Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Use Approval
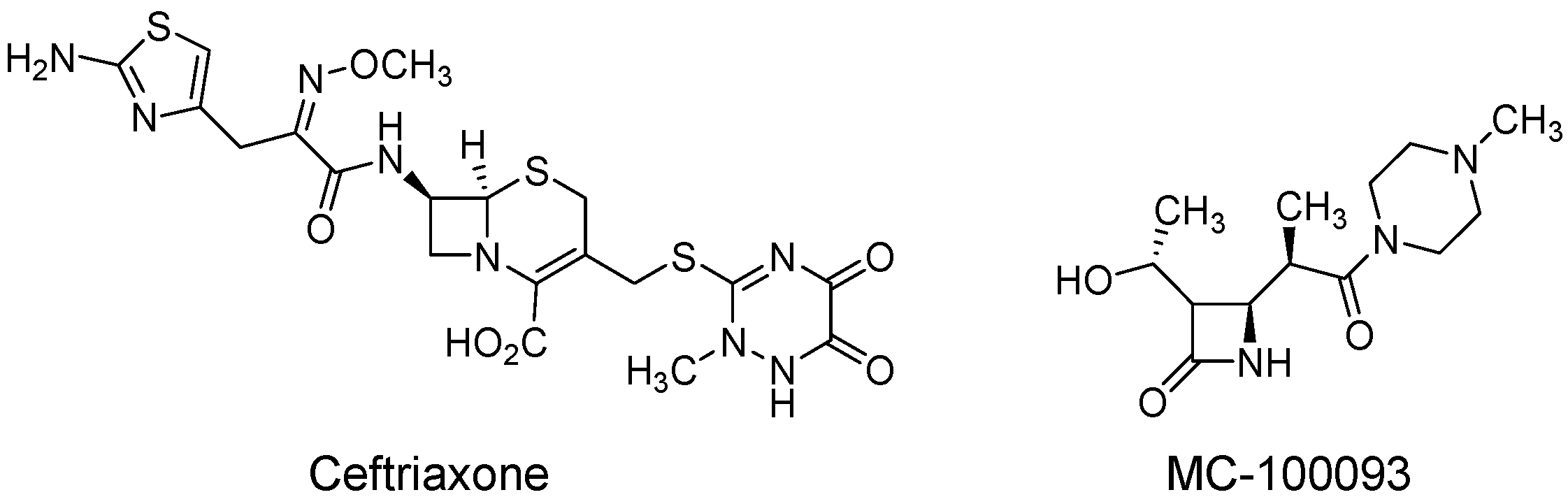
2.2. Study Design
2.3. Gas Chromatography–Mass Spectrometry (GC-MS)
2.3.1. Chemicals
2.3.2. Sample Preparation
2.3.3. Parameter Description
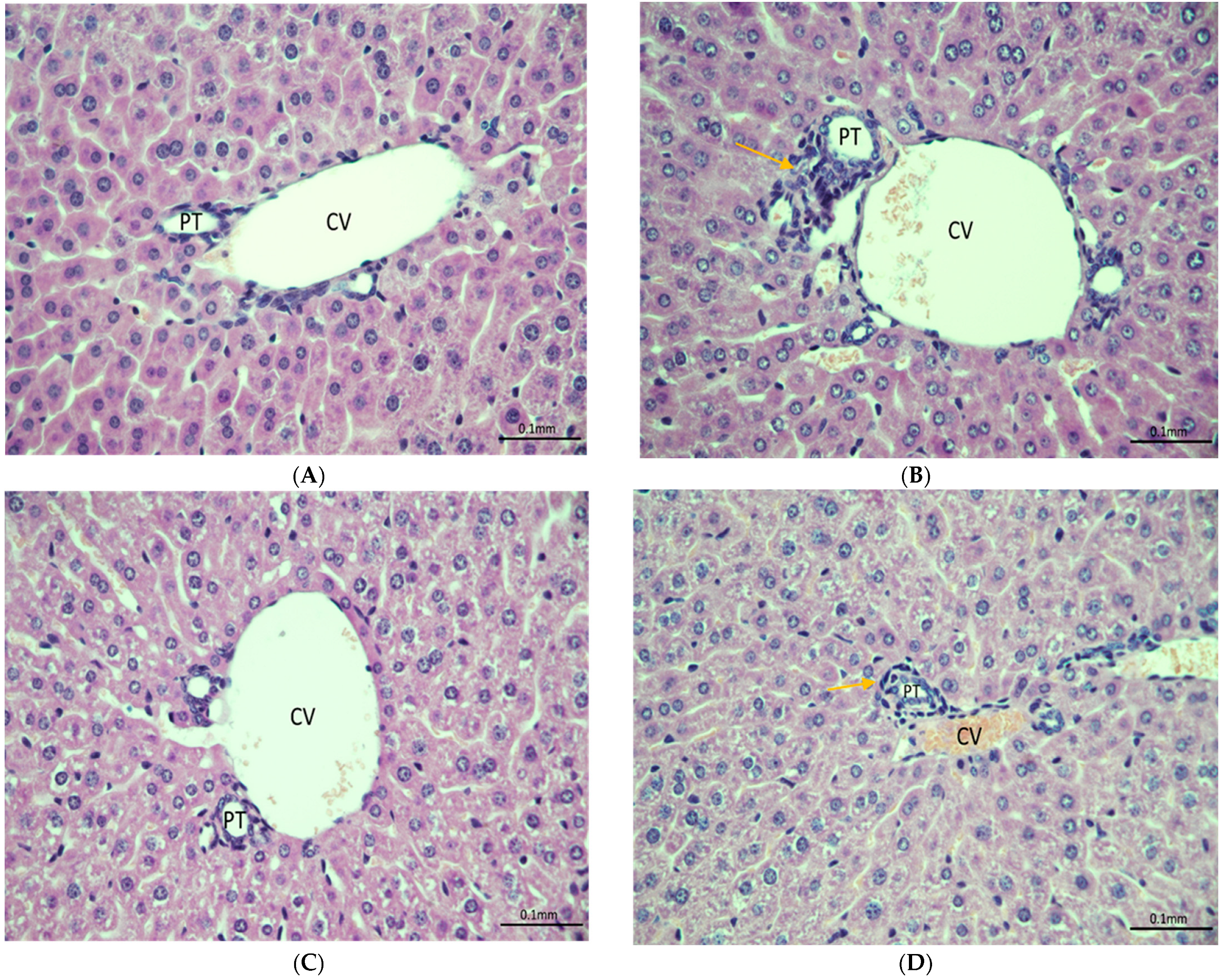
2.4. Histopathology
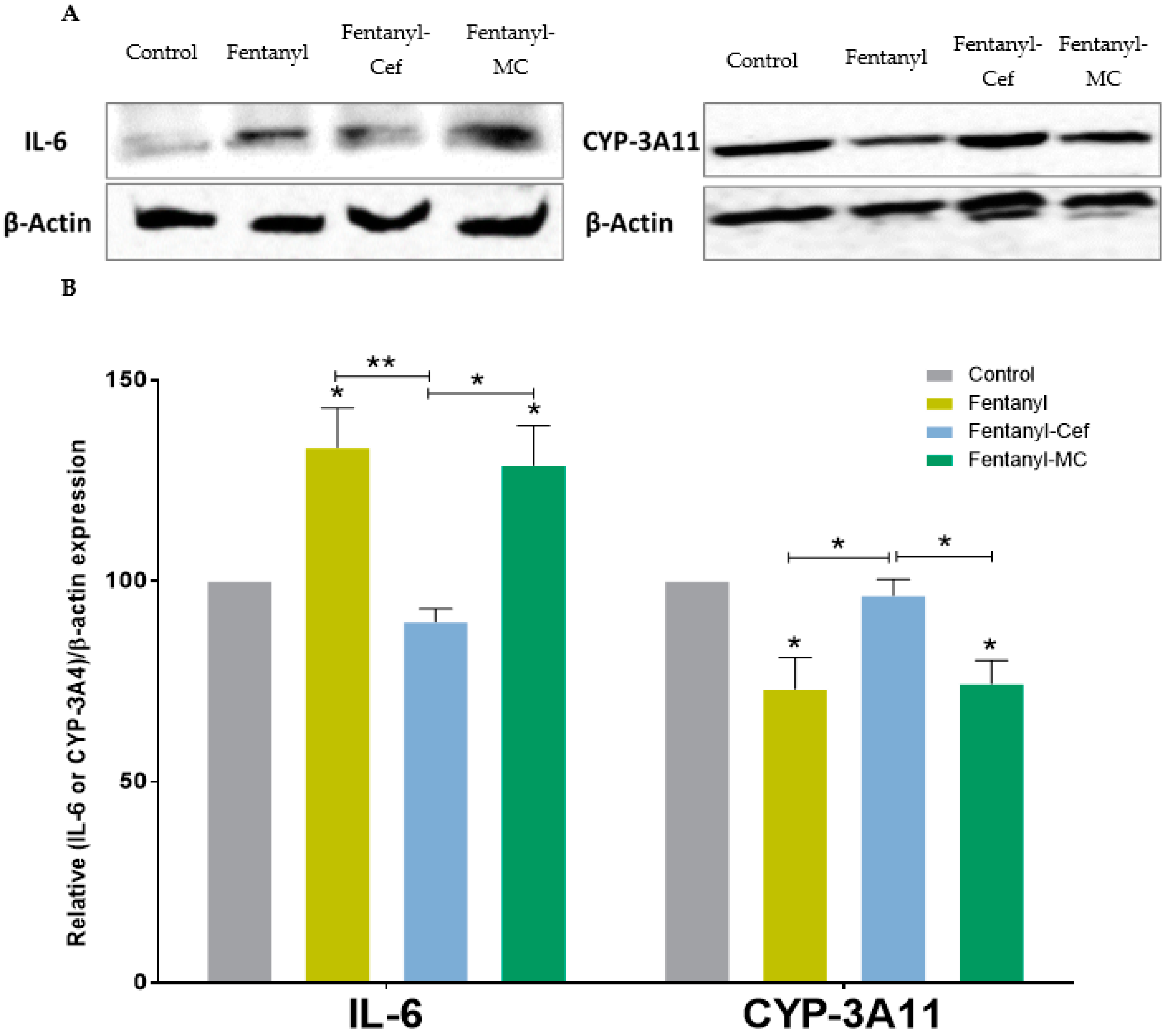
2.5. Western Blot Assay
2.6. Statistical Analysis
2.7. Heatmap and Enrichment Analysis Model
3. Results
3.1. Overall Metabolomic Profiling, Heatmap, Partial Least-Squares-Discriminant Analysis (PLSDA), Peak Ratio of Selected Metabolites of Fentanyl, Fentanyl–Ceftriaxone, and Fentanyl-MC-100093 Groups
3.1.1. Overall Metabolomic Profiling of Fentanyl, Fentanyl–Ceftriaxone, and Fentanyl-MC-100093 Groups
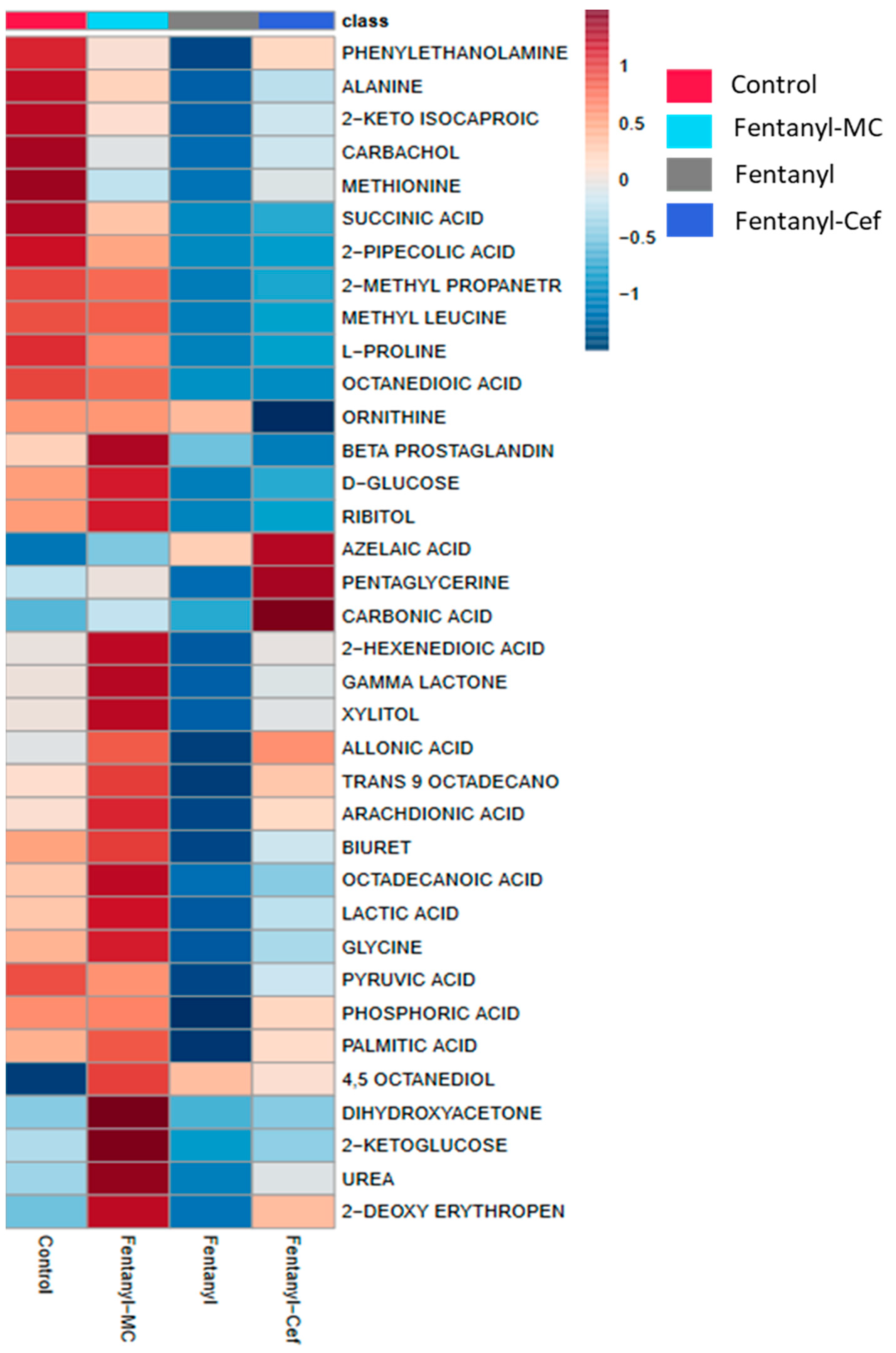
3.1.2. Heatmap of Fentanyl, Fentanyl–Ceftriaxone and Fentanyl-MC-100093 Groups
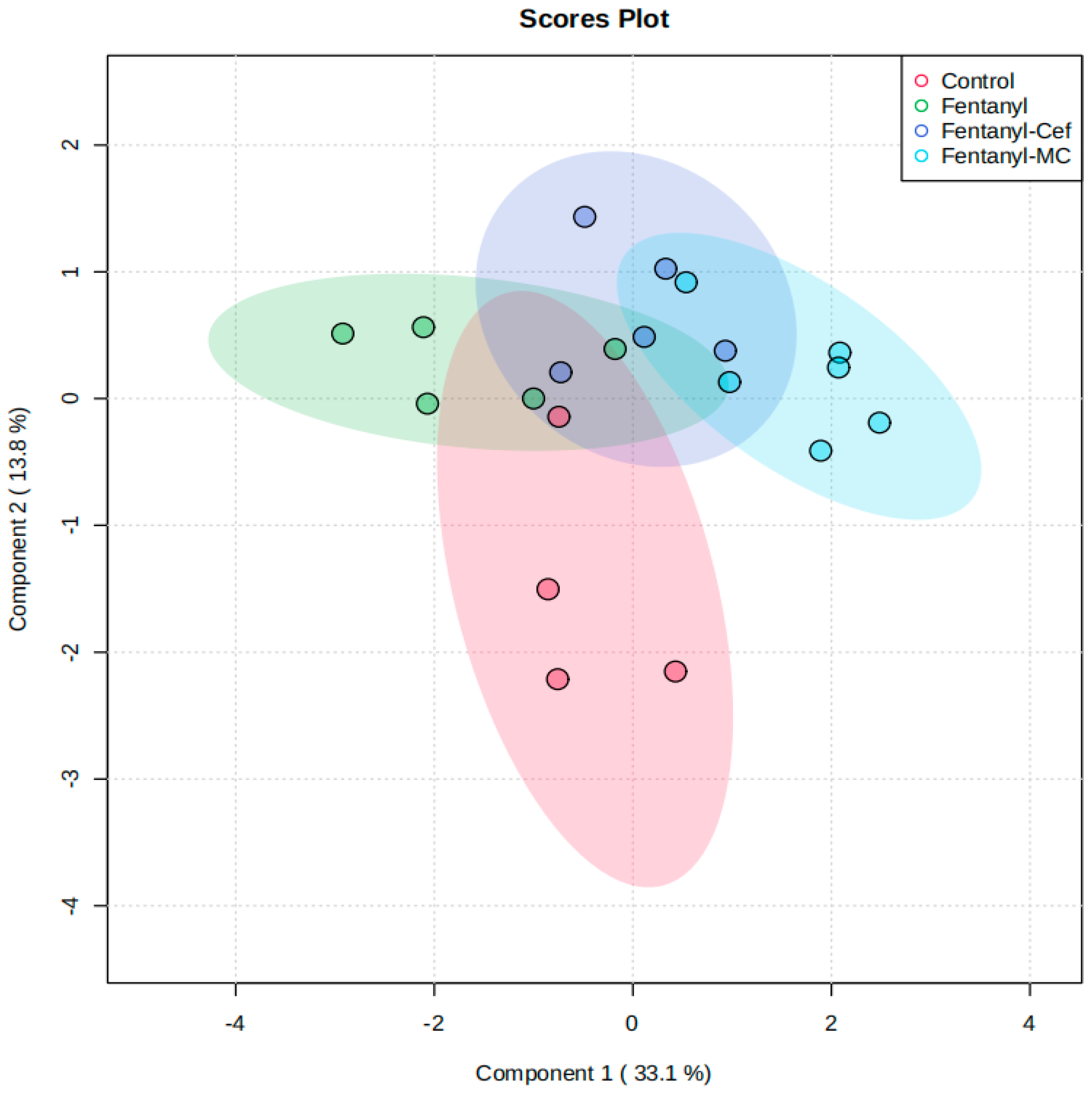
3.1.3. Partial Least-Squares-Discriminant Analysis (PLSDA) of Fentanyl, Fentanyl–Ceftriaxone, and Fentanyl-MC-100093 Groups
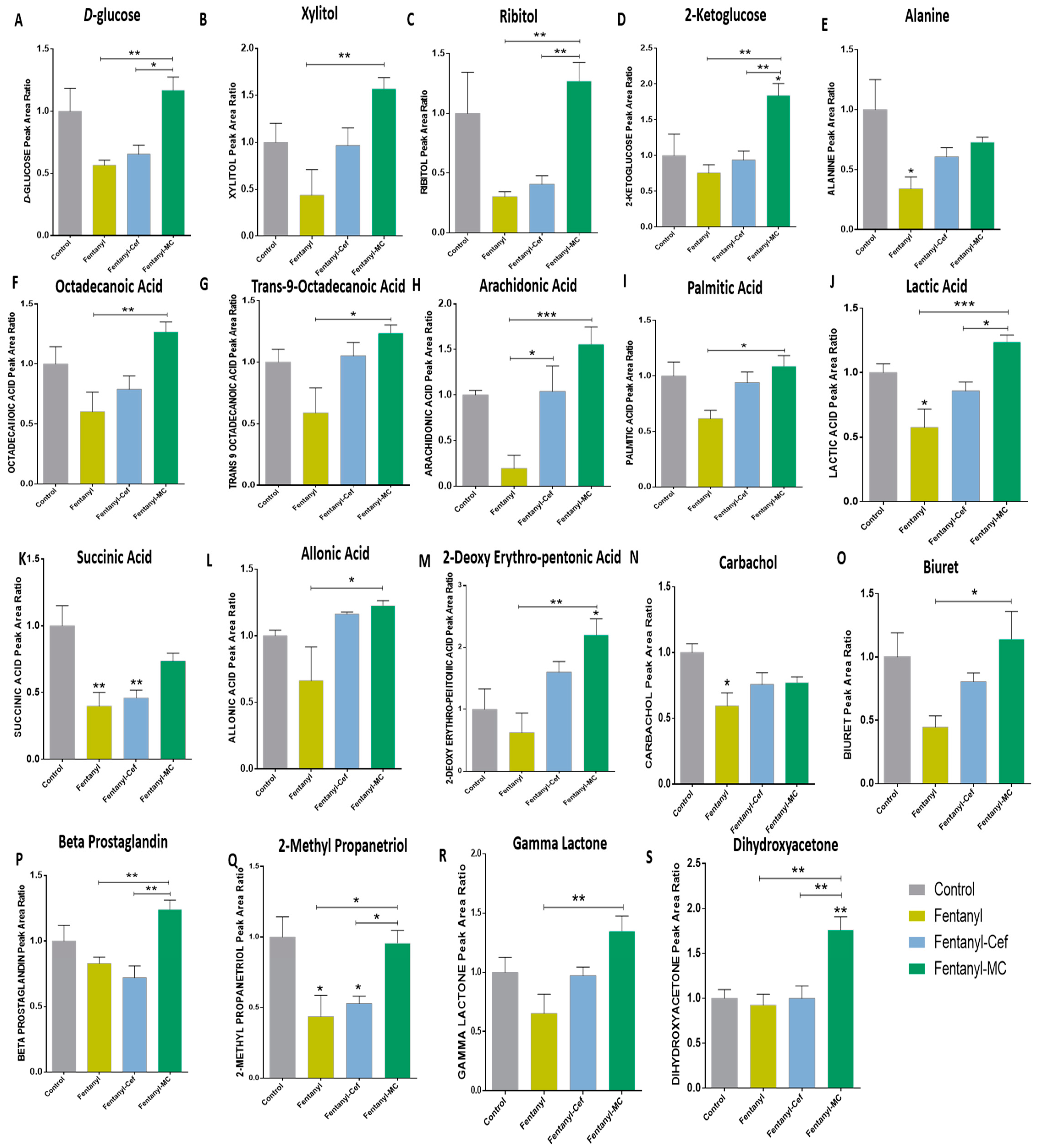
3.1.4. Peak Ratio of Selected Metabolites of Fentanyl, Fentanyl–Ceftriaxone and Fentanyl-MC-100093 Groups
3.2. Enrichment Analysis Model of Control, Fentanyl, Fentanyl–Ceftriaxone, and Fentanyl-MC-100093 Metabolomic Profiles
3.3. Histopathology Analysis and Protein Expression of IL-6 and CYP-3A4 in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams
3.4. Liver Protein Expression of IL-6 and CYP-3A4 in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams
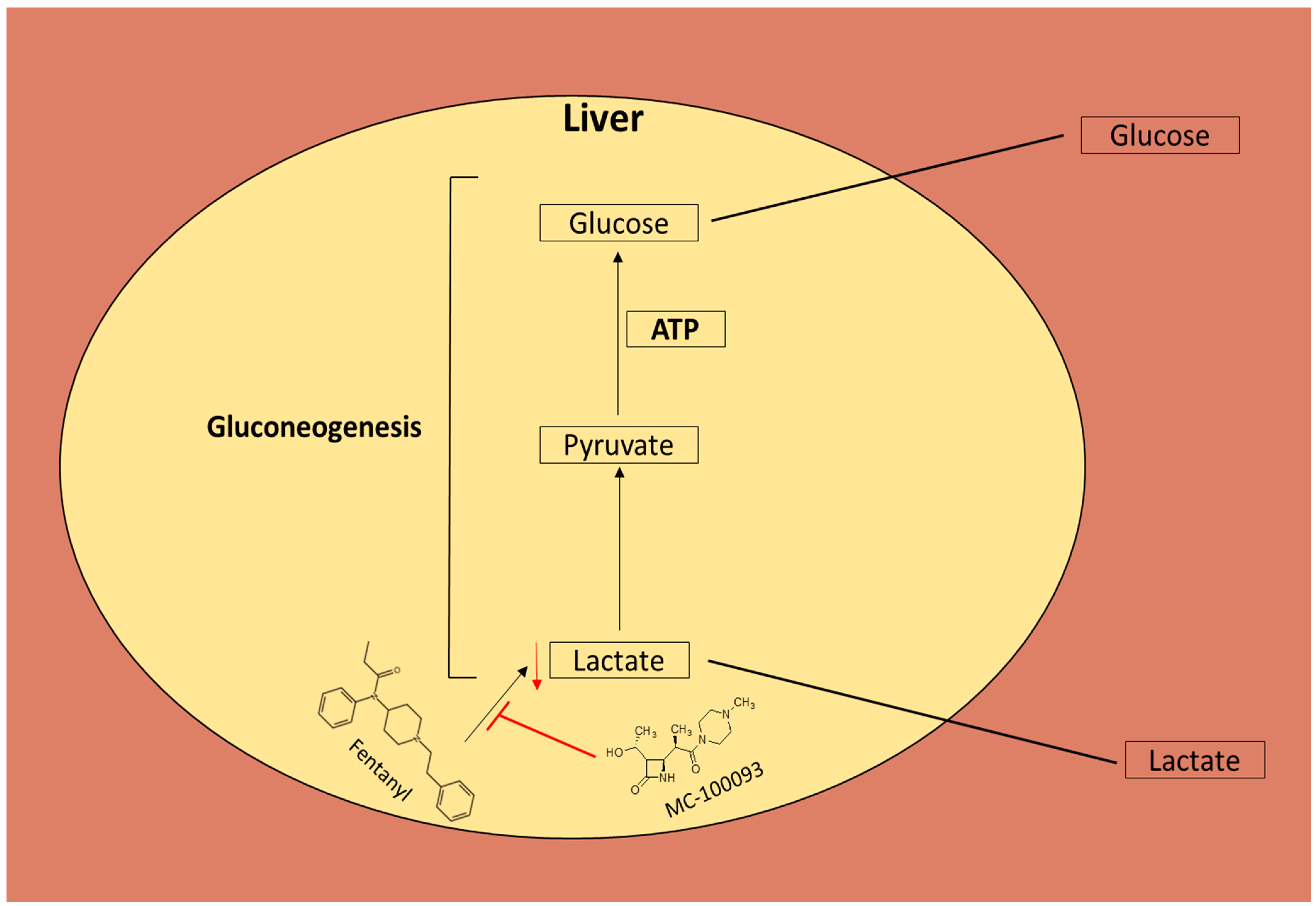
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobczak, M.; Sałaga, M.; Storr, M.A.; Fichna, J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: Current concepts and future perspectives. J. Gastroenterol. 2014, 49, 24–45. [Google Scholar] [CrossRef]
