Combined Activity of Saponin B Isolated from Dodonaea viscosa Seeds with Pesticide Azadirachtin against the Pest Spodoptera litura
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Insects
2.4. Cell Culture
2.5. Isolation of DVSB
2.6. Assays for Nonselective Antifeedant Activity
2.7. Co-Toxicity Coefficient Assays
2.8. Contact Angle Assays
2.9. Surface Tension Assays
2.10. Maximum Retention Assays
2.11. Cell Membrane Permeability Assays
2.12. Statistical Analysis
3. Results
3.1. Isolation of Dodonaea viscosa Saponin B
3.2. Evaluation of the Antifeedant Activities of DVSB and Azadirachtin
3.3. Evaluation of the Antifeedant Activities of Mixtures DVSB with Azadirachtin
3.4. Evaluation of the Antifeedant Activities Co-Toxicity of Mixtures DVSB with Azadirachtin
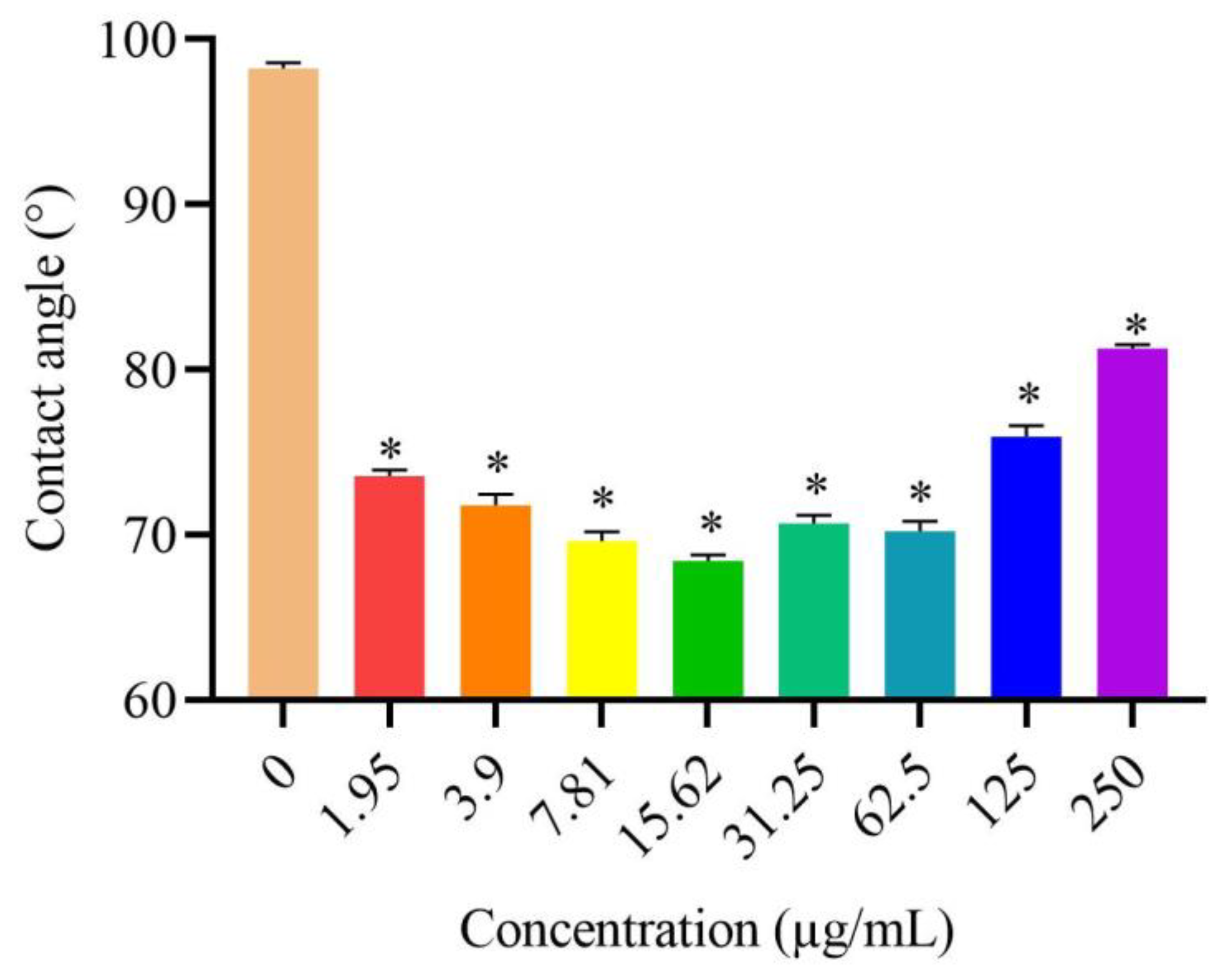
3.5. Effects of DVSB on Contact Angle
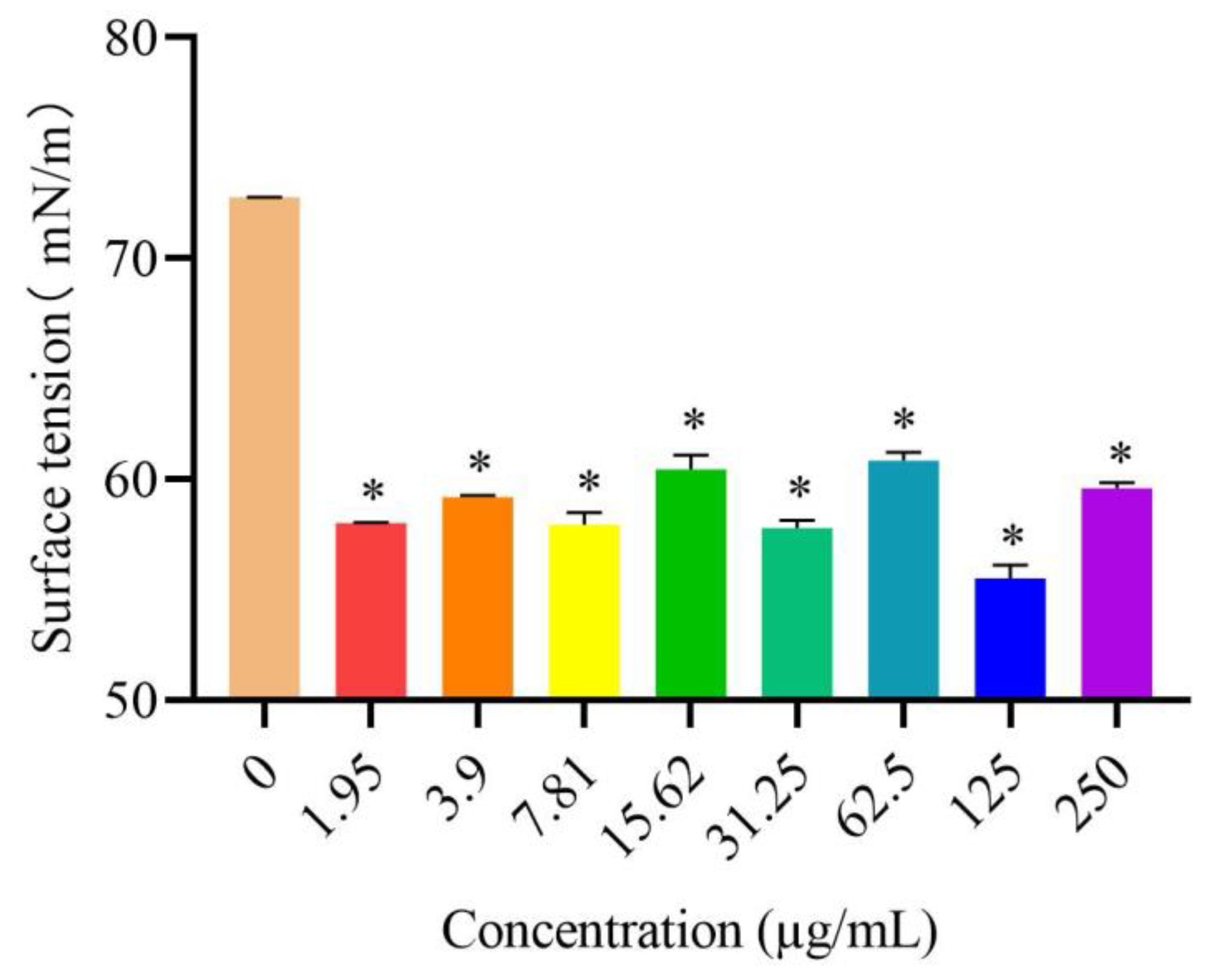
3.6. Effects of DVSB on Surface Tension
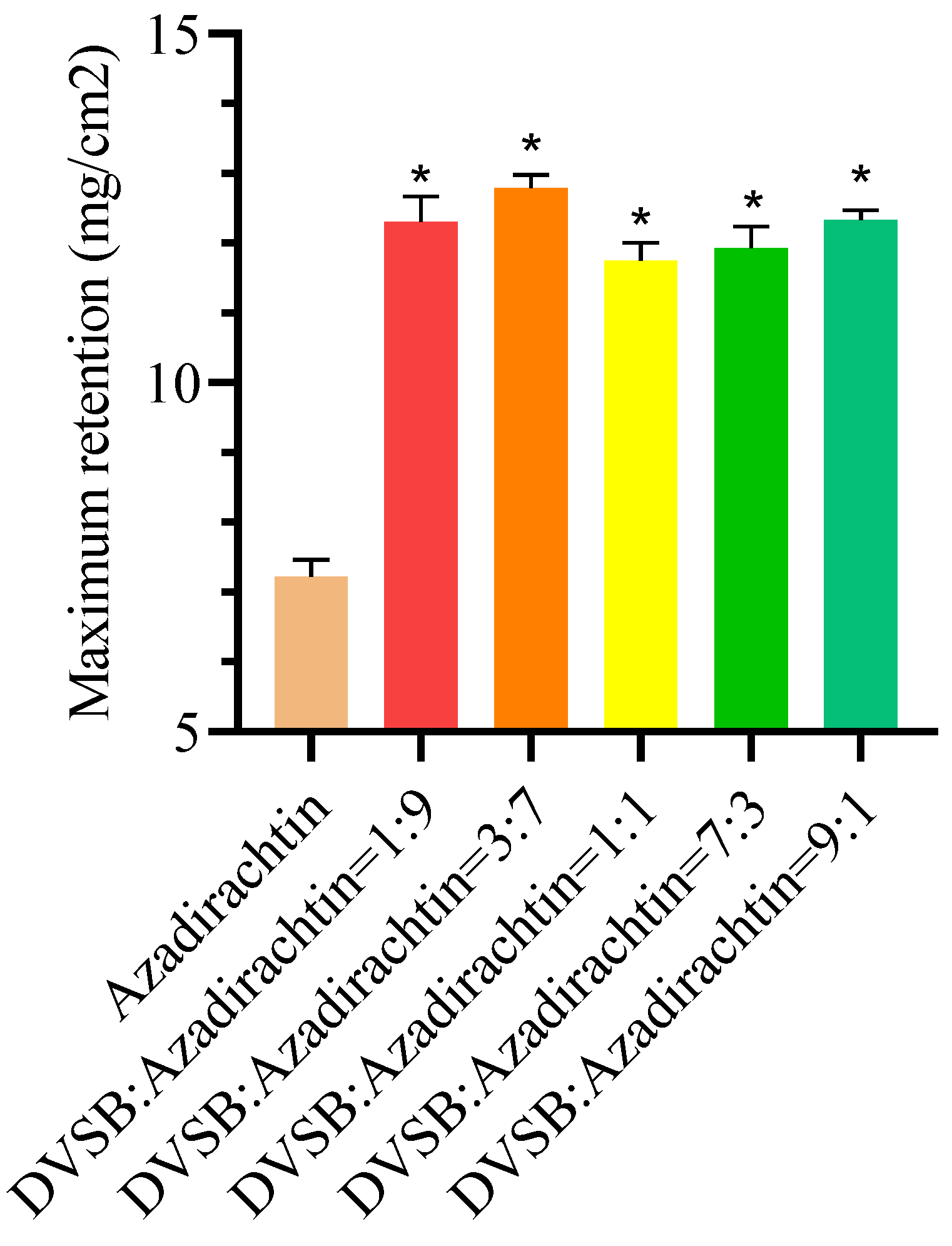
3.7. Effects of DVSB on Maximum Retention
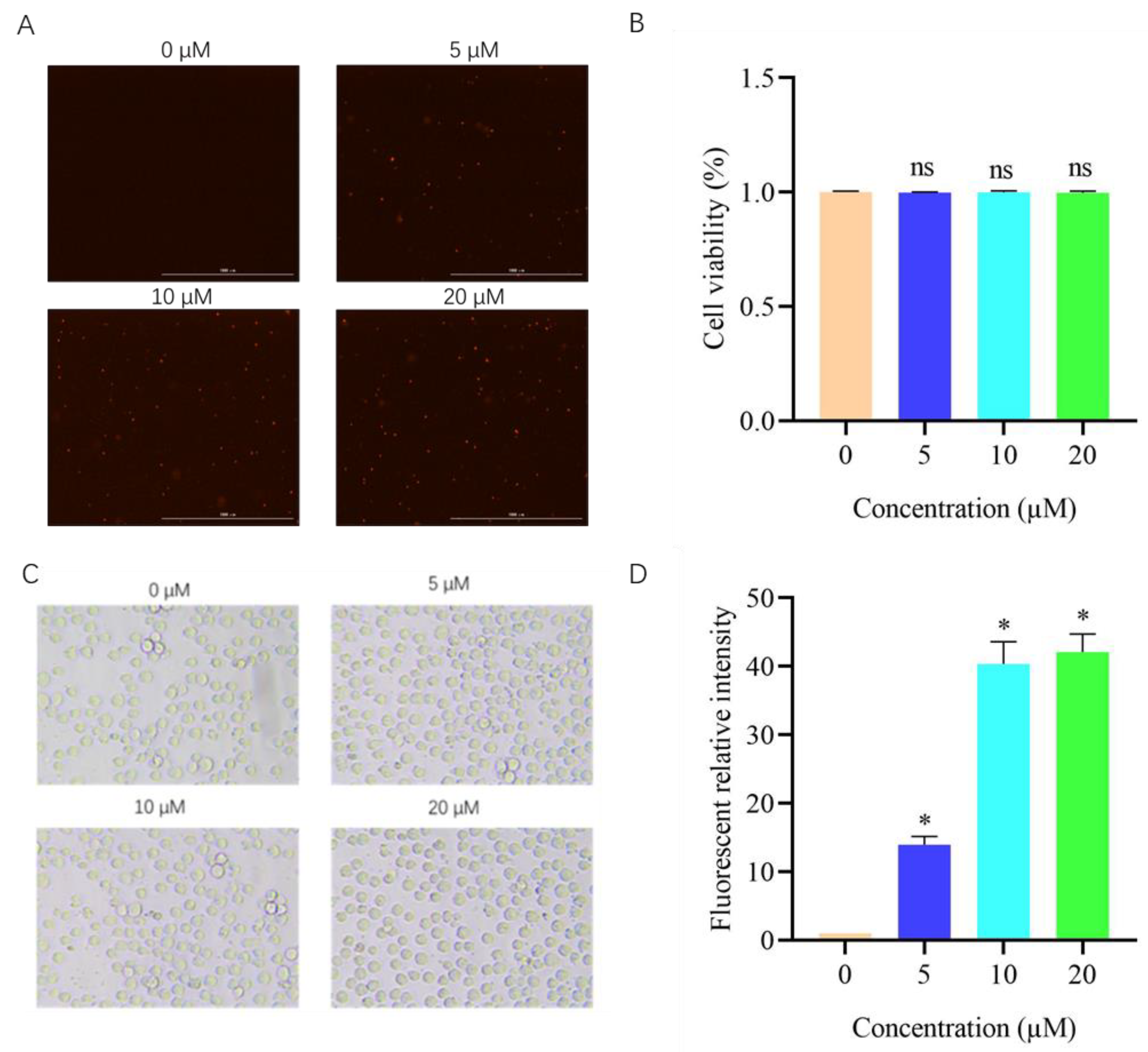
3.8. Effects of DVSB on Cell Membrane Permeability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Hummel, H.E.; Langner, S.S.; Leithold, G.; Schmutterer, H. Neem: Unusually versatile plant genus azadirachta with many useful and so far insufficiently exploited properties for agriculture, medicine, and industry. Commun. Agric. Appl. Biol. Sci. 2014, 79, 211–228. [Google Scholar] [PubMed]
- Ding, X.; Ouyang, M.A.; Liu, X.; Wang, R.Z. Acetylcholinesterase inhibitory activities of flavonoids from the leaves of Ginkgo biloba against brown planthopper. J. Chem. 2013, 2013, 645086. [Google Scholar]
- Li, M.Y.; Gao, X.; Lan, M.X.; Liao, X.B.; Su, F.W.; Fan, L.M.; Zhao, Y.H.; Hao, X.J.; Wu, G.X.; Ding, X. Inhibitory activities of flavonoids from Eupatorium adenophorum against acetylcholinesterase. Pestic. Biochem. Physiol. 2020, 170, 104701. