** |
| Abietic acid | 301.216 | 1.34 | *** |
| Adenosine 5′-diphosphoribose | 560.0761_582.0571 | −3.46 | **** |
| Adenosine 5′-monophosphate | 370.0487 | −0.61 | ** |
| Adenosine monophosphate | 695.1267 | −1.48 | ** |
| Adenosine-5-monophosphate | 315 | −1.2 | * |
| Adrenic acid (FA 22:4) | 331.2637 | 0.69 | *** |
| Arachidonic acid | 303.2328 | 1.13 | * |
| Arachidonic acid (FA 20:4) | 303.2333 | 0.77 | ** |
| Argininosuccinic acid | 291.127 | 0.8 | * |
| β-Glycerolphosphate | 243 | −0.76 | *** |
| Betaine aldehyde cation | 120.1019_102.0893 | 1.4 | *** |
| Cysteine | 220 | 0.71 | ** |
| Dehydroascorbic acid | 173 | −0.65 | ** |
| Docosahexaenoic acid (FA 22:6) | 327.2328 | 0.96 | *** |
| Docosapentaenoic acid (n-3, FA 22:5) | 329.2473 | 0.71 | ** |
| Eicosadienoic acid (FA 20:2) | 307.2637 | 0.72 | ** |
| Eicosapentaenoic acid (FA 20:5) | 301.2167 | 0.79 | ** |
| Eicosenoic acid (FA 20:1) | 309.2798 | 0.68 | ** |
| Epigallocatechin | 307.0835 | −0.59 | ** |
| Ethanolamine | 62.0596 | 0.76 | ** |
| Fatty acid 15:1 | 239.2009 | −0.92 | ** |
| Fructose | 307 | −1.21 | ** |
| Fructose-1-phosphate | 387 | −0.62 | ** |
| Fructose-6-phosphate | 315 | −1.13 | * |
| Galactolipid monogalactosyldiacylglycerol 34:1 | 774.605 | −0.64 | ** |
| Galactose-6-phosphate | 387 | −0.82 | * |
| Glucose | 319 | −1.11 | ** |
| Glucose-1-phosphate | 217 | −0.63 | ** |
| Glucose-6-phosphate | 387 | −1.9 | *** |
| Glycerol-alpha-phosphate | 357 | −1.97 | **** |
| Glycerophosphocholine | 515.2116_772.3096_ 280.0915_258.1106_ 296.0648 | −2.04 | **** |
| Guanine | 152.0564 | 0.64 | * |
| Guanosine | 324 | 1.16 | **** |
| His-Gly | 213.0949 | 1.01 | **** |
| Homo-γ-linolenic acid (FA 20:3) | 305.2487 | 0.86 | ** |
| Hypoxanthine | 265 | 0.8 | *** |
| Inosine | 230 | 0.6 | **** |
| Isovaleryl-L-carnitine | 246.17 | −0.98 | *** |
| Lactamide | 90.0544 | −1.25 | * |
| L-Cysteine-glutathione disulfide | 427.0924 | 0.81 | * |
| Lysophosphatidylcholine 16:0 | 540.3289 | 0.78 | ** |
| Lyso-phosphatidyl-ethanolamines 16:0 | 452.2776 | 0.62 | **** |
| Lyso-phosphatidyl-ethanolamines 22:6 | 526.2883 | 0.7 | ** |
| Lysophosphatidylinositol 16:0 | 571.2842 | 1.03 | ** |
| Lysophosphatidylinositol 18:0 | 599.3185 | 1.11 | ** |
| Maltose | 361 | −0.76 | * |
| N-Acetylalanine | 130.0515 | 0.79 | * |
| N-Acetylhistidine | 198.0888 | −1.96 | ** |
| N-Lauroylsarcosine | 272.2221 | −1.35 | * |
| Non-hydroxy-fatty acid sphingosine ceramides d42:3 | 704.6138 | 0.62 | *** |
| Oleic acid (FA 18:1) | 281.2491 | 0.75 | *** |
| Phenylacetylglycine | 192.0664 | 0.97 | ** |
| Phenylalanine | 164.0721 | 0.62 | * |
| Phosphatidylcholine 36:5 Isomer D | 780.555 | 0.88 | *** |
| Phosphoinositides 36:5 | 855.5001 | 0.6 | * |
| Phosphatidylethanol 18:1_18:1 | 727.5266 | 1.01 | ** |
| Propionylcarnitine | 218.1366 | 0.75 | *** |
| Prostaglandin E1 | 353.2316 | 1.07 | * |
| Riboflavin | 377.1473 | 0.69 | *** |
| Sedoheptulose 7-phosphate | 387 | −1.24 | ** |
| Serotonin | 174 | −0.91 | **** |
| sn-glycerol-3-phosphoethanolamine | 238.0433 | −0.96 | *** |
| Thiamine cation | 265.1075 | 0.65 | ** |
| Thiazolidine-4-carboxylic acid | 134.0252 | 0.66 | * |
| Triglyceride 52:4 | 872.7704 | −0.67 | *** |
| Trimethylamine N-oxide | 76.0748 | −0.8 | ** |
| Uracil | 241 | 0.89 | **** |
| Xanthine | 353 | 1.23 | *** |
| Xylose | 103 | −0.66 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-S.; Santana Maldonado, C.M.; Giulivi, C.; Rumbeiha, W.K. Metabolomic Signatures of Brainstem in Mice following Acute and Subchronic Hydrogen Sulfide Exposure. Metabolites 2024, 14, 53. https://doi.org/10.3390/metabo14010053
Kim D-S, Santana Maldonado CM, Giulivi C, Rumbeiha WK. Metabolomic Signatures of Brainstem in Mice following Acute and Subchronic Hydrogen Sulfide Exposure. Metabolites. 2024; 14(1):53. https://doi.org/10.3390/metabo14010053
Chicago/Turabian StyleKim, Dong-Suk, Cristina M. Santana Maldonado, Cecilia Giulivi, and Wilson Kiiza Rumbeiha. 2024. "Metabolomic Signatures of Brainstem in Mice following Acute and Subchronic Hydrogen Sulfide Exposure" Metabolites 14, no. 1: 53. https://doi.org/10.3390/metabo14010053
APA StyleKim, D.-S., Santana Maldonado, C. M., Giulivi, C., & Rumbeiha, W. K. (2024). Metabolomic Signatures of Brainstem in Mice following Acute and Subchronic Hydrogen Sulfide Exposure. Metabolites, 14(1), 53. https://doi.org/10.3390/metabo14010053