- Iwaszkiewicz, K.S.; Schneider, J.J.; Hua, S. Targeting peripheral opioid receptors to promote analgesic and anti-inflammatory actions. Front. Pharmacol. 2013, 4, 132. [Google Scholar] [CrossRef]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379. [Google Scholar] [CrossRef]
- Reeves, K.C.; Shah, N.; Muñoz, B.; Atwood, B.K. Opioid receptor-mediated regulation of neurotransmission in the brain. Front. Mol. Neurosci. 2022, 15, 919773. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Rasor, J., III; Harris, G. Opioid use for moderate to severe pain. J. Osteopath. Med. 2005, 105, 2–7. [Google Scholar]
- Radbruch, L.; Trottenberg, P.; Elsner, F.; Kaasa, S.; Caraceni, A. Systematic review of the role of alternative application routes for opioid treatment for moderate to severe cancer pain: An EPCRC opioid guidelines project. Palliat. Med. 2011, 25, 578–596. [Google Scholar] [CrossRef]
- Klepstad, P.; Kaasa, S.; Borchgrevink, P.C. Starting step III opioids for moderate to severe pain in cancer patients: Dose titration: A systematic review. Palliat. Med. 2011, 25, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.E.; Ahdieh, H.; Ma, T.; Rauck, R.; Group, O.E.S. Efficacy and safety of OPANA ER (oxymorphone extended release) for relief of moderate to severe chronic low back pain in opioid-experienced patients: A 12-week, randomized, double-blind, placebo-controlled study. J. Pain 2007, 8, 175–184. [Google Scholar] [CrossRef]
- Fala, L.; Welz, J.A. New perspectives in the treatment of opioid-induced respiratory depression. Am. Health Drug Benefits 2015, 8, S51. [Google Scholar]
- Baldwin, G.T.; Seth, P.; Noonan, R.K. Continued increases in overdose deaths related to synthetic opioids: Implications for clinical practice. JAMA 2021, 325, 1151–1152. [Google Scholar] [CrossRef]
