Abstract
Secondary metabolites are gaining an increasing importance in various industries, such as pharmaceuticals, dyes, and food, as is the need for reliable and efficient methods of procuring these compounds. To develop sustainable and cost-effective approaches, a comprehensive understanding of the biosynthetic pathways and the factors influencing secondary metabolite production is essential. These compounds are a unique type of natural product which recognizes the oxidative damage caused by stresses, thereby activating the defence mechanism in plants. Various methods have been developed to enhance the production of secondary metabolites in plants. The elicitor-induced in vitro culture technique is considered an efficient tool for studying and improving the production of secondary metabolites in plants. In the present review, we have documented various biosynthetic pathways and the role of secondary metabolites under diverse environmental stresses. Furthermore, a practical strategy for obtaining consistent and abundant secondary metabolite production via various elicitation agents used in culturing techniques is also mentioned. By elucidating the intricate interplay of regulatory factors, this review paves the way for future advancements in sustainable and efficient production methods for high-value secondary metabolites.
1. Introduction
Plant metabolites, both primary and secondary, play crucial roles in the growth and survival of plant species [1]. Primary metabolites, such as lipids, proteins, carbohydrates, amino acids, and vitamins, directly contribute to essential cellular processes like cell division, respiration, and photosynthesis, crucial for plant growth and development [2]. In contrast, secondary metabolites have multifunctional roles, primarily involved in defence and interactions with the environment [3]. Additionally, they contribute to plant colour, specific fragrances, flavours, and responses to various stresses. The concept of plant secondary metabolites in plant biology was introduced by Kossel et al. [4]. Secondary metabolites are highly reactive, and their accumulation is influenced by both biotic and abiotic stress conditions, which can have detrimental effects on physiological and morphological characteristics like leaf number, leaf area, plant height, and productivity [1].
Secondary metabolites play a crucial role in helping plants cope with different stress conditions. The response is initiated through the activation of plant defence mechanisms, triggered by the recognition of foreign agents through sensors and receptors in plants [5]. Moreover, plant survival and productivity rely on the expression of defensive transcriptional factors [3]. Detection of threat signals and increased production of secondary metabolites through elicitation contribute to the downstream expression of transcriptional factors [3]. For example, in response to abiotic stress, the expression of β-lycopene cyclase in Bixa orellana and phytoene synthase in Daucus carota is elevated, leading to the accumulation of carotenoids [5,6]. The production of secondary metabolites is highly dependent on the developmental stages and physiological conditions of the plant. In various interactions, such as mutualism observed in root nodules of legumes, or antagonistic relationships, such as pathogenicity and herbivory, secondary metabolites play a pivotal role by exerting irreversible effects [7].
Teoh [8] classified plant secondary metabolites into various groups based on functional groups and chemical structure. These groups include terpenes (including volatile compounds, sterols, and carotenoids), polysaccharides, phenolic compounds, phytoalexins (sulfur-containing compounds), alkaloids (nitrogen-containing compounds), flavonoids, and hydrocarbons. Almost all of these metabolites contribute significantly to defence against stressful situations. Plant hormones, such as abscisic acid (ABA), jasmonates (JA), polyamines, and salicylic acid (SA), are also involved in responding to environmental stresses, and their accumulation often results from various biotic and abiotic stresses and the response to elicitors and other signalling molecules [9].
The low-molecular-weight secondary metabolites have garnered significant interest among researchers due to their dramatic implications for pharmaceutical, nutritional, and industrial purposes. Recent advancements in the research of secondary metabolites have focused on finding a reliable source for production and extractions of important secondary metabolites for industrial use [10].
In vitro culture-based elicitation mechanisms are considered advantageous for the production of secondary metabolites as they offer independence from environmental conditions and reduced the risk of microbial contamination. In vitro micropropagation techniques are efficient for mass-producing secondary metabolites applicable to various industrial and pharmaceutical companies. Other natural products derived from plants, including steroids, codeine, morphine, pilocarpine, digitoxin, and quinine, are used in various pharmaceutical products [8].
Environmental and developmental factors influence the synthesis and accumulation of secondary metabolites in plants greatly. Therefore, this review aims to provide a comprehensive summary of how various environmental conditions influence the synthesis and accumulation of secondary metabolites. The qualitative and quantitative aspects of the environment can serve as tools to improve the accumulation of secondary metabolites in plants by modifying their growing conditions as well as the use of in vitro culture techniques for sustainable production and extraction of these secondary metabolites for industrial use.
2. Biosynthesis of Secondary Metabolites in Plants
Secondary metabolites in plants are categorized into distinct chemical groups based on their biosynthetic pathways: phenolic compounds, terpenes and steroids, and nitrogenous compounds. These chemical structures determine the function and stress adaptation of secondary metabolites. Various stresses such as drought, pathogenesis, herbicides, salinity, and heavy metals promote the accumulation of secondary metabolites [11].
Plants develop numerous adaptive strategies to overcome harsh conditions by upregulating the synthesis and accumulation of secondary metabolites. The production levels of these metabolites are greatly influenced by factors such as growing temperature and environmental constraints [12]. Under suitable conditions, more than 100,000 secondary metabolites are synthesized through various metabolic pathways. The interrelation between the synthesis of primary and secondary metabolites is fundamental for most plants.
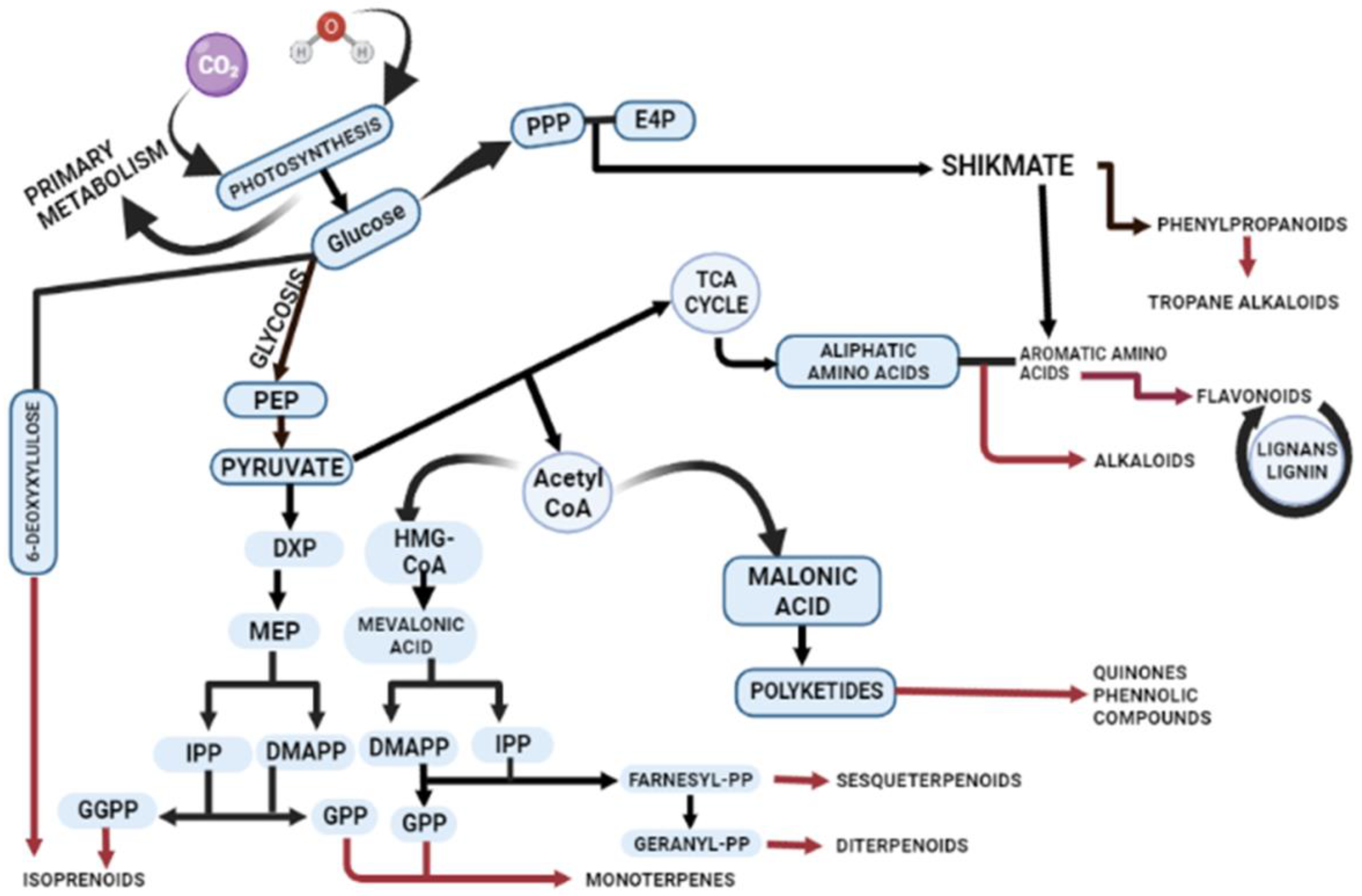
Primary and secondary metabolites are distinct in their distribution, chemical structure, and functional roles in plants. Figure 1 illustrates the production of secondary metabolites and their interconnections with primary metabolism within the plant cell. The alternative mechanisms involved in the biosynthesis of secondary metabolites lead to common products, such as phenols, flavonoids, and terpenes (Figure 1). However, the critical precursors for secondary metabolites are primary metabolites. The shikimic acid pathway and Krebs cycle produce essential precursors required for the production of phenolic metabolites [13]. The aromatic molecules synthesized by the shikimic acid pathway play featured roles in electron transport, antioxidants, wound response, structural agents, and defence systems [14]. The aromatic amino acids L-tryptophan (L-Trp), L-tyrosine (L-Tyr), and L-phenylalanine (L-Phe) serve as precursors for secondary metabolite synthesis produced by the shikimate pathway [15]. Chorismate is the final product in the seven-step pathway of shikimic acid, and it serves as the starting material for the biosynthesis of secondary metabolites. Chorismate mutase, aminodeoxychorismate synthase, and isochorismate synthase play regulatory roles in higher plants for chorismate, which is also the precursor of folate, phenylalanine, phylloquinone, and tryptophan [16]. In fungi, the AROM complex undergoes catalysis by enzymes, such as 3-dehydroquinate dehydratase (DHD) and shikimate dehydrogenase (SDH), which facilitate the third and fourth reactions within the shikimate pathway. In plants, SDH and DHD act bifunctionally, while they have single functions in Escherichia coli.
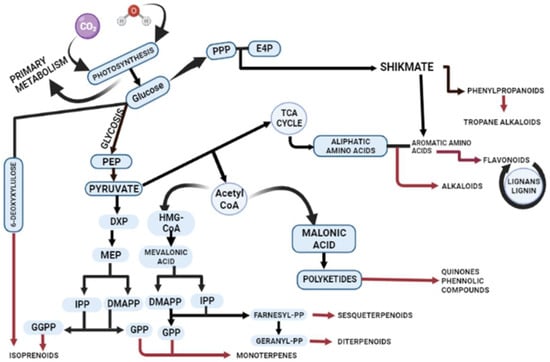
Figure 1.
Schematic illustration of biosynthetic pathways for secondary metabolite production. The figure demonstrates the intricate biosynthesis of secondary metabolites and their interconnections with primary metabolism within plants. Plant cells employ diverse mechanisms through major pathways including mevalonic acid (MVA) and the 2-C-methylerythritol 4-phosphate (MEP) and shikmate pathway to synthesize terpenes, phenols, flavonoids, and alkaloids. Abbreviations: phosphoenolpyruvate (PEP); 1-deoxy-d-xylulose 5-phosphate (DXP); 4-hydroxy-3-methylbut-2-enyl diphosphate (HMBPP); isopentenyl pyrophosphate (IPP); dimethylallyl pyrophosphate (DMAPP); geranyl pyrophosphate (GPP); 2-C-methyl-l-erythritol 4-phosphate (MEP); 1-deoxy-D-xylulose 5-phosphate (DXP); acetyl coenzyme A (Acetyl-CoA); β-hydroxy β-methylglutaryl-CoA (HMG-CoA); geranylgeranyl diphosphate (GGPP); erythrose 4-phosphate (E4P); and pentose phosphate pathway (PPP).
The synthesis of terpenes involves two other pathways: the MEP (2-C-methylerythritol 4-phosphate) and MVA (mevalonic acid) pathways, occurring in plastids and the cytosol, respectively. The MEP pathway is responsible for synthesizing isoprene, plastoquinone, terpenes, and phytol [17], while the MVA pathway is involved in the synthesis of triterpenes, sterols, and sesquiterpenes [18].
