From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches
Abstract
1. Introduction
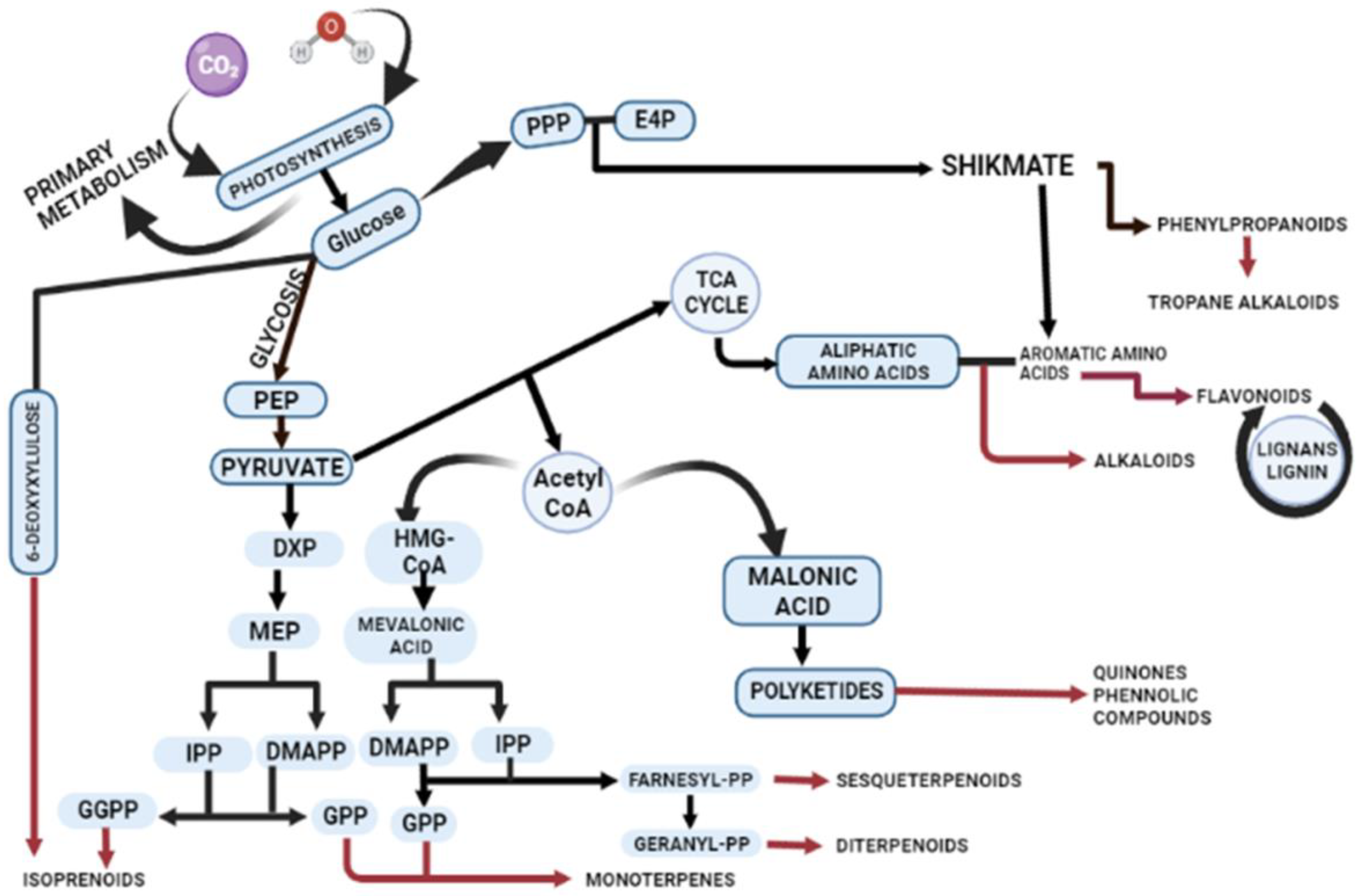
2. Biosynthesis of Secondary Metabolites in Plants
3. Secondary Metabolites as Regulators of Growth and Development
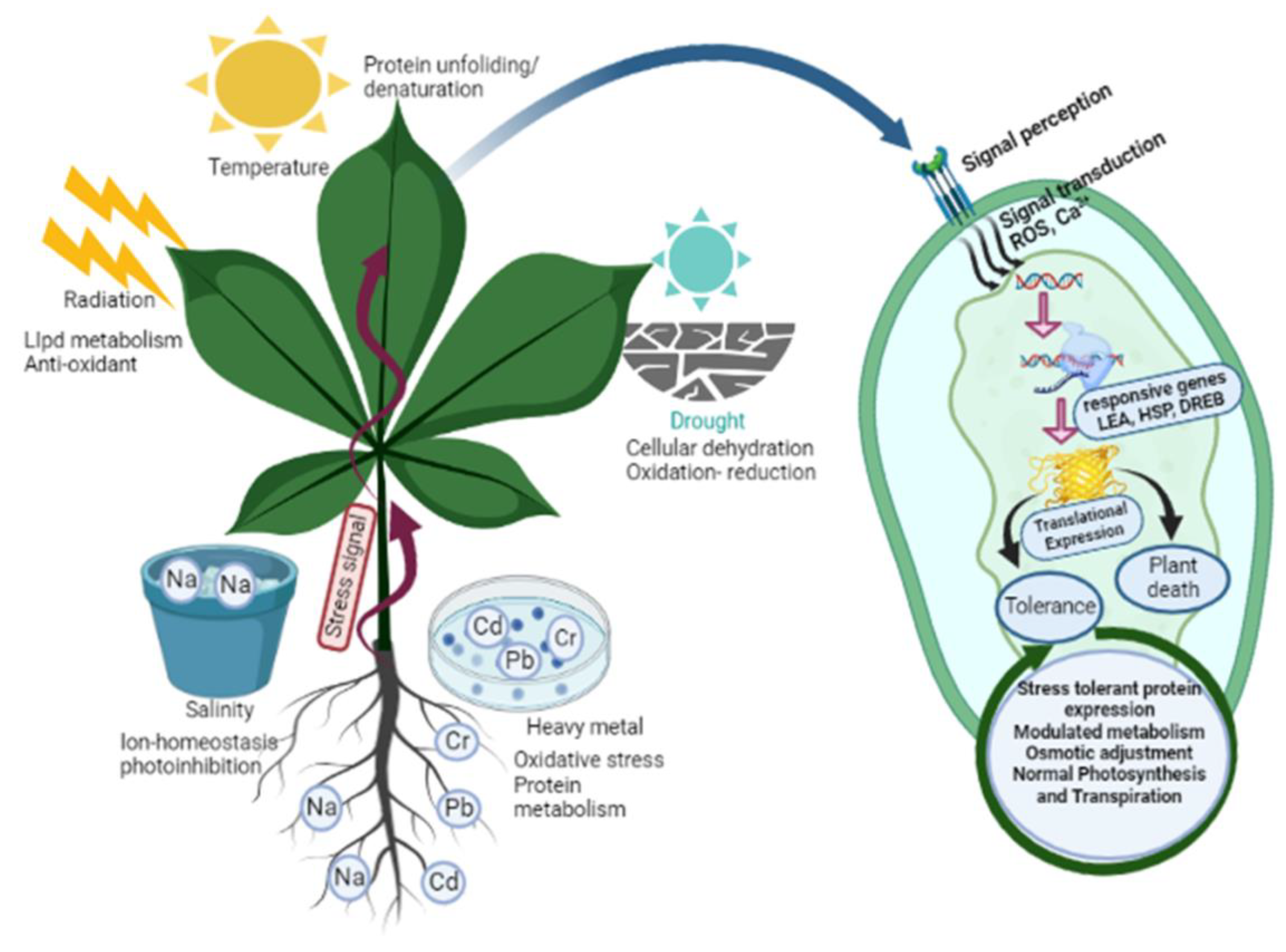
4. Secondary Metabolite Production in Plants in Response to Different Environmental Factors
4.1. Salt Stress
4.2. Drought Stress
4.3. Temperature Stress
4.4. Light, UV, and Ionization Radiation
4.5. Heavy Metal Stress
5. Other Factors Influencing Secondary Metabolism in Plants
6. Defence Action through Secondary Metabolism in Plants
7. Application of Plant Tissue Culture Techniques Associated with Plant Secondary Metabolites Production
| Plant Species | Medium + PGRs | Cultured Tisssue | Compound Name | Reference |
|---|---|---|---|---|
| Camellia sinensis L. | MS + 2,4-D + BAP | Callus | Catechin | [168] |
| Arbutus andrachne L. | WP + TDZ + NAA | Callus | Catechin | [169] |
| Rauwolfia serpentina | MS + Kn + BAP | Shoot | Phyllocladane diterpenoids | [170] |
| Eurycoma longifolia | MS + NAA +Kn | Cell suspension | Eurycomanone | [171] |
| Talinum paniculatum | MS + potassium nitrate | Hairy root | Saponin content | [172] |
| Momordica charantia | MS + sucrose | Hairy root | Flavonoids, phenolic acids | [173] |
| Eleutherococcus koreanum | ½ MS + IBA + TDZ | Adventitious root | Eleutheroside B and E | [174] |
| Eurycoma longifolia | 3/4 MS + IBA + NAA | Adventitious root | Flavonoids, phenolic content | [175] |
| Astragalus membranaceus | MS + IBA | Adventitious root | Saponin, flavonoid content | [176] |
| Coleus blumei | MMS + BA + NAA + sucrose | Callus and suspension | Rosmarinic acid | [177] |
| Spilanthes acmella | MS + BA + 2,4-D | Cell suspension | Scopoletin | [178] |
| Ajuga bracteosa | MS + BA + MeJ | Cell and callus | Monoterpene hydrocarbons | [179] |
| Fagonia indica | MS + TDZ | Callus | Gallic acid, quercetin | [180] |
| Rosa damascena | MS + BA + NAA | Callus | Tocopherols and β-carotene | [181] |
| Salvia dolomitica | MS + 2,4-D + Kn | Callus | α-Pinene, β-phellandrene | [182] |
| Corylus avellana L. | MS + BA + 2,4-D | Suspension | Taxol | [183] |
| Linum usitatsimum L. | MS + NAA | Callus | Lignans and neolignans | [184] |
| Morus alba L. | MS + Cefotaxime | Hairy root | Betulin and betulinic acid | [185] |
| Solanum trilobatum L. | MS + MeJ | Hairy root | Solasodine | [186] |
| Salvia miltiorrhiza | MS + MeJ + SA | Hairy root | Tanshinone | [187] |
| Caralluma tuberculata | MS + BA + 2,4-D | Callus | Phenolic and flavonoid content | [188] |
| Rhodiola imbricata | MS + BA + NAA | Callus | Phenylethanoids and phenylpropanoids | [189] |
| Plumbago zeylanica L. | MS + IBA | Root suspension | Plumbagin | [190] |
| Verbena officinalis L. | Schenk–Hildebrandt medium + 2,ip + TDZ | Shoot culture | Coumaran and hexadecenoic acid | [191] |
| Thevetia peruviana | Schenk–Hildebrandt medium + 2,4-D + Kn | Cell suspension | Phenolic compounds | [192] |
| Oldenlandia umbellata L. | MS + IBA + NAA | Adventitious root | Anthraquinones | [193] |
| Vitis vinifera | MS + IAA +GA3 | Callus | Resveratol | [194] |
7.1. Callus and Cell Culture
7.2. Hairy Root Culture
8. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023, 53, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Shahzad, S.M.; Hussain, M.; Alharby, H.F.; Irshad, M.K.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef] [PubMed]
- Kossel, A. Ueber Schleim und Schleimbildende Stoffe. Dtsch. Med. Wochenschr. 1891, 17, 1297–1299. [Google Scholar] [CrossRef][Green Version]
- Simpson, K.; Fuentes, P.; Quiroz-Iturra, L.F.; Flores-Ortiz, C.; Contreras, R.; Handford, M.; Stange, C. Unraveling the Induction of Phytoene Synthase 2 Expression by Salt Stress and Abscisic Acid in Daucus carota. J. Exp. Bot. 2018, 69, 4113–4126. [Google Scholar] [CrossRef]
- Sankari, M.; Hridya, H.; Sneha, P.; Doss, C.G.P.; Christopher, J.G.; Mathew, J.; Zayed, H.; Ramamoorthy, S. Implication of Salt Stress Induces Changes in Pigment Production, Antioxidant Enzyme Activity, and qRT-PCR Expression of Genes Involved in the Biosynthetic Pathway of Bixa orellana L. Funct. Integr. Genom. 2019, 19, 565–574. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant Secondary Metabolites: A Key Driver of Litter Decomposition and Soil Nutrient Cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary Metabolites of Plants. Med. Orchid. Asia 2015, 5, 59–73. [Google Scholar] [CrossRef]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 01463. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Fatani, S.H.; Mohamed Nour Eldin, E.E.; Wink, M. Natural Products Modulate the Multifactorial Multidrug Resistance of Cancer. Pharmaceuticals 2015, 6, 146–176. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. [Google Scholar] [CrossRef]
- Rahman, A.; Albadrani, G.M.; Waraich, E.A.; Awan, T.H.; Yavaş, İ.; Hussain, S. Plant Secondary Metabolites and Abiotic Stress Tolerance: Overview and Implications. In Plant Abiotic Stress Responses and Tolerance Mechanisms; Intechopen: London, UK, 2023. [Google Scholar] [CrossRef]
- Kaur, M.; Tak, Y.; Bhatia, S.; Kaur, H. Phenolics Biosynthesis, Targets, and Signaling Pathways in Ameliorating Oxidative Stress in Plants. In Plant Phenolics in Abiotic Stress Management; Springer: Singapore, 2023; pp. 149–171. [Google Scholar] [CrossRef]
- Weaver, L.M.; Herrmann, K.M. Dynamics of the Shikimate Pathway in Plants. Trends Plant Sci. 1997, 2, 346–351. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, E.; Mishra, N.; Mishra, P. Shikimic acid as an intermediary model for the production of drugs effective against influenza virus. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–256. [Google Scholar]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Ashraf, M.I.; Shu, S. Biosynthetic pathways of triterpenoids and strategies to improve their biosynthetic efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Gao, M.; Zhao, Y.; Wang, Y. Sir2 family proteins regulate terpenoid synthesis by deacetylation of 3-hydroxy-3-methylglutaryl-CoA synthase. Ind. Crops Prod. 2021, 170, 113770. [Google Scholar] [CrossRef]
- Zhang, X.K.; Wang, D.N.; Chen, J.; Liu, Z.J.; Wei, L.J.; Hua, Q. Metabolic engineering of β-carotene biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef]
