The Potential of the Flavonoid Content of Ipomoea batatas L. as an Alternative Analog GLP-1 for Diabetes Type 2 Treatment—Systematic Review
Abstract
1. Introduction
2. Materials and Methods
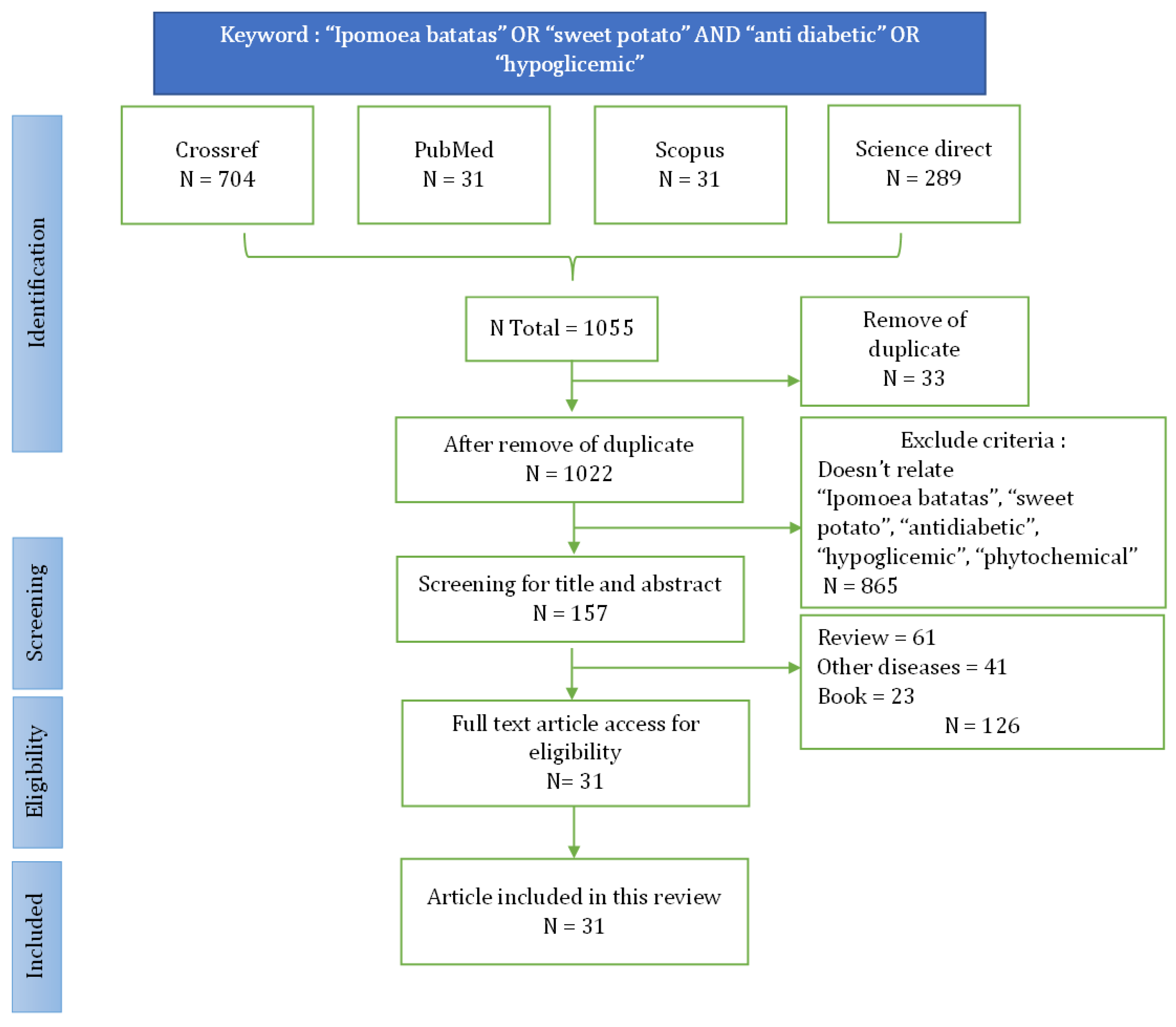
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction and Management
2.5. Data Extraction Strategy
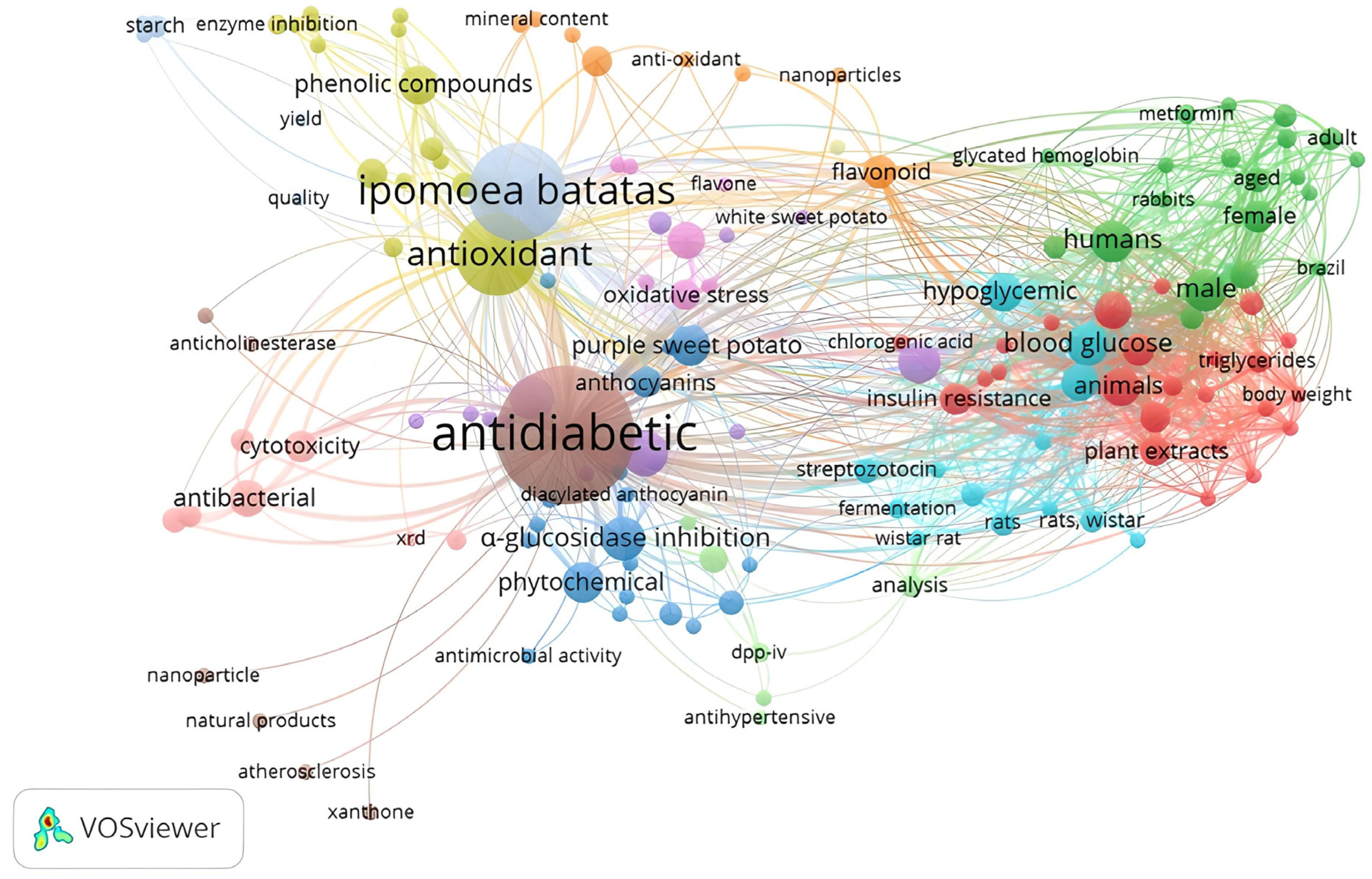
3. Results
The Literature Search
4. Discussion
4.1. Type or Cultivar
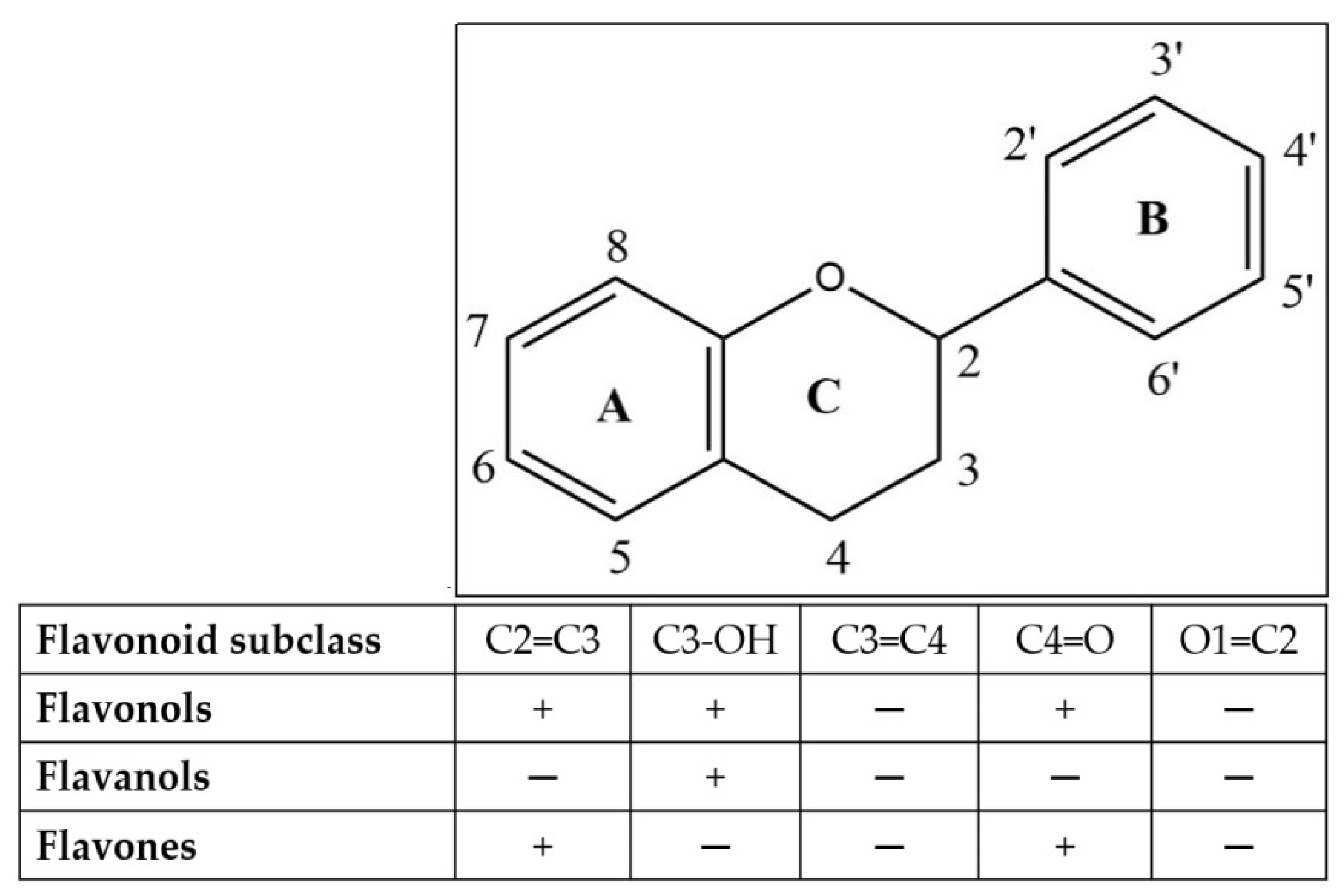
4.2. Parts of Plant and Phytochemical Identified of IBL
4.2.1. Site of Action
4.2.2. Gastrointestinal Tract
Regulation of Carbohydrate Metabolism
Increased Insulin Secretion
4.2.3. Pancreas
Inhibiting Apoptosis Beta Cell and Recovering the Islet Structure through Protective Cell Beta
Suppression of the Anti-Inflammatory Pathway
4.2.4. Liver
Improving Insulin Secretion and Insulin Sensitivity by Reducing Glucose Synthesis
4.2.5. Muscle
Enhancing the Absorption of Glucose, Secretion, and Insulin Sensitivity
4.2.6. Adiposa
Increasing Glucose Uptake and Insulin Secretion
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef]
- Sen Tseng, P.; Ande, C.; Moremen, K.W.; Crich, D. Influence of Side Chain Conformation on the Activity of Glycosidase Inhibitors. Angew. Chem.-Int. Ed. 2023, 62, 2–6. [Google Scholar] [CrossRef]
- Rajasekaran, P.; Ande, C.; Vankar, Y.D. Synthesis of (5,6 & 6,6)-oxa-oxa annulated sugars as glycosidase inhibitors from 2-formyl galactal using iodocyclization as a key step. Arkivoc 2022, 2022, 5–23. [Google Scholar]
- Arisanti, C.I.S.; Wirasuta, I.M.A.G.; Musfiroh, I.; Ikram, E.H.K.; Muchtaridi, M. Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review. Foods 2023, 12, 2810. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Andersen, A.; Christensen, A.S.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 receptor agonists for the treatment of Type 2 diabetes. Ugeskr. Laeger 2022, 181, 202–210. [Google Scholar] [CrossRef]
- Nagamine, R.; Ueno, S.; Tsubata, M.; Yamaguchi, K.; Takagaki, K.; Hira, T.; Hara, H.; Tsuda, T. Dietary sweet potato (Ipomoea batatas L.) leaf extract attenuates hyperglycaemia by enhancing the secretion of glucagon-like peptide-1 (GLP-1). Food Funct. 2014, 5, 2309–2316. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Lee, C.L.; Lee, S.L.; Chen, C.J.; Chen, H.C.; Kao, M.C.; Liu, C.H.; Wu, Y.C. Characterization of secondary metabolites from purple Ipomoea batatas leaves and their effects on glucose uptake. Molecules 2016, 21, 745. [Google Scholar] [CrossRef]
- Pal, S.; Gautam, S.; Mishra, A.; Maurya, R.; Srivastava, A.K. Antihyperglycemic and antidyslipidemic potential of ipomoea batatas leaves in validated diabetic animal models. Int. J. Pharm. Pharm. Sci. 2015, 7, 176–186. [Google Scholar]
- Yustiantara, P.S.; Yustiantara, P.S.; Warditiani, N.K.; Armita Sari, P.M.N.; Anita Dewi, N.L.K.A.; Ramona, Y.; Jawi, I.M.; Wirasuta, I.M.A.G. Determination of TLC fingerprint biomarker of Ipomoea batatas (L.) Lam leaves extracted with ethanol and its potential as antihyperglycemic agent. Pharmacia 2021, 68, 907–917. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.-C.; Yuan, T.; Wang, H.; Xie, X.; Fu, Z.-F. Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016, 208, 61–67. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Q.; Long, L.; Li, J.; Yang, R.; Gao, D. Antidiabetic activity of flavone from Ipomoea Batatas leaf in non-insulin dependent diabetic rats. Int. J. Food Sci. Technol. 2007, 42, 80–85. [Google Scholar] [CrossRef]