In the MVA pathway, mevalonate is formed from acetoacetyl-CoA through the enzymatic activity of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) [19,20]. Subsequently, mevalonate undergoes phosphorylation and decarboxylation to yield sterol and nonsterol isoprenoids. Phosphomevalonate kinase (PMK) catalyzes the phosphorylation of mevalonate, resulting in the formation of mevalonate-5-pyrophosphate [21,22]. The final step involves an ATP-dependent decarboxylation reaction, wherein mevalonate-5-pyrophosphate is converted to IPP by the enzymatic action of phosphomevalonate decarboxylase [23]. Terpenes are synthesized in distinct compartments of the cell through the action of terpene synthases [24]. Fungi and bacteria execute the malonic acid pathway for the synthesis of phenolic compounds [24]. Essential products of glycolysis, such as glyceraldehyde-3-phosphate and pyruvate, are required for the production of dimethylallyl diphosphate (DMADP) and pyrophosphate (IPP). DMADP and IPP serve as universal building blocks for all isoprenoids in numerous cellular compartments [18].
Amino acids such as lysine, tyrosine, and tryptophan act as precursors for nitrogenous secondary metabolites. Enzymes like chalcone synthase (CHS) and phenylalanine ammonia-lyase (PAL) regulate the phenolic content under different stress conditions. For example, plants respond to salt stress and UV rays by producing alkaloids through the regulation of hyoscyamine 6β-hydroxylase and tryptophan decarboxylase, respectively. Flavonoids are specifically synthesized through the phenylpropanoid pathway, where phenylalanine is transformed into 4-coumaroyl-CoA, which enters the biosynthetic pathway. Various biosynthetic enzymes, including reductases, hydroxylases, and Fe(II)/2OG-dependent oxygenases, catalyze oxidative transformations, leading to different flavonoid subclasses [25]. Flavonoids all originate from chalcone scaffolds produced by chalcone synthase, and transferases modify the flavonoid backbone, altering the physiological activity of the resultant flavonoid [26]. The phenylpropanoid pathway also plays a significant role in activating the antioxidant system in plants by eliciting phenolic compounds like vanillic acid, caffeic acid, cinnamyl acid, and gallic acid [27].
The biosynthesis of secondary metabolites in plants involves intricate pathways, connecting both primary and secondary metabolism. These compounds play crucial roles in stress adaptation and various physiological functions, making them essential for plant survival and defence.
3. Secondary Metabolites as Regulators of Growth and Development
Secondary metabolites, such as glucosinolates and flavonoids, play crucial roles in regulating the growth and development of plants, as well as responding to various environmental conditions.
Glucosinolates are involved in different mechanisms that modulate plant growth and development. For example, aliphatic 3-hydroxypropylglucosinolate inhibits meristematic root growth in Arabidopsis and other plant species through the rapamycin pathway [28,29]. However, the exact mechanism by which 3-hydroxypropylglucosinolate functions is yet to be fully understood. Aliphatic glucosinolates in Vicia faba and Arabidopsis regulate stomatal closure through a peroxidase-mediated receptor kinase [30]. Additionally, the enhanced expression of the myrosinase gene TGG1 in guard cells plays an active role in stomatal regulation through various signalling pathways [31]. Indole-3-carbinol, an immediate product of indole glucosinolate, negatively affects Arabidopsis root growth when applied after exogenous wounding. This effect is due to the binding of indole-3-carbinol at an allosteric enzyme site, mediating the regulation between auxin and its receptor TIR1 [32].
Flavonoids, a diverse group of phenolic compounds, also have significant regulatory roles in plant growth and development under different environmental conditions. Changes in the flavonoid biosynthetic pathways influence various physiological, growth, and developmental processes [33]. A study by Maloney et al. [34] demonstrated the effect of flavonols on the developmental stages of pollen in tomatoes. The study compared two mutant tomato varieties with a defective F3H gene and a wild-type variety. The mutation in the F3H gene resulted in decreased flavonoid accumulation in plants. This reduction in flavonoid content was critically associated with impaired pollen tube growth and germination, as well as compromised pollen tube integrity. Flavonoids also play a role in reducing reactive oxygen species (ROS) levels and affecting developmental processes in guard cells, leaves, and roots [35,36]. Exogenous application of flavonoids has been shown to influence auxin transport [37]. In vitro studies on Arabidopsis demonstrated that flavonoids act as potential inhibitors of auxin transport [38]. The study of the transparent testa (tt4) chalcone synthase mutant of Arabidopsis, compared to the wild-type, showed phenotypic differences such as decreased plant height, reduced root growth, and altered auxin transport due to the presence of naringenin, a precursor of flavonoid biosynthesis [38].
4. Secondary Metabolite Production in Plants in Response to Different Environmental Factors
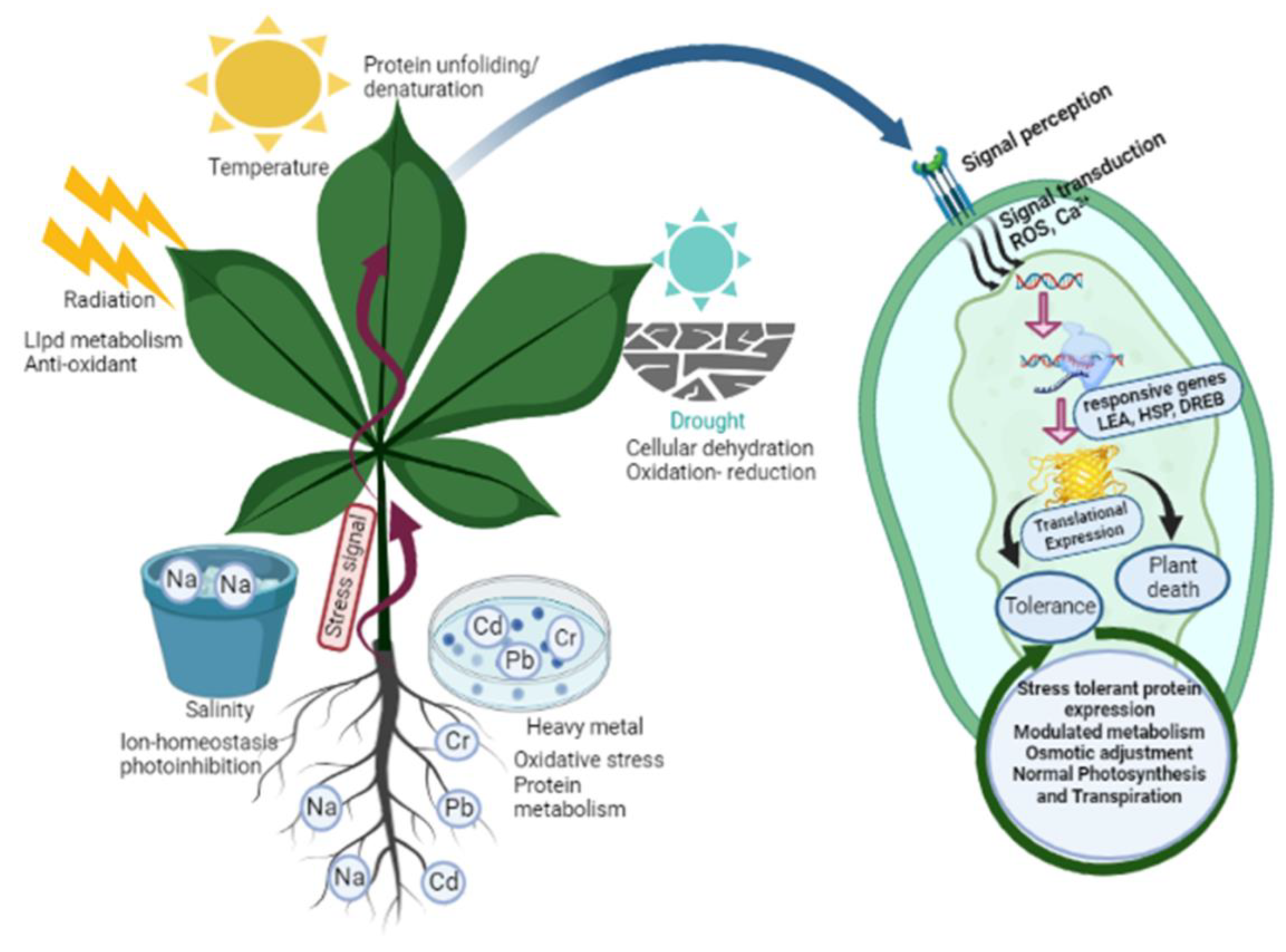
Plants, being sessile organisms, face potential threats from environmental or pathogenic stressors. These stresses can lead to osmotic imbalances, physiological and biochemical changes, and cellular dehydration, ultimately resulting in the death of the affected plant (Figure 2). To defend themselves, plants have evolved three different response mechanisms. Ephemeral desert plants respond by avoiding stress by regulating their life cycle, as the fragile plants lack effective mechanisms to survive stress, while resistant plants respond with efficient defensive mechanisms to counter various stresses. This defensive system is modulated by plants through alterations and modifications in membrane structure, cell cycle and division remodelling, changes in photosynthetic activity, conductance, and transpiration rates, which collectively affect growth, metabolic activity, and the physiology of metabolic compounds [39] (Figure 2). Signalling processes prompt primary metabolism, allowing for the accumulation of enhanced secondary metabolites that initiate a defensive mechanism against various environmental constraints [40] (Table 1). These secondary metabolites protect the plant against both biotic and abiotic stress conditions. Environmental stresses such as salinity, water availability, radiation, chemical exposure, and temperature fluctuations significantly impact the content of secondary metabolites. For instance, exposure to UV irradiation, herbicide treatment, and other environmental stresses often enhances the accumulation of phenylpropanoids [41]. The levels of magnesium, potassium, and iron can influence the increased accumulation of phenolic compounds in roots [41]. The antioxidant activity of polyamines and phenyl amides in tobacco and beans highlights the crucial role of secondary metabolites in coping with environmental constraints [42].
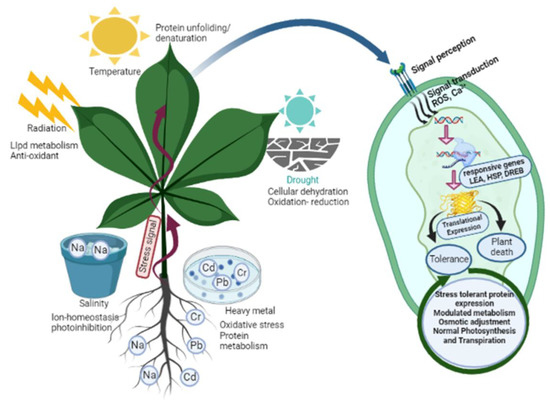
Figure 2.
The schematic presentation illustrates the diverse array of abiotic stresses, including drought, salinity, temperature, heavy metals, and radiation impacting plant growth and development. In response to these challenges, plants have evolved an array of remarkable adaptation mechanisms. These include the expression of stress-tolerant proteins, finely modulated metabolism, osmotic adjustment, stomatal closure, synthesis of vital secondary metabolites, activation of specific antioxidants, and meticulous maintenance of ionic balance. These strategies help plants confront and overcome environmental hardships. Abbreviations: late embryogenesis abundant (LEA); heat shock proteins(HSP); dehydration-responsive element binding (DREB); reactive oxygen species (ROS).

Table 1.
The effects of different environmental stresses on the content and accumulation of secondary metabolites in various plant species.
4.1. Salt Stress
Salt stress exerts a negative impact on plant growth and productivity [64]. Plants respond to salt stress by undergoing changes in their metabolic activity to counteract the toxicity induced by excessive salt levels [64]. Salinity often causes osmotic imbalance and ionic toxicity in plants, leading to membrane disruption and cellular dehydration in the cytoplasm [65]. These changes in ionic and osmotic components can result in either an increase or decrease in the accumulation of plant secondary metabolites. In vitro studies have revealed a decline in anthocyanin accumulation in salt-sensitive species [66], while the decrease in phenolic, anthocyanin, and flavonoid content under salt stress conditions was less pronounced in salt-tolerant clones [67]. Proline, which regulates stress signals and serves as an osmotic buffer, energy sink, and protector of membranes and proteins, shows increased accumulation in the roots of Alfalfa under salt stress [68]. Various studies have observed the accumulation of polyphenols and flavonoids under salinity stress in plants like Hordeum vulgare and Cakile maritma [69,70]. Transcription factors GmCAM4 and GmERFo57 play crucial roles in providing resistance to salinity stress in Glycine max [71]. Spinacia oleracea demonstrates resistance against saline soil by enhancing the production of 20-Hydroxyecdysone (20E) [72]. Furthermore, alkaloids such as vincristine and reserpine in Catharanthus roseus and Rauvolfia tetraphylla, respectively, increase under saline conditions [73].
