Abstract
Obesity, linked to chronic diseases, poses a global health challenge. While the role of the olfactory system in energy homeostasis is well-documented in rodents, its role in metabolism regulation and obesity in humans remains understudied. This review examines the interplay between olfactory function and metabolic alterations in human obesity and the effects of bariatric surgery on olfactory capabilities in humans. Adhering to PRISMA guidelines, a systematic review and meta-analysis was conducted, focusing exclusively on original human studies. From 51 articles, 14 were selected for the meta-analysis. It was found that variations in olfactory receptor genes influence the susceptibility to odors and predisposition to weight gain and poor eating habits. Bariatric surgery, particularly sleeve gastrectomy, shows significant improvements in olfactory function (SMD 2.37, 95% CI [0.96, 3.77], I = 92%, p = 0.001), especially regarding the olfactory threshold (SMD −1.65, 95% CI [−3.03, −0.27], I = 81%, p = 0.02). There is a bidirectional relationship between olfactory function and metabolism in humans. Bariatric surgery improves olfactory perception in obese patients, but it is still unclear if impacting the olfactory system directly affects eating behavior and the energy balance. However, these findings open novel avenues for future studies addressing the olfactory system as a novel target to alter systemic metabolism in humans.
1. Introduction
Obesity, resulting from a dysregulated balance between caloric intake and energy expenditure [1], represents a global socioeconomic health burden with epidemic dimensions worldwide [2,3,4]. Obesity is a major risk factor for chronic non-communicable diseases (NCDs) such as cardiovascular diseases, type 2 diabetes, fatty liver disease, and cancer [5], contributing to increased disease burden, mortality, and healthcare costs [6].
The brain, particularly the hypothalamus, is a master regulator of whole-body energy homeostasis [1]. Specialized neurons within the hypothalamus, such as anorexigenic proopiomelanocortin (POMC)- and orexigenic agouti-related peptide (AgRP)/neuropeptide Y (NPY)-expressing neurons, sense the nutritional status of the organism and integrate this information into a coordinated feedback regulation of food intake, glucose, and energy homeostasis [1,7]. However, metabolic regulation involves numerous interconnected systems beyond the hypothalamus, encompassing not only various other brain regions but other aspects such as digestive enzymes secretion, insulin release, and psychological factors influencing eating behavior [8].
The role of olfaction extends beyond mere sensory perception, deeply intertwining with hypothalamic function. Hunger enhances olfactory acuity, while satiety reduces it, indicating a reciprocal relationship between food intake and olfactory sensitivity [9,10,11,12]. Odors sensed by the olfactory system change the activation status of hypothalamic AgRP and POMC neurons, thereby modulating appetite and satiety circuits [13,14,15]. Furthermore, olfactory cues also influence thermogenesis and peripheral metabolism, possibly mediated by olfactomedin 2 (OLFM2), a protein that plays a significant role in energy balance modulation [16,17,18]. The integration of olfactory signals with hypothalamic regulation underscores a complex but crucial aspect of the energy balance, influencing feeding behavior, thermogenesis, and overall systemic metabolism. Understanding this intricate network opens new avenues for treatment strategies for overweight and obese individuals, as well as associated metabolic diseases, focusing on the sensory perception of food and its metabolic consequences [15,19].
Olfactory receptors (ORs), part of the G protein-coupled receptor family, play a crucial role in olfactory signal transduction. The binding of odorants leads to the activation of ORs and thus the G protein (Golf), resulting in increased intracellular cyclic AMP levels and neuronal depolarization [20,21]. The vast array of ORs, each detecting specific odorants, along with their combinatorial coding, underscores the complexity of olfactory perception. This molecular framework paves the way for hypothesizing potential alterations in human olfactory mechanisms, especially in disorders like obesity, where changes in olfactory perception are observed but not molecularly delineated [22].
Additionally, odor binding proteins (OBPs) in the nasal mucus, particularly OBPII2, play an important role in facilitating the interaction of odorants with ORs. The rs2590498 polymorphism in the OBPIIa gene, a specific variant of OBPII2, has been associated with variations in the olfactory threshold and the intensity of perceived odors in healthy individuals [23].
Growing evidence suggests that olfactory function, or the sense of smell, is altered in the obesity state. Specifically, olfactory performance seems to diminish linearly with an increase in body mass index (BMI) [24]. Patel et al. observed that 80% of people with obesity reported a decrease in olfactory function [2,4]. A recent meta-analysis reported an impaired odor threshold with increasing body mass index (BMI), while odor discrimination and sensitivity were not changed [25,26,27,28,29,30,31,32,33].
An altered olfactory performance might impact food choices, potentially fostering overeating and obesity in a modern food environment. Along this line, a study demonstrated an increased sensitivity to odors of energy-dense food in individuals with obesity, suggesting that the brain might amplify autonomic responses to high-rewarding food [34,35,36]. Furthermore, the concept of a sensory-specific appetite is worth mentioning, where the scent of specific foods increases the appetite for and the consumption of that particular food product, and even of other distinct food items [37,38,39]. For instance, exposure to the smell of a banana increases the desire to ingest a banana as well as the desire to eat chocolate and brownies [39,40]. Along the same line, exposure to the odor of pears before lunch leads to a preference for a fruit dessert [41]. It has been suggested that odors may contain information about nutrients and be perceived even before food ingestion [42]. Additionally, recent research highlights how factors such as odor exposure time and concentration can directly influence food intake in humans. In a study with healthy female participants, prolonged exposure (18 s) to a high concentration of tomato soup aroma resulted in a 9% reduction in food intake compared to shorter exposure times and lower concentrations. This suggests that manipulating the retronasal aroma release can significantly affect eating behavior, underlining the potential of olfactory cues in regulating satiety and food consumption [43]. Complementing these findings, another study revealed that the satiating effect of a beverage can be enhanced when its retronasal aroma release mimics that of solid foods, indicating the significant role of aroma profiles in influencing satiety [44].
In rodents, the visual and olfactory sensing of food, without any nutrient ingestion, is sufficient to reverse the effects of fasting on hypothalamic neuron activity [45,46,47]. This highlights the complex interplay between olfaction and metabolic processes, extending beyond the hypothalamus to digestive enzymes secretion, insulin release, and psychological influences on eating behavior [9]. Therefore, olfactory sensing-dependent changes in hypothalamic neuron activity may be influenced by a variety of variables, such as the feeding state [48], hormones [49], age [50], respiratory infection [51], and neurodegenerative diseases [52] (Figure 1).
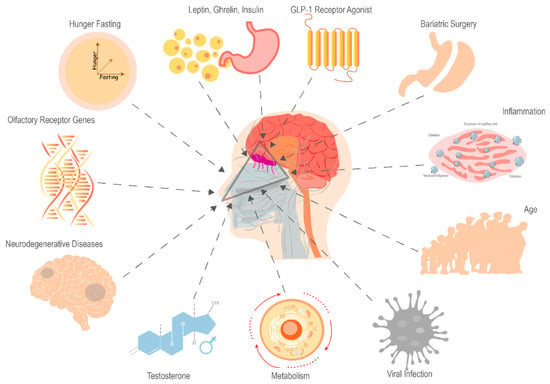
Figure 1.
Factors Impacting Olfactory Sensory Perception (Created using Adobe Illustrator).
Additionally, environmental and social factors, including cultural norms, food availability, and social settings, play a significant role in modulating eating behavior and metabolic outcomes in interaction with olfactory perception [53]. Genetic and epigenetic variations also impact how odors are perceived and influence eating behavior and metabolism [19]. The burgeoning research on the gut–brain axis and gut microbiota further underscores the complexity of these interactions in metabolic regulation [18].
Alterations in food preference and taste are widely reported after bariatric surgery (BS) [54,55]. BS is the gold standard for the treatment of morbid obesity as it is an efficient [56] and safe method [57] to reduce the BMI and associated comorbidities in the long-term [58], leading to an overall profound reduction in cardiovascular risk [59] and an increase in life expectancy [60]. One study reported that almost 97% of people who underwent bariatric surgery experienced changes in food-related taste, smell, or preferences [61,62]. However, only a few studies investigated the influence of BS on olfactory function. While some studies reported an overall improvement in olfactory function upon BS, others found effects depending on the type of BS or no effect at all [63]. However, also in regard to olfactory changes upon BS, the methods used to assess olfactory function varied significantly between the studies and ranged from self-reported questionnaires to validated olfactory function test batteries [56].
Human research in this area is critical due to notable differences in the olfactory system and eating behavior between humans and animals. For example, humans have a more complex relationship with food that encompasses cultural, emotional, and social dimensions, contrary to most laboratory animals. Additionally, the human olfactory system, while having fewer receptors than most animals, is able to process a vast array of complex odors, influencing dietary choices in a way that is not observed in animal models [53].
The objective of this systematic review and meta-analysis is to investigate the relationship between olfactory function and obesity, including associated metabolic alterations in humans, and to examine the effects of bariatric surgery on olfactory function. The analysis will differentiate outcomes based on the type of surgical procedure and the specific tools used for olfactory function assessment.
2. Materials and Methods
The protocol for this systematic review and meta-analysis was registered on PROSPERO (CRD42022355091), an international database of systematic reviews and meta-analyses. The literature review and reporting were conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA). The ROBINS-E instrument was used for non-randomized trials to define risk of bias [57]. The Rayyan.ai research collaboration platform was used by two independent reviewers to include studies in a systematic review in a blinded manner [64]. The Review Manager (RevMan) version 5.4 of the Cochrane Collaboration, 2020, was used for meta-analysis [65].