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.X.; Gao, X.; Duan, X.A.; Li, H.M.; Yu, H.; Li, J.L.; Zhao, Y.Q.; Hao, X.J.; Zhao, Y.H.; Ding, X.; et al. Nematicidal activity of tirotundin and parthenolide isolated from Tithonia diversifolia and Chrysanthemum parthenium. Environ. Sci. Health B 2022, 57, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Wang, H.T.; Shi, S.; Meng, Y.; Feng, J.C.; Wu, H.B. Sesquiterpenoids from Artemisia vestita and their antifeedant and antifungal activities. Molecules 2019, 24, 3671. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Chen, L.; Gao, F.; Zhou, X.L. Diterpenoid alkaloids from Delphinium naviculare var. lasiocarpum with their antifeedant activity on Spodoptera exigua. Nat. Prod. Res. 2019, 33, 3254–3259. [Google Scholar]
- Li, C.H.; Zhang, J.Y.; Tuong, T.M.L.; Liu, Y.; Hoang, X.N.; Gao, J.M. Cassane diterpenoids from the aerial parts of Caesalpinia pulcherrima and their antifeedant and insecticidal activities against Mythimna separate and Plutella xylostella. J. Agric. Food Chem. 2020, 68, 4227–4236. [Google Scholar] [CrossRef]
- Cui, G.F.; Yuan, H.Q.; He, W.; Deng, Y.K.; Sun, R.R.; Zhong, G.H. Synergistic effects of botanical curcumin-induced programmed cell death on the management of Spodoptera litura Fabricius with avermectin. Ecotoxicol. Environ. Saf. 2022, 229, 113097. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Li, D.T.; Wang, X.L.; Liu, Z.; Yu, X.P.; Shentu, X.P. Synergistic and additive interactions of zhongshengmycin to the chemical insecticide pymetrozine for controlling Nilaparvata lugens (Hemiptera: Delphacidae). Front. Physiol. 2022, 13, 875610. [Google Scholar] [CrossRef]
- Chen, H.T.; Zhang, Y.Q.; Zhang, H.C.; Ding, W. Synergistic effect of imidacloprid combined with synergistic agents (Beichuang, Jiexiaoli) on Myzus persicae (Hemiptera: Aphididae). Pest Manag. 2014, 60, 201–207. [Google Scholar]
