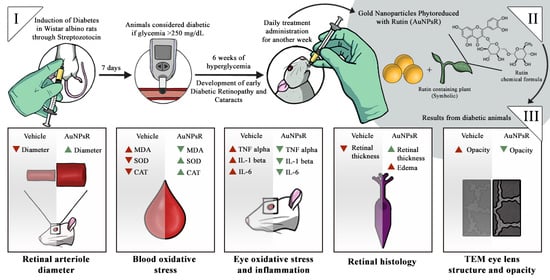
Effects of Gold Nanoparticles Phytoreduced with Rutin in an Early Rat Model of Diabetic Retinopathy and Cataracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Gold Nanoparticles Synthesis and Characterisation
2.3. Experimental Design
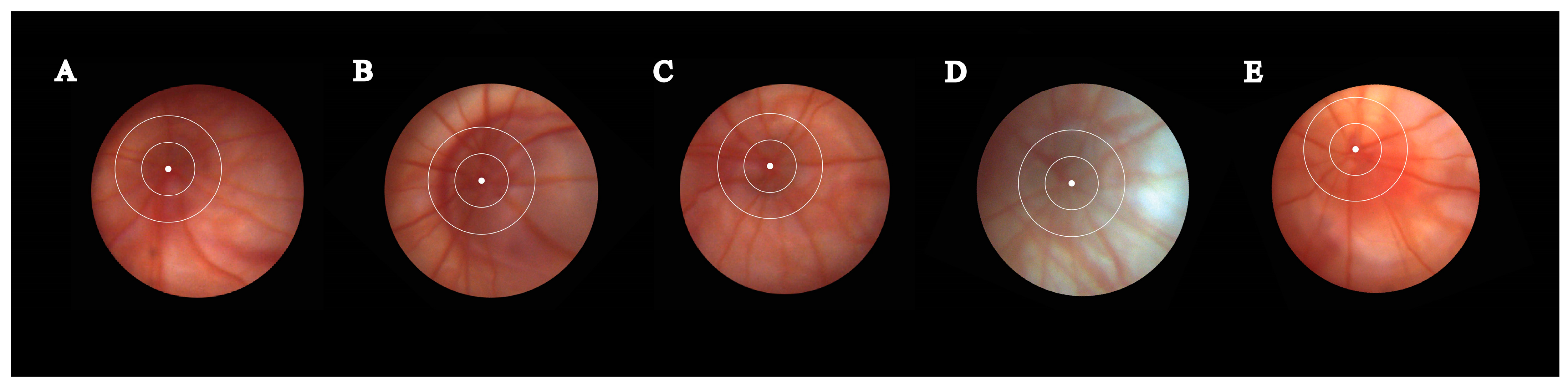
2.4. Fundoscopy Examination
2.5. Fundus Photography Processing and Analysis
2.6. Oxidative Stress Investigation and Inflammation Assessment
2.7. Retina Histopathological Examination
2.8. Eye Lens Transmission Electron Microscopy
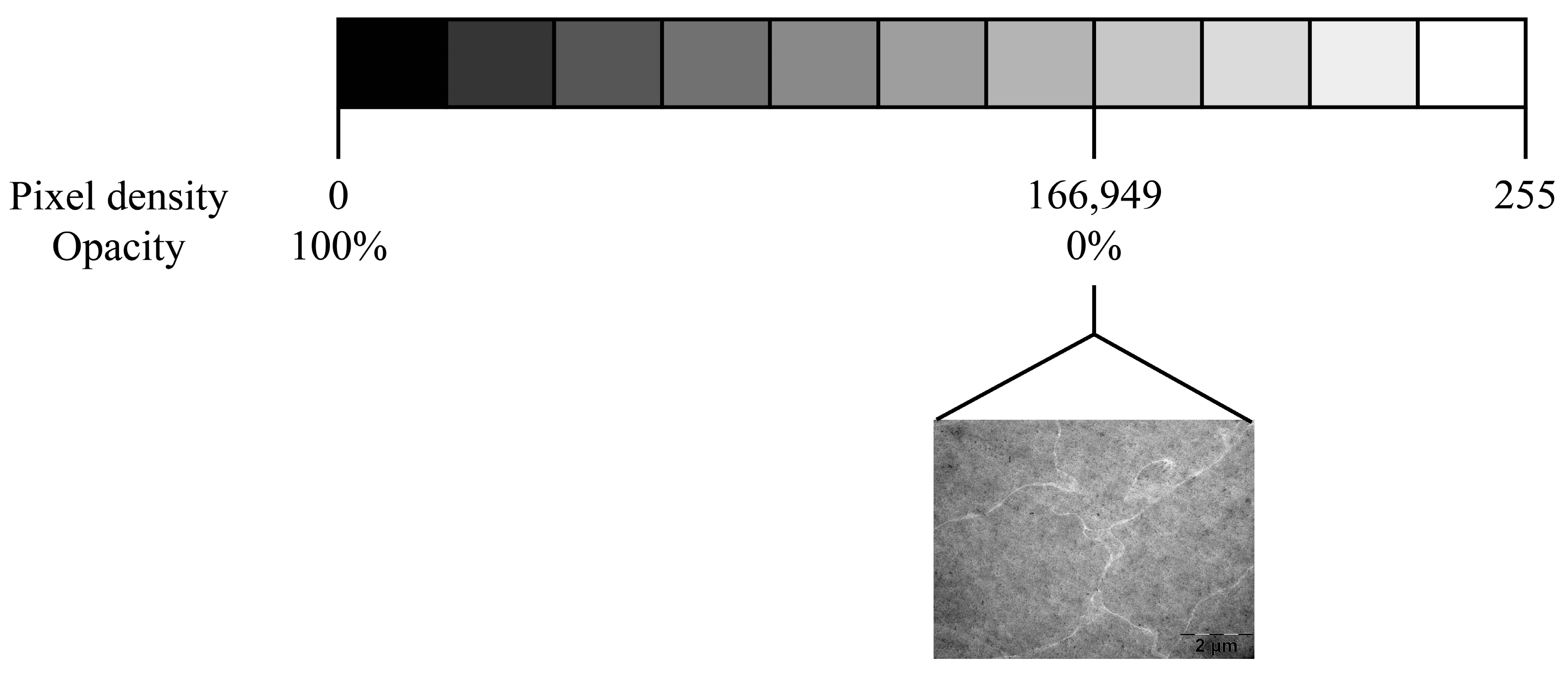
2.9. Eye Lens TEM Photography Processing and Analysis
2.10. Statistical Analysis
3. Results
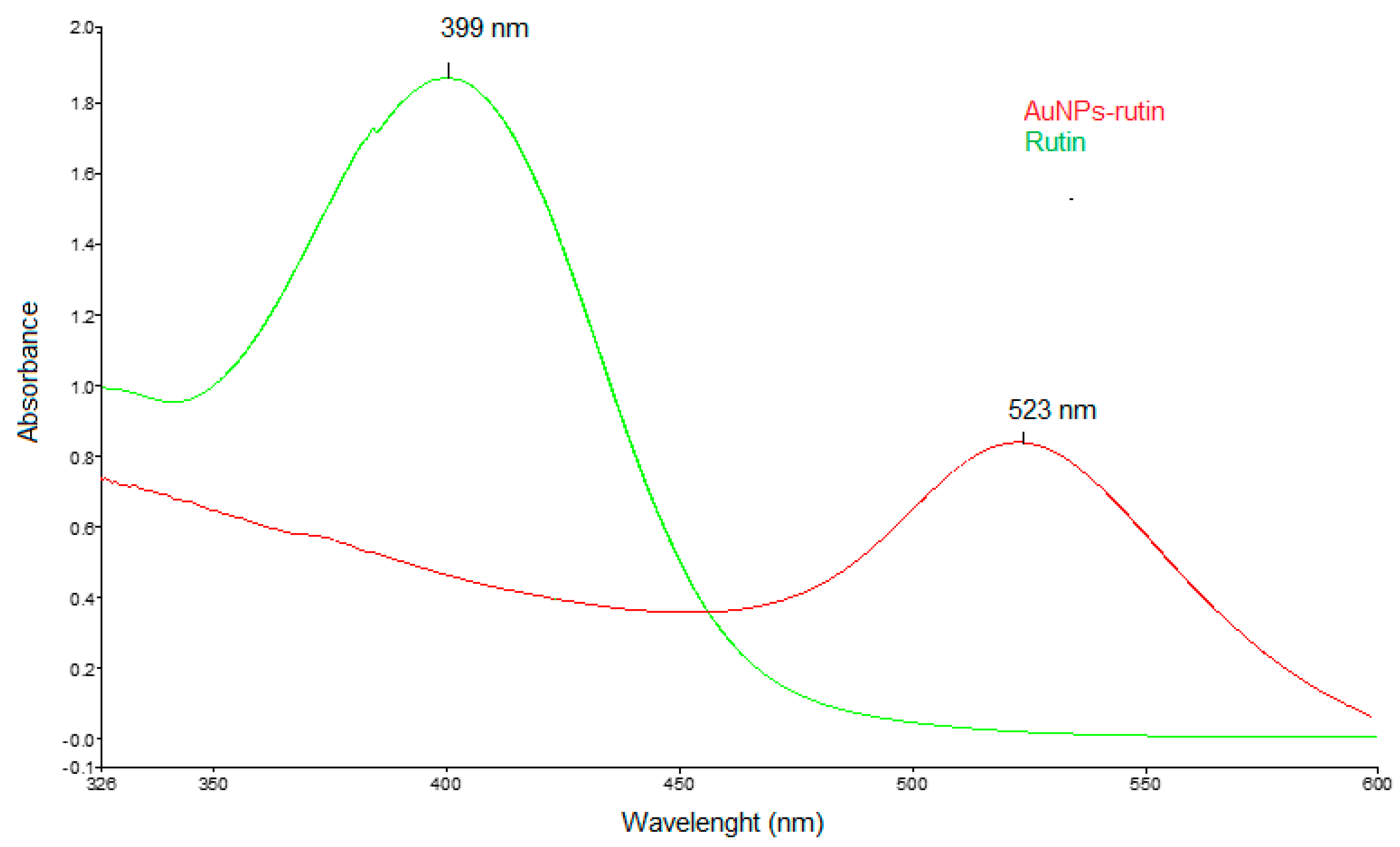
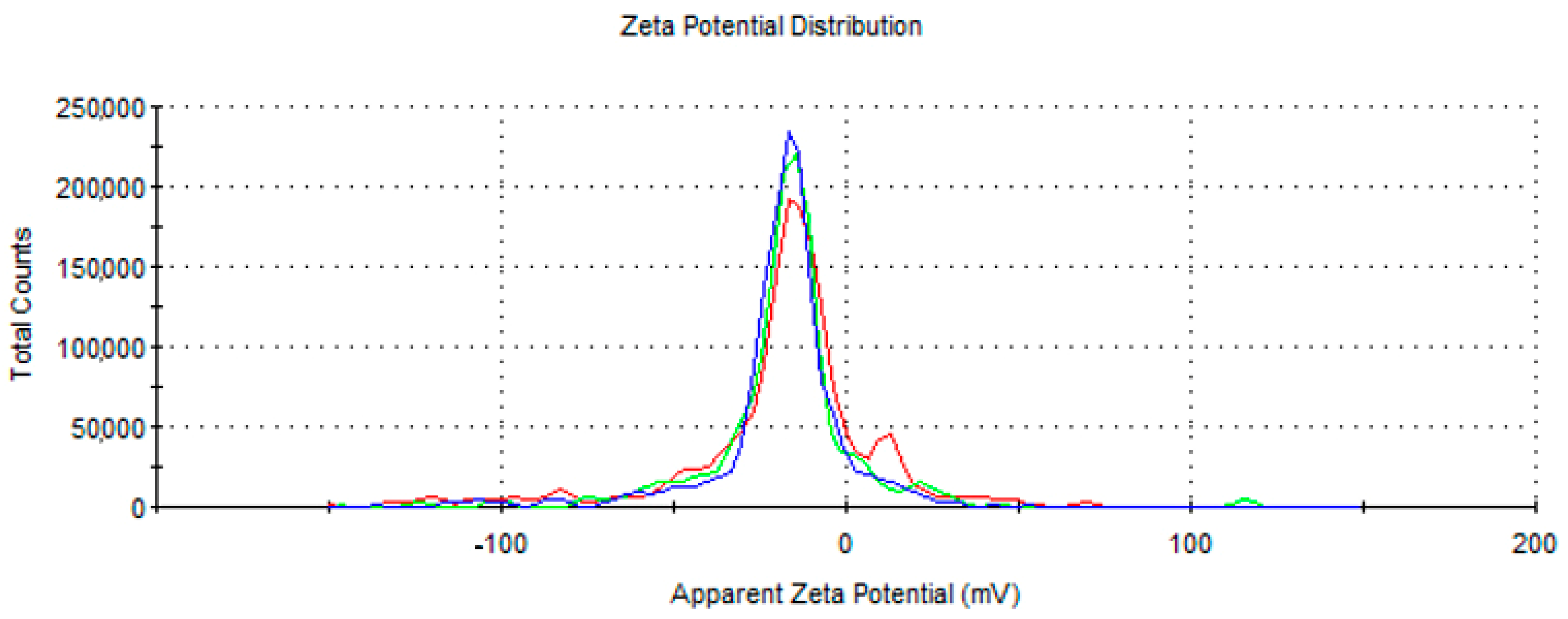
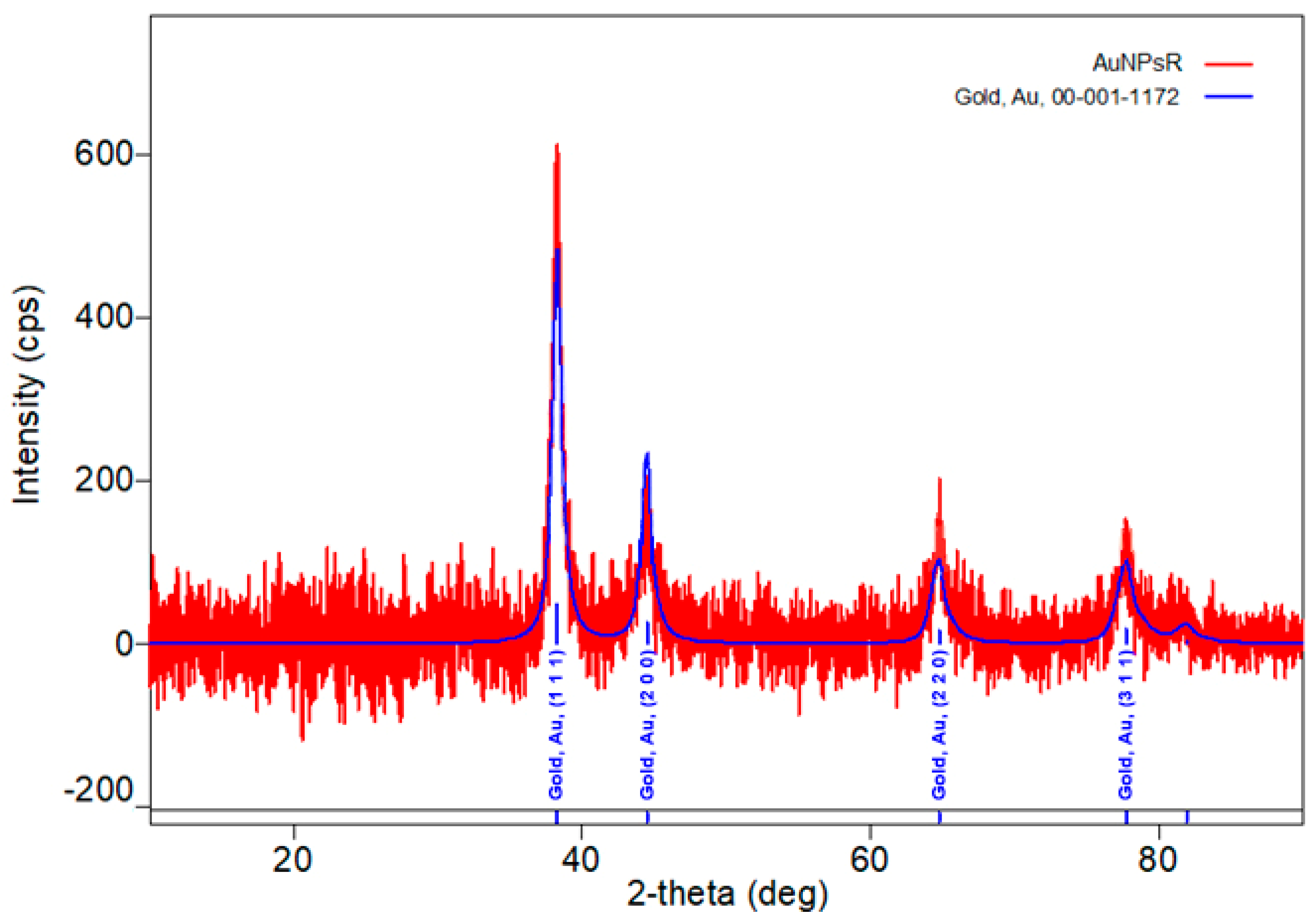
3.1. Characterisation of Gold Nanoparticles Functionalised with Rutin
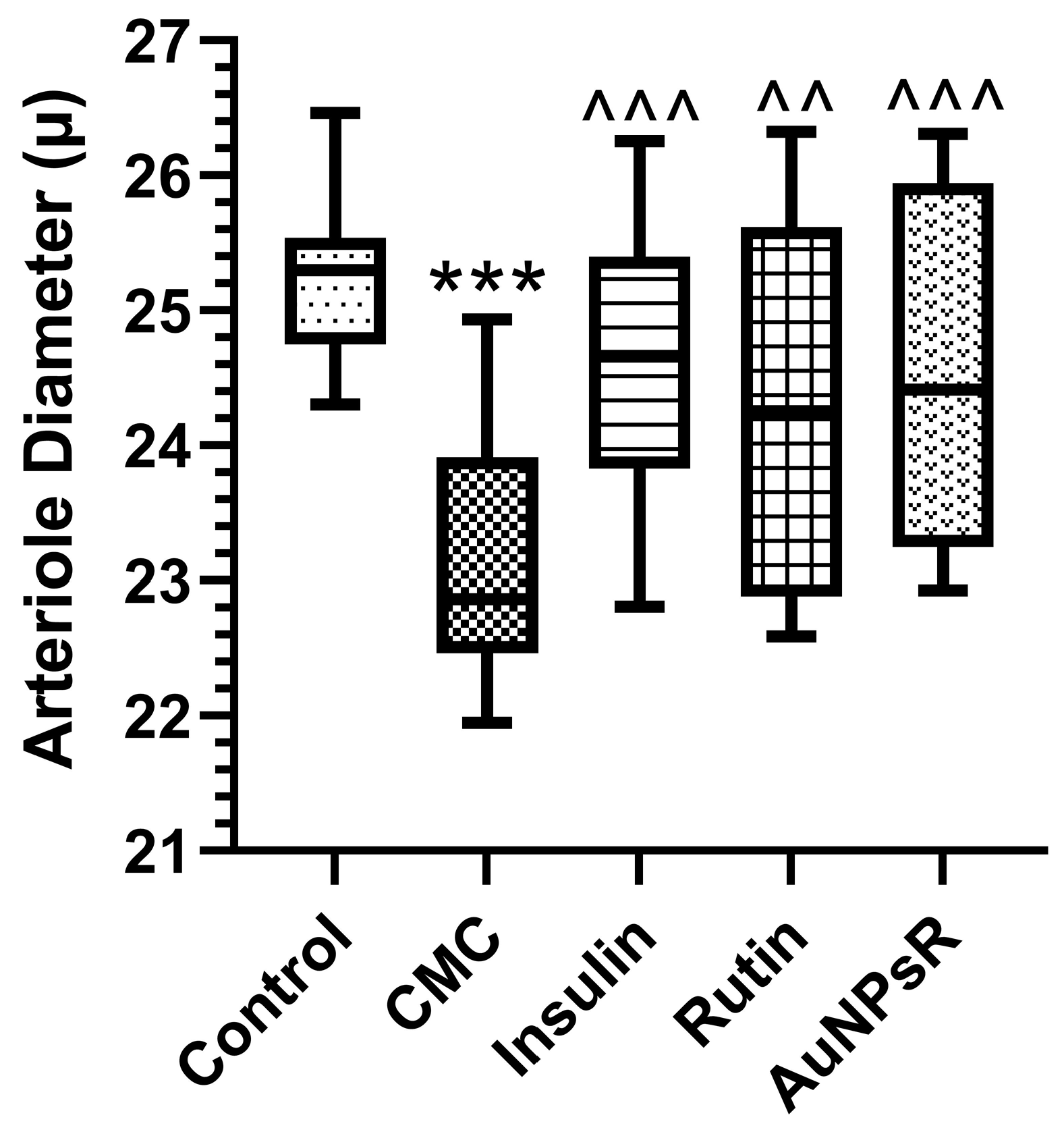
3.2. Fundoscopy Examination
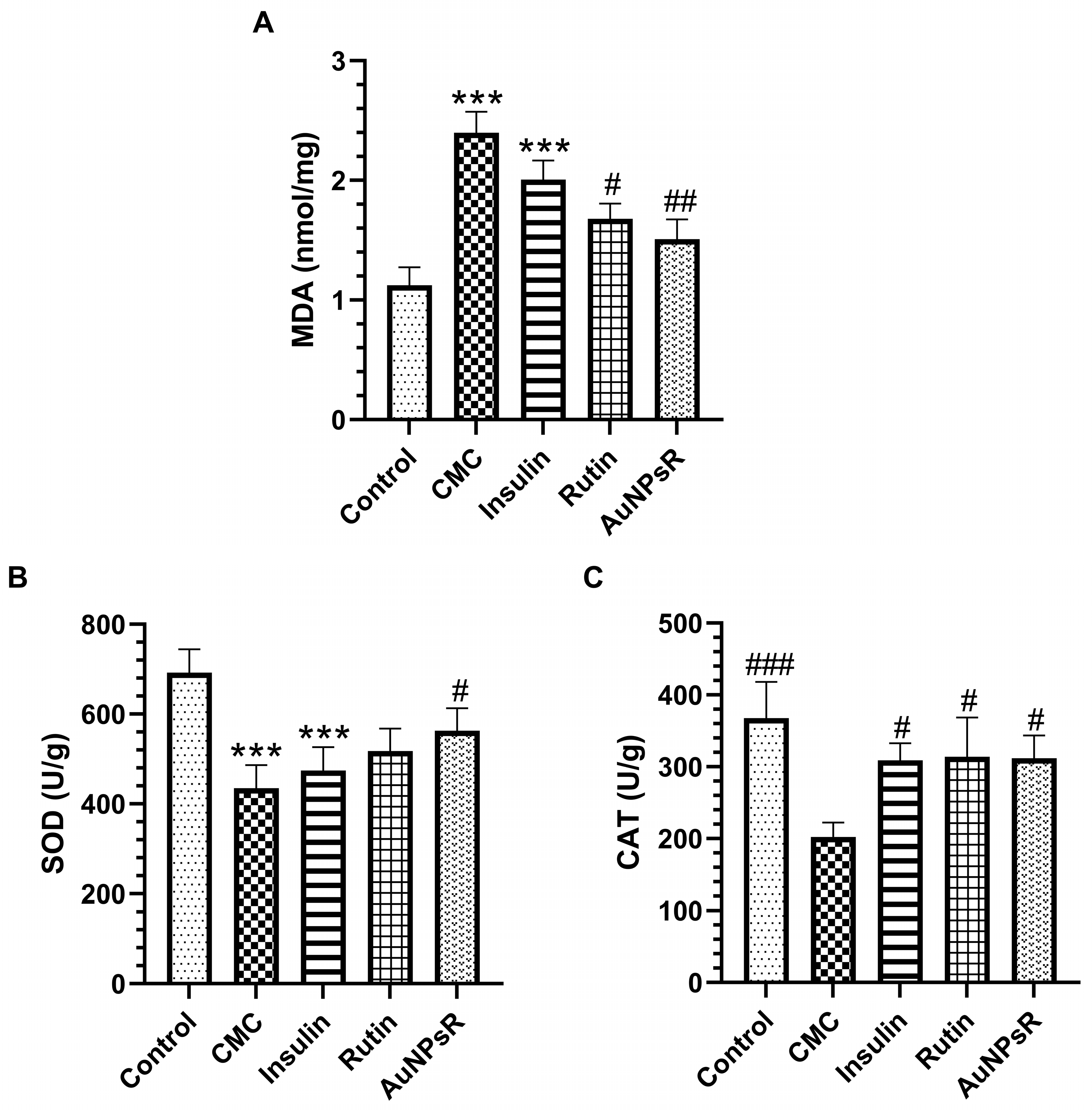
3.3. Blood and Eye Tissue Oxidative Stress Investigation
3.4. Eye Tissue Inflammation Investigation
3.5. Retina Histopathological Investigation
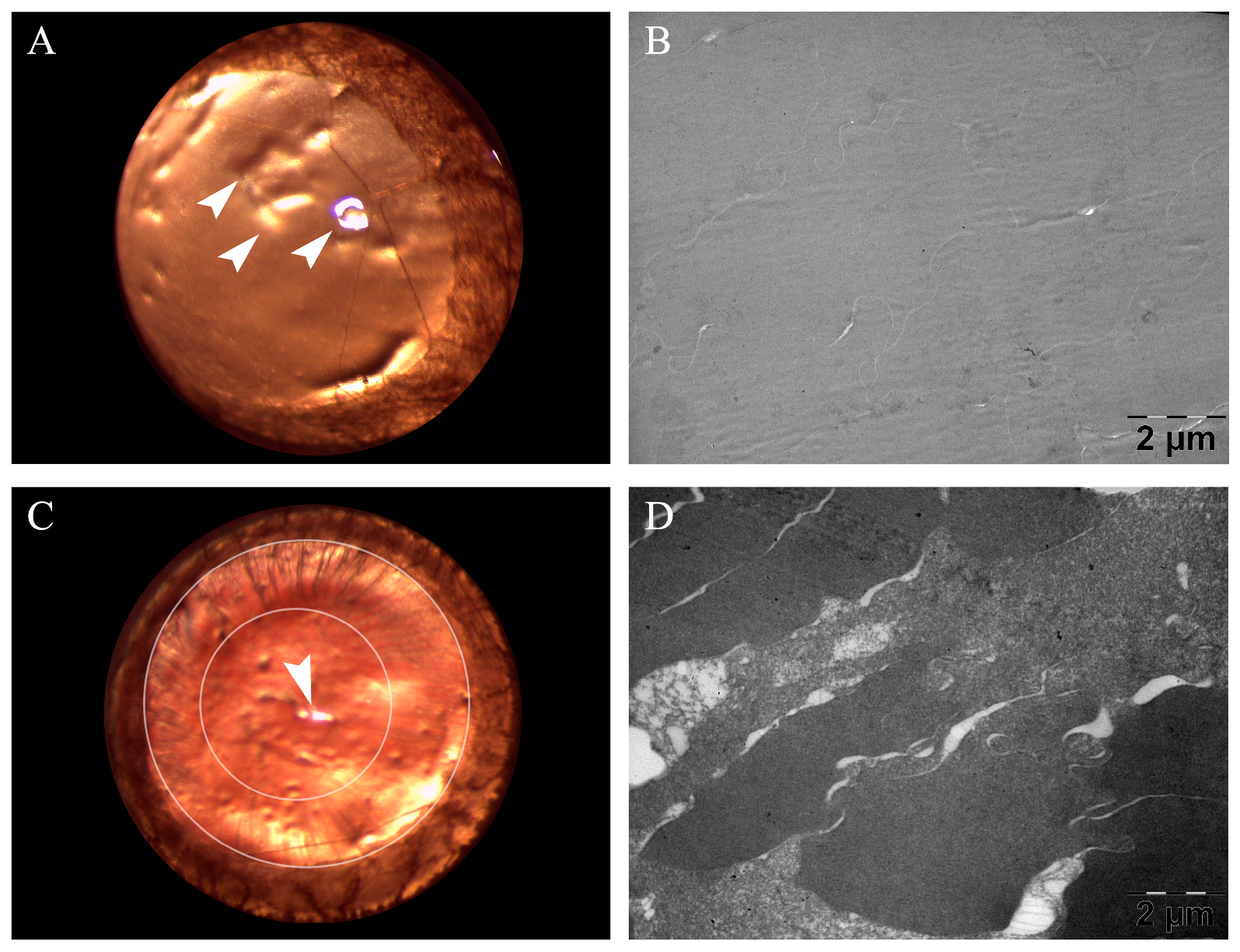
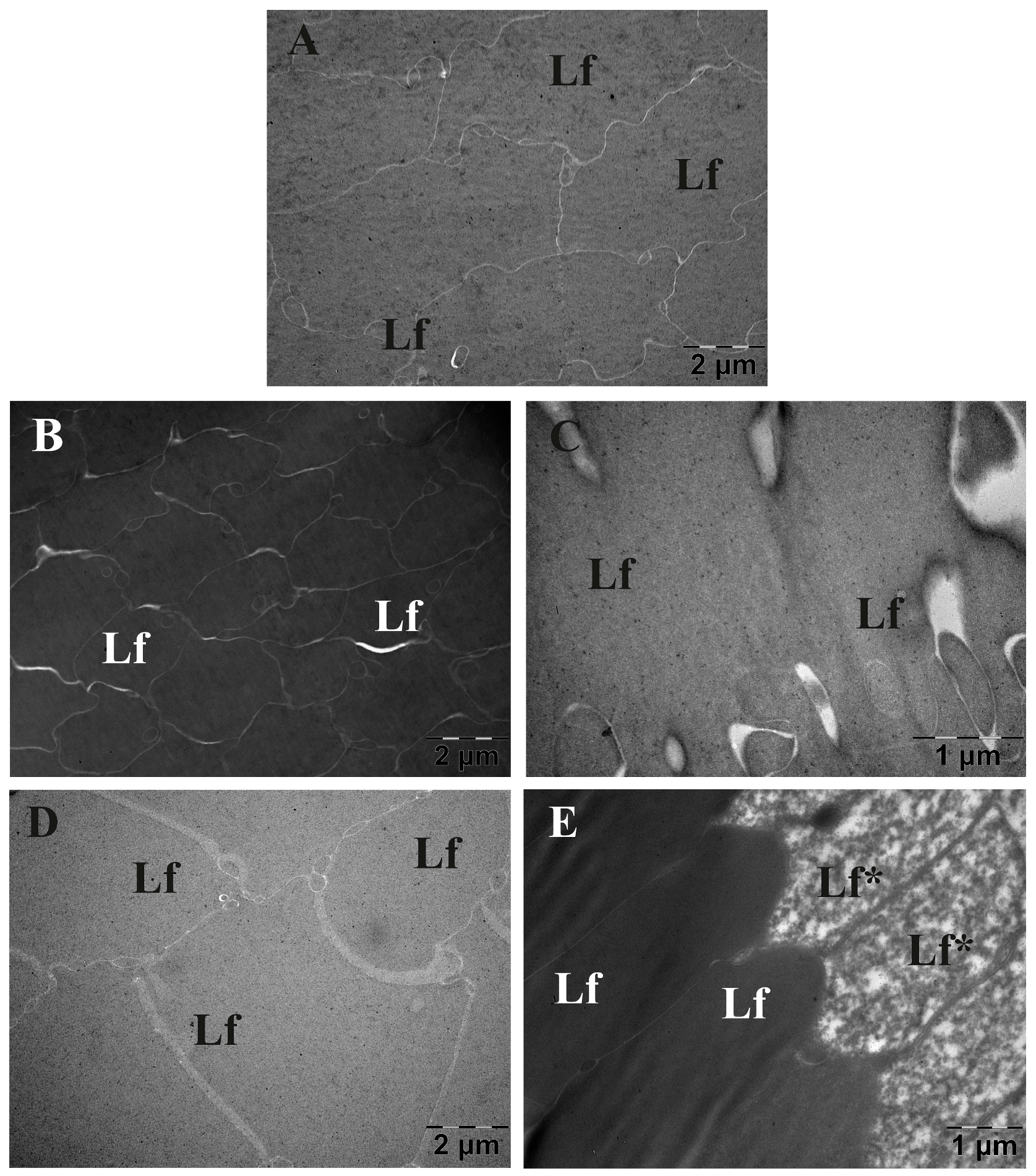
3.6. Eye Lens TEM Investigation
3.6.1. Morphology Investigation
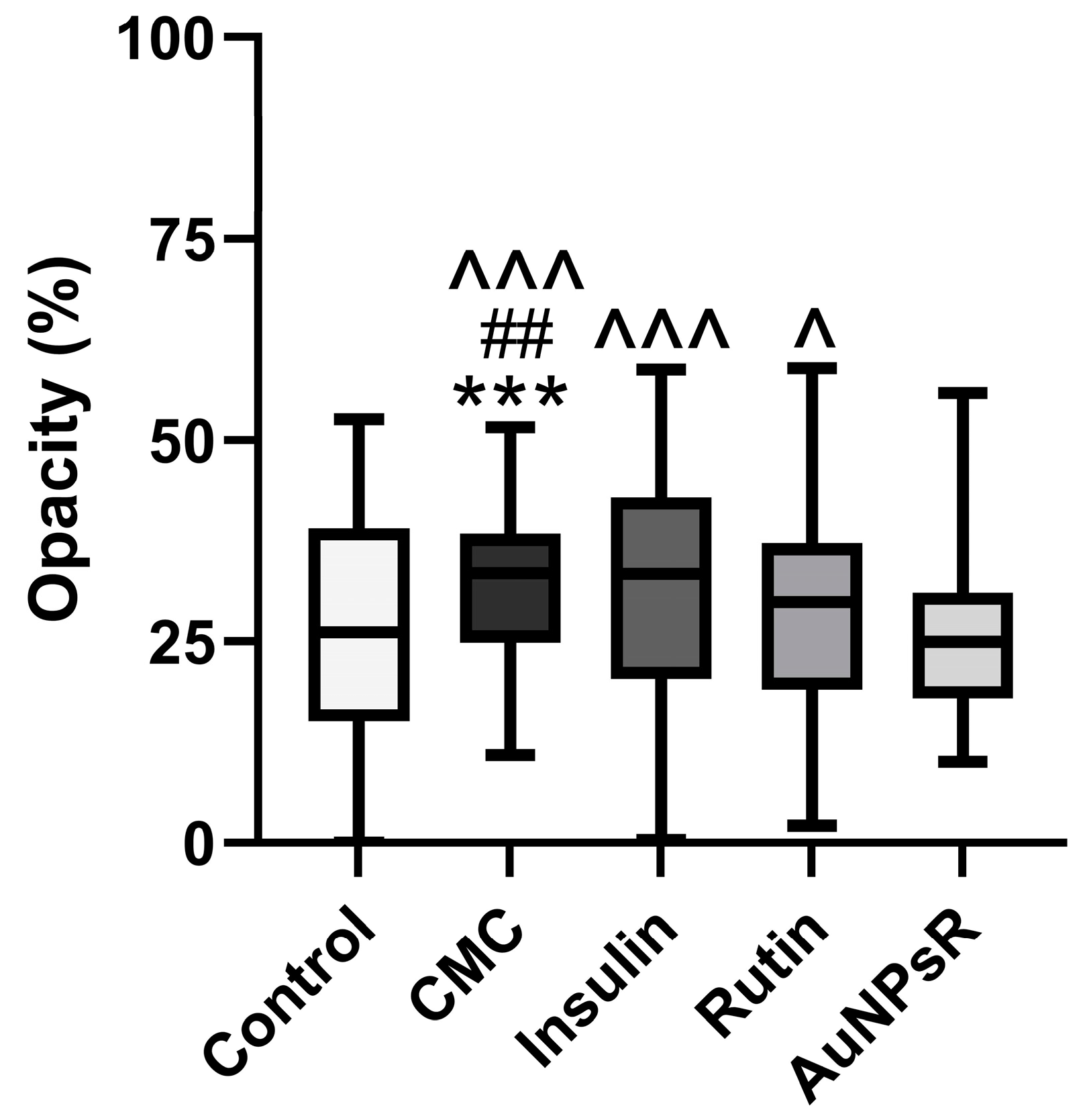
3.6.2. Opacity Investigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, L.; Yang, Y.J.; Liu, Q.; Peng, J.; Yan, J.F.; Peng, Q.H. Visualizing the intellectual structure and recent research trends of diabetic retinopathy. Int. J. Ophthalmol. 2021, 14, 1248–1259. [Google Scholar] [CrossRef]
- Bixler, J.E. Cataracts and Their Treatment in People with Diabetes. In Prevention and Management of Diabetes-Related Eye Disease; American Diabetes Association: Arlington, TX, USA, 2019; pp. 6–10. [Google Scholar]