4.2. Drought Stress
Drought severely impacts plants’ biochemical and physiological processes, causing disruptions in the electron transport chain and leading to the production of reactive oxygen species (ROS) like H2O2, OH−, and O2−. These ROS inflict oxidative damage on lipids, nucleic acids, and proteins, and also disrupt the photosynthetic mechanism of plants. Drought severity varies among plant species and is often accompanied by increased solar radiation and temperature [74]. The stress disturbs plant metabolism, reduces cell turgidity and signalling, and impairs energy storage, plasma membrane structure, and resource allocation [75].
To cope with drought stress, plants have developed response mechanisms that involve the accumulation of secondary metabolites such as phenolics, terpenes, and alkaloids. These secondary metabolites help regulate ionic balance and enzyme activity, repair oxidative damage, and maintain the connection between the phenotype and genotype of plants [76,77]. However, increased production of these metabolites can lead to reduced biomass in some plant species [78]. For instance, under drought stress, plants like Artemisia annua and Catharanthus roseus increase secondary metabolite production by several times [79]. Drought stress induces elevated expression of PAL genes, responsible for flavonoid synthesis, in roots of Scutellaria baicalensis [80]. In Chenopodium quinoa, the accumulation of saponin changes under different drought stress conditions [81]. Drought stress also affects the carotenoid and chlorophyll ratio in plants. Notably, Bellis perennis and Ophiorrhiza mungos show enhanced production of camptothecin, alkaloids, and phenolic compounds, respectively, under drought conditions [82,83].
Drought stress has varying effects on the concentrations of secondary metabolites in the roots and leaves of Bupleurum chinensis plants. For example, saikosaponin concentrations in the roots increase during vegetative and reproductive growth, while leaf rutin concentrations decrease significantly during these stages [84].
These studies underscore the intricate interplay between abiotic stresses and plant physiological responses, leading to diverse responses of secondary metabolites against drought stress.
4.3. Temperature Stress
Elevated temperatures lead to reduced development and early leaf senescence in plants. The impact of temperature on the production of secondary metabolites varies among different plant species [85,86]. Heat treatments can cause enzyme inactivation, and lipid and protein denaturation negatively affect membrane integrity. Under temperature stress, carotenoid and β-carotene accumulation in Brassicaceae are reduced [85]. Acidic pH combined with a high temperature in hairy root culture promotes the accumulation of flavonolignans in Silybum marianum [87]. Some plants, like Melastoma malabathricum, show increased anthocyanin and biomass production at lower temperatures compared to higher temperatures [88]. Daucus carota responds to heat shock by increasing terpene accumulation, while the production levels of α-terpinolene decrease at the same temperature. Short-term temperature treatment enhances isoprene production synthesized through the nonmevalonate pathway (MEP), which is believed to counteract the negative effects of heat shock [89,90]. Different temperature ranges can significantly affect the level of secondary metabolites in plants, as observed in Amaranthus cruentus [91]. Salicylic acid and phenolic content in flowering plants [92] and phenolic compounds in gymnosperms [93] were found to decrease with increasing temperature. In tea plants, the concentration of catechin levels increases when treated with higher temperatures [94].
4.4. Light, UV, and Ionization Radiation
Light plays a critical role in plant metabolism and the production of secondary metabolites. Stable light intensity regulates photosynthesis and dry matter accumulation. However, abnormal irradiation can lead to photodamage and negatively affect photosynthetic reaction centres, causing photoinhibition, which limits plant survival, growth, and development [95]. The production and accumulation of secondary metabolites from various precursor elements depend on light intensity and the lengths of the photoperiod [96,97]. Different plant species respond differently to the quality, intensity, and length of photoperiod (day length) [98]. Appropriate light intensity regulates the accumulation and quality of flavonoids, alkaloids, spermine, and hexadecanoic acid [97,99]. For example, Melastoma malabathricum cell cultures exposed to different light intensities and full irradiance or complete darkness showed varying anthocyanin yield and biomass accumulation [88]. In vitro cultured seedlings of Hyptid marrubiodes exhibited increased production of flavonoids in red light, while rutin accumulation increased in white and blue light [100]. UV light treatment generally has a positive effect on the synthesis of secondary metabolites; however, at higher irradiation doses of UV-C and UV-B, some plants may react with decreased growth, abnormal metabolic activity, and photosynthesis [101].
Exposure of Asparagus officinalis to UV-B results in higher activity of peroxidase and phenylalanine ammonia-lyase, which ultimately enhances the accumulation of quercetin-4′-O-monoglucoside [102]. In Catharanthus roseus, UV-B light exposure significantly influences the production of vinblastine and vincristine, both used in treating leukemia and lymphoma [103]. Moreover, a combination of UV light (280–320 nm) with red light stimulates anthocyanin production in Malus domestica [104], while UV irradiation on Fagopyrum esculentum increases the content of quercetin [105]. Several studies have shown that UV light treatment is associated with increased phenolic compounds and ROS scavenging systems [106].
Additionally, the duration of light exposure plays a role in affecting secondary metabolite concentration. For example, light intensity and wavelength had a considerable effect on the accumulation of secondary metabolites in the leaves of Flourensia cernua [107]. Similarly, Ipomea batatus demonstrated differential effects on the concentration of phenolic compounds and flavonoids with enhanced levels observed upon exposure to longer light duration [108]. In green algae, Dunaliella baradawil, the accumulation of indoleamines like melatonin and serotonin increased under different photoperiod treatments [109].
4.5. Heavy Metal Stress
Plants respond differently to metal toxicity, resulting in variations in the accumulation and production of secondary metabolites due to the differential mobility of heavy metals that alter plant components, leading to reduced biosynthesis of plant defence compounds [110]. The adaptation and tolerance of plants against heavy metal stress are associated with the signalling molecules responsible for the synthesis and accumulation of secondary metabolites [111]. For example, Hypericum perforatum showed decreased accumulation of hypericin and pseudohypericin when exposed to nickel stress [112]. Nickel can either increase or inhibit the synthesis and accumulation of anthocyanin content in different plant species [113,114]. Brassica juncea treated with Fe, Mn, and Cr showed increased plant oil content [115]. Toxicity under high copper chloride (CuCl2) conditions activates the defence mechanism in Viburnum ichangense, leading to the accumulation and biosynthesis of ichangoside and phenolic diglycoside [116]. Salix purpurea exhibits an increased accumulation of phenolic compounds and salicylic acid under Cu- and Ni-stress conditions [117].
5. Other Factors Influencing Secondary Metabolism in Plants
In addition to the stresses mentioned earlier, plants encounter various other stress conditions, and to cope with these challenges, they have developed improved mechanisms of avoidance, biological detoxification, accumulation, and exclusion [12]. Each plant genotype responds uniquely to different abiotic stress factors, necessitating the study of specific plant species considering their individual stress responses rather than a single stress condition. In response to these stresses, plants produce specific secondary metabolites to mitigate the negative effects induced by environmental factors [96].
Gaseous toxins significantly impact the production of plant secondary metabolites. For instance, gaseous pollutants like sulfur dioxide (SO2) can damage photosynthetic activity by entering plants through stomatal openings during the photosynthetic process, triggering defence mechanisms in plants [118]. Treating Brassica oleracea with sodium hydrosulfide at a lower concentration (0.5 and 1 mM) leads to an enhanced content of sinigrin, carotenoids, phenolic compounds, and anthocyanins [119].
Pesticides represent another potential abiotic stress factor that affects plant metabolism and biosynthetic pathways. However, their specific impact on the production of secondary metabolites is not fully understood. Some studies have shown that herbicides and fungicides can influence levels of flavonoids, tropane alkaloids, and anthocyanins [120,121]. Many plants have developed special mechanisms to degrade and neutralize the hazardous effects of pesticides [122].
Recent research has highlighted the positive role of mineral nutrients in biomass production and the biosynthesis of secondary metabolites. Mineral nutrition can either increase or decrease plant growth and the accumulation of secondary metabolites, depending on the genotype, developmental stage, and environmental conditions of the studied plant [123,124,125]. Deficiencies in phosphorus, an essential element in primary metabolism involving energy currency molecules like ATP and ADP, can lead to reduced development and increased production of anthocyanin compounds [126]. For instance, Satureja hortensis exhibited increased accumulation of rosmarinic acid, known for its antioxidant, antiviral, and anti-inflammatory activities, when supplemented with nitrogen fertilizer [127]. However, excessive nitrogen application negatively influenced secondary metabolite synthesis in Labisia pumila due to reduced phenylalanine ammonia-lyase (PAL) activity and low photosynthetic rates [128]. Copper can inhibit deaminase oxidase activity, which is crucial in the biosynthetic pathways of secondary metabolites like cadaverine and putrescine [129,130]. Other mineral nutrients such as sulfur, potassium, and phosphate also impact the synthetic pathways of phenolic compounds and phenylpropanoids in various plant species [123,124,125]. For example, potassium treatment enhances the synthesis of phenolic compounds in the leaves of Ocimum basilicum [131].
6. Defence Action through Secondary Metabolism in Plants
Plants face a wide range of environmental stresses such as temperature fluctuations, irradiation, heavy metals, salinity, and gaseous toxins [132]. In response to these abiotic stresses, plants accumulate and synthesize various secondary metabolites, with different species producing distinct compositions [133]. The production of secondary metabolites serves as a defence mechanism against abiotic stress and plays a crucial role in regulating the growth and productivity of plants [96]. When exposed to stress, plants induce gene networks involved in the regulation and production of cellular molecules like detoxifying enzymes and osmoprotectants, which act as the first line of defence [134,135]. Reactive oxygen species (ROS), known for their damaging effects, are rapidly generated upon sensing stress signals in plants [136]. ROS, acting as secondary messengers, stimulate defence genes and modulate protein structure [137,138]. They also impact signalling through respiratory burst oxidase homologue D (RBOHD) and disrupt cell-to-cell communication. ROS signalling activates stress-related genes in Arabidopsis by activating various transcription factors [30]. In salt-tolerant citrus plants, the accumulation of oxidized and S-nitrosylated proteins is enhanced by priming the antioxidant activity of H2O2 under NaCl stress, whereas control plants exhibit greater susceptibility to stress [139].