- Patel, M.; Srivastava, V.; Ahmad, A. Dodonaea viscosa var angustifolia derived 5,6,8-trihydroxy-7,4′ dimethoxy flavone inhibits ergosterol synthesis and the production of hyphae and biofilm in Candida albicans. J. Ethnopharmacol. 2020, 259, 112965. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, X.H.; Liu, Y.N.; Zhao, T.; Hu, Z.; Li, J.Y.; Hou, A.J. Clerodane diterpenoids from Dodonaea viscosa and their inhibitory effects on ATP citrate lyase. Phytochemistry 2021, 183, 112614. [Google Scholar] [CrossRef] [PubMed]
- Sagara, T.; Sugimoto, S.; Yamano, Y.; Nehira, T.; Masuda, K.; Otsuka, H.; Matsunami, K. Isolation of three new diterpenes from Dodonaea viscosa. Chem. Pharm. Bull. 2021, 69, 40–47. [Google Scholar] [CrossRef]
- Cao, S.G.; Brodie, P.; Callmander, M.; Randrianaivo, R.; Razafitsalama, J.; Rakotobe, E.; Rasamison, V.E.; TenDyke, K.; Shen, Y.C.; Suh, E.M.; et al. Antiproliferative triterpenoid saponins of Dodonaea viscosa from the Madagascar dry forest. Nat. Prod. 2009, 72, 1705–1707. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.L.; Wu, G.X.; Tang, Q.B.; Duan, X.A.; Liu, Q.J.; Lan, M.X.; Zhao, Y.H.; Hao, X.J.; Qin, X.P.; et al. Antifeedant mechanism of Dodonaea viscosa saponin A isolated from the seeds of Dodonaea viscosa. Molecules 2022, 27, 4464. [Google Scholar] [CrossRef]
- Herrmann, F.; Wink, M. Synergistic interactions of saponins and monoterpenes in HeLa cells, Cos7 cells and in erythrocytes. Phytomedicine 2011, 18, 1191–1196. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H. Synergistic interaction of tea saponin with mancozeb against Pestalotiopsis theae. Crop Prot. 2012, 40, 126–131. [Google Scholar] [CrossRef]
- Fields, P.G.; Woods, S.; Taylor, W.G. Triterpenoid saponins synergize insecticidal pea peptides: Effect on feeding and survival of Sitophilus oryzae (Coleoptera: Curculionidae). Can. Entomol. 2010, 142, 501–512. [Google Scholar] [CrossRef]
- Tuan, S.J.; Li, N.J.; Yeh, C.C.; Tang, L.C.; Chi, H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. Econ. Entomol. 2014, 107, 897–905. [Google Scholar] [CrossRef]