- Hemmerlin, A. Phosphorylation of Metabolites Involved in Salvage Pathways for Isoprenoid Biosynthesis in Plants. Kinases Phosphatases 2023, 1, 151–166. [Google Scholar] [CrossRef]
- Yap, P.G.; Choi, S.B.; Liong, M.T. Allantoin, a potential metabolite that promotes AMPK phosphorylation and suppresses cholesterol biosynthesis via the mevalonate pathway and Bloch pathway. Appl. Biochem. Biotechnol. 2020, 191, 226–244. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Zhang, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef]
- Martens, S.; Preuß, A.; Matern, U. Multifunctional Flavonoid Dioxygenases: Flavonol and Anthocyanin Biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic Metabolism and Related Heavy Metal Tolerance Mechanism in Kandelia obovata under Cd and Zn Stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, R.E.; Jimenez-Gomez, J.M.; Fulop, D.; Harmer, S.L.; Maloof, J.N.; Kliebensteina, D.J. Network Quantitative Trait Loci Mapping of Circadian Clock Outputs Identifies Metabolic Pathway-to-Clock Linkages in Arabidopsis. Plant Cell 2011, 23, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Thomsen, M.L.F.; Nintemann, S.J.; Jagd, L.M.; Bourgine, B.; Burow, M.; Kliebenstein, D.J. An Evolutionarily Young Defense Metabolite Influences the Root Growth of Plants via the Ancient TOR Signaling Pathway. eLife 2017, 6, e29353. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Ye, W.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate Degradation Products, Isothiocyanates, Nitriles, and Thiocyanates, Induce Stomatal Closure Accompanied by Peroxidase-Mediated Reactive Oxygen Species Production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Vyas, D. Myrosinase: Insights on Structural, Catalytic, Regulatory, and Environmental Interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The Glucosinolate Breakdown Product Indole-3-Carbinol Acts as an Auxin Antagonist in Roots of Arabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef]
- Gayomba, S.R.; Watkins, J.M.; Muday, G.K. Flavonols Regulate Plant Growth and Development through Regulation of Auxin Transport and Cellular Redox Status. Recent Adv. Polyphen. Res. 2016, 5, 143–170. [Google Scholar] [CrossRef]
- Maloney, G.S.; DiNapoli, K.T.; Muday, G.K. The Anthocyanin Reduced Tomato Mutant Demonstrates the Role of Flavonols in Tomato Lateral Root and Root Hair Development. Plant Physiol. 2014, 166, 614–631. [Google Scholar] [CrossRef]
- Buer, C.S.; Djordjevic, M.A. Architectural Phenotypes in the Transparent Testa Mutants of Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 751–763. [Google Scholar] [CrossRef]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the Study of the Function and Mechanism of the Action of Flavonoids in Plants under Environmental Stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Chalker-Scott, L.; Fuchigami, L.H. The Role of Phenolic Compounds in Plant Stress Responses. In Low Temperature Stress Physiology in Crops; Springer: Cham, Switzerland, 2018; pp. 67–79. [Google Scholar] [CrossRef]
- Wuddineh, W.; Minocha, R.; Minocha, S.C. Polyamines in the Context of Metabolic Networks. Methods Mol. Biol. 2018, 1694, 1–23. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Establishment of Cell Suspension Cultures of Ocimum basilicum L. and Enhanced Production of Pharmaceutical Active Ingredients. Ind. Crops Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.; Xie, D.Y.; Sebastiani, L. Salt Stress Induces Differential Regulation of the Phenylpropanoid Pathway in Olea europaea Cultivars Frantoio (Salt-Tolerant) and Leccino (Salt-Sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ahmad, P. Jasmonic Acid Application Triggers Detoxification of Lead (Pb) Toxicity in Tomato through the Modifications of Secondary Metabolites and Gene Expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef]
- Pandey, N.; Pandey-Rai, S. Short Term UV-B Radiation-Mediated Transcriptional Responses and Altered Secondary Metabolism of In Vitro Propagated Plantlets of Artemisia annua L. Plant Cell. Tissue Organ Cult. 2014, 116, 371–385. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of Secondary Metabolism in Citrus Plants Is Associated to Sensitivity to Combined Drought and High Temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, L.; Tůma, J. The Effect of UV Light on Isoflavonoid Production in Genista tinctoria Culture In Vitro. Acta Physiol. Plant. 2011, 33, 635–640. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant Growth Promoting Rhizobacteria (PGPR) Confer Drought Resistance and Stimulate Biosynthesis of Secondary Metabolites in Pennyroyal (Mentha pulegium L.) under Water Shortage Condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought Stress Adaptation Modulates Plant Secondary Metabolite Production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Podda, A.; Pollastri, S.; Bartolini, P.; Pisuttu, C.; Pellegrini, E.; Nali, C.; Cencetti, G.; Michelozzi, M.; Frassinetti, S.; Giorgetti, L.; et al. Drought Stress Modulates Secondary Metabolites in Brassica oleracea L. Convar. acephala (DC) Alef, var. sabellica L. J. Sci. Food Agric. 2019, 99, 5533–5540. [Google Scholar] [CrossRef]
- Ibrahim, W.; Zhu, Y.M.; Chen, Y.; Qiu, C.W.; Zhu, S.; Wu, F. Genotypic Differences in Leaf Secondary Metabolism, Plant Hormones and Yield under Alone and Combined Stress of Drought and Salinity in Cotton Genotypes. Physiol. Plant. 2019, 165, 343–355. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under In Vitro Salinity Stress. Plant Cell. Tissue Organ Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachâal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt Stress (NaCl) Affects Plant Growth and Branch Pathways of Carotenoid and Flavonoid Biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- Li, Q.; Lei, S.; Du, K.; Li, L.; Pang, X.; Wang, Z.; Wei, M.; Fu, S.; Hu, L.; Xu, L. RNA-Seq Based Transcriptomic Analysis Uncovers α-Linolenic Acid and Jasmonic Acid Biosynthesis Pathways Respond to Cold Acclimation in Camellia japonica. Sci. Rep. 2016, 6, 36463. [Google Scholar] [CrossRef]