2.1. Source and Methods of Data Retrieval
The electronic databases PubMed, Web of Science, and Scopus were searched for eligible studies without publication time limits on 1 August 2022. The language of original studies was restricted to English. The following keywords were used to search the databases: olfactory function OR smell OR odor OR odour OR hyposmia AND obesity OR extra weight OR weight OR metabolic disease OR metabolic function (detailed search strategy can be found in PROSPERO CRD42022355091).
Inclusion Criteria: The scope of this systematic review and meta-analysis was limited to original research studies involving human subjects, with no restrictions on age. The range of eligible study designs included randomized and non-randomized trials, as well as observational, cohort, and cross-sectional studies. Additionally, we established a defined population as a prerequisite for eligibility to ensure consistency and comparability across studies.
Exclusion Criteria: Studies were excluded from the analysis if they involved subjects with acute infections, including acute respiratory infection and COVID-19, or neoplastic diseases. Animal studies were not considered. Non-original studies, case reports, study protocols, and letters to editors were also deemed ineligible for inclusion.
2.2. Data Extraction
Two independent researchers conducted a comprehensive literature search. The identified studies were subsequently uploaded onto the Rayyan.ai software, designed to streamline the process of blind inclusion and exclusion by two independent reviewers. To ensure thorough data extraction, the reference lists of all included studies were manually examined for additional potential sources. Any discrepancies in the selection process were mitigated through discussion and, if necessary, a third senior author was consulted. The entire search and selection process was systematically documented using a PRISMA flow-chart (Figure 2) [66].
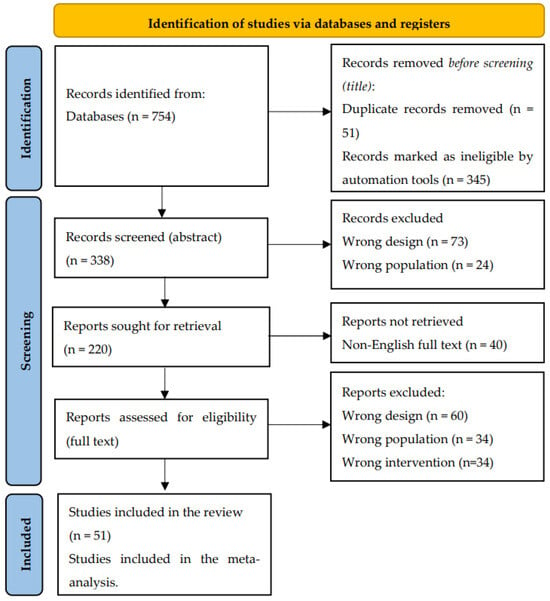
Figure 2.
Adapted from PRISMA Flow-Chart 2020 [66].
Following the selection of eligible studies, pertinent information was collated using the Rayyan.ai software. Details such as the lead author, publication year, country of origin, study design, population demographics, and reported outcomes were recorded. Additionally, specific information regarding the intervention’s content and components was analyzed. If required, information about certain intervention characteristics was sourced from earlier publications, as identified from the reference list of the original article.
When authors employed multiple instruments to measure identical outcomes, the data were primarily extracted from the most relevant instrument, as decided by consensus after scrutinizing the wording of each item. A parallel procedure was followed when multiple subscales of instruments were reported instead of global scores.
The quality and potential bias of each study were evaluated using the Cochrane ROBINS-E instrument [57]. This entailed assessing various factors such as selection bias, performance bias, detection bias, attrition bias, and reporting bias, among others. Each study was consequently categorized as having “low”, “high”, or “unclear” levels of bias.
2.3. Meta-Analysis
Data from bariatric surgery studies were processed using the Review Manager 5.4 software. We calculated the mean difference in olfactory function before and after intervention for each study, accompanied by a 95% confidence interval (CI), which was then juxtaposed with the control group. Data were stratified based on the olfactory function test employed (sniffin’ sticks test with threshold, discrimination and identification (TDI) scoring, visual analogue scale (VAS)) and the specific type of bariatric surgery (Sleeve Gastrectomy (SG) and Roux-en-Y Gastric Bypass (RYGB)).
For dichotomous variables, odds ratios were determined using the Mantel–Haenszel test, with a random-effects model for the analysis, and results were presented with a 95% confidence interval. Conversely, for continuous data, the hazard ratio was computed via the inverse variance method, applying a fixed-effects model, and using the standard mean deviation and effect size as the measure of effect. The choice of this measure was necessitated by the diverse tests used across studies, warranting standardization for effective comparison. Computed hazard ratios were similarly represented with a 95% confidence interval. Due to insufficient data, a subgroup analysis was not carried out.
The heterogeneity among studies was evaluated with a Chi-squared (χ²) test, and the overall effect of the interventions was calculated using the Z-test. This meta-analytic statistical approach aimed to deliver a thorough and standardized comparison of diverse olfactory function outcomes upon different bariatric surgical procedures.
Lastly, effect sizes were calculated based on the outcome data from the experimental and control groups of each study, facilitating a comprehensive comparison and analysis.
3. Results
The systematic identification and screening of electronic databases, following the methods described above, yielded 51 articles meeting the eligibility criteria. This systematic review includes studies which highlight (1) the connection between olfactory genes and their impact on olfactory function; and (2) the impact of metabolism on olfactory function; (3) the impact of obesity on olfactory function. The meta-analysis was limited to studies presenting quantitative evidence about the effect of bariatric surgery on olfactory function. Only these studies provided comparable quantitative data from olfactory function tests such as the “Sniffin’ Sticks” test and the VAS.
The most frequent form of bias identified across all articles was selection bias, which occurs when a participant’s eligibility for bariatric surgery is determined based on his/her condition, in accordance with specific guidelines. Performance bias was another common bias type, which emerged when a limited number of surgeons performed the surgery, introducing potential bias due to variation in surgeons’ skills and techniques. Many articles lacked enough information to accurately assess the risk of bias.
3.1. Variations in Olfactory Receptor Genes and Metabolism
The olfaction phenotype is determined based on variations in olfactory receptor genes, encoding a large number of olfactory receptors (ORs) [67]. We found 18 original studies reporting an association between variations in OR genes and systemic metabolism.
Currently, variations in 23 known OR genes are associated with changes in metabolism, body weight, visceral fat, and eating behavior (Table 1). In the Quebec Family Study, sequencing of the OR7D4 gene revealed seven single nucleotide polymorphisms (SNPs) associated with body weight and eating behavior. Specifically, rs2878329 and rs8109935 were associated with BMI, body fat percentage, waist circumference, susceptibility to hunger, and dietary restriction capacity [68]. The SNP of rs2878329 was also associated with the severity of adiposity, hunger, and conscious changes in dietary behavior. Both positive and negative associations with the amount of abdominal fat were observed in seven other OR7D4 SNPs. Additionally, the authors investigated six other OR genes; among them, OR7G3 exhibited a positive association with altered eating behavior and fat tissue mass. Specifically, the SNP rs10414255 of the M29V OR was related to increased hunger, BMI, cognitive dietary restraint, and percentage of body fat [68].
Furthermore, the Methyl Epigenome Network Association (MENA) project demonstrated the association of BMI and waist circumference (WC) with 15 CpG sites of olfactory pathway genes, four of which are OR genes. The analysis revealed that the methylation pattern of the OR4D2 and OR2Y1 genes was correlated with daily total energy and macronutrient intake [69].
In a genome-wide association study (GWAS), variants of three olfactory genes, OR4P4, OR4S2 and OR4C6, were found to be associated with obesity [70].
Conversely, specific diets have shown significant impact on olfactory signaling pathways, primarily through the modulation of distinct olfactory receptor (OR) genes. In the study by Vink et al. [71] (for analysis plasma was used), a very-low-calorie diet (VLCD) was associated with a substantial downregulation of various OR genes. Notably, this dietary intervention led to the differential expression of a significant number of genes (6135 in the VLCD group), including those involved in metabolism, mitochondrial functioning, and olfactory regulation. Particularly, gene sets related to oxidative phosphorylation, lipid metabolism, and olfactory signaling were found to be downregulated in the VLCD group compared to a low-calorie diet (LCD) group.
Furthermore, a genome-wide association study [72] explored the effects of protein quantity in diets during energy restriction on OR gene expression in white adipose tissue (WAT). This study revealed that high-protein energy restriction (HP-ER) diets resulted in distinct gene expression changes compared to normal-protein energy restriction (NP-ER) diets. A total of 1869 genes showed significant expression changes in the HP-ER group. Notably, upon HP-ER diets, gene sets involved in cell cycle upregulation, G protein-coupled receptor (GPCR) signaling, olfactory, and nitrogen metabolism-related pathways were observed. In contrast, the NP-ER diet led to a downregulation of pathways related to the inflammasome, adaptive immune response, immune cell infiltration, and cell cycle.
Overall, these findings underscore the complex interplay between dietary composition and genetic regulation in the context of olfactory signaling pathways. They also highlight the potential of dietary interventions in modulating gene expression related to olfaction, metabolism, and immune responses.
Genetic variations in OR genes may influence olfactory sensing, which in turn can drive food preferences, potentially contributing to obesity [40,73]. For instance, genetic variations in the OR7D4 gene not only relate to the ability to detect androsterone in cooked pork but are also linked to measures of total, visceral, and subcutaneous adipose tissue mass [74,75] (Table 2). In Table 3, an overview of variants in OR genes and related preferences for certain odors is presented.