- Ahmad, M.; Mehmood, R. Monitoring of resistance to new chemistry insecticides in Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Econ. Entomol. 2015, 108, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Lai, T.; Li, J. Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop Prot. 2012, 42, 217–222. [Google Scholar] [CrossRef]
- Ahmad, M.; Sayyed, A.H.; Saleem, M.A.; Ahmad, M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 2008, 27, 1367–1372. [Google Scholar] [CrossRef]
- Saleem, M.A.; Ahmad, M.; Ahmad, M.; Aslam, M.; Sayyed, A.H. Resistance to selected organochlorin, organophosphate, carbamate and pyrethroid, in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. J. Econ. Entomol. 2008, 101, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Shu, B.S.; Yi, X.; Liu, J.; Hu, M.Y.; Zhong, G.H. Cross-resistance and baseline susceptibility of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Manag. Sci. 2016, 72, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Su, Q.; Zhou, X.; Bai, L.Y. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. Pest Sci. 2013, 86, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.J.; Glendinning, J.I. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. Comp. Physiol. A 1996, 179, 255–261. [Google Scholar] [CrossRef]
- GB 5549-1990; State General Administration of the People’s Republic of China for Quality Supervision and Inspection and Quarantine; Standardization Administration of China. Surface Active Agents—Determination of Surface Tension by Drawing up Liquid Films. Standards Press of China: Beijing, China, 2011. [Google Scholar]
- Zhu, F.; Cao, C.; Cao, L.D.; Li, F.M.; Du, F.P.; Huang, Q.L. Wetting behavior and maximum retention of aqueous surfactant solutions on tea leaves. Molecules 2019, 24, 2094. [Google Scholar] [CrossRef]
- Wang, D.; Su, D.; Li, X.Z.; Liu, D. Xi, R.G.; Gao, H.Y.; Wang, X.B. Barrigenol triterpenes from the husks of Xanthoceras sorbifolia Bunge and their antitumor activities. RSC Adv. 2016, 6, 27434–27446. [Google Scholar] [CrossRef]