- Zovko, M.; Petlevski, R.; Kaloðera, Z.; Plantak, K. Antioxidant and antidiabetic activity of leaves of Ipomoea batatas grown in continental Croatia. Planta Medica 2008, 74, PA135. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Stefanucci, A.; Macedonio, G.; Novellino, E.; Mirzaie, S.; Dvorácskó, S.; Carradori, S.; Brunetti, L.; Orlando, G.; et al. Chemical characterization, antioxidant properties, anti-inflammatory activity, and enzyme inhibition of Ipomoea batatas L. leaf extracts. Int. J. Food Prop. 2017, 20, 1907–1919. [Google Scholar] [CrossRef]
- Novrial, D.; Soebowo, S.; Widjojo, P. Protective Effect of Ipomoea batatas L Leaves Extract on Histology of Pancreatic Langerhans Islet and Beta Cell Insulin Expression of Rats Induced by Streptozotocin. Molekul 2020, 15, 48. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic α-amylase. J. Funct. Foods 2016, 21, 50–57. [Google Scholar] [CrossRef]
- Ayeleso, T.; Ramachela, K.; Mukwevho, E. Aqueous-Methanol Extracts of Orange-Fleshed Sweet Potato (Ipomoea batatas) Ameliorate Oxidative Stress and Modulate Type 2 Diabetes Associated Genes in Insulin Resistant C2C12 Cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef]
- Shih, C.K.; Chen, C.M.; Varga, V.; Shih, L.C.; Chen, P.R.; Lo, S.F. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020, 64, 1–11. [Google Scholar] [CrossRef]
- Jiang, T.; Shuai, X.; Li, J.; Yang, N.; Deng, L.; Li, S.; He, J. Protein-Bound Anthocyanin Compounds of Purple Sweet Potato Ameliorate Hyperglycemia by Regulating Hepatic Glucose Metabolism in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. J. Agric. Food Chem. 2020, 68, 1596–1608. [Google Scholar] [CrossRef]
- Shen, L.; Yang, Y.; Zhang, J.; Feng, L.; Zhou, Q. Diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) attenuate hyperglycemia and hyperuricemia in mice induced by a high-fructose/high-fat diet. J. Zhejiang Univ. Sci. B 2023, 24, 587–601. [Google Scholar] [CrossRef]
- Okafor, C.S.; Ezekwesili, C.; Mbachu, N.; Onyewuchi, K.C.; Ogbodo, U.C. Anti-diabetic Effects of the Aqueous and Ethanol Extracts of Ipomoea batatas Tubers on Alloxan Induced Diabetes in Wistar Albino Rats. Int. J. Biochem. Res. Rev. 2021, 30, 1–13. [Google Scholar] [CrossRef]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K.; Sugita, K.; Terahara, N.; Matsumoto, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J. Agric. Food Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Basavegowda, N.; Vishnuprasad, C.N.; Shin, H.-S.H.-S.S. Comparative study on antidiabetic, cytotoxicity, antioxidant and antibacterial properties of biosynthesized silver nanoparticles using outer peels of two varieties of Ipomoea batatas (L.) Lam. Int. J. Nanomed. 2019, 14, 4741–4754. [Google Scholar] [CrossRef] [PubMed]