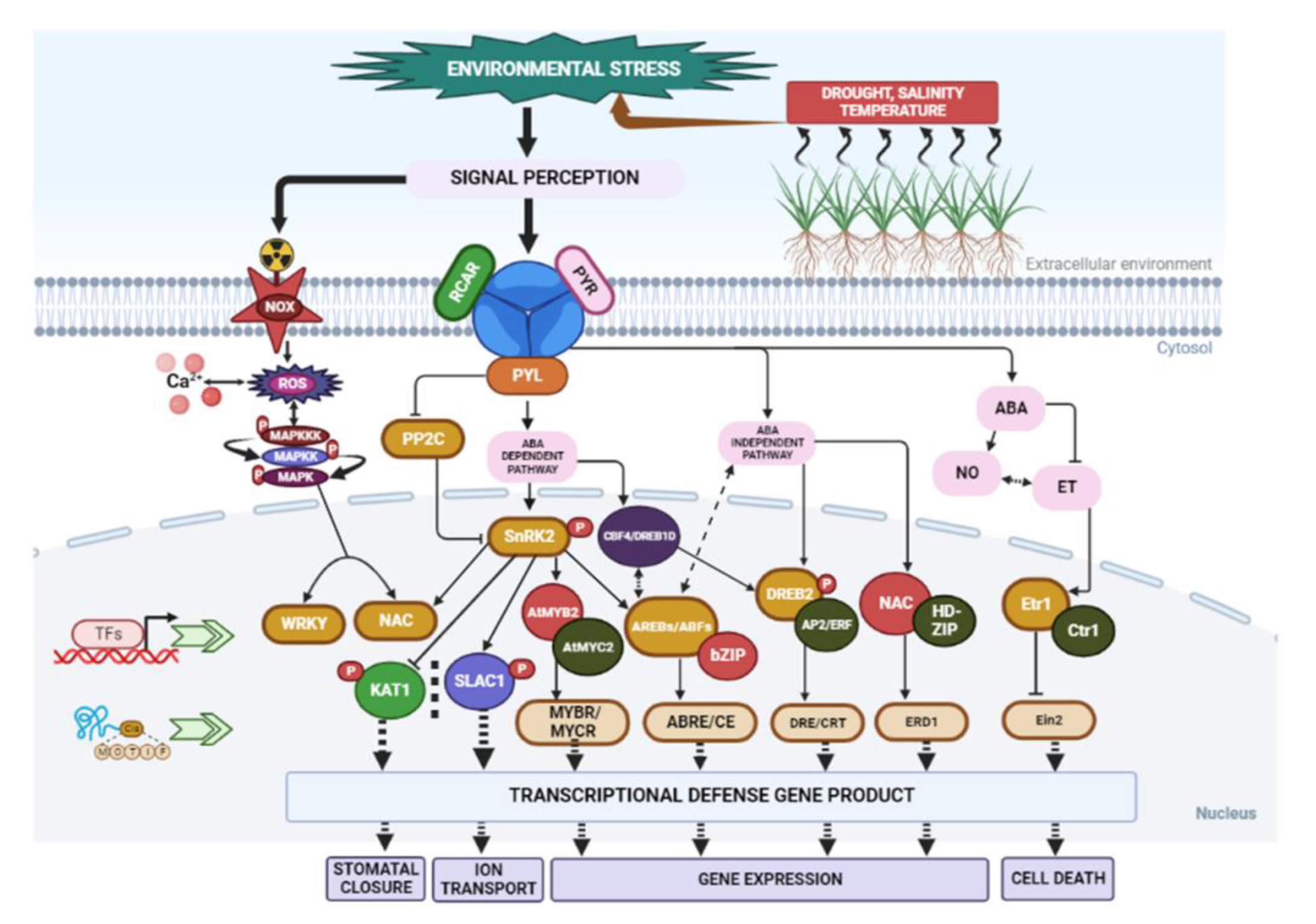
The plant response to abiotic stress involves cross-talk and interactions with various molecular pathways [140]. However, the responses to these abiotic conditions are dynamic and complex, and they can be reversible or irreversible [141,142]. Phytohormones play a critical role in regulating physiological processes and plant responses to abiotic stress. Hormones such as jasmonic acid (JA), abscisic acid (ABA), auxin, salicylic acid (SA), gibberellic acid (GA), and ethylene are essential for plant defence mechanisms [143]. These phytohormones activate signalling pathways that regulate downstream responses, mediating both growth and immunity against environmental stresses [144] (Figure 3). Among these hormones, ABA [145] and ethylene [146] play significant roles in abiotic stress, particularly in osmoregulation. ABA acts rapidly in signalling, at times independent of transcription factors. For example, ABA controls the stomatal aperture through mechanisms involving water transport and ion regulation [147]. Transcriptional regulation under salt-stress and water-deficit conditions has been studied in both ABA-dependent and ABA-independent mechanisms [148]. Water-deficit-induced cellular dehydration activates downstream signalling molecules, including metabolic enzymes and transcription factors, through increased endogenous ABA levels [148]. ABA-dependent mechanisms involve the expression of genes regulated by transcription factors like ABA-responsive element-binding proteins (AREBs) [148]. On the other hand, ABA-independent mechanisms involve the expression of transcription factors like dehydration-responsive element-binding proteins (DREB2) that regulate dehydration pathways [149]. In Arabidopsis thaliana, transcriptional control through the Abscisic acid-Insensitive 5 (ABI5) genes regulates plant response against various abiotic stresses [134]. Similarly, in Xerophyta viscosa, the regulatory expression of late embryogenesis abundant 4 (LEA4) genes plays a vital role in plant adaptation to stressful conditions [150]. The cascading activity of ABA signalling molecules enhances plant resistance against dehydration stress. In addition to ABA, SA also increases tolerance to water deficit in barley [151] and promotes drought resistance in other plant species [152].
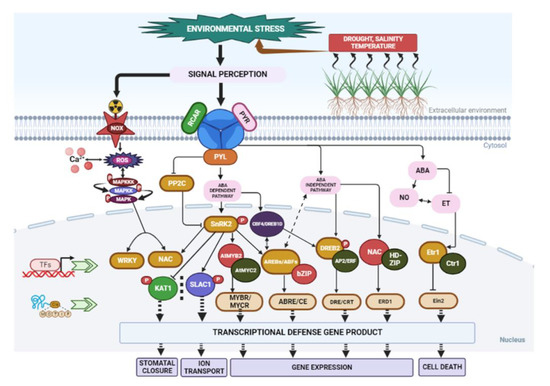
Figure 3.
Schematic overview of signal perception, transduction, and transcriptional regulatory networks under environmental stresses, including drought, heat, and other stresses. In plants with and without ABA, abiotic stress activates the ABA signalling system. In the absence of ABA, PP2C phosphatases (negative regulators) interact with SnRK2 kinases (positive regulators) and inhibit their activation. Signal transduction is blocked due to the inactivation of SnRK2s. The presence of ABA permits receptors to bind ABA and interact with PP2Cs, allowing SnRK2s to be released from binding. Autophosphorylation activates the SnRK2s, allowing for transduction to take place. SnRK2s that have been activated include phosphorylate downstream substrate proteins, such as transcription factors. Abbreviations: Abscisic acid (ABA); dehydration-response element-binding protein/C-repeat binding factor (DREB/CBF); ethylene response 1 (ETR1); Arabidopsis NAC domain-containing protein (ANAC); mitogen-activated protein kinase (MAPK); sucrose nonfermenting-1 related kinase(SnRK); transcription factors (TFs); reactive oxygen species (ROS); protein phosphatase 2C (PP2C); ABA receptors (PYR/PYL/RCAR); ethylene response factors (ERFs); nitric oxide (NO)-ethylene (ET) interplay; ABA-responsive element-binding proteins/ABA-responsive element -binding factor (AREB/ABF); Etr, ethylene-resistant; Ctr, constitutive triple response; Ein, ethylene-insensitive; basic-domain leucine zipper (bZIP); dehydration-responsive elements/C-repeat (DRE/CRT); ABA-responsive elements (ABREs); potassium channel protein in Arabidopsis thaliana 1 (KAT1); slow anion channel 1 (SLAC1).
Ethylene, along with ABA, regulates plant growth, survival, and defence against various environmental stresses [153]. The molecular response of ethylene to abiotic stress includes irradiation, drought, flooding, and temperature changes [154]. Ethylene-based responses depend on interactions with stress signals, metabolites, and other phytohormones [155]. The interplay between hormones like ABA and ethylene in drought response [154] has made the signalling pathways quite complex. Several membrane receptors, transcription factors, signalling protein kinases (PKs), and F-box proteins have been identified as signalling components in A. thaliana through bioinformatics and molecular genetics [156]. Among these, Ethylene Response 1 (ETR1) and Ethylene Sensor1 (ERS1) receptors, which possess conserved motifs of histidine kinases (HKs), play a crucial role in ethylene binding [157]. Constitutive Triple Response1 (CTR1), encoding MAPK kinase (MKKK), is associated with receptor protein complexes and negatively regulates these proteins [158]. Another receptor factor, Ethylene Insensitive 3 (EIN3), activates numerous responsive genes, including Ethylene Response Factor 1 (ERF1). This signalling cascade facilitates GCC binding, leading to the induction of ethylene-dependent secondary responses and the corresponding gene products that confer defence, growth, and survival in plants [159].
Primary metabolites such as sugars have been investigated for their active role in regulating plant metabolism and defence against abiotic stresses [12]. Soluble sugars, as signalling molecules, interact with ABA and ethylene to modulate plant growth and development. A study in grapevines demonstrated how interactive ABA and sugar signalling pathways control transport processes through enhanced expression of sugar transporter genes [160]. Lecourieux et al. [161] revealed increased expression of hexose transporters and protein kinase VVSK1 activity. Furthermore, sugar and light deficiency stimulate the activity of SnRK protein kinase [162]. Increased expression of SnRK2 protein kinase regulates plant metabolic activities and leads to higher sugar content in leaves [163]. Thus, sugar accumulation modulates plant metabolism and influences the accumulation of various secondary metabolites that are essential for adapting to environmental stresses.
7. Application of Plant Tissue Culture Techniques Associated with Plant Secondary Metabolites Production
While secondary metabolites can be obtained from plants grown under natural conditions, their production is influenced by environmental fluctuations [164]. Therefore, in vitro tissue culture serves as a crucial technique for enhancing the production of secondary metabolites [54]. In vitro culture for the production of these bioactive compounds offers a reliable resource and is not subject to quality fluctuations.
The synthesis of secondary metabolites through plant tissue culture is enhanced by identifying the optimal media composition, temperature, photoperiod, and plant growth regulators (PGRs) [165]. The use of suitable culture media, along with micronutrients, macronutrients, and plant hormones such as auxin, cytokinin, and gibberellins, plays a vital role in promoting the production of secondary metabolites [166] (Table 2).
Plant tissue culture involves the accumulation of biomass followed by the in vitro synthesis of secondary metabolites. Various approaches utilizing organized structures like callus, cell suspension, and shoot cultures have been employed for the synthesis of secondary metabolites [167].

Table 2.
The influence of different growth regulators, as well as differential culture regimes on secondary metabolite accumulation in various plant species.
Table 2.
The influence of different growth regulators, as well as differential culture regimes on secondary metabolite accumulation in various plant species.
| Plant Species | Medium + PGRs | Cultured Tisssue | Compound Name | Reference |
|---|---|---|---|---|
| Camellia sinensis L. | MS + 2,4-D + BAP | Callus | Catechin | [168] |
| Arbutus andrachne L. | WP + TDZ + NAA | Callus | Catechin | [169] |
| Rauwolfia serpentina | MS + Kn + BAP | Shoot | Phyllocladane diterpenoids | [170] |
| Eurycoma longifolia | MS + NAA +Kn | Cell suspension | Eurycomanone | [171] |
| Talinum paniculatum | MS + potassium nitrate | Hairy root | Saponin content | [172] |
| Momordica charantia | MS + sucrose | Hairy root | Flavonoids, phenolic acids | [173] |
| Eleutherococcus koreanum | ½ MS + IBA + TDZ | Adventitious root | Eleutheroside B and E | [174] |
| Eurycoma longifolia | 3/4 MS + IBA + NAA | Adventitious root | Flavonoids, phenolic content | [175] |
| Astragalus membranaceus | MS + IBA | Adventitious root | Saponin, flavonoid content | [176] |
| Coleus blumei | MMS + BA + NAA + sucrose | Callus and suspension | Rosmarinic acid | [177] |
| Spilanthes acmella | MS + BA + 2,4-D | Cell suspension | Scopoletin | [178] |
| Ajuga bracteosa | MS + BA + MeJ | Cell and callus | Monoterpene hydrocarbons | [179] |
| Fagonia indica | MS + TDZ | Callus | Gallic acid, quercetin | [180] |
| Rosa damascena | MS + BA + NAA | Callus | Tocopherols and β-carotene | [181] |
| Salvia dolomitica | MS + 2,4-D + Kn | Callus | α-Pinene, β-phellandrene | [182] |
| Corylus avellana L. | MS + BA + 2,4-D | Suspension | Taxol | [183] |
| Linum usitatsimum L. | MS + NAA | Callus | Lignans and neolignans | [184] |
| Morus alba L. | MS + Cefotaxime | Hairy root | Betulin and betulinic acid | [185] |
| Solanum trilobatum L. | MS + MeJ | Hairy root | Solasodine | [186] |
| Salvia miltiorrhiza | MS + MeJ + SA | Hairy root | Tanshinone | [187] |
| Caralluma tuberculata | MS + BA + 2,4-D | Callus | Phenolic and flavonoid content | [188] |
| Rhodiola imbricata | MS + BA + NAA | Callus | Phenylethanoids and phenylpropanoids | [189] |
| Plumbago zeylanica L. | MS + IBA | Root suspension | Plumbagin | [190] |
| Verbena officinalis L. | Schenk–Hildebrandt medium + 2,ip + TDZ | Shoot culture | Coumaran and hexadecenoic acid | [191] |
| Thevetia peruviana | Schenk–Hildebrandt medium + 2,4-D + Kn | Cell suspension | Phenolic compounds | [192] |
| Oldenlandia umbellata L. | MS + IBA + NAA | Adventitious root | Anthraquinones | [193] |
| Vitis vinifera | MS + IAA +GA3 | Callus | Resveratol | [194] |
MS—Murashige and Skoog, BA—6-Benzylaminopurine, IBA—indole-3-butyric acid, NAA—naphthalene acetic acid, 2,4-D—2,4-dichlorophenoxyacetic acid, GA3—gibberellic acid, TDZ—thidiazuron, Kn—kinetin, 2,iP—6-(γ,γ-Dimethylallylamino), MeJ—methyl jasmonate.