- Tagliaro, F.; De Battisti, Z.; Smith, F.P.; Marigo, M. Death from heroin overdose: Findings from hair analysis. Lancet 1998, 351, 1923–1925. [Google Scholar] [CrossRef]
- Seal, K.H.; Thawley, R.; Gee, L.; Bamberger, J.; Kral, A.H.; Ciccarone, D.; Downing, M.; Edlin, B.R. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: A pilot intervention study. J. Urban Health 2005, 82, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Rzasa Lynn, R.; Galinkin, J. Naloxone dosage for opioid reversal: Current evidence and clinical implications. Ther. Adv. Drug Saf. 2018, 9, 63–88. [Google Scholar] [CrossRef]
- Stanley, T.H. The fentanyl story. J. Pain 2014, 15, 1215–1226. [Google Scholar] [CrossRef]
- Kuczyńska, K.; Grzonkowski, P.; Kacprzak, Ł.; Zawilska, J.B. Abuse of fentanyl: An emerging problem to face. Forensic Sci. Int. 2018, 289, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692. [Google Scholar] [CrossRef]
- Ning, T.; Leng, C.; Chen, L.; Ma, B.; Gong, X. Metabolomics analysis of serum in a rat heroin self-administration model undergoing reinforcement based on 1H-nuclear magnetic resonance spectra. BMC Neurosci. 2018, 19, 4. [Google Scholar] [CrossRef]
- Alasmari, F.; Assiri, M.A.; Ahamad, S.R.; Aljumayi, S.R.; Alotaibi, W.H.; Alhamdan, M.M.; Alhazzani, K.; Alharbi, M.; Alqahtani, F.; Alasmari, A.F. Serum Metabolomic Analysis of Male Patients with Cannabis or Amphetamine Use Disorder. Metabolites 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Li, Y.; Pathmasiri, W.; McRitchie, S.; Etemadi, A.; Pollock, J.D.; Poustchi, H.; Rahimi-Movaghar, A.; Amin-Esmaeili, M.; Roshandel, G. Metabolomics reveals biomarkers of opioid use disorder. Transl. Psychiatry 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, R.; Sheng, W.; Feng, L.; Xu, P.; Wang, Y.; Xie, Y.; Xu, H.; Wang, G.; Aa, J. Identification of morphine and