In a study conducted by Ortega et al., an intriguing observation was made regarding olfactory sensitivity in non-smoking women. The study found that women with the AVI/AVI haplotypes of the TAS2R38 gene showed a negative correlation with olfactory odor sensitivity, marked by a decrease of 8.6% (p = 0.03). This finding was part of a broader investigation into the genetic variations of the TAS2R38 bitter taste receptor, which has revealed significant associations with obesity. These genetic variations are seen to influence not only taste perception but also phenotypic and clinical outcomes related to extreme weight conditions. The role of TAS2R38 variations extends beyond taste, affecting nutrient sensing and energy metabolism, and potentially impacting olfactory capacity and immune traits. However, it is important to note that the relationship between these TAS2R38 variants and BMI, particularly in the context of obesity, anorexia, or normal body weight, necessitates more in-depth research to unravel the underlying mechanisms. Overall, these findings highlight the complex interplay between genetics, sensory perception, and metabolic health, opening new avenues for the understanding of obesity and related conditions [76]. These findings were corroborated by a comprehensive review [25], which analyzed data from multiple studies assessing the olfactory function of individuals with different body weights. The review revealed that obesity is often associated with diminished olfactory detection and discrimination abilities. Interestingly, some studies observed an increase in odor sensitivity in individuals with a higher BMI. Moreover, bariatric surgery, particularly sleeve gastrectomy, was found to improve olfactory functions, suggesting a direct link between obesity-related metabolic changes and olfactory perception. These observations, coupled with the findings of another study [77], indicate a complex interaction between body weight, metabolic health, and olfactory sensory perception, further elucidating the intricate relationship between obesity and alterations in taste and smell perception [25,77].

Table 1.
Trials investigating variations in olfactory genes and their association with metabolic parameters.
Table 1.
Trials investigating variations in olfactory genes and their association with metabolic parameters.
| Study and Number of Participants (N) Age y.o. BMI kg/m2 | Method | Chromosome Gene Variations SNPs | Positive Association | Negative Association |
|---|---|---|---|---|
| Choquette [68] 2012 N = 890 Age = 43.7 ± 16.8 y.o. BMI = 27.9 ± 7.6 kg/m2 | Direct sequencing in the Quebec Family Study | 19p13 OR7D4 c.-466 | No association | |
| 19p13 OR7D4 rs56139543 | VAT | |||
| 19p13 OR7D4 rs1235784 | TAT, SAT | |||
| 19p13 OR7D4 rs10421711 | Hunger | |||
| 19p13 OR7D4 rs2878329 | BMI, WC, BF, hunger, restraint | |||
| 19p13 OR7D4 rs61729907 | VAT | |||
| 19p13 OR7D4 rs5020278 | ||||
| 19p13 OR7D4 rs8109935 | Restraint, hunger, BMI, BF | |||
| 19p13 OR7D4 rs61732676 | No association | |||
| 19p13 OR7G1 rs7246980 | VAT | |||
| 19p13 OR7G3 rs10414255 | Hunger, BMI, BF | Restraint, | ||
| 19p13 OR7E24 rs2240927 | Disinhibition | |||
| Jarick [70] 2010 N = 453 N = 435 control | Genotyping by the Affymetrix Genome-Wide Human SNP Array 6.0 (PCR) | 11q11 OR4P4 rs9804659 | Obesity | |
| 11q11 OR4S2 | ||||
| 11q11 OR4C6 | ||||
| Ortega [76] 2016 N = 210 women BMI = 34 ± 12 kg/m2 N 52 = 16.5 ± 1.3 y.o., N 86 = 21.5 ± 2.8 y.o., N 72 = 41.1 ± 7.7 y.o. | Genotyped by means of allelic discrimination assays, using a LightCyclerR | TAS2R38 AVI/AVI haplotypes arraying | Non-smoker women showed decreased smelling sensitivity. No connection to BMI | |
| Ramos-Lopez [69] 2019 N = 474 Age = 47.2 ± 14.1 y.o. BMI = 30.1 ± 5.6 kg/m2 | Nutriepigenomic analysis from Methyl Epigenome Network Association | OR4D2 cg02874396 | BMI, WC, daily intakes of total energy, carbohydrates, protein, fat | |
| OR51A7 cg00467296 | BMI, WC | |||
| OR2T34 cg13441213 | ||||
| OR2Y1 cg18482656 | BMI, WC, daily intakes of total energy, carbohydrates, protein, fat | |||
| SLC8A1 cg19302979 | BMI, WC | |||
| SLC8A1 cg12498094 | ||||
| ANO2 cg10610428 | ||||
| PDE2A cg07736155 | ||||
| CALML3 cg17283169 | ||||
| GNG7 cg02849894 | ||||
| CALML6 cg15102821 | ||||
| CALML6 cg15819352 | ||||
| PRKG1 cg16401207 | ||||
| PRKG1 cg24609819 | ||||
| CAMK2D cg13801347 | ||||
| Sun [78] 2022 N= 301 N = 307 control Age = 53.51 ± 11.1 y.o. Age = 51.20 ± 14.5 y.o. control | Bio Miao Biological Technology (PCR) | 17 OR4D1 rs8071251 rs7218964 rs9908511 rs1075009 | Obesity, smoking | |
| 11 OR52K1 rs96489 rs331508 rs331510 rs4468345 | Obesity | |||
| 1 OR2L8 rs4925583 rs4925792 | Obesity | |||
| 10 CALML3 rs1131482 rs2231413 rs1142825 rs4072071 rs4072070 rs4589188 rs4589189 | Smoking | Obesity |
VAT—visceral adipose tissue, BF—body fat, TAT—total adipose tissue, SAT—subcutaneous adipose tissue area (measurements were provided using underwater weighing and computed tomography), WC—waist circumference, BMI—body mass index, BW—body weight.

Table 2.
Overview of Olfactory Receptor Gene Variants. This table presents the percentages of specific pseudogene alleles in various olfactory receptors, the odors detectable by these receptors, and their corresponding natural agonists. The data elucidates the genetic diversity of olfactory receptors and their roles in odor perception.
Table 2.
Overview of Olfactory Receptor Gene Variants. This table presents the percentages of specific pseudogene alleles in various olfactory receptors, the odors detectable by these receptors, and their corresponding natural agonists. The data elucidates the genetic diversity of olfactory receptors and their roles in odor perception.
| Study and Number of Participants (N) | Method | Odorant Receptor Name | Pseudogene Allele Percentage | Resulting Odor to Be Detected | Natural Agonist |
|---|---|---|---|---|---|
| Mainland [75] 2013 N = 511 | For sequencing, human genomic DNA was amplified with HotStar Taq (Qiagen) | OR2B11 | 43% | 8-amino-acid protein | Cinnamaldehyde |
| OR4E2 | 30% | MAYDRY domain | Amyl acetate | ||
| OR8K3 | 24% | MAYDRY domain | (+)-menthol | ||
| OR10A6 | 22% | PMLNPLIY domain | 3-phenyl propyl propionate | ||
| OR2C1 | 4% | 272 amino acid protein | Octanethiol | ||
| OR4Q3 | 1.5% | 159 amino acid protein | Eugenol | ||
| OR10G7 | 1.4% | 191 amino acid protein | Eugenol | ||
| OR10G4 | guaiacol, vanillin and ethyl vanillin |

Table 3.
Comprehensive Overview of Multiple Studies on Olfactory Receptor Gene Variants and related Odor Perception: Correlating Genetic SNPs with Sensitivity for Specific Odors.
Table 3.
Comprehensive Overview of Multiple Studies on Olfactory Receptor Gene Variants and related Odor Perception: Correlating Genetic SNPs with Sensitivity for Specific Odors.
| Study and Number of Participants (N) | Method | Chromosome Odorant Receptor Variants (SNPs) | Related Odors to Be Detected |
|---|---|---|---|
| Eriksson [79] 2009 N = 22 studies | Genotyped on the Illumina HumanHap550+ BeadChip platform | 1 OR2M7 rs4481887 rs4309013 rs4244187 | Smell of asparagus metabolites in urine |
| Eriksson [80] 2012 N = 26691 | Genotyping by Beagle and Minimac | 11 OR6A2 rs72921001 | Aldehydes (cilantro) |
| Jaegaer [81] 2009 N = 48 | Microarray probe genotyping | 6 OR2W1 | Alcohols including 1-hexanol |
| 6 OR2J2 | |||
| 6 OR2J3 rs28757581 | Cis-3-hexen-1-ol (fruits, vegetables, white wine and processed foods) | ||
| 6 OR2J3 rs3749977 | |||
| Lunde [74] 2012 N = 23 | For sequencing, human genomic DNA was amplified with HotStar Taq (Qiagen) | OR7D4 rs61729907 | Androstenone (cooked pork) |
| OR7D4 rs5020278 | |||
| Menashe [82] 2007 N = 377 | Matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF | OR11H7 | Isovaleric acid (sweaty odor) |
3.2. Olfactory Sensory Perception and Metabolism
We found eight original studies reporting a connection between olfactory function and obesity. In a study by Massol et al., it was found that the odor of dark chocolate reduced the appetite and ghrelin levels in young women [83]. The study by Ketterer et al. added to our understanding of the metabolic influences on olfactory function by examining the effects of systemic insulin levels on olfactory thresholds in healthy individuals. Utilizing a hyperinsulinemic–euglycemic clamp approach, the study demonstrated an increase in the olfactory threshold (from 7.8 ± 1.2 to 6.2 ± 1.1, p = 0.0173) during hyperinsulinemia, while no significant change was noted in the fasting control group. These findings highlight the potential of insulin to adjust olfactory sensory perception in the postprandial state, characterized by a reduction in food seeking [84]. It is of note that insulin resistance is associated with impaired olfactory identification, although this interaction was not notably linked to the BMI and HOMA index [85]. To the contrary, the administration of intranasal insulin to patients with post-infection loss-of-smell improved olfactory sensitivity. Additionally, patients with a higher BMI also showed improvements in odor identification tasks upon intranasal insulin administration [86]. Another study indicated that the presence of insulin in the olfactory bulb could be connected with the process of satiation and the pathogenesis of obesity [72]. GLP-1 agonist treatment decreases the olfactory preference for sweet- and fat-enriched food [87] and improves olfactory sensitivity and odor-induced right parahippocampal activation [88]. In both cases, it remains unclear whether the observed effects were directly linked to GLP-1 analogue effects on the olfactory system or indirectly resulted from the reduction in body weight.