7.1. Callus and Cell Culture
The callus, which is an unorganized mass of cells, has proven to be highly efficient for the production of secondary metabolites due to its rapid growth and high reproducibility. In vitro callus culture has been successful in synthesizing various compounds such as anthocyanins, flavonoids, ajmaline alkaloids, α-tocopherol, serpentine, paclitaxel, scopolamine, and reserpine [195]. Glutathione and anthraquinones were produced through callus formation in Nicotiana tabacum and Morinda citrifolia [196]. Forskolin, a potent medicinal compound, has been found in calli obtained from the leaves of Coleus forskohlii [197]. Moreover, in vitro cell and callus cultures have been utilized to enhance the production of gymnemic acid in Gymnema sylvestre [198].
Elicitation by MJ (methyl jasmonate) was observed to be necessary for the enhanced synthesis of terpinolene and limonene monoterpenes under dark conditions in cell cultures of Rosa damascene [181]. Similarly, callus cultures of Mentha piperita have exhibited a higher accumulation of monoterpenes compared to naturally grown plants [199]. In Gingko biloba, cell suspension cultures recorded a higher accumulation of bilobalide and gingkolide [200]. Additionally, the combined effect of MJ and cyclodextrins in cell cultures of Catharanthus roseus has shown enhanced biosynthesis of alkaloids and terpenes [201].
7.2. Hairy Root Culture
Hairy root culture presents an alternative approach for enhancing biochemical properties and specific organic compounds, along with other classes of secondary metabolites [202]. This method involves transforming root cultures using Agrobacterium rhizogenes, resulting in the formation of hairy roots through the insertion of transfer DNA (T-DNA) [203]. The development of these hairy roots enables the accumulation of secondary metabolites in the newly grown plant roots [204].
Considerable attention has been given to optimize secondary metabolite production and selected plant species with favourable traits for hairy root (HR) induction. For instance, Barba-Espín et al. [205] demonstrated an increase in anthocyanin content in black carrot (Daucus carota ssp.) through HR induction by infecting it with an R. rhizogenes strain. Another example of specialized secondary metabolite production through hairy root culture involves introducing a gene of interest from Hyoscyamus muticus into Atropa belladonna using a binary vector system and A. rhizogenes [206]. Moreover, successful production of horhammericine alkaloids, tabersonine, and catharanthine compounds has been achieved from hairy root cultures of Catharanthus roseus transformed by the bacteria [207]. The potential of hairy root culture systems for optimal antiviral flavonoid production in Isatis tinctoria has also been demonstrated [208].
8. Conclusions and Future Prospects
Environmental factors have the ability to regulate the physiological and metabolic processes, consequently influencing the growth and productivity of plants. These factors, through abiotic stress, also impact the complex responses involved in the signal transduction of synthesized bioactive compounds. Therefore, the cascading signalling of stress factors has a profound effect on the regulation of phenotypic damage and metabolic functioning, which ultimately govern the plant’s inherent defence system.
The biosynthetic pathways responsible for producing secondary metabolites undergo modulation under different abiotic stresses and during various growing seasons, leading to either an increase or decrease in the content and accumulation of secondary metabolites. The adjustment of physiological and metabolic pathways under stress conditions is crucial for plants to adapt and develop an increased tolerance to such harsh environments. Studies have shown that specific secondary metabolites play roles as primary and regulator metabolites, intricately woven into plant metabolism. Researchers have endeavored to observe the dynamic and intricate plant responses to harsh conditions through cellular and molecular studies, but proteome analysis remains relatively unexplored.
To counter the influence of abiotic factors on the production of secondary metabolites, researchers have conducted studies involving a core group of genes to investigate the kinetics of plant responses under condition-dependent changes. Plant tissue cultures offer the advantage of synthesizing bioactive secondary metabolites regardless of temperature and other abiotic conditions. Therefore, there is substantial interest in large-scale production through in vitro cultures, necessitating comprehensive studies to explore the effects of environmental stimuli.
Investigations into the physiological and metabolic status of plants in response to stress stimuli could lay the foundation for integrating metabolic engineering and cell culture techniques in the synthesis of valuable plant secondary metabolites sustainably.
Author Contributions
Z.A.R.: study—conception, data collection, drafted the original manuscript, and editing; W.A.: prepared a draft of the figures; A.S.L.: edited and revised the manuscript and figures; S.B.J.: edited the final version of the manuscript and figures. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Zubair Altaf Reshi acknowledges financial support from CSIR-UGC (Council of Scientific & Industrial Research, University Grants Commission) New Delhi, India, as Junior Research fellowship under Ref. No. (560/CSIR-UGC NET) at Aligarh Muslim University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023, 53, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Shahzad, S.M.; Hussain, M.; Alharby, H.F.; Irshad, M.K.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef] [PubMed]
- Kossel, A. Ueber Schleim und Schleimbildende Stoffe. Dtsch. Med. Wochenschr. 1891, 17, 1297–1299. [Google Scholar] [CrossRef][Green Version]
- Simpson, K.; Fuentes, P.; Quiroz-Iturra, L.F.; Flores-Ortiz, C.; Contreras, R.; Handford, M.; Stange, C. Unraveling the Induction of Phytoene Synthase 2 Expression by Salt Stress and Abscisic Acid in Daucus carota. J. Exp. Bot. 2018, 69, 4113–4126. [Google Scholar] [CrossRef]
- Sankari, M.; Hridya, H.; Sneha, P.; Doss, C.G.P.; Christopher, J.G.; Mathew, J.; Zayed, H.; Ramamoorthy, S. Implication of Salt Stress Induces Changes in Pigment Production, Antioxidant Enzyme Activity, and qRT-PCR Expression of Genes Involved in the Biosynthetic Pathway of Bixa orellana L. Funct. Integr. Genom. 2019, 19, 565–574. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant Secondary Metabolites: A Key Driver of Litter Decomposition and Soil Nutrient Cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary Metabolites of Plants. Med. Orchid. Asia 2015, 5, 59–73. [Google Scholar] [CrossRef]
- Austen, N.; Walker, H.J.; Lake, J.A.; Phoenix, G.K.; Cameron, D.D. The Regulation of Plant Secondary Metabolism in Response to Abiotic Stress: Interactions between Heat Shock and Elevated CO2. Front. Plant Sci. 2019, 10, 01463. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Fatani, S.H.; Mohamed Nour Eldin, E.E.; Wink, M. Natural Products Modulate the Multifactorial Multidrug Resistance of Cancer. Pharmaceuticals 2015, 6, 146–176. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. [Google Scholar] [CrossRef]
- Rahman, A.; Albadrani, G.M.; Waraich, E.A.; Awan, T.H.; Yavaş, İ.; Hussain, S. Plant Secondary Metabolites and Abiotic Stress Tolerance: Overview and Implications. In Plant Abiotic Stress Responses and Tolerance Mechanisms; Intechopen: London, UK, 2023. [Google Scholar] [CrossRef]
- Kaur, M.; Tak, Y.; Bhatia, S.; Kaur, H. Phenolics Biosynthesis, Targets, and Signaling Pathways in Ameliorating Oxidative Stress in Plants. In Plant Phenolics in Abiotic Stress Management; Springer: Singapore, 2023; pp. 149–171. [Google Scholar] [CrossRef]
- Weaver, L.M.; Herrmann, K.M. Dynamics of the Shikimate Pathway in Plants. Trends Plant Sci. 1997, 2, 346–351. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, E.; Mishra, N.; Mishra, P. Shikimic acid as an intermediary model for the production of drugs effective against influenza virus. In Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 245–256. [Google Scholar]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Ashraf, M.I.; Shu, S. Biosynthetic pathways of triterpenoids and strategies to improve their biosynthetic efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Y.; Gao, M.; Zhao, Y.; Wang, Y. Sir2 family proteins regulate terpenoid synthesis by deacetylation of 3-hydroxy-3-methylglutaryl-CoA synthase. Ind. Crops Prod. 2021, 170, 113770. [Google Scholar] [CrossRef]
- Zhang, X.K.; Wang, D.N.; Chen, J.; Liu, Z.J.; Wei, L.J.; Hua, Q. Metabolic engineering of β-carotene biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef]
- Hemmerlin, A. Phosphorylation of Metabolites Involved in Salvage Pathways for Isoprenoid Biosynthesis in Plants. Kinases Phosphatases 2023, 1, 151–166. [Google Scholar] [CrossRef]
- Yap, P.G.; Choi, S.B.; Liong, M.T. Allantoin, a potential metabolite that promotes AMPK phosphorylation and suppresses cholesterol biosynthesis via the mevalonate pathway and Bloch pathway. Appl. Biochem. Biotechnol. 2020, 191, 226–244. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Zhang, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef]
- Martens, S.; Preuß, A.; Matern, U. Multifunctional Flavonoid Dioxygenases: Flavonol and Anthocyanin Biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic Metabolism and Related Heavy Metal Tolerance Mechanism in Kandelia obovata under Cd and Zn Stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, R.E.; Jimenez-Gomez, J.M.; Fulop, D.; Harmer, S.L.; Maloof, J.N.; Kliebensteina, D.J. Network Quantitative Trait Loci Mapping of Circadian Clock Outputs Identifies Metabolic Pathway-to-Clock Linkages in Arabidopsis. Plant Cell 2011, 23, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Thomsen, M.L.F.; Nintemann, S.J.; Jagd, L.M.; Bourgine, B.; Burow, M.; Kliebenstein, D.J. An Evolutionarily Young Defense Metabolite Influences the Root Growth of Plants via the Ancient TOR Signaling Pathway. eLife 2017, 6, e29353. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Ye, W.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate Degradation Products, Isothiocyanates, Nitriles, and Thiocyanates, Induce Stomatal Closure Accompanied by Peroxidase-Mediated Reactive Oxygen Species Production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Vyas, D. Myrosinase: Insights on Structural, Catalytic, Regulatory, and Environmental Interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The Glucosinolate Breakdown Product Indole-3-Carbinol Acts as an Auxin Antagonist in Roots of Arabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef]
- Gayomba, S.R.; Watkins, J.M.; Muday, G.K. Flavonols Regulate Plant Growth and Development through Regulation of Auxin Transport and Cellular Redox Status. Recent Adv. Polyphen. Res. 2016, 5, 143–170. [Google Scholar] [CrossRef]
- Maloney, G.S.; DiNapoli, K.T.; Muday, G.K. The Anthocyanin Reduced Tomato Mutant Demonstrates the Role of Flavonols in Tomato Lateral Root and Root Hair Development. Plant Physiol. 2014, 166, 614–631. [Google Scholar] [CrossRef]
- Buer, C.S.; Djordjevic, M.A. Architectural Phenotypes in the Transparent Testa Mutants of Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 751–763. [Google Scholar] [CrossRef]
- Watkins, J.M.; Chapman, J.M.; Muday, G.K. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Plant Physiol. 2017, 175, 1807–1825. [Google Scholar] [CrossRef]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the Study of the Function and Mechanism of the Action of Flavonoids in Plants under Environmental Stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef]