- Ayoola-Oresanya, I.O.; Sonibare, M.A.; Gueye, B.; Abberton, M.T.; Morlock, G.E. Elicitation of Antioxidant Metabolites in Musa Species In Vitro Shoot Culture Using Sucrose, Temperature and Jasmonic Acid. Plant Cell Tissue Organ Cult. 2021, 146, 225–236. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of Culture Medium Composition and Light Conditions on the Accumulation of Bioactive Compounds in Shoot Cultures of Scutellaria lateriflora L. (American Skullcap) Grown In Vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential Accumulation of Silymarin Induced by Exposure of Silybum marianum L. Callus Cultures to Several Spectres of Monochromatic Lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Krolicka, A.; Zabiegala, B.; Luczkiewicz, M. Elicitation Strategies for the Improvement of Essential Oil Content in Rhododendron tomentosum (Ledum Palustre) Bioreactor-Grown Microshoots. Ind. Crops Prod. 2018, 123, 461–469. [Google Scholar] [CrossRef]
- Farrokhzad, Y.; Rezaei, A. Aluminum Elicitation Improves Antioxidant Potential and Taxol Production in Hazelnut (Corylus avellana L.) Cell Suspension Culture. Agric. Conspec. Sci. 2020, 85, 229–236. [Google Scholar]
- Anjum, S.; Abbasi, B.H.; Doussot, J.; Favre-Réguillon, A.; Hano, C. Effects of Photoperiod Regimes and Ultraviolet-C Radiations on Biosynthesis of Industrially Important Lignans and Neolignans in Cell Cultures of Linum usitatissimum L. (Flax). J. Photochem. Photobiol. B Biol. 2017, 167, 216–227. [Google Scholar] [CrossRef]
- Ullrich, S.F.; Rothauer, A.; Hagels, H.; Kayser, O. Influence of Light, Temperature, and Macronutrients on Growth and Scopolamine Biosynthesis in Duboisia species. Planta Med. 2017, 83, 937–945. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Haider, M.Z.; Ashraf, M.A.; Rasheed, R.; Hussain, I.; Riaz, M.; Qureshi, F.F.; Hafeez, A. Impact of Salinity Stress on Medicinal Plants. In Medicinal Plants: Their Response to Abiotic Stress; Springer: Singapore, 2023; pp. 199–239. [Google Scholar] [CrossRef]
- Daneshmand, F.; Arvin, M.J.; Kalantari, K.M. Physiological Responses to NaCl Stress in Three Wild Species of Potato in Vitro. Acta Physiol. Plant. 2010, 32, 91–101. [Google Scholar] [CrossRef]
- Parmar, R.D.; Mali, S.C.; Patel, A.I.; Patel, P.K. In Vitro Response of Promising Sugarcane Varieties for Salinity Tolerance through Callus Culture. Int. J. Chem. Stud. 2017, 5, 1180–1186. [Google Scholar]
- Alagoz, S.M.; Lajayer, B.A.; Ghorbanpour, M. Proline and Soluble Carbohydrates Biosynthesis and Their Roles in Plants under Abiotic Stresses. In Plant Stress Mitigators; Academic Press: Cambridge, MA, USA, 2023; pp. 169–185. [Google Scholar] [CrossRef]
- Ali, R.M.; Abbas, H.M. Response of Salt Stressed Barley Seedlings to Phenylurea. Plant Soil Environ. 2003, 49, 158–162. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; El-Habbak, M.H.; Havens, W.M.; Singh, A.; Zheng, D.; Vaughn, L.; Haudenshield, J.S.; Hartman, G.L.; Korban, S.S.; Ghabrial, S.A. Overexpression of GmCaM4 in Soybean Enhances Resistance to Pathogens and Tolerance to Salt Stress. Mol. Plant Pathol. 2014, 15, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Muchate, N.S.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Evaluation of Spinacia oleracea (L.) for Phytodesalination and Augmented Production of Bioactive Metabolite, 20-Hydroxyecdysone. Int. J. Phytoremediation 2018, 20, 981–994. [Google Scholar] [CrossRef]
- Ahl, S.A.; Omer, E.A. Medicinal and Aromatic Plants Production under Salt Stress. A Review. Herba Pol. 2011, 57, 2. [Google Scholar]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Chang. Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The Use of Metabolomics to Dissect Plant Responses to Abiotic Stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Suhre, K.; Gieger, C. Genetic Variation in Metabolic Phenotypes: Study Designs and Applications. Nat. Rev. Genet. 2012, 13, 759–769. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.; Wu, C.; Chen, S.; Wang, Z.; Yang, Z.; Qin, S.; Huang, L. Water Deficit Affected Flavonoid Accumulation by Regulating Hormone Metabolism in Scutellaria baicalensis Georgi Roots. PLoS ONE 2012, 7, e42946. [Google Scholar] [CrossRef]
- Solíz-Guerrero, J.B.; De Rodriguez, D.J.; Rodríguez-García, R.; Angulo-Sánchez, J.L.; Méndez-Padilla, G. Quinoa Saponins: Concentration and Composition Analysis. In Trends New Crop. New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 110–114. [Google Scholar]
- Cingöz, G.; Pehlivan Karakaş, F. The Effects of Nutrient and Macronutrient Stress on Certain Secondary Metabolite Accumulations and Redox Regulation in Callus Cultures of Bellis perennis L. Turk. J. Biol. 2016, 40, 1328–1335. [Google Scholar] [CrossRef]
- Deepthi, S.; Satheeshkumar, K. Effects of Major Nutrients, Growth Regulators and Inoculum Size on Enhanced Growth and Camptothecin Production in Adventitious Root Cultures of Ophiorrhiza mungos L. Biochem. Eng. J. 2017, 117, 198–209. [Google Scholar] [CrossRef]
- Yang, L.-L.; Yang, L.; Yang, X.; Zhang, T.; Lan, Y.-M.; Zhao, Y.; Han, M.; Yang, L.-M. Drought Stress Induces Biosynthesis of Flavonoids in Leaves and Saikosaponins in Roots of Bupleurum chinense DC. Phytochemistry 2020, 177, 112434. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the relevance of high temperature and elevated CO2 in plant secondary metabolites (PSMs) production. Heliyon 2021, 7, e07975. [Google Scholar] [CrossRef]
- Rahimi, S.; Hasanloo, T. The Effect of Temperature and pH on Biomass and Bioactive Compounds Production in Silybum marianum Hairy Root Cultures. Res. J. Pharmacogn. 2016, 3, 53–59. [Google Scholar]
- Chan, L.K.; Koay, S.S.; Boey, P.L.; Bhatt, A. Effects of Abiotic Stress on Biomass and Anthocyanin Production in Cell Cultures of Melastoma malabathricum. Biol. Res. 2010, 43, 127–135. [Google Scholar] [CrossRef]
- Okereke, C.N.; Kaurilind, E.; Liu, B.; Kanagendran, A.; Pazouki, L.; Niinemets, Ü. Impact of heat stress of varying severity on papaya (Carica papaya) leaves: Major changes in stress volatile signatures, but surprisingly small enhancements of total emissions. Environ. Exp. Bot. 2022, 195, 104777. [Google Scholar] [CrossRef]
- Singsaas, E.L. Terpenes and the Thermotolerance of Photosynthesis. New Phytol. 2000, 146, 1–2. [Google Scholar] [CrossRef]