The interventional impairment of olfactory sensory perception using a specific intranasal device resulted in a reduction in body weight, improved insulin sensitivity, and decreased preferences for sweet foods in subjects aged ≤ 50 years with obesity on a low-calorie diet compared to a control group. Interestingly, no effect was observed in study participants > 50 years of age [89]. As olfactory function seems to deteriorate with aging [90],, this might explain why interventions to reduce olfactory sensory perception are not effective at an advanced age.
3.3. Olfactory Function and Obesity
We found 11 original studies reporting alterations in olfactory function in individuals with obesity. In a study by Velluzzi et al., only 34% of participants with overweight were normosmic, as opposed to 66% of healthy participants. When the overweight group was further divided into participants with overweight and obesitye, the authors observed that there was a negative correlation between the BMI and olfactory function [91]. In another population-based study, the data were similar, reporting hyposmia in 34.9% and anosmia in 31.7% of individuals with overweight and hyposmia in 34.7% and anosmia in 27.5% of individuals with obesity. It is important to note that the mean age in this study was 71.1 years, which could have influenced the result [92]. Campolo et al. further reported that, in subjects with obesity, olfactory impairment is highly common and associated with poor sleep quality and a lower cognition score [93]. Other studies showed that an increase in the BMI and visceral fat are linked to declining odor perception and olfactory capacity [24,31,77,94,95]. Rawal et al. also observed that self-reported olfactory dysfunction is positively correlated with BMI. Subjects with olfactory dysfunction were observed to have a BMI of 30.0 ± 0.3, while the normosmic group had a BMI of 29.2 ± 0.2 (p < 0.05). They also observed that the normosmic group consumed fewer calories per day, especially less additional sugar, and showed a healthier food preference than the hyposmic group [96]. A study by Patel et al. suggested that an increasing BMI may be a risk factor for anosmia and limited control of food consumption [97]. In a study examining the influence of odors on children’s food choices, 45 normal-weight and 29 obese children were enrolled. The study used pear and pound cake aromas as olfactory primes, selected for their appeal and recognition by children. The key finding was that the impact of these aromas on food choice differed according to their body weight. In normal-weight children, both pear and pound cake odors significantly decreased the likelihood of choosing fruit compared to the control condition. Conversely, in obese children, the pear odor increased the probability of selecting fruit, while the pound cake odor had no significant effect on their food choices. This differential response underscores that the non-conscious perception of specific olfactory cues—fruity (pear) and fatty-sweet (pound cake)—can influence food choice differently in children depending on their body weight status [98].
3.4. Olfactory Function and Bariatric Surgery
Following the inclusion of 51 articles, we selected 14 studies for the meta-analysis due to their comparable quantitative data. These studies examined the relationship between bariatric surgery and olfactory function. The characteristics of the included studies are presented in Table 4.

Table 4.
Characteristics of the 14 publications investigating the impact of bariatric surgery on olfactory function included in the meta-analysis. Sleeve Gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), mini gastric bypass (mGB), adjustable gastric band (AGB) Pocket Smell test (PST), threshold discrimination identification (TDI) test, visual analogue scale (VAS), Cross-Cultural Smell Identification test (CC-SIT).
In total, the studies encompassed data from 1402 participants. Among them, 724 underwent Roux-en-Y gastric bypass (RYGB), 537 received sleeve gastrectomy (SG), and 34 underwent another type of bariatric surgery. The median follow-up period was six months. Of the 14 studies, 5 either lacked a control group or did not specify the type of surgery. Different tools were applied to assess olfactory function. Seven studies used the Sniffin’ Sticks test combined with the threshold, discrimination, and identification (TDI) score; one used the Pocket Smell test (PCT); five employed a visual analogue scale (VAS); and one utilized the Cross-Cultural Smell Identification test (CC-SIT).
Four studies indicated improvements in OF post-bariatric surgery, assessed via the total TDI score using the Sniffin’ Sticks test (SMD 2.37, 95% CI [0.96, 3.77], I = 92%, p = 0.001) (Figure 3a) [99,104,105,109]. There was a significant improvement in the olfactory threshold post-bariatric surgery (SMD 3.44, 95% CI [1.16, 5.72], I = 96%, p = 0.003) (Figure 3b), while olfactory discrimination and identification remained unaffected (p > 0.05) (Figure 3c,d).
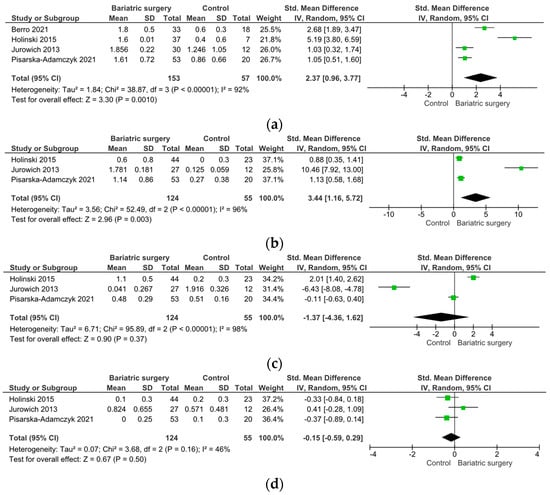
Figure 3.
Changes in olfactory function parameters upon bariatric surgery versus control group. (a) Total TDI, (b) threshold, (c) discrimination, and (d) identification.
Five studies compared the changes in olfactory function between RYGB and SG surgery. In two studies, olfactory function was assessed using the Sniffin’ Sticks test with TDI scoring, while three studies used the VAS score; therefore, the data were analyzed separately. Notably, the Sniffin’ Sticks test with TDI demonstrated a significantly stronger improvement in OF post-SG than post-RYGB surgery (SMD −4.76, 95% CI [−10.35, −0.82], I = 95%, p = 0.09) (Figure 4a). Upon SG, an improvement in the olfactory threshold (SMD −1.65, 95% CI [−3.03, −0.27], I = 81%, p = 0.02) (Figure 4b), no changes in olfactory discrimination (SMD −12.27, 95% CI [−36.33, −11.79], I = 99%, p = 0.32) (Figure 4c), and a decrease in olfactory identification (SMD 0.87, 95% CI [0.37, 0.33], I = 0%, p < 0.001) (Figure 4d) were observed.
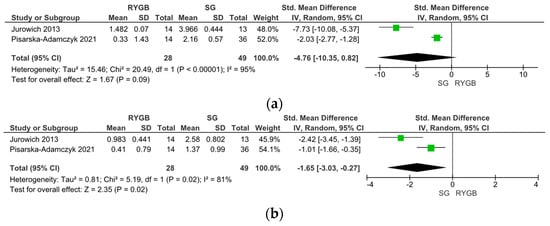
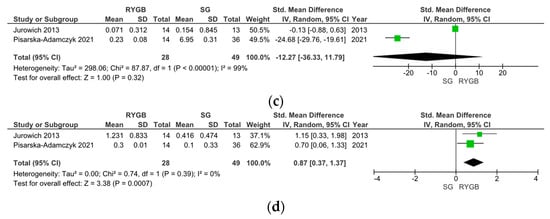
Figure 4.
Comparison of Roux-en-Y Gastric Bypass (RYGB) versus Sleeve Gastrectomy (SG). Changes in olfactory function according to olfactory identification (TDI).
However, an assessment via VAS scores revealed the opposite outcome, showing a stronger improvement of olfactory function parameters upon RYGB compared to SG (OR 1.58, 95% CI [1.06, 2.34], I = 0%, p = 0.02) (Figure 5).
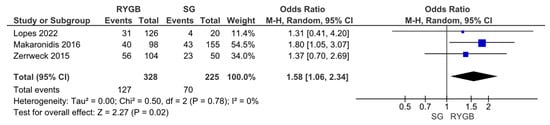
Figure 5.
Changes in olfactory function according to visual analogue scale (VAS) score upon Roux-en-Y Gastric Bypass (RYGB) versus Sleeve Gastrectomy (SG).
Four studies without a control group demonstrated an improvement in the total TDI score upon bariatric surgery [100,103,108,112]. Two other studies also lacking a control group showed an improvement in olfactory function following bariatric surgery, as assessed using VAS scores in combination with a questionnaire [101,102]. Only one study compared olfactory performance upon RYGB with a control group using VAS scores, showing a superiority of RYGB over the control group regarding the improvement of olfactory performance (OR 1.22, 95% CI [0.11, 14.09], p = 0.87) [110].
Figure 6 provides an overview of risk of bias of the studies investigated.
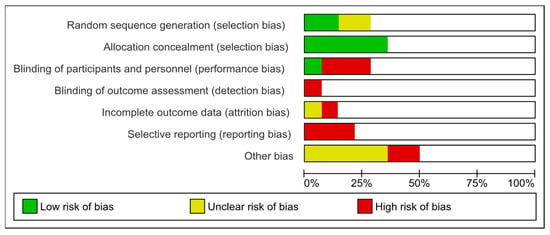
Figure 6.