- Yan, W.; Wang, W.; van Wijngaarden, P.; Mueller, A.; He, M. Longitudinal changes in global cataract surgery rate inequality and associations with socioeconomic indices. Clin. Exp. Ophthalmol. 2019, 47, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Denadai, L.; Mozetic, V.; Moore, R.A.; Yamada, V.H.; Riera, R. Pain control during panretinal photocoagulation for diabetic retinopathy. Cochrane Database Syst. Rev. 2022, 2022, CD014927. [Google Scholar] [CrossRef]
- Jeng, C.J.; Hsieh, Y.T.; Yang, C.M.; Yang, C.H.; Lin, C.L.; Wang, I.J. Development of diabetic retinopathy after cataract surgery. PLoS ONE 2018, 13, e0202347. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sabanayagam, C. The War on Diabetic Retinopathy: Where Are We Now? Asia Pac. J. Ophthalmol. 2019, 8, 448–456. [Google Scholar] [CrossRef]
- Manasvi, P.; Panimalar, V.; Veeramani, A.; Divya, N.; Bindu, B. Analysis of Cataract in Diabetic and Non-Diabetic Patients. Int. J. Curr. Res. Rev. 2021, 13, 67–71. [Google Scholar] [CrossRef]
- Vujosevic, S.; Toma, C.; Villani, E.; Gatti, V.; Brambilla, M.; Muraca, A.; Ponziani, M.C.; Aimaretti, G.; Nuzzo, A.; Nucci, P.; et al. Early Detection of Microvascular Changes in Patients with Diabetes Mellitus without and with Diabetic Retinopathy: Comparison between Different Swept-Source OCT-A Instruments. J. Diabetes Res. 2019, 2019, 2547216. [Google Scholar] [CrossRef]
- Rodriguez-Gutierrez, R.; Montori, V.M. Glycemic Control for Patients With Type 2 Diabetes Mellitus: Our Evolving Faith in the Face of Evidence. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 504–512. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Gomulka, K.; Ruta, M. The Role of Inflammation and Therapeutic Concepts in Diabetic Retinopathy—A Short Review. Int. J. Mol. Sci. 2023, 24, 24. [Google Scholar] [CrossRef]