- Daryanavard, H.; Postiglione, A.E.; Mühlemann, J.K.; Muday, G.K. Flavonols modulate plant development, signaling, and stress responses. Curr. Opin. Plant Biol. 2023, 72, 102350. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The Interaction of Plant Biotic and Abiotic Stresses: From Genes to the Field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar] [CrossRef]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Chalker-Scott, L.; Fuchigami, L.H. The Role of Phenolic Compounds in Plant Stress Responses. In Low Temperature Stress Physiology in Crops; Springer: Cham, Switzerland, 2018; pp. 67–79. [Google Scholar] [CrossRef]
- Wuddineh, W.; Minocha, R.; Minocha, S.C. Polyamines in the Context of Metabolic Networks. Methods Mol. Biol. 2018, 1694, 1–23. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Establishment of Cell Suspension Cultures of Ocimum basilicum L. and Enhanced Production of Pharmaceutical Active Ingredients. Ind. Crops Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.; Xie, D.Y.; Sebastiani, L. Salt Stress Induces Differential Regulation of the Phenylpropanoid Pathway in Olea europaea Cultivars Frantoio (Salt-Tolerant) and Leccino (Salt-Sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Bali, S.; Jamwal, V.L.; Kohli, S.K.; Kaur, P.; Tejpal, R.; Bhalla, V.; Ahmad, P. Jasmonic Acid Application Triggers Detoxification of Lead (Pb) Toxicity in Tomato through the Modifications of Secondary Metabolites and Gene Expression. Chemosphere 2019, 235, 734–748. [Google Scholar] [CrossRef]
- Pandey, N.; Pandey-Rai, S. Short Term UV-B Radiation-Mediated Transcriptional Responses and Altered Secondary Metabolism of In Vitro Propagated Plantlets of Artemisia annua L. Plant Cell. Tissue Organ Cult. 2014, 116, 371–385. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sales, C.; Beltrán, J.; Gómez-Cadenas, A.; Arbona, V. Activation of Secondary Metabolism in Citrus Plants Is Associated to Sensitivity to Combined Drought and High Temperatures. Front. Plant Sci. 2017, 7, 1954. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, L.; Tůma, J. The Effect of UV Light on Isoflavonoid Production in Genista tinctoria Culture In Vitro. Acta Physiol. Plant. 2011, 33, 635–640. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant Growth Promoting Rhizobacteria (PGPR) Confer Drought Resistance and Stimulate Biosynthesis of Secondary Metabolites in Pennyroyal (Mentha pulegium L.) under Water Shortage Condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought Stress Adaptation Modulates Plant Secondary Metabolite Production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Podda, A.; Pollastri, S.; Bartolini, P.; Pisuttu, C.; Pellegrini, E.; Nali, C.; Cencetti, G.; Michelozzi, M.; Frassinetti, S.; Giorgetti, L.; et al. Drought Stress Modulates Secondary Metabolites in Brassica oleracea L. Convar. acephala (DC) Alef, var. sabellica L. J. Sci. Food Agric. 2019, 99, 5533–5540. [Google Scholar] [CrossRef]
- Ibrahim, W.; Zhu, Y.M.; Chen, Y.; Qiu, C.W.; Zhu, S.; Wu, F. Genotypic Differences in Leaf Secondary Metabolism, Plant Hormones and Yield under Alone and Combined Stress of Drought and Salinity in Cotton Genotypes. Physiol. Plant. 2019, 165, 343–355. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The Effects of Chitosan and Salicylic Acid on Elicitation of Secondary Metabolites and Antioxidant Activity of Safflower under In Vitro Salinity Stress. Plant Cell. Tissue Organ Cult. 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachâal, M.; Karray-Bouraoui, N.; Hannoufa, A. Salt Stress (NaCl) Affects Plant Growth and Branch Pathways of Carotenoid and Flavonoid Biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- Li, Q.; Lei, S.; Du, K.; Li, L.; Pang, X.; Wang, Z.; Wei, M.; Fu, S.; Hu, L.; Xu, L. RNA-Seq Based Transcriptomic Analysis Uncovers α-Linolenic Acid and Jasmonic Acid Biosynthesis Pathways Respond to Cold Acclimation in Camellia japonica. Sci. Rep. 2016, 6, 36463. [Google Scholar] [CrossRef]
- Ayoola-Oresanya, I.O.; Sonibare, M.A.; Gueye, B.; Abberton, M.T.; Morlock, G.E. Elicitation of Antioxidant Metabolites in Musa Species In Vitro Shoot Culture Using Sucrose, Temperature and Jasmonic Acid. Plant Cell Tissue Organ Cult. 2021, 146, 225–236. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of Culture Medium Composition and Light Conditions on the Accumulation of Bioactive Compounds in Shoot Cultures of Scutellaria lateriflora L. (American Skullcap) Grown In Vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential Accumulation of Silymarin Induced by Exposure of Silybum marianum L. Callus Cultures to Several Spectres of Monochromatic Lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Krolicka, A.; Zabiegala, B.; Luczkiewicz, M. Elicitation Strategies for the Improvement of Essential Oil Content in Rhododendron tomentosum (Ledum Palustre) Bioreactor-Grown Microshoots. Ind. Crops Prod. 2018, 123, 461–469. [Google Scholar] [CrossRef]
- Farrokhzad, Y.; Rezaei, A. Aluminum Elicitation Improves Antioxidant Potential and Taxol Production in Hazelnut (Corylus avellana L.) Cell Suspension Culture. Agric. Conspec. Sci. 2020, 85, 229–236. [Google Scholar]
- Anjum, S.; Abbasi, B.H.; Doussot, J.; Favre-Réguillon, A.; Hano, C. Effects of Photoperiod Regimes and Ultraviolet-C Radiations on Biosynthesis of Industrially Important Lignans and Neolignans in Cell Cultures of Linum usitatissimum L. (Flax). J. Photochem. Photobiol. B Biol. 2017, 167, 216–227. [Google Scholar] [CrossRef]
- Ullrich, S.F.; Rothauer, A.; Hagels, H.; Kayser, O. Influence of Light, Temperature, and Macronutrients on Growth and Scopolamine Biosynthesis in Duboisia species. Planta Med. 2017, 83, 937–945. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Haider, M.Z.; Ashraf, M.A.; Rasheed, R.; Hussain, I.; Riaz, M.; Qureshi, F.F.; Hafeez, A. Impact of Salinity Stress on Medicinal Plants. In Medicinal Plants: Their Response to Abiotic Stress; Springer: Singapore, 2023; pp. 199–239. [Google Scholar] [CrossRef]
- Daneshmand, F.; Arvin, M.J.; Kalantari, K.M. Physiological Responses to NaCl Stress in Three Wild Species of Potato in Vitro. Acta Physiol. Plant. 2010, 32, 91–101. [Google Scholar] [CrossRef]
- Parmar, R.D.; Mali, S.C.; Patel, A.I.; Patel, P.K. In Vitro Response of Promising Sugarcane Varieties for Salinity Tolerance through Callus Culture. Int. J. Chem. Stud. 2017, 5, 1180–1186. [Google Scholar]
- Alagoz, S.M.; Lajayer, B.A.; Ghorbanpour, M. Proline and Soluble Carbohydrates Biosynthesis and Their Roles in Plants under Abiotic Stresses. In Plant Stress Mitigators; Academic Press: Cambridge, MA, USA, 2023; pp. 169–185. [Google Scholar] [CrossRef]
- Ali, R.M.; Abbas, H.M. Response of Salt Stressed Barley Seedlings to Phenylurea. Plant Soil Environ. 2003, 49, 158–162. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; El-Habbak, M.H.; Havens, W.M.; Singh, A.; Zheng, D.; Vaughn, L.; Haudenshield, J.S.; Hartman, G.L.; Korban, S.S.; Ghabrial, S.A. Overexpression of GmCaM4 in Soybean Enhances Resistance to Pathogens and Tolerance to Salt Stress. Mol. Plant Pathol. 2014, 15, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Muchate, N.S.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Evaluation of Spinacia oleracea (L.) for Phytodesalination and Augmented Production of Bioactive Metabolite, 20-Hydroxyecdysone. Int. J. Phytoremediation 2018, 20, 981–994. [Google Scholar] [CrossRef]
- Ahl, S.A.; Omer, E.A. Medicinal and Aromatic Plants Production under Salt Stress. A Review. Herba Pol. 2011, 57, 2. [Google Scholar]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Chang. Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The Use of Metabolomics to Dissect Plant Responses to Abiotic Stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Suhre, K.; Gieger, C. Genetic Variation in Metabolic Phenotypes: Study Designs and Applications. Nat. Rev. Genet. 2012, 13, 759–769. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural Product Discovery: Past, Present, and Future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.; Wu, C.; Chen, S.; Wang, Z.; Yang, Z.; Qin, S.; Huang, L. Water Deficit Affected Flavonoid Accumulation by Regulating Hormone Metabolism in Scutellaria baicalensis Georgi Roots. PLoS ONE 2012, 7, e42946. [Google Scholar] [CrossRef]
- Solíz-Guerrero, J.B.; De Rodriguez, D.J.; Rodríguez-García, R.; Angulo-Sánchez, J.L.; Méndez-Padilla, G. Quinoa Saponins: Concentration and Composition Analysis. In Trends New Crop. New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 110–114. [Google Scholar]
- Cingöz, G.; Pehlivan Karakaş, F. The Effects of Nutrient and Macronutrient Stress on Certain Secondary Metabolite Accumulations and Redox Regulation in Callus Cultures of Bellis perennis L. Turk. J. Biol. 2016, 40, 1328–1335. [Google Scholar] [CrossRef]
- Deepthi, S.; Satheeshkumar, K. Effects of Major Nutrients, Growth Regulators and Inoculum Size on Enhanced Growth and Camptothecin Production in Adventitious Root Cultures of Ophiorrhiza mungos L. Biochem. Eng. J. 2017, 117, 198–209. [Google Scholar] [CrossRef]
- Yang, L.-L.; Yang, L.; Yang, X.; Zhang, T.; Lan, Y.-M.; Zhao, Y.; Han, M.; Yang, L.-M. Drought Stress Induces Biosynthesis of Flavonoids in Leaves and Saikosaponins in Roots of Bupleurum chinense DC. Phytochemistry 2020, 177, 112434. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the relevance of high temperature and elevated CO2 in plant secondary metabolites (PSMs) production. Heliyon 2021, 7, e07975. [Google Scholar] [CrossRef]
- Rahimi, S.; Hasanloo, T. The Effect of Temperature and pH on Biomass and Bioactive Compounds Production in Silybum marianum Hairy Root Cultures. Res. J. Pharmacogn. 2016, 3, 53–59. [Google Scholar]
- Chan, L.K.; Koay, S.S.; Boey, P.L.; Bhatt, A. Effects of Abiotic Stress on Biomass and Anthocyanin Production in Cell Cultures of Melastoma malabathricum. Biol. Res. 2010, 43, 127–135. [Google Scholar] [CrossRef]
- Okereke, C.N.; Kaurilind, E.; Liu, B.; Kanagendran, A.; Pazouki, L.; Niinemets, Ü. Impact of heat stress of varying severity on papaya (Carica papaya) leaves: Major changes in stress volatile signatures, but surprisingly small enhancements of total emissions. Environ. Exp. Bot. 2022, 195, 104777. [Google Scholar] [CrossRef]
- Singsaas, E.L. Terpenes and the Thermotolerance of Photosynthesis. New Phytol. 2000, 146, 1–2. [Google Scholar] [CrossRef]
- Cawood, M.E.; Allemann, I.; Allemann, J. Impact of Temperature Stress on Secondary Metabolite Profile and Phytotoxicity of Amaranthus cruentus L. Leaf Extracts. Acta Agric. Slov. 2018, 111, 609–620. [Google Scholar] [CrossRef]
- Sivadasan, U.; Chenhao, C.; Nissinen, K.; Randriamanana, T.; Nybakken, L.; Julkunen-Tiitto, R. Growth and Defence of Aspen (Populus tremula) after Three Seasons under Elevated Temperature and Ultraviolet-B Radiation. Can. J. For. Res. 2018, 48, 629–641. [Google Scholar] [CrossRef]
- Zhang, Y.; Virjamo, V.; Du, W.; Yin, Y.; Nissinen, K.; Nybakken, L.; Guo, H.; Julkunen-Tiitto, R. Effects of Soil Pyrene Contamination on Growth and Phenolics in Norway Spruce (Picea abies) Are Modified by Elevated Temperature and CO2. Environ. Sci. Pollut. Res. 2018, 25, 12788–12799. [Google Scholar] [CrossRef]