- Gilabert-Oriol, R.; Mergel, K.; Thakur, M.; Von Mallinckrodt, B.; Melzig, M.F.; Fuchs, H.; Weng, A. Real-time analysis of membrane permeabilizing effects of oleanane saponins. Bioorgan. Med. Chem. 2013, 21, 2387–2395. [Google Scholar] [CrossRef]
- Yin, S.; Li, L.; Su, L.; Li, H.F.; Zhao, Y.; Wu, Y.D.; Liu, R.A.; Zou, F.; Ni, G.H. Synthesis and in vitro synergistic antifungal activity of analogues of Panax stipulcanatus saponin against fluconazole-resistant Candida albicans. Carbohyd. Res. 2022, 517, 108575. [Google Scholar] [CrossRef] [PubMed]
- Del Socorro, A.P.; Gregg, P.C.; Alter, D.; Moore, C.J. Development of a synthetic plant volatile-based attracticide for female noctuid moths. I. Potential sources of volatiles attractive to Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 10–20. [Google Scholar] [CrossRef]
- Aheer, G.M.; Aziz, M.A.; Hameed, A.; ALI, A. Evaluation of resistance to different insecticides in field strains of Helicoverpa armigera (Lepidoptera: Noctuidae) in Punjab, Pakistan. Entomol. Res. 2009, 39, 159–167. [Google Scholar] [CrossRef]
- Wang, H.; Dong, H.Y.; He, Q.M.; Liang, J.L.; Zhao, T.; Zhao, L. Characterization of limonoids isolated from the fruits of Melia toosendan and their antifeedant activity against Pieris rapae. Chem. Biodivers. 2020, 17, e1900674. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, K. Isolation of insecticidal components in Inula salsoloides Ostenf. and characterisation of their activities. Nat. Prod. Res. 2017, 31, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wang, B.J.; Wang, M.R.; Peng, Y.C.; Cao, H.Q.; Sheng, C.W. Control efficacy and joint toxicity of metaflumizone mixed with chlorantraniliprole or indoxacarb against the fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2022, 79, 1094–1101. [Google Scholar] [CrossRef]
- Chen, J.X.; Jiang, W.L.; Hu, H.Y.; Ma, X.Y.; Li, Q.; Song, X.P.; Ren, X.L.; Ma, Y. Joint toxicity of methoxyfenozide and lufenuron on larvae of Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Asia-Pac. Entomol. 2019, 22, 795–801. [Google Scholar] [CrossRef]