- Sakuramata, Y.; Oe, H.; Kusano, S.; Aki, O. Effects of combination of Caiapo® with other plant-derived substance on anti-diabetic efficacy in KK-Ay mice. Biofactors 2004, 22, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Neuffer, B.; Pacini, G. Efficacy of Ipomoea batatas (Caiapo) on Diabetes Control in Type 2 Diabetic. Diabetes Care 2004, 27, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Kusano, S.; Tamasu, S.; Nakatsugawa, S. Effects of the White-Skinned Sweet Potato (Ipomoea batatas L.) on the Expression of Adipocytokine in Adipose Tissue of Genetic Type 2 Diabetic Mice. Food Sci. Technol. Res. 2005, 11, 369–372. [Google Scholar] [CrossRef][Green Version]
- Kinoshita, A.; Nagata, T.; Furuya, F.; Nishizawa, M.; Mukai, E. White-skinned sweet potato (Ipomoea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon 2023, 9, e14719. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Kim, H.; Kim, S. In vitro and in vivo hypoglycemic effects of cyanidin 3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside, an anthocyanin isolated from purple-fleshed sweet potato. Food Chem. 2019, 272, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Koo, K.A.; Park, W.S.; Kang, D.-M.; Kim, H.S.; Lee, B.Y.; Goo, Y.-M.; Kim, J.-H.; Lee, M.K.; Woo, D.K.; et al. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. J. Food Biochem. 2020, 44, e13438. [Google Scholar] [CrossRef] [PubMed]
- Niwa, A.; Tajiri, T.; Higashino, H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2011, 48, 194–202. [Google Scholar] [CrossRef]
- Oki, N.; Nonaka, S.; Ozaki, S. The effects of an arabinogalactan-protein from the white-skinned sweet potato (Ipomoea batatas L.) on blood glucose in spontaneous diabetic mice. Biosci. Biotechnol. Biochem. 2011, 75, 596–598. [Google Scholar] [CrossRef]
- Wicaksono, L.A.; Yunianta; Widyaningsih, T.D. Anthocyanin extraction from purple sweet potato cultivar antin-3 (Ipomoea batatas L.) using maceration, microwave assisted extraction, ultrasonic assisted extraction and their application as anti-hyperglycemic agents in alloxan-induced wistar rats. Int. J. PharmTech Res. 2016, 9, 181–192. [Google Scholar]
- Kamal, S.; Akhter, N.; Khan, S.G.; Kiran, S.; Farooq, T.; Akram, M.; Zaheer, J. Anti-diabetic activity of aqueous extract of Ipomoea batatas L. in alloxan induced diabetic Wistar rats and its effects on biochemical parameters in diabetic rats. Pak. J. Pharm. Sci. 2018, 31, 1539–1548. [Google Scholar]
- Akhtar, N.; Akram, M.; Daniyal, M.; Ahmad, S. Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418814678. [Google Scholar] [CrossRef]
- Nurdjanah, S.; Astuti, S.; Yuliana, N. Inhibition Activity of α-amylase by Crude Acidic Water Extract from Fresh Purple Sweet Potato (Ipomoea batatas L.) and its Modified Flours. Asian J. Sci. Res. 2020, 13, 190–196. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Mastaloudis, A.; Oteiza, P.I. (−)-Epicatechin and Anthocyanins Modulate GLP-1 Metabolism: Evidence from C57BL/6J Mice and GLUTag Cells. J. Nutr. 2021, 151, 1497–1506. [Google Scholar] [CrossRef]