Risk of bias of the studies investigated.
4. Discussion
In this systematic review and meta-analysis, we observed a correlation between increased BMI and changes in olfactory function. This association was characterized by a higher incidence of anosmia and hyposmia among individuals with overweight and obesity. However, it is important to note that these findings indicate a relationship, not a causal link. Additionally, individuals with overweight, but not obesity, who developed hyposmia reported a reduced food consumption and a lower preference for high-calorie foods [113].
For decades it was believed that humans can distinguish up to 10,000 different odors. However, in 2014 Bushdid et al. challenged this view by testing and calculating the capacity of humans to discriminate odor mixtures, revealing that at least one trillion different odors can be recognized by humans [114], although the results were debated [115]. While humans have around 400 different olfactory receptors (ORs), mice have more than 1000 ORs [116]. OR genes represent some of the largest gene families among vertebrates [117] and code G-protein-coupled receptors (GPCRs), each of which can detect different odor molecules [20,21]. Copy number variations, which are common in OR genes, lead to a high diversity in odor perception capability [22]. Variations in distinct ORs genes are associated with obesity, BMI, waist circumference, and daily calorie intake. Although the direct link and underlying mechanisms of genetic variations in OR genes in regard to metabolism regulation remains to be demonstrated, these findings indicate a link between olfactory performance and systemic energy homeostasis, and possibly eating behavior in humans [118]. Notably, ORs are not only expressed by olfactory sensory neurons, but by almost every tissue in the rodent and human organism, whereby the relevance is unclear [119]. At the level of intestinal enterocytes, ORs may direct food choice depending on the fat content of the food [120].
Other studies have shown that temporary alterations in the general health condition of individuals, including nasal infections or allergic rhinitis, may have concomitant, yet temporary, effects on olfactory function and subsequently on systemic metabolism [121,122,123]. On the other hand, changes in systemic metabolism impact olfactory function. In this review and meta-analysis, we found that bariatric surgery has a positive effect on olfactory function. During a follow-up period of at least 6 months, an improvement in smell and olfactory sensitivity was detected in ten studies assessing olfactory performance via a Sniffin’ Sticks test combined with TDI scoring or VAS. One study showed that while participants with obesity had a decreased olfactory performance, as assessed via Sniffin Sticks test and TDI scoring, participants with anorexia had an improved overall olfactory function [26]. The latter finding was contradicted in the study by Enck et al., although the normal weight control group was not age-matched in their study [100].
A separate analysis of the odor threshold, discrimination, and identification using the TDI score revealed that the distinct dimensions of olfactory sensory perception are differentially impacted upon bariatric surgery. The odor threshold was significantly improved after bariatric surgery, especially upon SG. These findings build upon previous studies reporting a lowered odor threshold with increasing body weight [31,91,124,125], while olfactory discrimination and identification does not seem to be impacted by body weight or obesity in humans [25,33,126], although these findings are still a subject of debate [91]. This impairment in the olfactory threshold might be mediated via proinflammatory cytokines or leptin [95,126]. In contrast to the olfactory threshold, olfactory discrimination and identification did not change upon bariatric surgery when compared to control groups. However, comparing RYGB and SG surgery, olfactory discrimination and identification scores were significantly better upon RYGB compared to SG.
The majority of studies used the widely accepted and validated ‘Sniffin’ Sticks’ test battery [127] combined with TDI scoring. In addition, there is a wide variety of unstandardized tests to determine olfactory function, such as the Smell Identification Test (UP-SIT), which is based on the identification of scents on each page of four booklets [128], the Pocket Smell Test (PST), which is based on the ability to determine the correct smell from the proposed options [129], and the CC-SIT, which is based on the same principles as the previous two tests with adaptions to a specific cultural background usually consisting of 12 questions [130]. Moreover, olfactory function is widely assessed via VAS, which is based on a person’s subjective self-assessment [131] and thus is prone to bias. Interestingly, olfactory function, as assessed via the Sniffin’ sticks test, seems to improve after bariatric surgery, specifically after SG [99,100,103], but not after RYGB [110]. This might be explained by the stimulation of the vagus–insula–olfactory cortex pathway which is suppressed in obesity and restored upon SG, but not upon RYGB, despite comparable weight loss [104,105,106,107,108,111].
It is interesting and somewhat puzzling that olfactory performance is significantly more improved upon RYGB compared to SG when assessed via VAS. VAS is validated as a reliable method for the assessment of several health conditions [132,133]. However, changes in olfactory performance might be too small to be correctly captured via VAS. Moreover, VAS is not able to assess the different dimensions of olfactory sensory perception. Therefore, olfactory function outcomes based on VAS should be interpreted with caution.
The studies incorporated in this systematic review present several limitations. Firstly, five of the studies lacked control groups. Additionally, all of the included studies were non-randomized and often involved only a small cohort of participants. A notable concern was the absence of standardized methods to evaluate olfactory function, which complicated direct comparisons between studies. Although many studies employed the TDI test—considered by many as the objective gold standard—others utilized the VAS score. The latter introduces a greater potential for bias and poses challenges when attempting to compare results across individuals and distinct therapeutic procedures.
5. Conclusions
In summary, our review and meta-analysis revealed that variants in OR genes are associated with metabolic changes at the systemic level in humans where underlying mechanisms are elusive. Moreover, we found that olfactory performance is negatively correlated with BMI. Our findings suggest that the most prevalent bariatric surgery procedures, RYBG and SG, result in an improved olfactory sensory perception, particularly regarding the olfactory threshold, among patients with obesity. When assessed via the VAS score, RYBG led to a more pronounced improvement in olfactory function compared to SG. However, when evaluated with the Sniffin’ Sticks test, SG resulted in a significantly improved olfactory performance compared to RYGB.
Author Contributions
L.M. was responsible for writing the protocol of the review. She conducted the initial literature search, managed the blind inclusion and exclusion of studies, and was instrumental in writing the results section and formulating the statistical approach. A.L.H. served as the second researcher. She independently conducted a literature search, carried out the blind inclusion and exclusion of studies, and contributed to writing the results section and statistical analysis. K.T. acted as the supervisor for this review. She was involved in the final revision of included studies and played a key role in correcting and ensuring adherence to manuscript guidelines throughout the development of the review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by internal funds of K.T. K.T. received research grants from Fondation Machaon (Geneva); the Goldschmidt-Jacobson Foundation (Basel); the Gottfried and Julia Bangerter-Rhyner Foundation; the Novartis Foundation for Medical-Biological Research; Novo Nordisk; the Olga Mayenfisch Foundation; the propatient Research Foundation of University Hospital Basel; the Swiss Diabetes Association; the Swiss National Science Foundation (PCEFP3_194627); the Vontobel Research Foundation; and the University of Basel.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study were derived from previously published articles and are available in the public domain. Details of the specific studies included in this systematic review and meta-analysis can be found in the referenced articles and within the PROSPERO registration (CRD42022355091). No new data were created or analyzed in this study.
Acknowledgments
The authors would like to acknowledge the contribution of all researchers whose studies were included in this systematic review and meta-analysis. Their dedication to advancing knowledge in the field has been instrumental in compiling this comprehensive analysis. Additionally, we extend our gratitude to the reviewers for their insightful comments and suggestions, which significantly enhanced the quality of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Timper, K.; Brüning, J.C. Hypothalamic Circuits Regulating Appetite and Energy Homeostasis: Pathways to Obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 8 June 2022).