- Tang, T.; Zhao, M.P.; Wang, P.; Huang, S.K.; Fu, W. Control efficacy and joint toxicity of thiamethoxam mixed with spirotetramat against the Asian citrus psyllid, Diaphorina citri Kuwayama. Pest Manag. Sci. 2021, 77, 168–176. [Google Scholar] [CrossRef]
- Chen, J. Molecular Mechansim Underlying Natural Borneol Potentiated Curcuminoids Inhibiting Hepg2 Human Hepatocellular Carcinoma Cells Growth; South China University of Technology: Guangzhou, China, 2015. [Google Scholar]
- Zdybicka-Barabas, A.; Stączek, S.; Mak, P.; Skrzypiec, K.; Mendyk, E.; Cytrynska, M. Synergistic action of Galleria mellonella apolipophorin III and lysozyme against Gram-negative bacteria. Biochim. Biophys. Acta (BBA)—Biomembr. 2013, 1828, 1449–1456. [Google Scholar] [CrossRef]

| NO. | δH J (Hz) | δC |
|---|---|---|
| 1 | 0.88, m, 1.45, m | 39.4 |
| 2 | 2.12, m, 1.87, m | 27.0 |
| 3 | 3.3 (dd, 3.9, 11.5) | 89.6 |
| 4 | − | 40.0 |
| 5 | 0.79 (d, 12.2) | 56.0 |
| 6 | 1.32, m, 1.55 (d, 12.2) | 19.2 |
| 7 | 2.12, m, 2.03, m | 37.4 |
| 8 | − | 41.9 |
| 9 | 1.70, m | 47.6 |
| 10 | − | 37.2 |
| 11 | 1.80, m, 1.89, m | 24.5 |
| 12 | 5.50, s | 125.8 |
| 13 | − | 144.2 |
| 14 | − | 48.2 |
| 15 | 4.21, m | 67.9 |
| 16 | 4.43, m | 73.4 |
| 17 | − | 49.0 |
| 18 | 3.07, m | 41.4 |
| 19 | 3.07, m, 1.42 (d, 9.6) | 47.2 |
| 20 | − | 36.8 |
| 21 | 6.66 (d, 10.2) | 78.9 |
| 22 | 6.24 (d, 10.2) | 73.6 |
| 23 | 1.29, s | 28.5 |
| 24 | 1.11, s | 17.2 |
| 25 | 0.86, s | 16.3 |
| 26 | 1.03, s | 18.0 |
| 27 | 1.84, s | 21.7 |
| 28 | 3.76 (d, 9.6) 3.48 (d, 9.6) | 63.6 |
| 29 | 1.09, m | 29.9 |
| 30 | 1.30, s | 20.7 |
| C-3-GlcA | ||
| 1′ | 5.0, s | 105.8 |
| 2′ | 4.26, m | 84.2 |
| 3′ | 4.32 (t, 9.0) | 77.9 |
| 4′ | 4.43, m | 73.3 |
| 5′ | 4.06, m | 77.5 |
| 6′ | - | 171.0 |
| C-6′-OMe | 3.70, s | 52.5 |
| GlcA -2-Gal | ||
| 1″ | 5.23 (d, 7.6) | 107.7 |
| 2″ | 4.58, m | 75.2 |
| 3″ | 4.18, m | 75.4 |
| 4″ | 4.70, s | 69.9 |
| 5″ | 4.54 (d, 9.7) | 77.2 |
| 6″ | 4.41, m, 4.62, m | 61.7 |
| C-21-Ang | ||
| 1 | − | 167.9 |
| 2 | − | 129.3 |
| 3 | 6.06, m | 139.2 |
| 4 | 2.16 (d, 7.2) | 16.6 |