- Hjørne, A.P.; Modvig, I.M.; Holst, J.J. The Sensory Mechanisms of Nutrient-Induced GLP-1 Secretion. Metabolites 2022, 12, 420. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Frost, C.R.; Svendsen, B.; Holst, J.J. Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 2015, 64, 370–382. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Ma, B.; Fan, L.; Yi, N.; Lu, B.; Wang, Q.; Liu, R. GLP-1 improves adipocyte insulin sensitivity following induction of endoplasmic reticulum stress. Front. Pharmacol. 2018, 9, 1168. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, M.; Li, T.; Dong, N.; Yi, L.; Zhang, Q.; Mi, M. GLP-1 regulates exercise endurance and skeletal muscle remodeling via GLP-1R/AMPK pathway. Biochim. Biophys. Acta-Mol. Cell Res. 2022, 1869, 119300. [Google Scholar] [CrossRef]
- Omotuyi, O.I.; Nash, O.; Inyang, O.K.; Ogidigo, J.; Enejoh, O.; Okpalefe, O.; Hamada, T. Flavonoid-rich extract of Chromolaena odorata modulate circulating GLP-1 in Wistar rats: Computational evaluation of TGR5 involvement. 3 Biotech 2018, 8, 124. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, A.; Alkhalidy, H.; Luo, J.; Moomaw, E.; Neilson, A.P.; Liu, D. Flavone Hispidulin Stimulates Glucagon-Like peptide-1 secretion and Ameliorates Hyperglycemia in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2020, 64, e1900978. [Google Scholar] [CrossRef]
- de Castilho, T.S.; Matias, T.B.; Nicolini, K.P.; Nicolini, J. Study of interaction between metal ions and quercetin. Food Sci. Hum. Wellness 2018, 7, 215–219. [Google Scholar] [CrossRef]
- Horáková, L. Flavonoids in prevention of diseases with respect to modulation of Ca-pump function. Interdiscip. Toxicol. 2011, 4, 114–124. [Google Scholar] [CrossRef]
- de Almeida, L.F.; Dos Santos, E.C.F.; Machado, J.C.B.; de Oliveira, A.M.; Napoleão, T.H.; Ferreira, M.R.A.; Soares, L.A.L. Phytochemical profile, in vitro activities, and toxicity of optimized Eugenia uniflora extracts. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2023, 22, 130–144. [Google Scholar] [CrossRef]
- Oh, Y.S.; Jun, H.S. Effects of glucagon-like peptide-1 on oxidative stress and Nrf2 signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as human intestinal α-glucosidase inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Zhang, C.; Yang, W.; Liu, H.; Lv, Z.; Liu, J.; Jiao, Z. Inhibition of Dipeptidyl Peptidase-4 by Flavonoids: Structure–Activity Relationship, Kinetics and Interaction Mechanism. Front. Nutr. 2022, 9, 892426. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, A.M.; Gannon, M. Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 2007, 38, 193–206. [Google Scholar] [CrossRef]
- Wirasuta, I.M.A.G. Chemical profiling of ecstasy recovered from around Jakarta by High Performance Thin Layer Chromatography (HPTLC)-densitometry. Egypt. J. Forensic Sci. 2012, 2, 97–104. [Google Scholar] [CrossRef][Green Version]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Arisanti, C.; Sukawati, C.; Prasetia, I.G.N.J.A.; Wirasuta, I. Stability of Anthocyanins Encapsulated from Purple Sweet Potato Extract Affected by Maltodextrin Concentration. Macromol. Symp. 2020, 391, 1900127. [Google Scholar] [CrossRef]