heroin-treatment in mice using metabonomics. Metabolites 2021, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Hammad, A.M.; Swiss, G.M.; Hall, F.S.; Hikmat, S.; Sari, Y.; Al-Qirim, T.; Amawi, H. Ceftriaxone reduces waterpipe tobacco smoke withdrawal-induced anxiety in rats via modulating the expression of TNF-α/NFĸB, Nrf2, and GLT-1. Neuroscience 2021, 463, 128–142. [Google Scholar] [CrossRef]
- Hammad, A.M.; Amawi, H.; Hall, F.S.; Tiwari, A.K.; Al-Trad, B. Effect of amoxicillin/clavulanic acid in attenuating pregabalin-induced condition place preference. Behav. Brain Res. 2023, 439, 114244. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Dykes Hoberg, M.; Vidensky, S.; Chung, D.S. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-E.; Kim, S.-K.; Cho, K.-O.; Kim, S.Y. Pre-ischemic treatment with ampicillin reduces neuronal damage in the mouse hippocampus and neostriatum after transient forebrain ischemia. Korean J. Physiol. Pharmacol. 2008, 12, 287–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, K.-E.; Cho, K.-O.; Choi, Y.-S.; Kim, S.Y. The neuroprotective mechanism of ampicillin in a mouse model of transient forebrain ischemia. Korean J. Physiol. Pharmacol. 2016, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Thöne-Reineke, C.; Neumann, C.; Namsolleck, P.; Schmerbach, K.; Krikov, M.; Schefe, J.H.; Lucht, K.; Hörtnagl, H.; Godes, M.; Müller, S. The β-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J. Hypertens. 2008, 26, 2426–2435. [Google Scholar] [CrossRef]
- Zumkehr, J.; Rodriguez-Ortiz, C.J.; Cheng, D.; Kieu, Z.; Wai, T.; Hawkins, C.; Kilian, J.; Lim, S.L.; Medeiros, R.; Kitazawa, M. Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer's disease. Neurobiol. Aging 2015, 36, 2260–2271. [Google Scholar] [CrossRef]