- Cawood, M.E.; Allemann, I.; Allemann, J. Impact of Temperature Stress on Secondary Metabolite Profile and Phytotoxicity of Amaranthus cruentus L. Leaf Extracts. Acta Agric. Slov. 2018, 111, 609–620. [Google Scholar] [CrossRef]
- Sivadasan, U.; Chenhao, C.; Nissinen, K.; Randriamanana, T.; Nybakken, L.; Julkunen-Tiitto, R. Growth and Defence of Aspen (Populus tremula) after Three Seasons under Elevated Temperature and Ultraviolet-B Radiation. Can. J. For. Res. 2018, 48, 629–641. [Google Scholar] [CrossRef]
- Zhang, Y.; Virjamo, V.; Du, W.; Yin, Y.; Nissinen, K.; Nybakken, L.; Guo, H.; Julkunen-Tiitto, R. Effects of Soil Pyrene Contamination on Growth and Phenolics in Norway Spruce (Picea abies) Are Modified by Elevated Temperature and CO2. Environ. Sci. Pollut. Res. 2018, 25, 12788–12799. [Google Scholar] [CrossRef]
- Yao, L.; Caffin, N.; D’Arcy, B.; Jiang, Y.; Shi, J.; Singanusong, R.; Liu, X.; Datta, N.; Kakuda, Y.; Xu, Y. Seasonal Variations of Phenolic Compounds in Australia-Grown Tea (Camellia sinensis). J. Agric. Food Chem. 2005, 53, 6477–6483. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and Biochemical Responses to High Light and Temperature Stress in Plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Kong, D.X.; Li, Y.Q.; Wang, M.L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of Light Intensity on Leaf Photosynthetic Characteristics, Chloroplast Structure, and Alkaloid Content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant. 2016, 38, 120. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Aust. J. Plant Physiol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Liang, H.L.; Wu, H. Alkaloid Content and Essential Oil Composition of Mahonia breviracema Cultivated under Different Light Environments. J. Appl. Bot. Food Qual. 2018, 91, 171–179. [Google Scholar] [CrossRef]
- Pedroso, R.C.N.; Branquinho, N.A.A.; Hara, A.C.B.A.M.; Costa, A.C.; Silva, F.G.; Pimenta, L.P.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Januario, A.H. Impact of Light Quality on Flavonoid Production and Growth of Hyptis marrubioides Seedlings Cultivated in Vitro. Rev. Bras. Farmacogn. 2017, 27, 466–470. [Google Scholar] [CrossRef]
- Katerova, Z.; Todorova, D.; Sergiev, I. Plant Secondary Metabolites and Some Plant Growth Regulators Elicited by UV Irradiation, Light and/or Shade. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–121. [Google Scholar] [CrossRef]
- Eichholz, I.; Rohn, S.; Gamm, A.; Beesk, N.; Herppich, W.B.; Kroh, L.W.; Ulrichs, C.; Huyskens-Keil, S. UV-B-Mediated Flavonoid Synthesis in White Asparagus (Asparagus officinalis L.). Food Res. Int. 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Binder, B.Y.K.; Peebles, C.A.M.; Shanks, J.V.; San, K.Y. The Effects of UV-Stress on the Production of Terpenoid Indole Alkaloids in Catharanthus roseus Hairy Roots. Biotechnol. Prog. 2009, 25, 861–865. [Google Scholar] [CrossRef]
- Arakawa, O.; Hori, Y.; Ogata, R. Relative Effectiveness and Interaction of Ultraviolet-B, Red and Blue Light in Anthocyanin Synthesis of Apple Fruit. Physiol. Plant. 1985, 64, 323–327. [Google Scholar] [CrossRef]
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B Radiation Affects Flavonoids and Fungal Colonisation in Fagopyrum esculentum and F. tataricum. Cent. Eur. J. Biol. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Secondary Metabolite Profiles in Flowering Heads of Arnica montana cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef]
- Estell, R.E.; Fredrickson, E.L.; James, D.K. Effect of Light Intensity and Wavelength on Concentration of Plant Secondary Metabolites in the Leaves of Flourensia cernua. Biochem. Syst. Ecol. 2016, 65, 108–114. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of Photoperiod on Flavonoid Pathway Activity in Sweet Potato (Ipomoea batatas (L.) Lam.) Leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Dayananda, C.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Photoperiod Influences Endogenous Indoleamines in Cultured Green Alga Dunaliella bardawil. Indian J. Exp. Biol. 2011, 49, 234–240. [Google Scholar]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021, 11, 968. [Google Scholar] [CrossRef]
- Murch, S.J.; Haq, K.; Rupasinghe, H.P.V.; Saxena, P.K. Nickel contamination affects growth and secondary metabolite composition of St. John’s wort (Hypericum perforatum L.). Environ. Exp. Bot. 2003, 49, 251–257. [Google Scholar] [CrossRef]
- dos Reis, A.R.; de Queiroz Barcelos, J.P.; de Souza Osório, C.R.W.; Santos, E.F.; Lisboa, L.A.M.; Santini, J.M.K.; dos Santos, M.J.D.; Junior, E.F.; Campos, M.; de Figueiredo, P.A.M.; et al. A Glimpse into the Physiological, Biochemical and Nutritional Status of Soybean Plants under Ni-Stress Conditions. Environ. Exp. Bot. 2017, 144, 76–87. [Google Scholar] [CrossRef]
- Merlot, S. Understanding Nickel Responses in Plants: More than Just an Interaction with Iron Homeostasis. Plant Cell Physiol. 2020, 61, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sinha, S. Accumulation of metals and its effects in Brassica juncea (L.) Czern. (cv. Rohini) grown on various amendments of tannery waste. Ecotoxicol. Environ. Saf. 2005, 62, 118–127. [Google Scholar] [CrossRef]
- Wu, B.; Wang, K.; Wu, X. A new phenolic diglycoside produced in response to copper toxicity and a new flavan dimer from the leaves of Viburnum ichangense (Hemsl.). Rehd. Helv. Chim. Acta 2011, 94, 1677–1684. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and Nickel Co-Treatment Alters Metal Uptake and Stress Parameters of Salix purpurea × viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- da Silva, L.C.; de Araújo, T.O.; Martinez, C.A.; de Almeida Lobo, F.; Azevedo, A.A.; Oliva, M.A. Differential Responses of C3 and CAM Native Brazilian Plant Species to a SO2- and SPMFe-Contaminated Restinga. Environ. Sci. Pollut. Res. 2015, 22, 14007–14017. [Google Scholar] [CrossRef]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Baenas, N.; Moreno, D.A.; Sánchez-Rodríguez, E.; Blasco, B.; Ruiz, J.M. Evaluation of Hydrogen Sulfide Supply to Biostimulate the Nutritive and Phytochemical Quality and the Antioxidant Capacity of Cabbage (Brassica oleracea L. ’Bronco’). J. Appl. Bot. Food Qual. 2016, 89, 290–298. [Google Scholar] [CrossRef]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, Herbicides and Bees: A Systematic Review of Existing Research and Methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef]