- Olivier, D.K.; van Wyk, B.E.; van Heerden, F.R. The chemotaxonomic and medicinal significance of phenolic acids in Arctopus and Alepidea (Apiaceae subfamily Saniculoideae). Biochem. Syst. Ecol. 2008, 36, 724–729. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2021, 146, 112560. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ding, Y.; Zhang, Z.; Cai, X.; Li, Y. Quercetin and quercitrin protect against cytokine-induced injuries in RINm5F β-cells via the mitochondrial pathway and NF-κB signaling. Int. J. Mol. Med. 2012, 31, 265–271. [Google Scholar] [CrossRef]
- Wirasuta, I.M.A.G.; Dewi, N.M.A.R.; Cahyadi, K.D.; Dewi, L.P.M.K.; Astuti, N.M.W.; Widjaja, I.N.K. Studying systematic errors on estimation decision, detection, and quantification limit on micro-TLC. Chromatographia 2013, 76, 1261–1269. [Google Scholar] [CrossRef]
- Ben-Shlomo, S.; Zvibel, I.; Shnell, M.; Shlomai, A.; Chepurko, E.; Halpern, Z.; Barzilai, N.; Oren, R.; Fishman, S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J. Hepatol. 2011, 54, 1214–1223. [Google Scholar] [CrossRef]
- Solverson, P. Anthocyanin Bioactivity in Obesity and Diabetes: And Periphery. Cells 2020, 9, 2515. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, H.; Cao, S.; Chen, Q.; Cui, M.; Wang, Z.; Li, D.; Zhou, J.; Wang, T.; Qiu, F.; et al. Baicalin and its metabolites suppresses gluconeogenesis through activation of AMPK or AKT in insulin resistant HepG-2 cells. Eur. J. Med. Chem. 2017, 141, 92–100. [Google Scholar] [CrossRef]
- Chen, C.; Tan, S.; Ren, T.; Wang, H.; Dai, X.; Wang, H. Polyphenol from Rosaroxburghii Tratt Fruit Ameliorates the Symptoms of Diabetes by Activating the P13K/AKT Insulin Pathway in db/db Mice. Foods 2022, 11, 636. [Google Scholar] [CrossRef]
- Warditiani, N.K.; Astuti, N.M.W.; Sari, P.M.N.A.; Swastini, D.A.; Wirasuta, I.M.A.G. Analysis of lipid profile and atherogenic index (Aip) in dyslipidemia rats given ipomea batatas tuber extract (ibte). Res. J. Pharm. Technol. 2021, 14, 4999–5002. [Google Scholar] [CrossRef]
- Song, W.Y.; Aihara, Y.; Hashimoto, T.; Kanazawa, K.; Mizuno, M. (−)-Epigallocatechin-3-gallate induces secretion of anorexigenic gut hormones. J. Clin. Biochem. Nutr. 2015, 57, 164–169. [Google Scholar] [CrossRef]
- Warditiani, N.K.T.; Astuti, K.W.; Sari, P.M.N.A.; Wirasuta, I.M.A.G. Antidyslipidemic Activity of Methanol, Ethanol and Ethyl Acetate Mangosteen rind (Garcinia mangostana L). Res. J. Pharm. Technol. 2020, 13, 261. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, R.W.; Kunutsor, S.K.; Chowdhury, R.; Yuan, J.M.; Koh, W.P.; Pan, A. Plasma adiponectin levels and type 2 diabetes risk: A nested case-control study in a Chinese population and an updated meta-analysis. Sci. Rep. 2018, 8, 406. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
| No | Type/Cultivar | Part of Plant | Identified Compound | Predictive Bioactive Compound | Analytical Method | Site of Action | Mechanism Pharmacology | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | IBL from cultivar Simon (Beijing, China) | Leaves | 1-Caffeoylquinic acid; Neochlorogenic acid; Esculin; Protocatechualdehyde; Chlorogenic acid; Cryptochlorogenic acid; Caffeic acid hydroxycoumarin; Isochlorogenic acid A, B, and C, 3,4,5-Tricaffeoylquinic acid; Rutin, Hyperoside; Isoquercitrin; Astragalin; Quercetin; KAE; Diosmetin; Jaceosidin; Chrysin; and Pectolinarigenin | - | UHPLC-hybrid quadrupole-orbitrap/MS | Pancreas |
| [11] |
| - | Liver |
| ||||||
| - | Muscle |