- Yao, L.; Caffin, N.; D’Arcy, B.; Jiang, Y.; Shi, J.; Singanusong, R.; Liu, X.; Datta, N.; Kakuda, Y.; Xu, Y. Seasonal Variations of Phenolic Compounds in Australia-Grown Tea (Camellia sinensis). J. Agric. Food Chem. 2005, 53, 6477–6483. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and Biochemical Responses to High Light and Temperature Stress in Plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Kong, D.X.; Li, Y.Q.; Wang, M.L.; Bai, M.; Zou, R.; Tang, H.; Wu, H. Effects of Light Intensity on Leaf Photosynthetic Characteristics, Chloroplast Structure, and Alkaloid Content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiol. Plant. 2016, 38, 120. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to Sun and Shade: A Whole-Plant Perspective. Aust. J. Plant Physiol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Liang, H.L.; Wu, H. Alkaloid Content and Essential Oil Composition of Mahonia breviracema Cultivated under Different Light Environments. J. Appl. Bot. Food Qual. 2018, 91, 171–179. [Google Scholar] [CrossRef]
- Pedroso, R.C.N.; Branquinho, N.A.A.; Hara, A.C.B.A.M.; Costa, A.C.; Silva, F.G.; Pimenta, L.P.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Januario, A.H. Impact of Light Quality on Flavonoid Production and Growth of Hyptis marrubioides Seedlings Cultivated in Vitro. Rev. Bras. Farmacogn. 2017, 27, 466–470. [Google Scholar] [CrossRef]
- Katerova, Z.; Todorova, D.; Sergiev, I. Plant Secondary Metabolites and Some Plant Growth Regulators Elicited by UV Irradiation, Light and/or Shade. In Medicinal Plants and Environmental Challenges; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–121. [Google Scholar] [CrossRef]
- Eichholz, I.; Rohn, S.; Gamm, A.; Beesk, N.; Herppich, W.B.; Kroh, L.W.; Ulrichs, C.; Huyskens-Keil, S. UV-B-Mediated Flavonoid Synthesis in White Asparagus (Asparagus officinalis L.). Food Res. Int. 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Binder, B.Y.K.; Peebles, C.A.M.; Shanks, J.V.; San, K.Y. The Effects of UV-Stress on the Production of Terpenoid Indole Alkaloids in Catharanthus roseus Hairy Roots. Biotechnol. Prog. 2009, 25, 861–865. [Google Scholar] [CrossRef]
- Arakawa, O.; Hori, Y.; Ogata, R. Relative Effectiveness and Interaction of Ultraviolet-B, Red and Blue Light in Anthocyanin Synthesis of Apple Fruit. Physiol. Plant. 1985, 64, 323–327. [Google Scholar] [CrossRef]
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B Radiation Affects Flavonoids and Fungal Colonisation in Fagopyrum esculentum and F. tataricum. Cent. Eur. J. Biol. 2012, 7, 275–283. [Google Scholar] [CrossRef]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Secondary Metabolite Profiles in Flowering Heads of Arnica montana cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef]
- Estell, R.E.; Fredrickson, E.L.; James, D.K. Effect of Light Intensity and Wavelength on Concentration of Plant Secondary Metabolites in the Leaves of Flourensia cernua. Biochem. Syst. Ecol. 2016, 65, 108–114. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of Photoperiod on Flavonoid Pathway Activity in Sweet Potato (Ipomoea batatas (L.) Lam.) Leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Dayananda, C.; Giridhar, P.; Rajasekaran, T.; Ravishankar, G.A. Photoperiod Influences Endogenous Indoleamines in Cultured Green Alga Dunaliella bardawil. Indian J. Exp. Biol. 2011, 49, 234–240. [Google Scholar]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy. 2021, 11, 968. [Google Scholar] [CrossRef]
- Murch, S.J.; Haq, K.; Rupasinghe, H.P.V.; Saxena, P.K. Nickel contamination affects growth and secondary metabolite composition of St. John’s wort (Hypericum perforatum L.). Environ. Exp. Bot. 2003, 49, 251–257. [Google Scholar] [CrossRef]
- dos Reis, A.R.; de Queiroz Barcelos, J.P.; de Souza Osório, C.R.W.; Santos, E.F.; Lisboa, L.A.M.; Santini, J.M.K.; dos Santos, M.J.D.; Junior, E.F.; Campos, M.; de Figueiredo, P.A.M.; et al. A Glimpse into the Physiological, Biochemical and Nutritional Status of Soybean Plants under Ni-Stress Conditions. Environ. Exp. Bot. 2017, 144, 76–87. [Google Scholar] [CrossRef]
- Merlot, S. Understanding Nickel Responses in Plants: More than Just an Interaction with Iron Homeostasis. Plant Cell Physiol. 2020, 61, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sinha, S. Accumulation of metals and its effects in Brassica juncea (L.) Czern. (cv. Rohini) grown on various amendments of tannery waste. Ecotoxicol. Environ. Saf. 2005, 62, 118–127. [Google Scholar] [CrossRef]
- Wu, B.; Wang, K.; Wu, X. A new phenolic diglycoside produced in response to copper toxicity and a new flavan dimer from the leaves of Viburnum ichangense (Hemsl.). Rehd. Helv. Chim. Acta 2011, 94, 1677–1684. [Google Scholar] [CrossRef]
- Drzewiecka, K.; Mleczek, M.; Gąsecka, M.; Magdziak, Z.; Budka, A.; Chadzinikolau, T.; Kaczmarek, Z.; Goliński, P. Copper and Nickel Co-Treatment Alters Metal Uptake and Stress Parameters of Salix purpurea × viminalis. J. Plant Physiol. 2017, 216, 125–134. [Google Scholar] [CrossRef]
- da Silva, L.C.; de Araújo, T.O.; Martinez, C.A.; de Almeida Lobo, F.; Azevedo, A.A.; Oliva, M.A. Differential Responses of C3 and CAM Native Brazilian Plant Species to a SO2- and SPMFe-Contaminated Restinga. Environ. Sci. Pollut. Res. 2015, 22, 14007–14017. [Google Scholar] [CrossRef]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Baenas, N.; Moreno, D.A.; Sánchez-Rodríguez, E.; Blasco, B.; Ruiz, J.M. Evaluation of Hydrogen Sulfide Supply to Biostimulate the Nutritive and Phytochemical Quality and the Antioxidant Capacity of Cabbage (Brassica oleracea L. ’Bronco’). J. Appl. Bot. Food Qual. 2016, 89, 290–298. [Google Scholar] [CrossRef]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, Herbicides and Bees: A Systematic Review of Existing Research and Methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef]
- Geetha, A. Chapter-2 Phytotoxicity Due to Fungicides and Herbicides and Its Impact in Crop Physiological Factors. In Advances in Agriculture Sciences; Naresh, R.K., Ed.; AkiNik Publications: New Delhi, India, 2019; p. 29. [Google Scholar]
- Chaudhary, N.; Choudhary, K.K.; Agrawal, S.B.; Agrawal, M. Pesticides Usage, Uptake and Mode of Action in Plants with Special Emphasis on Photosynthetic Characteristics. In Pesticides in Crop Production; Wiley: Hoboken, NJ, USA, 2020; pp. 159–180. [Google Scholar] [CrossRef]
- Hassan, A. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 105–110. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Zykin, P.A.; Andreeva, E.A.; Lykholay, A.N.; Tsvetkova, N.V.; Voylokov, A.V. Anthocyanin composition and content in rye plants with different grain color. Molecules 2018, 23, 948. [Google Scholar] [CrossRef]
- Luciano, Á.J.; Irineo, T.P.; Virginia, O.V.R.; Feregrino-Pérez, A.A.; Hernández, A.C.; Gerardo, G.G.R. Integrating plant nutrients and elicitors for production of secondary metabolites, sustainable crop production and human health: A review. Int. J. Agric. Biol. 2017, 19, 391–402. [Google Scholar] [CrossRef]
- Babalar, M.; Mumivand, H.; Hadian, J.; Tabatabaei, S.M.F. Effects of Nitrogen and Calcium Carbonate on Growth, Rosmarinic Acid Content and Yield of Satureja hortensis L. J. Agric. Sci. 2010, 2, 92–98. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of kacip fatimah (Labisia pumila blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef]
- Han, B.; Gao, J.; Han, X.; Deng, H.; Wu, T.; Li, C.; Jiang, B.; You, Y. Hanseniaspora uvarum FS35 degrade putrescine in wine through the direct oxidative deamination pathway of copper amine oxidase 1. Food Res. Int. 2022, 162, 111923. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in plant growth and development: Roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Stanius, Ž. Effect of External and Internal Factors on Secondary Metabolites Accumulation in St. John’s Worth. Bot. Lith. 2013, 18, 101–108. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bera, K.; Sadhukhan, S.; Dutta, P. Bio-priming of seeds: Plant stress management and its underlying cellular, biochemical and molecular mechanisms. Plant Stress 2022, 3, 100052. [Google Scholar] [CrossRef]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive Oxygen Species Function as Signaling Molecules in Controlling Plant Development and Hormonal Responses. Curr. Opin. Plant Biol. 2022, 69, 102293. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Tada, Y.; Loake, G.J. Post-Translational Protein Modification as a Tool for Transcription Reprogramming. New Phytol. 2010, 186, 333–339. [Google Scholar] [CrossRef]
- Shayeghan, M.; Ansari, A.M.; Forouzesh, F.; Javidi, M.A. Reactive Oxygen Species, the Trident of Neptune in the Hands of Hecate; Role in Different Diseases, Signaling Pathways, and Detection Methods. Arch. Biochem. Biophys. 2022, 728, 109357. [Google Scholar] [CrossRef]
- Tanou, G.; Molassiotis, A.; Diamantidis, G. Hydrogen Peroxide- and Nitric Oxide-Induced Systemic Antioxidant Prime-Like Activity under NaCl-Stress and Stress-Free Conditions in Citrus Plants. J. Plant Physiol. 2009, 166, 1904–1913. [Google Scholar] [CrossRef]
- Joshi, H.; Bisht, N.; Mishra, S.K.; Prasad, V.; Chauhan, P.S. Bacillus amyloliquefaciens Modulate Carbohydrate Metabolism in Rice-PGPR Cross-Talk Under Abiotic Stress and Phytohormone Treatments. J. Plant Growth Regul. 2023, 42, 4466–4483. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from Less: Plant Growth under Limited Water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Dutta, D.; Mondal, S.; Karmakar, S. Phytohormones and Nitric Oxide Cross-Talk in Regulation of Stress Tolerance in Plants. In Nitric Oxide in Plants: A Molecule with Dual Roles; Springer: Singapore, 2022; pp. 179–200. [Google Scholar] [CrossRef]
- Shigenaga, A.M.; Argueso, C.T. No Hormone to Rule Them All: Interactions of Plant Hormones during the Responses of Plants to Pathogens. Semin. Cell Dev. Biol. 2016, 56, 174–189. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Poór, P.; Nawaz, K.; Gupta, R.; Ashfaque, F.; Khan, M.I.R. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2022, 41, 675–698. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular Phosphorylation Signaling and Gene Expression in Drought Stress Responses: Aba-Dependent and Aba-Independent Regulatory Systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 Are Master Transcription Factors That Cooperatively Regulate ABRE-Dependent ABA Signaling Involved in Drought Stress Tolerance and Require ABA for Full Activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Costa, M.C.D.; Artur, M.A.S.; Maia, J.; Jonkheer, E.; Derks, M.F.L.; Nijveen, H.; Williams, B.; Mundree, S.G.; Jiménez-Gómez, J.M.; Hesselink, T.; et al. A Footprint of Desiccation Tolerance in the Genome of Xerophyta viscosa. Nat. Plants 2017, 3, 17038. [Google Scholar] [CrossRef]
- Malaga, S.; Janeczko, A.; Janowiak, F.; Waligórski, P.; Oklestkova, J.; Dubas, E.; Biesaga-Koscielniak, J.; Kuta, E.; Kościelniak, J.; Żur, I. Involvement of homocastasterone, salicylic and abscisic acids in the regulation of drought and freezing tolerance in doubled haploid lines of winter barley. Plant Growth Regul. 2020, 90, 173–188. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Ahmad, M.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kaçar, Y.A. An Overview and Recent Progress of Plant Growth Regulators (PGRs) in the Mitigation of Abiotic Stresses in Fruits: A Review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Rezayian, M.; Mousavi, S.H. Drought stress: Involvement of Plant Hormones in Perception, Signaling, and Response. In Plant Hormones and Climate Change; Springer: Singapore, 2023; pp. 227–250. [Google Scholar]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Azhar, B.J.; Zulfiqar, A.; Shakeel, S.N.; Schaller, G.E. Amplification and adaptation in the ethylene signaling pathway. Small Methods 2020, 4, 1900452. [Google Scholar] [CrossRef]