- Geetha, A. Chapter-2 Phytotoxicity Due to Fungicides and Herbicides and Its Impact in Crop Physiological Factors. In Advances in Agriculture Sciences; Naresh, R.K., Ed.; AkiNik Publications: New Delhi, India, 2019; p. 29. [Google Scholar]
- Chaudhary, N.; Choudhary, K.K.; Agrawal, S.B.; Agrawal, M. Pesticides Usage, Uptake and Mode of Action in Plants with Special Emphasis on Photosynthetic Characteristics. In Pesticides in Crop Production; Wiley: Hoboken, NJ, USA, 2020; pp. 159–180. [Google Scholar] [CrossRef]
- Hassan, A. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 105–110. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Zykin, P.A.; Andreeva, E.A.; Lykholay, A.N.; Tsvetkova, N.V.; Voylokov, A.V. Anthocyanin composition and content in rye plants with different grain color. Molecules 2018, 23, 948. [Google Scholar] [CrossRef]
- Luciano, Á.J.; Irineo, T.P.; Virginia, O.V.R.; Feregrino-Pérez, A.A.; Hernández, A.C.; Gerardo, G.G.R. Integrating plant nutrients and elicitors for production of secondary metabolites, sustainable crop production and human health: A review. Int. J. Agric. Biol. 2017, 19, 391–402. [Google Scholar] [CrossRef]
- Babalar, M.; Mumivand, H.; Hadian, J.; Tabatabaei, S.M.F. Effects of Nitrogen and Calcium Carbonate on Growth, Rosmarinic Acid Content and Yield of Satureja hortensis L. J. Agric. Sci. 2010, 2, 92–98. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of kacip fatimah (Labisia pumila blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef]
- Han, B.; Gao, J.; Han, X.; Deng, H.; Wu, T.; Li, C.; Jiang, B.; You, Y. Hanseniaspora uvarum FS35 degrade putrescine in wine through the direct oxidative deamination pathway of copper amine oxidase 1. Food Res. Int. 2022, 162, 111923. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in plant growth and development: Roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Stanius, Ž. Effect of External and Internal Factors on Secondary Metabolites Accumulation in St. John’s Worth. Bot. Lith. 2013, 18, 101–108. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive Oxygen Species Function as Signaling Molecules in Controlling Plant Development and Hormonal Responses. Curr. Opin. Plant Biol. 2022, 69, 102293. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Tada, Y.; Loake, G.J. Post-Translational Protein Modification as a Tool for Transcription Reprogramming. New Phytol. 2010, 186, 333–339. [Google Scholar] [CrossRef]
- Shayeghan, M.; Ansari, A.M.; Forouzesh, F.; Javidi, M.A. Reactive Oxygen Species, the Trident of Neptune in the Hands of Hecate; Role in Different Diseases, Signaling Pathways, and Detection Methods. Arch. Biochem. Biophys. 2022, 728, 109357. [Google Scholar] [CrossRef]
- Tanou, G.; Molassiotis, A.; Diamantidis, G. Hydrogen Peroxide- and Nitric Oxide-Induced Systemic Antioxidant Prime-Like Activity under NaCl-Stress and Stress-Free Conditions in Citrus Plants. J. Plant Physiol. 2009, 166, 1904–1913. [Google Scholar] [CrossRef]
- Joshi, H.; Bisht, N.; Mishra, S.K.; Prasad, V.; Chauhan, P.S. Bacillus amyloliquefaciens Modulate Carbohydrate Metabolism in Rice-PGPR Cross-Talk Under Abiotic Stress and Phytohormone Treatments. J. Plant Growth Regul. 2023, 42, 4466–4483. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from Less: Plant Growth under Limited Water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Dutta, D.; Mondal, S.; Karmakar, S. Phytohormones and Nitric Oxide Cross-Talk in Regulation of Stress Tolerance in Plants. In Nitric Oxide in Plants: A Molecule with Dual Roles; Springer: Singapore, 2022; pp. 179–200. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Argueso, C.T. No Hormone to Rule Them All: Interactions of Plant Hormones during the Responses of Plants to Pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Poór, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2022, 41, 675–698. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular Phosphorylation Signaling and Gene Expression in Drought Stress Responses: Aba-Dependent and Aba-Independent Regulatory Systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 Are Master Transcription Factors That Cooperatively Regulate ABRE-Dependent ABA Signaling Involved in Drought Stress Tolerance and Require ABA for Full Activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Costa, M.C.D.; Artur, M.A.S.; Maia, J.; Jonkheer, E.; Derks, M.F.L.; Nijveen, H.; Williams, B.; Mundree, S.G.; Jiménez-Gómez, J.M.; Hesselink, T.; et al. A Footprint of Desiccation Tolerance in the Genome of Xerophyta viscosa. Nat. Plants 2017, 3, 17038. [Google Scholar] [CrossRef]
- Malaga, S.; Janeczko, A.; Janowiak, F.; Waligórski, P.; Oklestkova, J.; Dubas, E.; Biesaga-Koscielniak, J.; Kuta, E.; Kościelniak, J.; Żur, I. Involvement of homocastasterone, salicylic and abscisic acids in the regulation of drought and freezing tolerance in doubled haploid lines of winter barley. Plant Growth Regul. 2020, 90, 173–188. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Ahmad, M.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kaçar, Y.A. An Overview and Recent Progress of Plant Growth Regulators (PGRs) in the Mitigation of Abiotic Stresses in Fruits: A Review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Rezayian, M.; Mousavi, S.H. Drought stress: Involvement of Plant Hormones in Perception, Signaling, and Response. In Plant Hormones and Climate Change; Springer: Singapore, 2023; pp. 227–250. [Google Scholar]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Azhar, B.J.; Zulfiqar, A.; Shakeel, S.N.; Schaller, G.E. Amplification and adaptation in the ethylene signaling pathway. Small Methods 2020, 4, 1900452. [Google Scholar] [CrossRef]
- Gao, Z.; Wen, C.K.; Binder, B.M.; Chen, Y.F.; Chang, J.; Chiang, Y.H.; Kerris, R.J.; Chang, C.; Schaller, G.E. Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J. Biol. Chem. 2008, 283, 23801–23810. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Miao, Y.; Liu, Y.; Botella, J.R.; Li, W.; Li, K.; Song, C.P. Function of Protein Kinases in Leaf Senescence of Plants. Front. Plant Sci. 2022, 13, 864215. [Google Scholar] [CrossRef]