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of All-Cause Mortality with Overweight and Obesity Using Standard Body Mass Index Categories. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Li, C.S.R.; Mantzoros, C.S. Central Nervous System Regulation of Eating: Insights from Human Brain Imaging. Metabolism 2016, 65, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and Neuromodulation Approaches to Study Eating Behavior and Prevent and Treat Eating Disorders and Obesity. Neuroimage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef]
- Weingarten, H.P. Conditioned Cues Elicit Feeding in Sated Rats: A Role for Learning in Meal Initiation. Science 1983, 220, 431–433. [Google Scholar] [CrossRef]
- Su, Z.; Alhadeff, A.L.; Betley, J.N. Nutritive, Post-Ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell Rep. 2017, 21, 2724–2736. [Google Scholar] [CrossRef]
- Petrovich, G.D.; Setlow, B.; Holland, P.C.; Gallagher, M. Amygdalo-Hypothalamic Circuit Allows Learned Cues to Override Satiety and Promote Eating. J. Neurosci. 2002, 22, 8748–8753. [Google Scholar] [CrossRef]
- Jovanovic, P.; Riera, C.E. Olfactory System and Energy Metabolism: A Two-Way Street. Trends Endocrinol. Metab. 2022, 33, 281–291. [Google Scholar] [CrossRef]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC Neuronal Heterogeneity in Energy Balance and beyond: An Integrated View. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef]
- Pager, J. Ascending Olfactory Information and Centrifugal Influxes Contributing to a Nutritional Modulation of the Rat Mitral Cell Responses. Brain Res. 1978, 140, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal-Hypothalamic Circuitry and Food Motivation: Integration of Energy, Action and Reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Barrientos-Riosalido, A.; Bertran, L.; Vilaró-Blay, M.; Aguilar, C.; Martínez, S.; Paris, M.; Sabench, F.; Riesco, D.; Binetti, J.; Del Castillo, D.; et al. The Role of Olfactomedin 2 in the Adipose Tissue–Liver Axis and Its Implication in Obesity-Associated Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 5221. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Nakaya, N.; Dong, L.; Abu-Asab, M.; Qian, H.; Tomarev, S.I. Deletion of Olfactomedin 2 Induces Changes in the AMPA Receptor Complex and Impairs Visual, Olfactory, and Motor Functions in Mice. Exp. Neurol. 2014, 261, 802–811. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Freire-Agulleiro, Ó.; Nakaya, N.; Ortega, F.J.; Garrido-Gil, P.; Liñares-Pose, L.; Fernø, J.; Labandeira-Garcia, J.L.; Diéguez, C.; Sultana, A.; et al. Olfactomedin 2 Deficiency Protects against Diet-Induced Obesity. Metabolism 2022, 129, 155122. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Fernández-Real, J.M.; Tomarev, S.I. Obesity Wars: May the Smell Be with You. Am. J. Physiol. Metab. 2023, 324, E569–E576. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A Novel Multigene Family May Encode Odorant Receptors: A Molecular Basis for Odor Recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Olender, T.; Lancet, D.; Nebert, D.W. Update on the Olfactory Receptor (OR) Gene Superfamily. Hum. Genom. 2008, 3, 87–97. [Google Scholar] [CrossRef]
- Conrad, D.F.; Andrews, T.D.; Carter, N.P.; Hurles, M.E.; Pritchard, J.K. A High-Resolution Survey of Deletion Polymorphism in the Human Genome. Nat. Genet. 2006, 38, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Melis, M.; Tomassini Barbarossa, I.; Crnjar, R. A Polymorphism in the Human Gene Encoding OBPIIa Affects the Perceived Intensity of Smelled Odors. Behav. Brain Res. 2022, 427, 113860. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; vander Woude, E.A.; Sudan, R.; Thompson, J.S.; Leopold, D.A. Altered Olfactory Acuity in the Morbidly Obese. Obes. Surg. 2004, 14, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Coutts, D.; Wang, T.; Cakmak, Y.O. Systematic Review of Olfactory Shifts Related to Obesity. Obes. Rev. 2019, 20, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aranda, F.; Agüera, Z.; Fernández-García, J.C.; Garrido-Sanchez, L.; Alcaide-Torres, J.; Tinahones, F.J.; Giner-Bartolomé, C.; Baños, R.M.; Botella, C.; Cebolla, A.; et al. Smell–Taste Dysfunctions in Extreme Weight/Eating Conditions: Analysis of Hormonal and Psychological Interactions. Endocrine 2016, 51, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Obrebowski, A.; Obrebowska-Karsznia, Z.; Gawliński, M. Smell and Taste in Children with Simple Obesity. Int. J. Pediatr. Otorhinolaryngol. 2000, 55, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.J.F. Odour and Taste Sensitivity Is Associated with Body Weight and Extent of Misreporting of Body Weight. Eur. J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [CrossRef]
- Guild, A.A. Olfactory Acuity in Normal and Obese Human Subjects: Diurnal Variations and the Effect of D-Amphetamine Sulphate. J. Laryngol. Otol. 1956, 70, 408–414. [Google Scholar] [CrossRef]
- Thompson, D.A.; Moskowitz, H.R.; Campbell, R.G. Taste and Olfaction in Human Obesity. Physiol. Behav. 1977, 19, 335–337. [Google Scholar] [CrossRef]
- Stafford, L.D.; Welbeck, K. High Hunger State Increases Olfactory Sensitivity to Neutral but Not Food Odors. Chem. Senses 2011, 36, 189–198. [Google Scholar] [CrossRef]
- Stafford, L.D.; Whittle, A. Obese Individuals Have Higher Preference and Sensitivity to Odor of Chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Skrandies, W.; Zschieschang, R. Olfactory and Gustatory Functions and Its Relation to Body Weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.; Wardle, J. Behavioral Susceptibility to Obesity: Gene-Environment Interplay in the Development of Weight. Physiol. Behav. 2015, 152, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Cecchetto, C.; Dal Bò, E.; Aiello, M.; Fischmeister, F.P.S.; Gentili, C.; Osimo, S.A. Alexithymia Modulates the Attitudes towards Odors but Not the Olfactory Abilities or the Affective Reactions to Odors. PLoS ONE 2023, 18, e0278496. [Google Scholar] [CrossRef] [PubMed]
- Zoon, H.F.A.; de Graaf, C.; Boesveldt, S. Food Odours Direct Specific Appetite. Foods 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, I.; Polivy, J.; Peter Herman, C. The Specificity of Restrained versus Unrestrained Eaters’ Responses to Food Cues: General Desire to Eat, or Craving for the Cued Food? Appetite 2003, 41, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, M.G.; Boesveldt, S.; Gort, G.; Lakemond, C.M.M.; van Boekel, M.A.J.S.; Luning, P.A. Sensory-Specific Appetite Is Affected by Actively Smelled Food Odors and Remains Stable Over Time in Normal-Weight Women. J. Nutr. 2014, 144, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; de Graaf, K. The Differential Role of Smell and Taste for Eating Behavior. Perception 2017, 46, 307–319. [Google Scholar] [CrossRef]
- Yeomans, M.R. Olfactory Influences on Appetite and Satiety in Humans. Physiol. Behav. 2006, 87, 800–804. [Google Scholar] [CrossRef]
- Gaillet-Torrent, M.; Sulmont-Rossé, C.; Issanchou, S.; Chabanet, C.; Chambaron, S. Impact of a Non-Attentively Perceived Odour on Subsequent Food Choices. Appetite 2014, 76, 17–22. [Google Scholar] [CrossRef]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of Deep Brain Stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef]
- Ramaekers, M.G.; Luning, P.A.; Ruijschop, R.M.A.J.; Lakemond, C.M.M.; Bult, J.H.F.; Gort, G.; Van Boekel, M.A.J.S. Aroma Exposure Time and Aroma Concentration in Relation to Satiation. Br. J. Nutr. 2014, 111, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Ruijschop, R.M.A.J.; Boelrijk, A.E.M.; de Ru, J.A.; De Graaf, C.; Westerterp-Plantenga, M.S. Effects of Retro-Nasal Aroma Release on Satiation. Br. J. Nutr. 2008, 99, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Betley, J.N.; Xu, S.; Cao, Z.F.H.; Gong, R.; Magnus, C.J.; Yu, Y.; Sternson, S.M. Neurons for Hunger and Thirst Transmit a Negative-Valence Teaching Signal. Nature 2015, 521, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.; Nolte, H.; Henschke, S.; Engström Ruud, L.; Awazawa, M.; Morgan, D.A.; Gabel, P.; Sprenger, H.G.; Hess, M.E.; Günther, S.; et al. Food Perception Primes Hepatic ER Homeostasis via Melanocortin-Dependent Control of MTOR Activation. Cell 2018, 175, 1321–1335.e20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Y.C.; Kuo, T.W.; Knight, Z.A. Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell 2015, 160, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Horio, N.; Liberles, S.D. Hunger Enhances Food-Odour Attraction through a Neuropeptide Y Spotlight. Nature 2021, 592, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Janet, R.; Fournel, A.; Fouillen, M.; Derrington, E.; Corgnet, B.; Bensafi, M.; Dreher, J.C. Cognitive and Hormonal Regulation of Appetite for Food Presented in the Olfactory and Visual Modalities. Neuroimage 2021, 230, 117811. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste and Smell Processing in the Brain. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 97–118. [Google Scholar]
- Kırgezen, T.; Yücetaş, U.; Server, E.A.; Övünç, O.; Yiğit, Ö. Possible Effects of Low Testosterone Levels on Olfactory Function in Males. Braz. J. Otorhinolaryngol. 2021, 87, 702–710. [Google Scholar] [CrossRef]
- Jung, H.J.; Shin, I.-S.; Lee, J.-E. Olfactory Function in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. Laryngoscope 2019, 129, 362–369. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, Olfactory, and Food Reward Value Processing in the Brain. Prog. Neurobiol. 2015, 127–128, 64–90. [Google Scholar] [CrossRef] [PubMed]
- Ochner, C.N.; Kwok, Y.; Conceição, E.; Pantazatos, S.P.; Puma, L.M.; Carnell, S.; Teixeira, J.; Hirsch, J.; Geliebter, A. Selective Reduction in Neural Responses to High Calorie Foods Following Gastric Bypass Surgery. Ann. Surg. 2011, 253, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; Hackenberg, T.; Spector, A.C.; le Roux, C.W. Gastric Bypass Surgery for Obesity Decreases the Reward Value of a Sweet-Fat Stimulus as Assessed in a Progressive Ratio Task. Am. J. Clin. Nutr. 2012, 96, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.K. (Ed.) Essentials and Controversies in Bariatric Surgery; InTech: London, UK, 2014; ISBN 978-953-51-1726-1. [Google Scholar]
- Risk of Bias Tools—ROBINS-E Tool. Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 31 August 2023).