| 5 | 2.03, s | 21.6 |
| C-22-2MB | ||
| 1 | − | 176.9 |
| 2 | 2.09, m | 42.2 |
| 3 | 1.64, m 1.22, m | 27.2 |
| 4 | 0.74 (t, 7.4) | 12.3 |
| 5 | 0.86, s | 17.5 |
| Concentration (μg/mL) | Feeding Area (mm2) | Inhibition Rate (%) |
|---|---|---|
| Control check (CK) | 1075.14 ± 4.87 a | |
| 62.5 | 703.36 ± 3.42 b | 34.57 |
| 125 | 703.36 ± 3.93 b | 34.57 |
| 250 | 663.17 ± 10.72 c | 40.18 |
| 500 | 343.83 ± 9.84 d | 68.02 |
| 1000 | 160.77 ± 3.93 e | 85.05 |
| Concentration (μg/mL) | Feeding Area (mm2) | Inhibition Rate (%) |
|---|---|---|
| Control check (CK) | 1075.14 ± 4.87 a | |
| 12.34 | 559.58 ± 4.64 c | 47.95 |
| 37.03 | 556.92 ± 5.81 d | 48.2 |
| 111 | 496.26 ± 6.38 e | 53.84 |
| 333 | 291.83 ± 4.39 f | 72.85 |
| 1000 | 6.02 ± 0.82 g | 99.43 |
| Concentration (μg/mL) | Feeding Area (mm2) | Inhibition Rate (%) |
|---|---|---|
| Control check (CK) | 1075.14 ± 4.87 a | |
| 5.44 | 813.13 ± 20.68 b | 24.37 |
| 10.87 | 719.05 ± 10.04 c | 33.12 |
| 21.74 | 598.21 ± 12.60 d | 44.36 |
| 43.5 | 454.14 ± 7.04 e | 57.76 |
| 87 | 266.63 ± 11.23 f | 75.2 |
| Concentration (μg/mL) | Feeding Area (mm2) | Inhibition Rate (%) |
|---|---|---|
| Control check (CK) | 1075.14 ± 4.87 a | |
| 9.31 | 602.88 ± 15.05 b | 25.93 |
| 18.61 | 562.69 ± 10.55 c | 30.86 |
| 37.22 | 502.40 ± 10.59 d | 38.27 |
| 74.43 | 422.02 ± 11.58 e | 48.14 |
| 148.85 | 301.44 ± 7.20 f | 63 |
| Concentration (μg/mL) | Feeding Area (mm2) | Inhibition Rate (%) |
|---|---|---|
| Control check (CK) | 1075.14 ± 4.87 a | |
| 13.18 | 683.26 ± 10.92 b | 16.05 |
| 26.36 | 602.88 ± 7.34 c | 25.93 |
| 52.72 | 462.21 ± 15.70 d | 43.21 |
| 105.44 | 361.73 ± 15.02 e | 55.56 |
| 210.87 | 221.06 ± 10.02 f | 72.84 |
| Concentration (μg/mL) | Feeding Area (mm2) | Inhibition Rate (%) |
|---|---|---|
| Control check (CK) | 1075.14 ± 4.87 a | |
| 17.06 | 884.22 ± 11.95 b | 32.31 |
| 34.11 | 844.03 ± 14.23 bc | 35.38 |
| 68.23 | 803.84 ± 10.69 c | 38.46 |
| 136.47 | 643.07 ± 12.04 d | 50.77 |
| 272.93 | 321.54 ± 11.34 e | 60.49 |