- Fan, S.; Xian, X.; Li, L.; Yao, X.; Hu, Y.; Zhang, M.; Li, W. Ceftriaxone improves cognitive function and upregulates GLT-1-related glutamate-glutamine cycle in APP/PS1 mice. J. Alzheimer's Dis. 2018, 66, 1731–1743. [Google Scholar] [CrossRef]
- Alshehri, F.S.; Hakami, A.Y.; Althobaiti, Y.S.; Sari, Y. Effects of ceftriaxone on hydrocodone seeking behavior and glial glutamate transporters in P rats. Behav. Brain Res. 2018, 347, 368–376. [Google Scholar] [CrossRef]
- Das, S.C.; Yamamoto, B.K.; Hristov, A.M.; Sari, Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 2015, 97, 67–74. [Google Scholar] [CrossRef]
- Alhaddad, H.; Wong, W.; Abou-Gharbia, M.; Childers, W.; Melenski, E.; Bell, R.L.; Sari, Y. Effects of a Novel Beta Lactam Compound, MC-100093, on the Expression of Glutamate Transporters/Receptors and Ethanol Drinking Behavior of Alcohol-Preferring Rats. J. Pharmacol. Exp. Ther. 2022, 383, 208–216. [Google Scholar] [CrossRef]
- Knackstedt, L.A.; Wu, L.; Rothstein, J.; Vidensky, S.; Gordon, J.; Ramanjulu, M.; Dunman, P.; Blass, B.; Childers, W.; Abou-Gharbia, M. MC-100093, a novel β-lactam glutamate transporter-1 enhancer devoid of antimicrobial properties, attenuates cocaine relapse in rats. J. Pharmacol. Exp. Ther. 2021, 378, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Granero, L.; Santiago, M.; Cano, J.; Machado, A.; Peris, J.-E. Analysis of ceftriaxone and ceftazidime distribution in cerebrospinal fluid of and cerebral extracellular space in awake rats by in vivo microdialysis. Antimicrob. Agents Chemother. 1995, 39, 2728–2731. [Google Scholar] [CrossRef]
- Uddin, O.; Jenne, C.; Fox, M.E.; Arakawa, K.; Keller, A.; Cramer, N. Divergent profiles of fentanyl withdrawal and associated pain in mice and rats. Pharmacol. Biochem. Behav. 2021, 200, 173077. [Google Scholar] [CrossRef]