- Kendrick, M.D.; Chang, C. Ethylene signaling: New levels of complexity and regulation. Curr. Opin. Plant Biol. 2008, 11, 479–485. [Google Scholar] [CrossRef]
- Owen, S.J.; Lafond, M.D.; Bowen, P.; Bogdanoff, C.; Usher, K.; Abrams, S.R. Profiles of abscisic acid and its catabolites in developing Merlot grape (Vitis vinifera) berries. Am. J. Enol. Vitic. 2009, 60, 277–284. [Google Scholar] [CrossRef]
- Lecourieux, F.; Lecourieux, D.; Vignault, C.; Delrot, S. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in grapevine cells. Plant Physiol. 2010, 152, 1096–1106. [Google Scholar] [CrossRef]
- Zirngibl, M.E.; Araguirang, G.E.; Kitashova, A.; Jahnke, K.; Rolka, T.; Kühn, C.; Sorgatz, A.; Friedrichs, S.; Müller, M.; Höhne, M.; et al. Triose Phosphate Export from Chloroplasts and Cellular Sugar Content Regulate Anthocyanin Biosynthesis during High Light Acclimation. Plant Commun. 2023, 4, 100423. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, X.; Crosley, R.A.; Greenwalt, S.A.; Sun, Y.; Blakeslee, B.; Wang, L.; Ni, W.; Sopko, M.S.; Yao, C.; et al. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010, 153, 99–113. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Cardoso, J.C.; de Oliveira, M.E.B.S.; de Cardoso, F.C.I. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Sutini, W.; Widiwurjani; Ardianto, C.; Khotib, J.; Purwanto, D.A.; Muslihatin, W. Production of the secondary metabolite catechin by in vitro cultures of Camellia sinensis L. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–7. [Google Scholar] [CrossRef]
- Aljabari, Z.; Alzeer, J.; Arafeh, R. Catechin detection in callus and in vitro cultures of the Eastern strawberry tree, Arbutus andrachne L., an endangered medicinal tree in Palestine. Glob. J. Res. Med. Plants Indig. Med. (GJRMI) 2014, 3, 196–205. [Google Scholar]
- Goel, M.K.; Kukreja, A.K.; Singh, A.K.; Khanuja, S.P.S. In vitro plant growth promoting activity of phyllocladane diterpenoids isolated from Callicarpa macrophylla Vahl. in shoot cultures of Rauwolfia serpentina. Nat. Prod. Commun. 2007, 2, 799–802. [Google Scholar] [CrossRef]
- Nhan, N.H.; Loc, N.H. Production of eurycomanone from cell suspension culture of Eurycoma longifolia. Pharm. Biol. 2017, 55, 2234–2239. [Google Scholar] [CrossRef]
- Manuhara, Y.S.W.; Kristanti, A.N.; Utami, E.S.W.; Yachya, A. Effect of sucrose and potassium nitrate on biomass and saponin content of Talinum paniculatum Gaertn. hairy root in balloon-type bubble bioreactor. Asian Pac. J. Trop. Biomed. 2015, 5, 1027–1032. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Maria John, K.M.; Yang, Y.S.; Kim, S.H.; Chung, I.M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell. Tissue Organ Cult. 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Lee, E.J.; Paek, K.Y. Enhanced productivity of biomass and bioactive compounds through bioreactor cultures of Eleutherococcus koreanum Nakai adventitious roots affected by medium salt strength. Ind. Crops Prod. 2012, 36, 460–465. [Google Scholar] [CrossRef]
- Lulu, T.; Park, S.Y.; Ibrahim, R.; Paek, K.Y. Production of biomass and bioactive compounds from adventitious roots by optimization of culturing conditions of Eurycoma longifolia in balloon-type bubble bioreactor system. J. Biosci. Bioeng. 2015, 119, 712–717. [Google Scholar] [CrossRef]
- Wu, S.Q.; Lian, M.L.; Gao, R.; Park, S.Y.; Piao, X.C. Bioreactor application on adventitious root culture of Astragalus membranaceus. Vitr. Cell. Dev. Biol. Plant 2011, 47, 719–724. [Google Scholar] [CrossRef]
- Qian, J.; Guiping, L.; Xiujun, L.; Xincai, H.; Hongmei, L. Influence of growth regulators and sucrose concentrations on growth and rosmarinic acid production in calli and suspension cultures of Coleus blumei. Nat. Prod. Res. 2009, 23, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Abyari, M.; Nasr, N.; Soorni, J.; Sadhu, D. Enhanced Accumulation of Scopoletin in Cell Suspension Culture of Spilanthes acmella Murr. Using Precursor Feeding. Braz. Arch. Biol. Technol. 2016, 59, 1–7. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Ullah, N.; Khan, R.S. Impacts of Hormonal Elicitors and Photoperiod Regimes on Elicitation of Bioactive Secondary Volatiles in Cell Cultures of Ajuga bracteosa. J. Photochem. Photobiol. B Biol. 2018, 183, 242–250. [Google Scholar] [CrossRef]
- Khan, T.; Abbasi, B.H.; Khan, M.A.; Shinwari, Z.K. Differential Effects of Thidiazuron on Production of Anticancer Phenolic Compounds in Callus Cultures of Fagonia indica. Appl. Biochem. Biotechnol. 2016, 179, 46–58. [Google Scholar] [CrossRef]
- Olgunsoy, P.; Ulusoy, S.; Çelikkol Akçay, U. Metabolite Production and Antibacterial Activities of Callus Cultures from Rosa damascena Mill. Petals. Marmara Pharm. J. 2017, 21, 590. [Google Scholar] [CrossRef]
- Bassolino, L.; Giacomelli, E.; Giovanelli, S.; Pistelli, L.; Cassetti, A.; Damonte, G.; Bisio, A.; Ruffoni, B. Tissue Culture and Aromatic Profile in Salvia dolomitica Codd. Plant Cell. Tissue Organ Cult. 2015, 121, 83–95. [Google Scholar] [CrossRef]
- Rahpeyma, S.A.; Moieni, A.; Jalali Javaran, M. Paclitaxel Production Is Enhanced in Suspension-Cultured Hazel (Corylus avellana L.) Cells by Using a Combination of Sugar, Precursor, and Elicitor. Eng. Life Sci. 2015, 15, 234–242. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, W.; Zahir, A.; Hano, C.; Abbasi, B.H. Salicylic Acid-Enhanced Biosynthesis of Pharmacologically Important Lignans and Neo Lignans in Cell Suspension Culture of Linum ussitatsimum L. Eng. Life Sci. 2019, 19, 168–174. [Google Scholar] [CrossRef]