- Phipps, J.A.; Vessey, K.A.; Brandli, A.; Nag, N.; Tran, M.X.; Jobling, A.I.; Fletcher, E.L. The Role of Angiotensin II/AT1 Receptor Signaling in Regulating Retinal Microglial Activation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.A.; Jobling, A.I.; Dixon, M.A.; Bui, B.V.; Vessey, K.A.; Phipps, J.A.; Greferath, U.; Venables, G.; Wong, V.H.Y.; Wong, C.H.Y.; et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2112561118. [Google Scholar] [CrossRef] [PubMed]
- Shree, J.; Choudhary, R.; Bodakhe, S.H. Losartan delays the progression of streptozotocin-induced diabetic cataracts in albino rats. J. Biochem. Mol. Toxicol. 2019, 33, e22342. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Bolaji-Alabi, F.B.; Ajibade, T.O.; Adejumobi, O.A.; Ajani, O.S.; Jarikre, T.A.; Omobowale, T.O.; Ola-Davies, O.E.; Soetan, K.O.; Aro, A.O.; et al. Novel antihypertensive action of rutin is mediated via inhibition of angiotensin converting enzyme/mineralocorticoid receptor/angiotensin 2 type 1 receptor (ATR1) signaling pathways in uninephrectomized hypertensive rats. J. Food. Biochem. 2020, 44, e13534. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; Eros, D.A. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (BCS). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef]
- Frutos-Rincon, L.; Gomez-Sanchez, J.A.; Inigo-Portugues, A.; Acosta, M.C.; Gallar, J. An Experimental Model of Neuro-Immune Interactions in the Eye: Corneal Sensory Nerves and Resident Dendritic Cells. Int. J. Mol. Sci. 2022, 23, 2997. [Google Scholar] [CrossRef]
- Addo, R.T. Ocular Drug Delivery: Advances, Challenges and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 53–74. [Google Scholar]