- Ahamad, S.R.; Alhaider, A.Q.; Raish, M.; Shakeel, F. Metabolomic and elemental analysis of camel and bovine urine by GC–MS and ICP–MS. Saudi J. Biol. Sci. 2017, 24, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, S.R.; Raish, M.; Ahmad, A.; Shakeel, F. Potential health benefits and metabolomics of camel milk by GC-MS and ICP-MS. Biol. Trace Elem. Res. 2017, 175, 322–330. [Google Scholar] [CrossRef]
- Hammad, A.M.; Alhusban, A.A.; Alzaghari, L.F.; Alasmari, F.; Sari, Y. Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters’ Tissue Content in Rats’ Hippocampus and Amygdala. Metabolites 2023, 13, 515. [Google Scholar] [CrossRef]
- Nakhaee, S.; Farrokhfall, K.; Miri-Moghaddam, E.; Askari, M.; Amirabadizadeh, A.; Foadoddini, M.; Mehrpour, O. Effects of naloxone and diazepam on blood glucose levels in tramadol overdose using generalized estimating equation (GEE) model; (an experimental study). BMC Endocr. Disord. 2021, 21, 180. [Google Scholar] [CrossRef]
- Koekkoek, L.L.; van der Gun, L.L.; Serlie, M.J.; la Fleur, S.E. The clash of two epidemics: The relationship between opioids and glucose metabolism. Curr. Diabetes Rep. 2022, 22, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Gheni, G.; Ogura, M.; Iwasaki, M.; Yokoi, N.; Minami, K.; Nakayama, Y.; Harada, K.; Hastoy, B.; Wu, X.; Takahashi, H. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014, 9, 661–673. [Google Scholar] [CrossRef]
- Dashty, M. A quick look at biochemistry: Carbohydrate metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef]
- Walker, M.C.; van der Donk, W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016, 43, 419–430. [Google Scholar] [CrossRef]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Kuhnt, K.; Baehr, M.; Rohrer, C.; Jahreis, G. Trans fatty acid isomers and the trans-9/trans-11 index in fat containing foods. Eur. J. Lipid Sci. Technol. 2011, 113, 1281–1292. [Google Scholar] [CrossRef]