- Park, C.H.; Zhao, S.; Yeo, H.J.; Park, Y.E.; Baska, T.B.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Comparison of Different Strains of Agrobacterium rhizogenes for Hairy Root Induction and Betulin and Betulinic Acid Production in Morus alba. Nat. Prod. Commun. 2017, 12, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Shilpha, J.; Satish, L.; Kavikkuil, M.; Joe Virgin Largia, M.; Ramesh, M. Methyl Jasmonate Elicits the Solasodine Production and Antioxidant Activity in Hairy Root Cultures of Solanum trilobatum L. Ind. Crops Prod. 2015, 71, 54–64. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of Methyl Jasmonate and Salicylic Acid on Tanshinone Production and Biosynthetic Gene Expression in Transgenic Salvia miltiorrhiza Hairy Roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver Nanoparticles Elicited in Vitro Callus Cultures for Accumulation of Biomass and Secondary Metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of Light Quality on Growth, Secondary Metabolites Production and Antioxidant Activity in Callus Culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018, 183, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bharadvaja, N. Establishment of Root Suspension Culture of Plumbago zeylanica and Enhanced Production of Plumbagin. Ind. Crops Prod. 2019, 137, 419–427. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Zabiegala, B.; Kubica, P.; Szopa, A.; Bucinski, A.; Ekiert, H.; Luczkiewicz, M. Accumulation of Volatile Constituents in Agar and Bioreactor Shoot Cultures of Verbena officinalis L. Plant Cell. Tissue Organ Cult. 2021, 144, 671–679. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of Salicylic Acid and Methyl Jasmonate in the Production of Phenolic Compounds in Plant Cell Suspension Cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef]
- Krishnan, S.R.S.; Siril, E.A. Elicitor Mediated Adventitious Root Culture for the Large-Scale Production of Anthraquinones from Oldenlandia umbellata L. Ind. Crops Prod. 2018, 114, 173–179. [Google Scholar] [CrossRef]
- Keskin, N.; Kunter, B. The Effects of Callus Age, UV Irradiation and Incubation Time on Trans-Resveratrol Production in Grapevine Callus Culture. Tarim Bilim. Derg. 2009, 15, 9–13. [Google Scholar] [CrossRef][Green Version]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Biotechnology, In Vitro Production of Natural Bioactive Compounds, Herbal Preparation, and Disease Management (Treatment and Prevention). In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2; Springer: Berlin/Heidelberg, Germany, 2018; pp. 585–664. [Google Scholar] [CrossRef]
- Mastan, A.; Bharadwaj, R.; Kushwaha, R.K.; Vivek Babu, C.S. Functional Fungal Endophytes in Coleus forskohlii Regulate Labdane Diterpene Biosynthesis for Elevated Forskolin Accumulation in Roots. Microb. Ecol. 2019, 78, 914–926. [Google Scholar] [CrossRef]
- Veerashree, V.; Anuradha, C.M.; Kumar, V. Elicitor-Enhanced Production of Gymnemic Acid in Cell Suspension Cultures of Gymnema sylvestre R. Br. Plant Cell. Tissue Organ Cult. 2012, 108, 27–35. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, T.; Ali, H. Plant Cell Culture Strategies for the Production of Terpenes as Green Solvents. Ind. Appl. Green Solvents 2019, 50, 1–20. [Google Scholar]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Karigar, C.S.; Kim, S.W.; Goo, G.H.; Choi, M.S. Effect of Biotic Elicitors on the Accumulation of Bilobalide and Ginkgolides in Ginkgo biloba Cell Cultures. J. Biotechnol. 2009, 139, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gutierrez, J.; Pedreño, M.A.; Sottomayor, M. Synergistic and Additive Influence of Cyclodextrins and Methyl Jasmonate on the Expression of the Terpenoid Indole Alkaloid Pathway Genes and Metabolites in Catharanthus roseus Cell Cultures. Plant Cell. Tissue Organ Cult. 2014, 119, 543–551. [Google Scholar] [CrossRef]
- Shilpha, J.; Largia, M.J.V.; Kumar, R.R.; Satish, L.; Swamy, M.K.; Ramesh, M. Hairy Root Cultures: A Novel Way to Mass Produce Plant Secondary Metabolites. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer Nature: Singapore, 2023; pp. 417–445. [Google Scholar] [CrossRef]
- Palazón, J.; Navarro-Ocaña, A.; Hernandez-Vazquez, L.; Mirjalili, M.H. Application of Metabolic Engineering to the Production of Scopolamine. Molecules 2008, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, Y.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. Soybean Hairy Roots Produced in Vitro by Agrobacterium rhizogenes-Mediated Transformation. Crop J. 2018, 6, 162–171. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Chen, S.T.; Agnolet, S.; Hegelund, J.N.; Stanstrup, J.; Christensen, J.H.; Müller, R.; Lütken, H. Ethephon-Induced Changes in Antioxidants and Phenolic Compounds in Anthocyanin-Producing Black Carrot Hairy Root Cultures. J. Exp. Bot. 2020, 71, 7030–7045. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A. Hairy roots: An untapped potential for production of plant products. Front. Plant Sci. 2022, 13, 2808. [Google Scholar] [CrossRef]
- Li, M.; Peebles, C.A.M.; Shanks, J.V.; San, K.Y. Effect of Sodium Nitroprusside on Growth and Terpenoid Indole Alkaloid Production in Catharanthus roseus Hairy Root Cultures. Biotechnol. Prog. 2011, 27, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Gai, Q.Y.; Wang, W.; Zang, Y.P.; Niu, L.L.; Fu, Y.J.; Wang, X. Remarkable Enhancement of Flavonoid Production in a Co-Cultivation System of Isatis tinctoria L. Hairy Root Cultures and Immobilized Aspergillus niger. Ind. Crops Prod. 2018, 112, 252–261, Erratum in Ind. Crops Prod. 2019, 138, 111434. [Google Scholar] [CrossRef] [PubMed]

| Plant Species | Abiotic Stress | Secondary Metabolite and Nature of Response | Reference |
|---|---|---|---|
| Ocimum basilicum (common basil) | AgNO3 | The highest values of linalool and estragole compared with the control culture were obtained as 4.37 μg/g DW (25 μM treatment) and 3.30 μg/g DW (5 μM treatment), respectively. | [43] |
| Chrysanthemum morifoilum (garden mum) | Water | Anthocyanin content was observed to show an increasing trend by 1.71 and 3.5 times on days 7 and 5 of the stress period, respectively. | [44] |
| Olea europaea (common olive) cultivars | NaCl | Under 60 and 120 mM NaCl, the content of kaempferol in old leaves of Frantoio increased by three times (25.1 and 27.4 g g−1 FW, respectively). However, in Leccino, kaempferol remains unchanged at 60 mM NaCl and reduced 16 times (0.5 g g−1 FW) at 120 mM NaCl, when compared to control. Similarly, quercetin did not change up to 60 mM NaCl in both cultivars, while at the highest NaCl treatment (120 mM), it slightly increased in Frantoio. | [45] |
| Lycopersicon esculentum (garden tomato) | Pb | The treatment of Pb enhances the production of phenol, flavonoid, and anthocyanin content by 79.25%, 47.73%, and 58.25%, respectively, at 0.75 mM concentration. | [46] |