- Rocha, S.; Lucas, M.; Ribeiro, D.; Corvo, M.L.; Fernandes, E.; Freitas, M. Nano-based drug delivery systems used as vehicles to enhance polyphenols therapeutic effect for diabetes mellitus treatment. Pharmacol. Res. 2021, 169, 105604. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, P.; Liu, X.; Hu, S.; Zheng, H.; Liu, T.; Li, C. Gold Nanoparticles Encapsulated Resveratrol as an Anti-Aging Agent to Delay Cataract Development. Pharmaceuticals 2022, 16, 26. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Chockalingam, S.; Thada, R.; Dhandapani, R.K.; Panchamoorthy, R. Biogenesis, characterization, and the effect of vicenin-gold nanoparticles on glucose utilization in 3T3-L1 adipocytes: A bioinformatic approach to illuminate its interaction with PTP 1B and AMPK. Biotechnol. Prog. 2015, 31, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Muranov, K.; Poliansky, N.; Winkler, R.; Rieger, G.; Schmut, O.; Horwath-Winter, J. Protection by iodide of lens from selenite-induced cataract. Graefes. Arch. Clin. Exp. Ophthalmol. 2004, 242, 146–151. [Google Scholar] [CrossRef] [PubMed]
- McLenachan, S.; Magno, A.L.; Ramos, D.; Catita, J.; McMenamin, P.G.; Chen, F.K.; Rakoczy, E.P.; Ruberte, J. Angiography reveals novel features of the retinal vasculature in healthy and diabetic mice. Exp. Eye Res. 2015, 138, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.; Amini, Z.; Rabbani, H.; Kafieh, R. A Comprehensive Study of Retinal Vessel Classification Methods in Fundus Images. J. Med. Signals Sens. 2017, 7, 59–70. [Google Scholar] [PubMed]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved fluorometric determination of malonaldehyde. Clin. Chem. 1991, 37, 1273–1275. [Google Scholar] [CrossRef]
- Pippenger, C.E.; Browne, R.W.; Armstrong, D. Regulatory antioxidant enzymes. Methods. Mol. Biol. 1998, 108, 299–313. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Wirahadikesuma, I.; Santoso, K.; Maheshwari, H.; Akhiruddin, M. Determining Image Opacity in Broiler Respiratory Radiographic Using ImageJ and Ansel Adam’s Zone System. J. Indones. Vet. Res. 2020, 4, 22–35. [Google Scholar] [CrossRef]