| Mixture (Mass Ratio) | Regression Equation | AFC50 (µg/mL) | Correlation Coefficient | Active Toxicity Index | Theoretical Toxicity Index | Co-Toxicity Coefficient |
|---|---|---|---|---|---|---|
| DVSB | y = 0.0006x + 0.3025 | 329.17 | 0.919 | 100 | ||
| Azadirachtin | y = 0.000527x + 0.4877 | 23.33 | 0.9702 | 1410.93 | ||
| DVSB: aza-dirachtin =1:4 (3.4:1) | y = 0.0059x + 0.2507 | 38.86 | 0.9443 | 847.07 | 397.93 | 212.87 |
| DVSB: aza-dirachtin =2:3 (9:1) | y = 0.0026x + 0.2644 | 90.62 | 0.9732 | 363.24 | 231.1 | 157.18 |
| DVSB: aza-dirachtin =3:2 (20.3:1) | y = 0.0027x + 0.2078 | 108.22 | 0.9016 | 304.17 | 161.54 | 188.29 |
| DVSB: aza-dirachtin =4:1 (54.3:1) | y = 0.0011x + 0.3169 | 166.46 | 0.9643 | 197.75 | 123.7 | 159.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Liu, B.; Zhao, Y.; Li, J.; Wu, G.; Ma, J.; Gui, F.; Tao, F.; Hao, X.; Ding, X.; et al. Combined Activity of Saponin B Isolated from Dodonaea viscosa Seeds with Pesticide Azadirachtin against the Pest Spodoptera litura. Metabolites 2024, 14, 15. https://doi.org/10.3390/metabo14010015
Yu H, Liu B, Zhao Y, Li J, Wu G, Ma J, Gui F, Tao F, Hao X, Ding X, et al. Combined Activity of Saponin B Isolated from Dodonaea viscosa Seeds with Pesticide Azadirachtin against the Pest Spodoptera litura. Metabolites. 2024; 14(1):15. https://doi.org/10.3390/metabo14010015
Chicago/Turabian StyleYu, Hang, Boyu Liu, Yuhan Zhao, Jinliang Li, Guoxing Wu, Junhong Ma, Furong Gui, Feng Tao, Xiaojiang Hao, Xiao Ding, and et al. 2024. "Combined Activity of Saponin B Isolated from Dodonaea viscosa Seeds with Pesticide Azadirachtin against the Pest Spodoptera litura" Metabolites 14, no. 1: 15. https://doi.org/10.3390/metabo14010015
APA StyleYu, H., Liu, B., Zhao, Y., Li, J., Wu, G., Ma, J., Gui, F., Tao, F., Hao, X., Ding, X., & Qin, X. (2024). Combined Activity of Saponin B Isolated from Dodonaea viscosa Seeds with Pesticide Azadirachtin against the Pest Spodoptera litura. Metabolites, 14(1), 15. https://doi.org/10.3390/metabo14010015