- Gao, Z.; Wen, C.K.; Binder, B.M.; Chen, Y.F.; Chang, J.; Chiang, Y.H.; Kerris, R.J.; Chang, C.; Schaller, G.E. Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. J. Biol. Chem. 2008, 283, 23801–23810. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Miao, Y.; Liu, Y.; Botella, J.R.; Li, W.; Li, K.; Song, C.P. Function of Protein Kinases in Leaf Senescence of Plants. Front. Plant Sci. 2022, 13, 864215. [Google Scholar] [CrossRef]
- Kendrick, M.D.; Chang, C. Ethylene signaling: New levels of complexity and regulation. Curr. Opin. Plant Biol. 2008, 11, 479–485. [Google Scholar] [CrossRef]
- Owen, S.J.; Lafond, M.D.; Bowen, P.; Bogdanoff, C.; Usher, K.; Abrams, S.R. Profiles of abscisic acid and its catabolites in developing Merlot grape (Vitis vinifera) berries. Am. J. Enol. Vitic. 2009, 60, 277–284. [Google Scholar] [CrossRef]
- Lecourieux, F.; Lecourieux, D.; Vignault, C.; Delrot, S. A sugar-inducible protein kinase, VvSK1, regulates hexose transport and sugar accumulation in grapevine cells. Plant Physiol. 2010, 152, 1096–1106. [Google Scholar] [CrossRef]
- Zirngibl, M.E.; Araguirang, G.E.; Kitashova, A.; Jahnke, K.; Rolka, T.; Kühn, C.; Sorgatz, A.; Friedrichs, S.; Müller, M.; Höhne, M.; et al. Triose Phosphate Export from Chloroplasts and Cellular Sugar Content Regulate Anthocyanin Biosynthesis during High Light Acclimation. Plant Commun. 2023, 4, 100423. [Google Scholar] [CrossRef]
- Zheng, Z.; Xu, X.; Crosley, R.A.; Greenwalt, S.A.; Sun, Y.; Blakeslee, B.; Wang, L.; Ni, W.; Sopko, M.S.; Yao, C.; et al. The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol. 2010, 153, 99–113. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Cardoso, J.C.; de Oliveira, M.E.B.S.; de Cardoso, F.C.I. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Sutini, W.; Widiwurjani; Ardianto, C.; Khotib, J.; Purwanto, D.A.; Muslihatin, W. Production of the secondary metabolite catechin by in vitro cultures of Camellia sinensis L. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–7. [Google Scholar] [CrossRef]
- Aljabari, Z.; Alzeer, J.; Arafeh, R. Catechin detection in callus and in vitro cultures of the Eastern strawberry tree, Arbutus andrachne L., an endangered medicinal tree in Palestine. Glob. J. Res. Med. Plants Indig. Med. (GJRMI) 2014, 3, 196–205. [Google Scholar]
- Goel, M.K.; Kukreja, A.K.; Singh, A.K.; Khanuja, S.P.S. In vitro plant growth promoting activity of phyllocladane diterpenoids isolated from Callicarpa macrophylla Vahl. in shoot cultures of Rauwolfia serpentina. Nat. Prod. Commun. 2007, 2, 799–802. [Google Scholar] [CrossRef]
- Nhan, N.H.; Loc, N.H. Production of eurycomanone from cell suspension culture of Eurycoma longifolia. Pharm. Biol. 2017, 55, 2234–2239. [Google Scholar] [CrossRef]
- Manuhara, Y.S.W.; Kristanti, A.N.; Utami, E.S.W.; Yachya, A. Effect of sucrose and potassium nitrate on biomass and saponin content of Talinum paniculatum Gaertn. hairy root in balloon-type bubble bioreactor. Asian Pac. J. Trop. Biomed. 2015, 5, 1027–1032. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Maria John, K.M.; Yang, Y.S.; Kim, S.H.; Chung, I.M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell. Tissue Organ Cult. 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Lee, E.J.; Paek, K.Y. Enhanced productivity of biomass and bioactive compounds through bioreactor cultures of Eleutherococcus koreanum Nakai adventitious roots affected by medium salt strength. Ind. Crops Prod. 2012, 36, 460–465. [Google Scholar] [CrossRef]
- Lulu, T.; Park, S.Y.; Ibrahim, R.; Paek, K.Y. Production of biomass and bioactive compounds from adventitious roots by optimization of culturing conditions of Eurycoma longifolia in balloon-type bubble bioreactor system. J. Biosci. Bioeng. 2015, 119, 712–717. [Google Scholar] [CrossRef]
- Wu, S.Q.; Lian, M.L.; Gao, R.; Park, S.Y.; Piao, X.C. Bioreactor application on adventitious root culture of Astragalus membranaceus. Vitr. Cell. Dev. Biol. Plant 2011, 47, 719–724. [Google Scholar] [CrossRef]
- Qian, J.; Guiping, L.; Xiujun, L.; Xincai, H.; Hongmei, L. Influence of growth regulators and sucrose concentrations on growth and rosmarinic acid production in calli and suspension cultures of Coleus blumei. Nat. Prod. Res. 2009, 23, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Abyari, M.; Nasr, N.; Soorni, J.; Sadhu, D. Enhanced Accumulation of Scopoletin in Cell Suspension Culture of Spilanthes acmella Murr. Using Precursor Feeding. Braz. Arch. Biol. Technol. 2016, 59, 1–7. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Ullah, N.; Khan, R.S. Impacts of Hormonal Elicitors and Photoperiod Regimes on Elicitation of Bioactive Secondary Volatiles in Cell Cultures of Ajuga bracteosa. J. Photochem. Photobiol. B Biol. 2018, 183, 242–250. [Google Scholar] [CrossRef]
- Khan, T.; Abbasi, B.H.; Khan, M.A.; Shinwari, Z.K. Differential Effects of Thidiazuron on Production of Anticancer Phenolic Compounds in Callus Cultures of Fagonia indica. Appl. Biochem. Biotechnol. 2016, 179, 46–58. [Google Scholar] [CrossRef]
- Olgunsoy, P.; Ulusoy, S.; Çelikkol Akçay, U. Metabolite Production and Antibacterial Activities of Callus Cultures from Rosa damascena Mill. Petals. Marmara Pharm. J. 2017, 21, 590. [Google Scholar] [CrossRef]
- Bassolino, L.; Giacomelli, E.; Giovanelli, S.; Pistelli, L.; Cassetti, A.; Damonte, G.; Bisio, A.; Ruffoni, B. Tissue Culture and Aromatic Profile in Salvia dolomitica Codd. Plant Cell. Tissue Organ Cult. 2015, 121, 83–95. [Google Scholar] [CrossRef]
- Rahpeyma, S.A.; Moieni, A.; Jalali Javaran, M. Paclitaxel Production Is Enhanced in Suspension-Cultured Hazel (Corylus avellana L.) Cells by Using a Combination of Sugar, Precursor, and Elicitor. Eng. Life Sci. 2015, 15, 234–242. [Google Scholar] [CrossRef]
- Nadeem, M.; Ahmad, W.; Zahir, A.; Hano, C.; Abbasi, B.H. Salicylic Acid-Enhanced Biosynthesis of Pharmacologically Important Lignans and Neo Lignans in Cell Suspension Culture of Linum ussitatsimum L. Eng. Life Sci. 2019, 19, 168–174. [Google Scholar] [CrossRef]
- Park, C.H.; Zhao, S.; Yeo, H.J.; Park, Y.E.; Baska, T.B.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Comparison of Different Strains of Agrobacterium rhizogenes for Hairy Root Induction and Betulin and Betulinic Acid Production in Morus alba. Nat. Prod. Commun. 2017, 12, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Shilpha, J.; Satish, L.; Kavikkuil, M.; Joe Virgin Largia, M.; Ramesh, M. Methyl Jasmonate Elicits the Solasodine Production and Antioxidant Activity in Hairy Root Cultures of Solanum trilobatum L. Ind. Crops Prod. 2015, 71, 54–64. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of Methyl Jasmonate and Salicylic Acid on Tanshinone Production and Biosynthetic Gene Expression in Transgenic Salvia miltiorrhiza Hairy Roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver Nanoparticles Elicited in Vitro Callus Cultures for Accumulation of Biomass and Secondary Metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of Light Quality on Growth, Secondary Metabolites Production and Antioxidant Activity in Callus Culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018, 183, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bharadvaja, N. Establishment of Root Suspension Culture of Plumbago zeylanica and Enhanced Production of Plumbagin. Ind. Crops Prod. 2019, 137, 419–427. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Zabiegala, B.; Kubica, P.; Szopa, A.; Bucinski, A.; Ekiert, H.; Luczkiewicz, M. Accumulation of Volatile Constituents in Agar and Bioreactor Shoot Cultures of Verbena officinalis L. Plant Cell. Tissue Organ Cult. 2021, 144, 671–679. [Google Scholar] [CrossRef]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of Salicylic Acid and Methyl Jasmonate in the Production of Phenolic Compounds in Plant Cell Suspension Cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar] [CrossRef]
- Krishnan, S.R.S.; Siril, E.A. Elicitor Mediated Adventitious Root Culture for the Large-Scale Production of Anthraquinones from Oldenlandia umbellata L. Ind. Crops Prod. 2018, 114, 173–179. [Google Scholar] [CrossRef]
- Keskin, N.; Kunter, B. The Effects of Callus Age, UV Irradiation and Incubation Time on Trans-Resveratrol Production in Grapevine Callus Culture. Tarim Bilim. Derg. 2009, 15, 9–13. [Google Scholar] [CrossRef][Green Version]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Biotechnology, In Vitro Production of Natural Bioactive Compounds, Herbal Preparation, and Disease Management (Treatment and Prevention). In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2; Springer: Berlin/Heidelberg, Germany, 2018; pp. 585–664. [Google Scholar] [CrossRef]
- Mastan, A.; Bharadwaj, R.; Kushwaha, R.K.; Vivek Babu, C.S. Functional Fungal Endophytes in Coleus forskohlii Regulate Labdane Diterpene Biosynthesis for Elevated Forskolin Accumulation in Roots. Microb. Ecol. 2019, 78, 914–926. [Google Scholar] [CrossRef]
- Veerashree, V.; Anuradha, C.M.; Kumar, V. Elicitor-Enhanced Production of Gymnemic Acid in Cell Suspension Cultures of Gymnema sylvestre R. Br. Plant Cell. Tissue Organ Cult. 2012, 108, 27–35. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, T.; Ali, H. Plant Cell Culture Strategies for the Production of Terpenes as Green Solvents. Ind. Appl. Green Solvents 2019, 50, 1–20. [Google Scholar]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Karigar, C.S.; Kim, S.W.; Goo, G.H.; Choi, M.S. Effect of Biotic Elicitors on the Accumulation of Bilobalide and Ginkgolides in Ginkgo biloba Cell Cultures. J. Biotechnol. 2009, 139, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Almagro, L.; Gutierrez, J.; Pedreño, M.A.; Sottomayor, M. Synergistic and Additive Influence of Cyclodextrins and Methyl Jasmonate on the Expression of the Terpenoid Indole Alkaloid Pathway Genes and Metabolites in Catharanthus roseus Cell Cultures. Plant Cell. Tissue Organ Cult. 2014, 119, 543–551. [Google Scholar] [CrossRef]
- Shilpha, J.; Largia, M.J.V.; Kumar, R.R.; Satish, L.; Swamy, M.K.; Ramesh, M. Hairy Root Cultures: A Novel Way to Mass Produce Plant Secondary Metabolites. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer Nature: Singapore, 2023; pp. 417–445. [Google Scholar] [CrossRef]
- Palazón, J.; Navarro-Ocaña, A.; Hernandez-Vazquez, L.; Mirjalili, M.H. Application of Metabolic Engineering to the Production of Scopolamine. Molecules 2008, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, Y.; Liu, X.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. Soybean Hairy Roots Produced in Vitro by Agrobacterium rhizogenes-Mediated Transformation. Crop J. 2018, 6, 162–171. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Chen, S.T.; Agnolet, S.; Hegelund, J.N.; Stanstrup, J.; Christensen, J.H.; Müller, R.; Lütken, H. Ethephon-Induced Changes in Antioxidants and Phenolic Compounds in Anthocyanin-Producing Black Carrot Hairy Root Cultures. J. Exp. Bot. 2020, 71, 7030–7045. [Google Scholar] [CrossRef]
- Morey, K.J.; Peebles, C.A. Hairy roots: An untapped potential for production of plant products. Front. Plant Sci. 2022, 13, 2808. [Google Scholar] [CrossRef]
- Li, M.; Peebles, C.A.M.; Shanks, J.V.; San, K.Y. Effect of Sodium Nitroprusside on Growth and Terpenoid Indole Alkaloid Production in Catharanthus roseus Hairy Root Cultures. Biotechnol. Prog. 2011, 27, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Gai, Q.Y.; Wang, W.; Zang, Y.P.; Niu, L.L.; Fu, Y.J.; Wang, X. Remarkable Enhancement of Flavonoid Production in a Co-Cultivation System of Isatis tinctoria L. Hairy Root Cultures and Immobilized Aspergillus niger. Ind. Crops Prod. 2018, 112, 252–261, Erratum in Ind. Crops Prod. 2019, 138, 111434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).