- Guzman-Hernandez, D.S.; Palomar-Pardave, M.; Sanchez-Perez, F.; Juarez-Gomez, J.; Corona-Avendano, S.; Romero-Romo, M.; Ramirez-Silva, M.T. Spectro-electrochemical characterization and quantification of Rutin in aqueous media. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 228, 117814. [Google Scholar] [CrossRef]
- Clichici, S.; David, L.; Moldovan, B.; Baldea, I.; Olteanu, D.; Filip, M.; Nagy, A.; Luca, V.; Crivii, C.; Mircea, P.; et al. Hepatoprotective effects of silymarin coated gold nanoparticles in experimental cholestasis. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111117. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Antiochia, R. Biocide Activity of Green Quercetin-Mediated Synthesized Silver Nanoparticles. Nanomaterials 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Nahdi, A.; John, A.; Raza, H. Elucidation of Molecular Mechanisms of Streptozotocin-Induced Oxidative Stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic beta-Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7054272. [Google Scholar] [CrossRef]
- Aung, M.H.; Kim, M.K.; Olson, D.E.; Thule, P.M.; Pardue, M.T. Early visual deficits in streptozotocin-induced diabetic long evans rats. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Majaw, S.; Challam, S.K.; Syiem, D. Effect of Potentilla fulgens L. onselected enzyme activities and altered tissue morphology in diabetic mice. J. Morphol. Sci. 2018, 35, 153–160. [Google Scholar] [CrossRef]
- Lai, A.K.; Lo, A.C. Animal models of diabetic retinopathy: Summary and comparison. J. Diabetes Res. 2013, 2013, 106594. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.J.; Chen, Y.N.; Tsao, Y.T.; Cheng, C.M.; Wu, W.C.; Chen, H.C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Kisic, B.; Miric, D.; Zoric, L.; Ilic, A.; Dragojevic, I. Antioxidant capacity of lenses with age-related cataract. Oxid. Med. Cell. Longev. 