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H1060–H1076. [Google Scholar] [CrossRef]
- Cori, C.F. The glucose-lactic acid cycle and gluconeogenesis. Curr. Top. Cell. Regul. 1981, 18, 377–387. [Google Scholar] [CrossRef]
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic acid: No longer an inert and end-product of glycolysis. Physiology 2017, 32, 453–463. [Google Scholar] [CrossRef]
- Porporato, P.E.; Payen, V.L.; De Saedeleer, C.J.; Préat, V.; Thissen, J.-P.; Feron, O.; Sonveaux, P. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012, 15, 581–592. [Google Scholar] [CrossRef]
- Idborg, H.; Pawelzik, S.-C. Prostanoid metabolites as biomarkers in human disease. Metabolites 2022, 12, 721. [Google Scholar] [CrossRef]
- Kregiel, D. Succinate dehydrogenase of Saccharomyces cerevisiae–the unique enzyme of TCA cycle–current knowledge and new perspectives. In Dehydrogenases; Canuto, R.A., Ed.; INTECH: Cheadle Hulme, UK, 2012; pp. 211–234. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Dihydroxyacetone: An updated insight into an important bioproduct. ChemistryOpen 2018, 7, 233–236. [Google Scholar] [CrossRef]
- Fassoulaki, A.; Andreopoulou, K.; Williams, G.; Pateras, C. The effect of single and repeated doses of thiopentone and fentanyl on liver function in the rat. Anaesth. Intensive Care 1986, 14, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Sakamoto, M.; Makino, K.; Tayama, K.; Tada, Y.; Nakagawa, Y.; Nakajima, J.i.; Suzuki, J.; Suzuki, T.; Takahashi, H. Hepatic and renal toxicities and metabolism of fentanyl analogues in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2023, 396, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Baden, J.M.; Kundomal, Y.R.; Luttropp Jr, M.E.; Maze, M.; Kosek, J.C. Effects of volatile anesthetics or fentanyl on hepatic function in cirrhotic rats. Anesth. Analg. 