| ||||||
| Leaves | - | 3,4,5-Tricaffeoylquinic acid; Cryptochlorogenic acid; Chlorogenic acid; Isochlorogenic acid A, B and C; Neochlorogenic acid; Esculin; Protocatechualdehyde; Caffeic acid; 7-hydroxycoumarin; Ethyl Caffeate; Rutin; Hyperoside; Isoquercitrin; Astragalin; Quercetin; Kampferol; Diosmetin; Jaceosidin; Chrysin; Pectolinarigenin; Hysperidin; Luteolin; and Catechin | UHPLC-hybrid quadrupole-orbitrap/MS | Gastrointestinal |
| [12] | ||
| Pancreas |
| |||||||
| 2 | Purple IBL from in Luzhu District, Taoyuan City, Taiwan | Leaves | Methyl decanoate: Quercetin 3-O-β-D sophoroside; 4-Hydroxy-3-methoxy benzaldehyde; Quercetin; and Benzyl β-d-glucoside | GC-MS | Adipose |
| [13] | |
| 3 | IBL from the local market, India | Leaves | Acidic glycoprotein | - | Gastrointestinal |
| [14] | |
| 4 | IBL from Aan Village, Klungkung Regency, Bali Province, Indonesia | Leaves | - | Peonidin-caffeoyl-p-hydroxybenzoylsophorside-5-glucoside; Cyanidin 3-O-rutinoside (C3OR); Peonidin dirhamnoside; Cyanidin-3-glucoside isomer (C3G); Pelargonidin glucoside or cyanidin 3-O-rutinoside; and Peonidin dirhamnosaloyl-glucoside isomer | ESI-MS | Pancreas |
| [15] |
| 5 | Fresh orange-fleshed SPL (Jishu No. 16) collected from a farm in Yichun | Leaves | Trans-N-(p-coumaroyl) tyramine; 7,3′-Dimethylquercetin; 7-Hydroxy-5-methoxycoumarin; Caffeic acid ethyl ester; Trans-N-feruloyltyramine; Cis-N-feruloyltyramine; 3,4,5-Tricaffeoylquinic acid; 3,4-Dicaffeoylquinic acid; 4,5-Dicaffeoylquinic acid; 4,5-Feruloylcourmaoylquinic acid; Caffeic acid; Quercetin-3-O-a-D-glucopyranoside; and Indole-3-carboxaldehyde | 3,4,5-Tricaffeoylquinic acid; 4,5-Dicaffeoylquinic acid; 3,4-Dicaffeoylquinic acid; Caffeic acid Quercetin-3-O-α-D-glucopyranoside; and 7,3′-dimethylquercetin | HPLC, 1D NMR, 2D NMR, ESI-MS | Pancreas |
| [16] |
| Trans-N-(p-coumaroyl) tyramine; Trans-N-feruloyltyramine; 7-Hydroxy-5-methoxycoumarin; Cis-N-feruloyltyramine; Caffeic acid ethyl ester; 3,4,5-Tricaffeoylquinic acid; and Indole-3-carboxaldehyde | Gastrointestinal |
| ||||||
| 6 | IBL leaves from (Hebei province) in Autumn | Leaves | Flavone | - | Pancreas |
| [17] | |
| 7 | IBL from Slatina (central Croatia) | Leaves | Flavonoid; Phenol | - | Pancreas |
| [18] | |
| 8 | IBL from Anguillara Veneta (Northern Italy) | Leaves | Catechin; Naringin; Epicatechin; Chlorogenic acid; p-OH benzoic acid; Vanillic acid; t-Ferulic acid; and o-Coumaric acid | Chlorogenic acid and Epicatechin | HPLC-PDA | Gastrointestinal |
| [19] |
| Pancreas |
| |||||||
| 9 | Fresh leaves of IBL (family of clones B 00593) from Bandungan, Central Java Indonesia. | Leaves | Anthocyanins; Catechins; Quercetin; Proanthocyanidins; and Caffeic acid | - | Pancreas |
| [20] | |
| 10 | ‘Suioh,’ a IBL cultivar from Kumamoto prefecture, Japan | Leaves | Chlorogenic acid; 3,4,5-Tricaffeoylquinic acid; 3,4-Dicaffeoylquinic acid; 4,5-Dicaffeoylquinic acid; and 3,5-Dicaffeoylquinic acid | 3,4,5-Tricaffeoylquinic acid | - | Gastrointestinal |
| [9] |
| 11 | Purple IBL | - | Peonidin-3-glucoside (P3G); Cyanidin-3-rutinoside C3R); Cyanidin-3-glucoside (C3G); and Cyanidin-3,5-glucoside (C35G) | P3G; C3R; C3G; and C35G | - | Gastrointestinal |