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-Analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, T.; Guidozzi, N.; Welbourn, R.; Ahmed, A.R.; Markar, S.R. Association of Bariatric Surgery with All-Cause Mortality and Incidence of Obesity-Related Disease at a Population Level: A Systematic Review and Meta-Analysis. PLoS Med. 2020, 17, e1003206. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists—Executive Summary. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Althumiri, N.A.; Basyouni, M.H.; Al-Qahtani, F.S.; Zamakhshary, M.; Bindhim, N.F. Food Taste, Dietary Consumption, and Food Preference Perception of Changes Following Bariatric Surgery in the Saudi Population: A Cross-Sectional Study. Nutrients 2021, 13, 3401. [Google Scholar] [CrossRef]
- Jennifer Traub, R. Taste Changes after Bariatric Surgery: What to Do When Your Patients Cannot Stand the Taste of Their Food. Bariatr. Times 2012, 9, 14–15. [Google Scholar]
- Reges, O.; Greenland, P.; Dicker, D.; Leibowitz, M.; Hoshen, M.; Gofer, I.; Rasmussen-Torvik, L.J.; Balicer, R.D. Association of Bariatric Surgery Using Laparoscopic Banding, Roux-En-Y Gastric Bypass, or Laparoscopic Sleeve Gastrectomy vs Usual Care Obesity Management with All-Cause Mortality. JAMA 2018, 319, 279–290. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- RevMan|Cochrane Training. Available online: https://training.cochrane.org/online-learning/core-software/revman (accessed on 31 August 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Hasin-Brumshtein, Y.; Lancet, D.; Olender, T. Human Olfaction: From Genomic Variation to Phenotypic Diversity. Trends Genet. 2009, 25, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Choquette, A.C.; Bouchard, L.; Drapeau, V.; Lemieux, S.; Tremblay, A.; Bouchard, C.; Vohl, M.C.; Pérusse, L. Association between Olfactory Receptor Genes, Eating Behavior Traits and Adiposity: Results from the Quebec Family Study. Physiol. Behav. 2012, 105, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Zulet, M.A.; Santos, J.L.; Martinez, J.A.; MENA Project. Associations between Olfactory Pathway Gene Methylation Marks, Obesity Features and Dietary Intakes. Genes. Nutr. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Jarick, I.; Vogel, C.I.G.; Scherag, S.; Schäfer, H.; Hebebrand, J.; Hinney, A.; Scherag, A. Novel Common Copy Number Variation for Early Onset Extreme Obesity on Chromosome 11q11 Identified by a Genome-Wide Analysis. Hum. Mol. Genet. 2011, 20, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.G.; Roumans, N.J.; Fazelzadeh, P.; Tareen, S.H.K.; Boekschoten, M.V.; Van Baak, M.A.; Mariman, E.C. Adipose Tissue Gene Expression Is Differentially Regulated with Different Rates of Weight Loss in Overweight and Obese Humans. Int. J. Obes. 2017, 41, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Van Bussel, I.P.G.; Backx, E.M.P.; De Groot, C.P.G.M.; Tieland, M.; Müller, M.; Afman, L.A. The Impact of Protein Quantity during Energy Restriction on Genome-Wide Gene Expression in Adipose Tissue of Obese Humans. Int. J. Obes. 2017, 41, 1114–1120. [Google Scholar] [CrossRef]
- Spinelli, S.; Monteleone, E. Food Preferences and Obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef]
- Lunde, K.; Egelandsdal, B.; Skuterud, E.; Mainland, J.D.; Lea, T.; Hersleth, M.; Matsunami, H. Genetic Variation of an Odorant Receptor OR7D4 and Sensory Perception of Cooked Meat Containing Androstenone. PLoS ONE 2012, 7, e35259. [Google Scholar] [CrossRef]
- Mainland, J.D.; Keller, A.; Li, Y.R.; Zhou, T.; Trimmer, C.; Snyder, L.L.; Moberly, A.H.; Adipietro, K.A.; Liu, W.L.L.; Zhuang, H.; et al. The Missense of Smell: Functional Variability in the Human Odorant Receptor Repertoire. Nat. Neurosci. 2013, 17, 114–120. [Google Scholar] [CrossRef]
- Ortega, F.J.; Agüera, Z.; Sabater, M.; Moreno-Navarrete, J.M.; Alonso-Ledesma, I.; Xifra, G.; Botas, P.; Delgado, E.; Jimenez-Murcia, S.; Fernández-García, J.C.; et al. Genetic Variations of the Bitter Taste Receptor TAS2R38 Are Associated with Obesity and Impact on Single Immune Traits. Mol. Nutr. Food Res. 2016, 60, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.; Fernández-Aranda, F.; Fitó, M.; Jiménez-Murcia, S.; Botella, C.; Fernández-Real, J.M.; Frühbeck, G.; Tinahones, F.J.; Fagundo, A.B.; Rodriguez, J.; et al. A Lower Olfactory Capacity Is Related to Higher Circulating Concentrations of Endocannabinoid 2-Arachidonoylglycerol and Higher Body Mass Index in Women. PLoS ONE 2016, 11, e0148734. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, S.; Ning, F.; Zhang, L.; Wang, W.; Duan, H.; Wu, Y. Association between Olfactory Pathway Gene Variants and Obesity in Chinese Han Population: A Case-Control Study Based on Genetic Score. Gene 2022, 825, 146442. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, N.; Macpherson, J.M.; Tung, J.Y.; Hon, L.S.; Naughton, B.; Saxonov, S.; Avey, L.; Wojcicki, A.; Pe’er, I.; Mountain, J. Web-Based, Participant-Driven Studies Yield Novel Genetic Associations for Common Traits. PLoS Genet. 2010, 6, e1000993. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, N.; Wu, S.; Do, C.B.; Kiefer, A.K.; Tung, J.Y.; Mountain, J.L.; Hinds, D.A.; Francke, U. A Genetic Variant near Olfactory Receptor Genes Influences Cilantro Preference. Flavour 2012, 1, 22. [Google Scholar] [CrossRef]
- Jaeger, S.R.; McRae, J.F.; Salzman, Y.; Williams, L.; Newcomb, R.D. A Preliminary Investigation into a Genetic Basis for Cis-3-Hexen-1-Ol Odour Perception: A Genome-Wide Association Approach. Food Qual. Prefer. 2010, 21, 121–131. [Google Scholar] [CrossRef]
- Menashe, I.; Abaffy, T.; Hasin, Y.; Goshen, S.; Yahalom, V.; Luetje, C.W.; Lancet, D. Genetic Elucidation of Human Hyperosmia to Isovaleric Acid. PLoS Biol. 2007, 5, e284. [Google Scholar] [CrossRef]
- Massolt, E.T.; van Haard, P.M.; Rehfeld, J.F.; Posthuma, E.F.; van der Veer, E.; Schweitzer, D.H. Appetite Suppression through Smelling of Dark Chocolate Correlates with Changes in Ghrelin in Young Women. Regul. Pept. 2010, 161, 81–86. [Google Scholar] [CrossRef]
- Ketterer, C.; Heni, M.; Thamer, C.; Herzberg-Schäfer, S.A.; Häring, H.-U.; Fritsche, A. Acute, Short-Term Hyperinsulinemia Increases Olfactory Threshold in Healthy Subjects. Int. J. Obes. 2011, 35, 1135–1138. [Google Scholar] [CrossRef]
- Poessel, M.; Morys, F.; Breuer, N.; Villringer, A.; Hummel, T.; Horstmann, A. Brain Response to Food Odors Is Not Associated with Body Mass Index and Obesity-Related Metabolic Health Measures. Appetite 2022, 168, 105774. [Google Scholar] [CrossRef]
- Schöpf, V.; Kollndorfer, K.; Pollak, M.; Mueller, C.A.; Freiherr, J. Intranasal Insulin Influences the Olfactory Performance of Patients with Smell Loss, Dependent on the Body Mass Index: A Pilot Study. Rhinology 2015, 53, 371–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brindisi, M.C.; Brondel, L.; Meillon, S.; Barthet, S.; Grall, S.; Fenech, C.; Liénard, F.; Schlich, P.; Astruc, K.; Mouillot, T.; et al. Proof of Concept: Effect of GLP-1 Agonist on Food Hedonic Responses and Taste Sensitivity in Poor Controlled Type 2 Diabetic Patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, B.; Wang, X.; Zhang, X.; Yang, Q.X.; Qing, Z.; Zhang, W.; Zhu, D.; Bi, Y. Olfactory Dysfunction Mediates Adiposity in Cognitive Impairment of Type 2 Diabetes: Insights from Clinical and Functional Neuroimaging Studies. Diabetes Care 2019, 42, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Dicker, D.; Beck, A.; Markel, A.; Marcovicu, D.; Mazzawi, S.; Sarid, M.; Greenberg, E.; Atkinson, R.L. Weight Loss, Dietary Preferences, and Reduction in the Sense of Smell with the Use of a Novel Nasal Device. Obes. Facts 2020, 13, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Shone, G.R. Effects of Ageing on Smell and Taste. Postgrad. Med. J. 2006, 82, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Velluzzi, F.; Deledda, A.; Onida, M.; Loviselli, A.; Crnjar, R.; Sollai, G. Relationship between Olfactory Function and BMI in Normal Weight Healthy Subjects and Patients with Overweight or Obesity. Nutrients 2022, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.; Liu, K.; Liu, R.; Tang, S.; Zhang, Q.; Ekström, I.; Laukka, E.J.; Du, Y.; Qiu, C. Olfactory Impairment Among Rural-Dwelling Chinese Older Adults: Prevalence and Associations with Demographic, Lifestyle, and Clinical Factors. Front. Aging Neurosci. 2021, 13, 621619. [Google Scholar] [CrossRef] [PubMed]
- Campolo, J.; Corradi, E.; Rizzardi, A.; Parolini, M.; Dellanoce, C.; Di Guglielmo, M.L.; Tarlarini, P.; Cattaneo, M.; Trivella, M.G.; De Maria, R. Correlates of Olfactory Impairment in Middle-Aged Non-Diabetic Caucasian Subjects with Stage I-II Obesity. Eur. Arch. Otorhinolaryngol. 2021, 278, 2047–2054. [Google Scholar] [CrossRef]