| Artemisia annua L. (sweet sagewort) | UV-B | UV-B-treated plants observed significant induction of 92 and 100% in total phenolic and flavonoid content after 3 h, respectively. However, artemisinin observed concentration up to 100%. | [47] |
| Citrus genotypes | Drought and heat stress | In Carrizo, impact of heat stress on secondary metabolite composition was 26.3%, whereas water stress had a lower contribution 18.8%. In Cleopatra, however, both heat and water stress influenced the secondary metabolite accumulation (21.1%), although the stress combination had a stronger contribution (26.2%). | [48] |
| Genista tinctoria (dyer’s greenweed) | UV radiation | The highest genistin content (3.03%) was demonstrated after 300 s of UV 254 nm treatment, followed by 2.06% accumulation after 120 s of UV 366 nm radiation. Similarly, 0.16–0.17% genistein content was observed after UV 254 nm treatment. | [49] |
| Mentha pulegium L. (pennyroyal) | Drought | The moderate and severe drought stress treatments increased TPC (17.2 and 30.3%, respectively) and TFC (35.9 and 33.7%, respectively). | [50] |
| Salvia dolomitica Codd (dolomite sage) | Drought | These total phenols and flavonoids were substantially reduced by moderate and severe drought stress compared with control (305.2, 53.2, and 20.5 mg GAE g−1, 105.7, 17.1, and 5.3 mg g−1, respectively). | [51] |
| Brassica oleracea L. convar. acephala (DC) Alef, var. sabellica L. (cabbage) | Drought | The content of proline and phytol in drought-stressed plants when compared to well-watered plants showed an increased trend of more than 22%, with values of 7.56 to 22.7 mg/plant for proline, respectively, and 22.1 to 35.6 mg/plant for phytol, respectively. | [52] |
| Cotton genotypes | Drought and Salinity | In Zhongmian 23, TPC increased significantly under drought and/or salinity compared to their controls, while under salinity, it remained unaffected at 10% SMC. However, in Zhongmian 41, TPC was significantly increased under D + S relative to control and remained unchanged under drought, but it was significantly decreased under salinity when compared to control. Both drought and/or salinity stress increased TFC content. However, under salinity, it decreased in Zhongmian 23 and increased in Zhongmian 41 at 10% SMC, while at 4% SMC it remained unaffected compared to control. | [53] |
| Carthamus tinctorius L. (safflower) | Salinity | Significant increase of 34% in TPC, 13% in TFC, and 12% in TFL was observed in salinity-stressed plants when compared to nonstressed plants. | [54] |
| Solanum nigrum L. (black nightshade) | NaCl | Significant increases were observed in both lutein and β-carotene at 100 mM NaCl, while at 50 and 150 mM NaCl, both compounds showed reduced accumulation. Quercetin levels increased 2.6-fold compared to quercetin 3-β-D-glucoside at 0 and 50 mM NaCl treatments, whereas they were the same for the 100 mM treatment and 2-fold lower at 150 mM NaCl treatment. | [55] |
| Camellia japonica cultivars (Japanese camellia) | Temp. | In ‘Jiangxue’, the content of palmitic acid, stearic acid, and oleic acid gradually decreased during cold treament, while α-linolenic acid increased significantly. Similar tendencies were found in ‘Desire’ and ‘Nuccio’s Bella Rossa’, but the changes were not as significant as in ‘Jiangxue’. | [56] |
| Musa spp. (banana) | Temp. | The TPC of Simili radjah increased 2.9-fold, from 6.3 (26 °C) to 18.5 ng/g GAE (20 °C), whereas it increased 4.8-fold from 8.6 (26 °C) to 41.6 ng/g GAE (20 °C) for Dole. | [57] |
| Scutellaria lateriflora (mad dog skullcap) | Light | The accumulation of baicalin was promoted by blue light, 0.96–2.00 times higher than under white light. Flavonoids showed 0.93–1.54 times accumulation in blue light than under white light. However, 3,4-dihydroxyphenylacetic acid observed the highest concentration of (33.56 mg 100 g−1 DW) in the presence of white light. | [58] |
| Silybum marianum L. (milk thistle) | Light | Under red light, silymarin content (18.67 mg/g DW) was almost double compared to control (9.17 mg/g DW). Conversely, taxifolin accumulation (0.480 mg/g DW) was found to be maximum under continuous white light, which was almost eightfold higher than control (0.063 mg/g DW). | [59] |
| Rhododendron tomentosum (marsh Labrador tea) | Cu and Ni | About a twofold increase in pcymene and sabinene was observed in Cu- and Ni-treated plants compared with control. Pcymene and sabinene contents were about 23.0% and 17.7% vs. 6.9% (control), respectively; and 5.4% and 3.0% vs. 1.7% (control), respectively. On the other hand, δ-cadinene was decreased by 1.0% and 1.1% vs. 4.1% (control), respectively, in the total level of sesquiterpene hydrocarbons. | [60] |
| Corylus avellana L. (common hazel) | Al | Extracellular taxol content showed an upward trend at 100 µM of Al treatment and was 38-fold higher than that of the control medium. However, cell-associated taxol content at 50 and 100 µM of Al concentration was enhanced by 11.4- and 8.3-fold, respectively, compared to control cells. | [61] |
| Linum usitatissimum L. (flax) | UV + photoperiod | Under UV + dark and UV + photoperiod, 1.12- and 2.82-fold enhancement in TPP was noted, respectively, in response to 3.6 kJ/m2 of UV-C radiations. However, at similar conditions, TFP showed 1.42- and 2.94-fold enhancement compared to their respective controls. | [62] |
| Duboisia Species (corkwood) | Light and temperature | Scopolamineproduction showed a negative trend by increased light intensity up to 350 μmol/m2 × s, light exposure up to 24 h/d, and temperature (28 °C). | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. https://doi.org/10.3390/metabo13080895
Reshi ZA, Ahmad W, Lukatkin AS, Javed SB. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites. 2023; 13(8):895. https://doi.org/10.3390/metabo13080895
Chicago/Turabian StyleReshi, Zubair Altaf, Waquar Ahmad, Alexander S. Lukatkin, and Saad Bin Javed. 2023. "From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches" Metabolites 13, no. 8: 895. https://doi.org/10.3390/metabo13080895
APA StyleReshi, Z. A., Ahmad, W., Lukatkin, A. S., & Javed, S. B. (2023). From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites, 13(8), 895. https://doi.org/10.3390/metabo13080895