| [21] |
| 12 | ‘Bophelo’ orange-fleshed IBL cultivar | Tubers and leaves | Isovanillic acid; Protocatechuic acid; Quercetin; Caffeic acid; Catechin; Hyperoside; Kaempferol; Rutin; and Vanillic acid | Isovanillic acid; Kaempferol; Protocatechuic acid; Caffeic acid; Catechin; Hyperoside; Rutin; Quercetin; and Vanillic acid | HPLC-MS | Pancreas |
| [22] |
| Muscle |
| |||||||
| 13 | White potato Tainung No.10 | Tubers and leaves | Arabinogalactan; and Epigallocatechin gallate | - | Muscle |
| [23] | |
| 14 | Purple IBL (Cultivar Eshu No.12) from the Institute of Food Crops, Hubei Academy of Agricultural Sciences | Tubers | Peonidin-3-sophoroside-5-glucoside (P3S5G); Cyanidin-3-sophoroside-5-glucoside (C3S5G); Anthocyanins (containing one or two p-hydroxybenzoic, caffeic and/or ferulic acid); and 17 proteins (consisted of group: Acetylesterase, Proteinase inhibitor, Sporamin A, Superoxide dismutase [Cu-Zn], Beta-amylase, Sporamin B, preprosporamin, Polyphenol oxidase I chloroplastic, Purple acid phosphatase, and NBS-LRR protein and pectin) | P3S5G; C3S5G; Anthocyanins (containing one or two p-hydroxybenzoic, caffeic and/or ferulic acid); and 17 proteins (consisted of the group: Acetylesterase, Proteinase inhibitor, Sporamin A, Superoxide dismutase [Cu-Zn], Beta-amylase, Sporamin B, preprosporamin, Polyphenol oxidase I chloroplastic, Purple acid phosphatase, and NBS-LRR protein and pectin) | HPLC-DAD/ESI-MS | Pancreas |
| [24] |
| Liver |
| |||||||
| 15 | Purple IBL powder (cultivar Eshu No. 8) | Tubers | Diacylated anthocyanins | Peonidin-3-caffeoylferuloyl sophoroside-5-glucoside | - | Liver | Enhancing the secretion and sensitivity of the insulin elucidates mechanism: (i) inhibitor of liver XO activity; (ii) activation of the expression of SGLT2, GLUT-5, and GLUT-2; (iii) the suppression of the NF-κB pathway leads to a decrease in the expression of IL-1ß and iNOS | [25] |
| 16 | IBL (Linn.) Lam from Western Research Farm, National Root Crop Research Institute, Umudike, Abia state | Tubers | Flavonoid; Terpenoid; Tannin; Phenol | - | Pancreas |
| [26] | |
| Gastrointestinal |
| |||||||
| Adipose |
| |||||||
| 17 | Purple IBL cv. Ayamurasaki from the Kyushu National Agricultural Experiment Station in Miyazaki prefecture (Japan) | Tubers | Peonidin 3-O-[2-O-(6-O-E-feruloyl-β-D-glucopyranosyl)-6-O-E-caffeoyl-β-D-glucopyranoside]-5-O-β-D-glucopyranoside | - | Gastrointestinal |
| [27] | |
| 18 | Korean red skin IBL (Ib 1)and Korean pumpkin IBL (Ib 2) from the market in Goyang, Republic of Korea | Peel-off tuber | α-carotene; ß-carotene; zeaxanthin; and lutein | - | Gastrointestinal |
| [28] | |
| 19 | White IBL (Caiapo) | Tubers | Acidic glycoprotein | - | Gastrointestinal |
| [29] | |
| Adipose |
| [30] | ||||||
| 20 | White-skinned sweet potato (WSSP) purchased from Kagawa, Japan, Prefectural Cooperative | Tubers | Caffeic acid | - | Adipose |
| [31] | |
| WSPP fraction consists of >50 kDa, 10–50 kDa, and ≤10 kDa | ≤10 kDa fraction | - | Muscle |
| [32] | |||
| >50 kDa fraction | Liver |
| ||||||