- Besser, G.; Erlacher, B.; Aydinkoc-tuzcu, K.; Liu, D.T.; Pablik, E.; Niebauer, V.; Koenighofer, M.; Renner, B.; Mueller, C.A. Body-Mass-Index Associated Differences in Ortho- and Retronasal Olfactory Function and the Individual Significance of Olfaction in Health and Disease. J. Clin. Med. 2020, 9, 366. [Google Scholar] [CrossRef]
- Fernandez-Garcia, J.C.; Alcaide, J.; Santiago-Fernandez, C.; Roca-Rodriguez, M.M.; Aguera, Z.; Baños, R.; Botella, C.; de La Torre, R.; Fernandez-Real, J.M.; Fruhbeck, G.; et al. An Increase in Visceral Fat Is Associated with a Decrease in the Taste and Olfactory Capacity. PLoS ONE 2017, 12, e0171204. [Google Scholar] [CrossRef]
- Rawal, S.; Duffy, V.B.; Berube, L.; Hayes, J.E.; Kant, A.K.; Li, C.M.; Graubard, B.I.; Hoffman, H.J. Self-Reported Olfactory Dysfunction and Diet Quality: Findings from the 2011-2014 National Health and Nutrition Examination Survey (NHANES). Nutrients 2021, 13, 4561. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.M.; Delgaudio, J.M.; Wise, S.K. Higher Body Mass Index Is Associated with Subjective Olfactory Dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef] [PubMed]
- Marty, L.; Bentivegna, H.; Nicklaus, S.; Monnery-Patris, S.; Chambaron, S. Non-Conscious Effect of Food Odors on Children’s Food Choices Varies by Weight Status. Front. Nutr. 2017, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Berro, C.; Pendolino, A.L.; Foletto, M.; Facciolo, M.C.; Maculan, P.; Prevedello, L.; Giuntoli, D.G.; Scarpa, B.; Pavan, C.; Andrews, P.J.; et al. Olfactory and Gustatory Function before and after Laparoscopic Sleeve Gastrectomy. Medicina 2021, 57, 913. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Rieber, N.; Sauer, H.; Klosterhalfen, S.; Mack, I.; Zipfel, S.; Teufel, M. Almost Nothing—Not Even Bariatric Surgery for Obesity—Changes Olfactory Sensitivity. J. Res. Obes. 2014, 2014, 491890. [Google Scholar] [CrossRef][Green Version]
- Graham, L.; Murty, G.; Bowrey, D.J. Taste, Smell and Appetite Change After Roux-En-Y Gastric Bypass Surgery. Obes. Surg. 2014, 24, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Guyot, E.; Dougkas, A.; Robert, M.; Nazare, J.-A.; Iceta, S.; Disse, E. Food Preferences and Their Perceived Changes Before and After Bariatric Surgery: A Cross-Sectional Study. Obes. Surg. 2021, 31, 3075–3082. [Google Scholar] [CrossRef]
- Hancı, D.; Altun, H.; Altun, H.; Batman, B.; Karip, A.B.; Serin, K.R. Laparoscopic Sleeve Gastrectomy Improves Olfaction Sensitivity in Morbidly Obese Patients. Obes. Surg. 2016, 26, 558–562. [Google Scholar] [CrossRef]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and Gustatory Function After Bariatric Surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef]
- Jurowich, C.F.; Seyfried, F.; Miras, A.D.; Bueter, M.; Deckelmann, J.; Fassnacht, M.; Germer, C.-T.; Thalheimer, A. Does Bariatric Surgery Change Olfactory Perception? Results of the Early Postoperative Course. Int. J. Color. Dis. 2014, 29, 253–260. [Google Scholar] [CrossRef]
- Lopes, K.G.; dos Santos, G.P.; Romagna, E.C.; Mattos, D.M.F.; Braga, T.G.; Cunha, C.B.; Maranhão, P.A.; Kraemer-Aguiar, L.G. Changes in Appetite, Taste, Smell, and Food Aversion in Post-Bariatric Patients and Their Relations with Surgery Time, Weight Loss and Regain. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2022, 27, 1679–1686. [Google Scholar] [CrossRef]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.-H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported Appetite, Taste and Smell Changes Following Roux-En-Y Gastric Bypass and Sleeve Gastrectomy: Effect of Gender, Type 2 Diabetes and Relationship to Post-Operative Weight Loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pintus, S.; Mastinu, M.; Fantola, G.; Moroni, R.; Pepino, M.Y.; Tomassini Barbarossa, I. Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Pisarska-Adamczyk, M.; Tylec, P.; Gajewska, N.; Wierzbicka, J.; Przęczek, K.; Małczak, P.; Wysocki, M.; Pędziwiatr, M.; Wierdak, M.; Major, P. Postoperative Olfaction Alteration following Laparoscopic Bariatric Surgery. J. Clin. Med. 2021, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Vanderwoude, E.A.; Sudan, R.; Leopold, D.A.; Thompson, J.S. Gastric Bypass Does Not Influence Olfactory Function in Obese Patients. Obes. Surg. 2012, 22, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Zerrweck, C.; Zurita, L.; Álvarez, G.; Maydón, H.G.; Sepúlveda, E.M.; Campos, F.; Caviedes, A.; Guilbert, L. Taste and Olfactory Changes Following Laparoscopic Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2016, 26, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Zerrweck, C.; Gallardo, V.C.; Calleja, C.; Sepúlveda, E.; Guilber, L. Gross Olfaction Before and After Laparoscopic Gastric Bypass. Obes. Surg. 2017, 27, 2988–2992. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.S.; Hummel, T. The Influence of Olfactory Loss on Dietary Behaviors. Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans Can Discriminate More than 1 Trillion Olfactory Stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef]
- Gerkin, R.C.; Castro, J.B. The Number of Olfactory Stimuli That Humans Can Discriminate Is Still Unknown. eLife 2015, 4, e08127. [Google Scholar] [CrossRef]
- Niimura, Y. Olfactory Receptor Multigene Family in Vertebrates: From the Viewpoint of Evolutionary Genomics. Curr. Genom. 2012, 13, 103–114. [Google Scholar] [CrossRef]
- Niimura, Y.; Nei, M. Comparative Evolutionary Analysis of Olfactory Receptor Gene Clusters between Humans and Mice. Gene 2005, 346, 13–21. [Google Scholar] [CrossRef]
- Mariman, E.C.M.; Szklarczyk, R.; Bouwman, F.G.; Aller, E.E.J.G.; van Baak, M.A.; Wang, P. Olfactory Receptor Genes Cooperate with Protocadherin Genes in Human Extreme Obesity. Genes. Nutr. 2015, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.N.; Koo, J.H. Olfactory Receptors in Non-Chemosensory Tissues. BMB Rep. 2012, 45, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Allerton, T.D.; Primeaux, S.D. High-Fat Diet Differentially Regulates Metabolic Parameters in Obesity-Resistant S5B/Pl Rats and Obesity-Prone Osborne-Mendel Rats. Can. J. Physiol. Pharmacol. 2016, 94, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Simola, M.; Malmberg, H. Sense of Smell in Allergic and Nonallergic Rhinitis. Allergy Eur. J. Allergy Clin. Immunol. 1998, 53, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kutlug, S.; Gunbey, E.; Sogut, A.; Celiksoy, M.H.; Kardas, S.; Yildirim, U.; Karli, R.; Murat, N.; Sancak, R. Evaluation of Olfactory Function in Children with Allergic Rhinitis and Nonallergic Rhinitis. Int. J. Pediatr. Otorhinolaryngol. 2016, 86, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Luo, F.; Han, Y.; Lou, H.; Tang, X.; Zhang, L. Obesity/Overweight and Risk of Allergic Rhinitis: A Meta-Analysis of Observational Studies. Allergy 2020, 75, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Cain, W.S.; Gent, J.F. Olfactory Sensitivity: Reliability, Generality, and Association with Aging. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Velluzzi, F.; Deledda, A.; Lombardo, M.; Fosci, M.; Crnjar, R.; Grossi, E.; Sollai, G. Application of Artificial Neural Networks (ANN) to Elucidate the Connections among Smell, Obesity with Related Metabolic Alterations, and Eating Habit in Patients with Weight Excess. Metabolites 2023, 13, 206. [Google Scholar] [CrossRef]
- Trellakis, S.; Tagay, S.; Fischer, C.; Rydleuskaya, A.; Scherag, A.; Bruderek, K.; Schlegl, S.; Greve, J.; Canbay, A.E.; Lang, S.; et al. Ghrelin, Leptin and Adiponectin as Possible Predictors of the Hedonic Value of Odors. Regul. Pept. 2011, 167, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odour Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Shaman, P.; Kimmelman, C.P.; Dann, M.S. University of Pennsylvania Smell Identification Test: A Rapid Quantitative Olfactory Function Test for the Clinic. Laryngoscope 1984, 94, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Duff, K.; McCaffrey, R.J.; Solomon, G.S. The Pocket Smell Test. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Marcus, A.; William Lee, W. Development of the 12-Item Cross-Cultural Smell Identification Test(CC-SIT). Laryngoscope 1996, 106, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Feng, L.; Yang, F.; Ma, W. Effects of Age on Recovery of Olfactory Function After Endoscopic Sinus Surgery and Related Risk Factors. Ear Nose Throat J. 2021, 102, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, A.; Maxwell, L.J.; Ostelo, R.W.; Boers, M.; Tugwell, P.; Terwee, C.B. Measurement Properties of Visual Analogue Scale, Numeric Rating Scale, and Pain Severity Subscale of the Brief Pain Inventory in Patients with Low Back Pain: A Systematic Review. J. Pain 2019, 20, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Facco, E.; Stellini, E.; Bacci, C.; Manani, G.; Pavan, C.; Cavallin, F.; Zanette, G. Validation of Visual Analogue Scale for Anxiety (VAS-A) in Preanesthesia Evaluation. Minerva Anestesiol. 2013, 79, 1389–1395. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).