2012, 2012, 467130. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Zahoor, A.F.; Bilal, M.; Hussain, S.M.; Irfan, M.; Akhtar, R.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Sharpless Asymmetric Dihydroxylation: An Impressive Gadget for the Synthesis of Natural Products: A Review. Molecules 2023, 28, 2722. [Google Scholar] [CrossRef]
- Muir, E.R.; Renteria, R.C.; Duong, T.Q. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6488–6494. [Google Scholar] [CrossRef]
- Eshaq, R.S.; Watts, M.N.; Carter, P.R.; Leskova, W.; Aw, T.Y.; Alexander, J.S.; Harris, N.R. Candesartan Normalizes Changes in Retinal Blood Flow and p22phox in the Diabetic Rat Retina. Pathophysiology 2021, 28, 86–97. [Google Scholar] [CrossRef]
- Manrique, C.; Lastra, G.; Sowers, J.R. New insights into insulin action and resistance in the vasculature. Ann. N. Y. Acad. Sci. 2014, 1311, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, D.T.; Bothou, C.; Skarmoutsos, F.; Papaevangelou, V.; Papadimitriou, A. Acute Bilateral Cataract in Type 1 Diabetes Mellitus. Ann. Pediatr. Child. Health 2015, 3, 1080. [Google Scholar] [CrossRef]
- Meng, D.; Mei, A.; Liu, J.; Kang, X.; Shi, X.; Qian, R.; Chen, S. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PLoS ONE 2012, 7, e48393. [Google Scholar] [CrossRef]
- Nien, C.W.; Lee, C.Y.; Chen, H.C.; Chao, S.C.; Hsu, H.J.; Tzeng, S.H.; Yang, S.J.; Huang, J.Y.; Yang, S.F.; Lin, H.Y. The elevated risk of sight-threatening cataract in diabetes with retinopathy: A retrospective population-based cohort study. BMC Ophthalmol. 2021, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Bain, S.C.; Klufas, M.A.; Ho, A.; Matthews, D.R. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes. Metab. 2019, 21, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, F.; Sone, H.; Nonoyama, T.; Hommura, S. Refractive changes in diabetic patients during intensive glycaemic control. Br. J. Ophthalmol. 2000, 84, 1097–1102. [Google Scholar] [CrossRef]