| 21 | Korean purple IBL (Shinzami, Saeungbone9, Gyeyae2469, Gyebone108, Saeungyae33, and Gyeyae2258) | Tubers | 3-Caffeoyl-phydroxybenzoylsophoroside-5-glucoside; Peonidin 3-caffeoyl sophoroside-5-glucoside; Peonidin 3-(6″-caffeoyl-6‴-feruloyl sophoroside)-5-glucoside; and Peonidin 3-caffeoyl-phydroxybenzoylsophoroside-5-glucoside | Cyanidin 3-caffeoyl-p-hydroxybenzolsophoroside-5-glucoside and Peonidin 3-(6″-caffeoyl-6‴-feruloyl sophoroside)-5-glucoside | LC-DAD-ESI/MS | Liver |
| [33] |
| 22 | Color-fleshed potatoes (Sinjami and Sinhwangmi) | Tubers | Lutein; Peonidin 3-(6″-caffeoyl-6″-feruloyl sophoroside)5-glucoside; Zeaxanthin; Cryptoxanthi; 13Z-ß-carotene; Peonidin 3-sophoroside-5-glucoside; Peonidin 3-p-hydroxybenzoyl sophoroside-5-glucoside; Cyanidin 3-p-hydroxybenzoyl sophoroside-5-glucoside; Cyanidin3-(6″-feruloyl sophoroside)-5-glucoside; Peonidin 3-(6″-feruloyl sophoroside)-5-glucoside; Cyanidin 3-(6″,6″-dicaffeoyl sophoroside)-5-glucoside; Cyanidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside; Cyanidin3-(6″-caffeoyl6″-feruloyl sophoroside)-5-glucoside; Peonidin 3-caffeoyl sophoroside-5-glucoside; Peonidin 3-(6″,6″-dicaffeoyl sophoroside)-5glucoside; Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside; all-trans-ß-carotene; and 9Z-ß-carotene | Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | UPLC-MS/MS (Q-TOF-ESI) | Adipose |
| [34] |
| 23 | IBL from Kagawa Prefecture, Japan | Tubers | Chlorogenic acid; and Caffeic acid and its derivatives | - | Pancreas |
| [35] | |
| 24 | White-skinned sweet potato (WSSP) | Tubers | Arabinogalactan protein | - | Liver |
| [36] | |
| 25 | Purple IBL Antin-3 cultivar from the BALITKABI Malang | Tubers | Anthocyanin group | - | Pancreas |
| [37] | |
| 26 | White-skinned sweet potatoes (WSSP) from the local market Faisalabad (Pakistan) | Tubers | Carotenoid | - | Pancreas |
| [38] | |
| Glicoprotein; Flavonoid; and Carotenoid | - | Liver |
| [39] | ||||
| 27 | Purple IBL from Padang, West Sumatra, Indonesia | Tubers | Peonidin; and Cyanidin | - | Gastrointestinal |
| [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, N.K.S.M.; Ramona, Y.; Saraswati, M.R.; Wihandani, D.M.; Wirasuta, I.M.A.G. The Potential of the Flavonoid Content of Ipomoea batatas L. as an Alternative Analog GLP-1 for Diabetes Type 2 Treatment—Systematic Review. Metabolites 2024, 14, 29. https://doi.org/10.3390/metabo14010029
Dewi NKSM, Ramona Y, Saraswati MR, Wihandani DM, Wirasuta IMAG. The Potential of the Flavonoid Content of Ipomoea batatas L. as an Alternative Analog GLP-1 for Diabetes Type 2 Treatment—Systematic Review. Metabolites. 2024; 14(1):29. https://doi.org/10.3390/metabo14010029
Chicago/Turabian StyleDewi, Ni Kadek Santi Maha, Yan Ramona, Made Ratna Saraswati, Desak Made Wihandani, and I Made Agus Gelgel Wirasuta. 2024. "The Potential of the Flavonoid Content of Ipomoea batatas L. as an Alternative Analog GLP-1 for Diabetes Type 2 Treatment—Systematic Review" Metabolites 14, no. 1: 29. https://doi.org/10.3390/metabo14010029
APA StyleDewi, N. K. S. M., Ramona, Y., Saraswati, M. R., Wihandani, D. M., & Wirasuta, I. M. A. G. (2024). The Potential of the Flavonoid Content of Ipomoea batatas L. as an Alternative Analog GLP-1 for Diabetes Type 2 Treatment—Systematic Review. Metabolites, 14(1), 29. https://doi.org/10.3390/metabo14010029