1985, 64, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- de Jong, L.M.; Jiskoot, W.; Swen, J.J.; Manson, M.L. Distinct effects of inflammation on cytochrome P450 regulation and drug metabolism: Lessons from experimental models and a potential role for pharmacogenetics. Genes 2020, 11, 1509. [Google Scholar] [CrossRef]
- Aitken, A.E.; Morgan, E.T. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab. Dispos. 2007, 35, 1687–1693. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmari, F.; Alasmari, M.S.; Assiri, M.A.; Alswayyed, M.; Rizwan Ahamad, S.; Alhumaydhi, A.I.; Arif, B.I.; Aljumayi, S.R.; AlAsmari, A.F.; Ali, N.; et al. Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams. Metabolites 2023, 13, 965. https://doi.org/10.3390/metabo13080965
Alasmari F, Alasmari MS, Assiri MA, Alswayyed M, Rizwan Ahamad S, Alhumaydhi AI, Arif BI, Aljumayi SR, AlAsmari AF, Ali N, et al. Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams. Metabolites. 2023; 13(8):965. https://doi.org/10.3390/metabo13080965
Chicago/Turabian StyleAlasmari, Fawaz, Mohammed S. Alasmari, Mohammed A. Assiri, Mohammed Alswayyed, Syed Rizwan Ahamad, Abdulrahman I. Alhumaydhi, Bandar I. Arif, Sahar R. Aljumayi, Abdullah F. AlAsmari, Nemat Ali, and et al. 2023. "Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams" Metabolites 13, no. 8: 965. https://doi.org/10.3390/metabo13080965
APA StyleAlasmari, F., Alasmari, M. S., Assiri, M. A., Alswayyed, M., Rizwan Ahamad, S., Alhumaydhi, A. I., Arif, B. I., Aljumayi, S. R., AlAsmari, A. F., Ali, N., Childers, W. E., Abou-Gharbia, M., & Sari, Y. (2023). Liver Metabolomics and Inflammatory Profiles in Mouse Model of Fentanyl Overdose Treated with Beta-Lactams. Metabolites, 13(8), 965. https://doi.org/10.3390/metabo13080965