- Rojas, M.A.; Zhang, W.; Xu, Z.; Nguyen, D.T.; Caldwell, R.W.; Caldwell, R.B. Interleukin 6 has a Critical Role in Diabetes-induced Retinal Vascular Inflammation and Permeability. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1003. [Google Scholar]
- Schmidt, M.; Matsumoto, Y.; Tisdale, A.; Lowden, P.; Kovalchin, J.; Wu, P.; Golden, K.; Dombrowski, C.; Lain, B.; Furfine, E.S. Optimized intravitreal IL-6 antagonist for the treatment of diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3488. [Google Scholar]
- Cosert, K.M.; Kim, S.; Jalilian, I.; Chang, M.; Gates, B.L.; Pinkerton, K.E.; Van Winkle, L.S.; Raghunathan, V.K.; Leonard, B.C.; Thomasy, S.M. Metallic Engineered Nanomaterials and Ocular Toxicity: A Current Perspective. Pharmaceutics 2022, 14, 981. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Zhao, G.; Liu, J.X. Silver nanoparticles affect lens rather than retina development in zebrafish embryos. Ecotoxicol. Environ. Saf. 2018, 163, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Antoszyk, A.N.; Blodi, B.A.; Jampol, L.M.; Marcus, D.M.; Martin, D.F.; Melia, M.; Salehi-Had, H.; et al. Effect of Intravitreous Anti-Vascular Endothelial Growth Factor vs Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy: The Protocol W Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.P.; Li, Y.T.; Huang, C.X.; Cai, C.S.; Zhu, Y.J.; Wang, L.; Zhu, X.B. Evans blue staining to detect deep blood vessels in peripheral retina for observing retinal pathology in early-stage diabetic rats. Int. J. Ophthalmol. 2021, 14, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Zhong, H.; Xiong, L.; Yang, L.; Xu, Y.; Wen, S.; Shao, Y.; Zhou, Q. Sequential and Dynamic Variations of IL-6, CD18, ICAM, TNF-alpha, and Microstructure in the Early Stage of Diabetic Retinopathy. Dis. Markers 2022, 2022, 1946104. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.M. Retinal Neurodegeneration in Diabetes: An Emerging Concept in Diabetic Retinopathy. Curr. Diab. Rep. 2021, 21, 65. [Google Scholar] [CrossRef]
- Baptista, F.I.; Aveleira, C.A.; Castilho, A.F.; Ambrosio, A.F. Elevated Glucose and Interleukin-1beta Differentially Affect Retinal Microglial Cell Proliferation. Mediat. Inflamm. 2017, 2017, 4316316. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, M.; Păpurică, A.-M.; Muntean, M.; Bungărdean, R.M.; Gheban, D.; Moldovan, B.; Katona, G.; David, L.; Filip, G.A. Effects of Gold Nanoparticles Phytoreduced with Rutin in an Early Rat Model of Diabetic Retinopathy and Cataracts. Metabolites 2023, 13, 955. https://doi.org/10.3390/metabo13080955
Moldovan M, Păpurică A-M, Muntean M, Bungărdean RM, Gheban D, Moldovan B, Katona G, David L, Filip GA. Effects of Gold Nanoparticles Phytoreduced with Rutin in an Early Rat Model of Diabetic Retinopathy and Cataracts. Metabolites. 2023; 13(8):955. https://doi.org/10.3390/metabo13080955
Chicago/Turabian StyleMoldovan, Mădălina, Ana-Maria Păpurică, Mara Muntean, Raluca Maria Bungărdean, Dan Gheban, Bianca Moldovan, Gabriel Katona, Luminița David, and Gabriela Adriana Filip. 2023. "Effects of Gold Nanoparticles Phytoreduced with Rutin in an Early Rat Model of Diabetic Retinopathy and Cataracts" Metabolites 13, no. 8: 955. https://doi.org/10.3390/metabo13080955
APA StyleMoldovan, M., Păpurică, A.-M., Muntean, M., Bungărdean, R. M., Gheban, D., Moldovan, B., Katona, G., David, L., & Filip, G. A. (2023). Effects of Gold Nanoparticles Phytoreduced with Rutin in an Early Rat Model of Diabetic Retinopathy and Cataracts. Metabolites, 13(8), 955. https://doi.org/10.3390/metabo13080955