The Impact of Acute Low-Dose Gamma Irradiation on Biomass Accumulation and Secondary Metabolites Production in Cotinus coggygria Scop. and Fragaria × ananassa Duch. Red Callus Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Irradiation of Callus Cultures Using Gamma Rays
2.3. Biomass Production Evaluation
2.4. Biochemical Analysis
2.4.1. Total Phenolic Content (TPC) Evaluation
2.4.2. Total Flavonoid Content (TFC) Evaluation
2.4.3. Total Monomeric Anthocyanins’ Content (TAC) Evaluation
- MW = molecular weight (449.2 g/mol C-3-G);
- DF = dilution factor;
- 1 = optical path (1 cm);
- ε = molar absorptivity (26,900 L × mol−1 × cm−1 for C-3-G).
- Results were expressed as micrograms of cyanidin-3-glucoside equivalents (C-3-GE)/g DW.
2.4.4. Index of Anthocyanins Polymerization (Degradation or Oxidation)
2.4.5. Antioxidant Activity (DPPH) Evaluation
2.4.6. Preparation of Callus Extracts for UPLC-HRMS Analysis
2.4.7. UPLC-HRMS Analysis
2.4.8. Chromatographic Data Processing
2.5. Statistical Analysis
3. Results
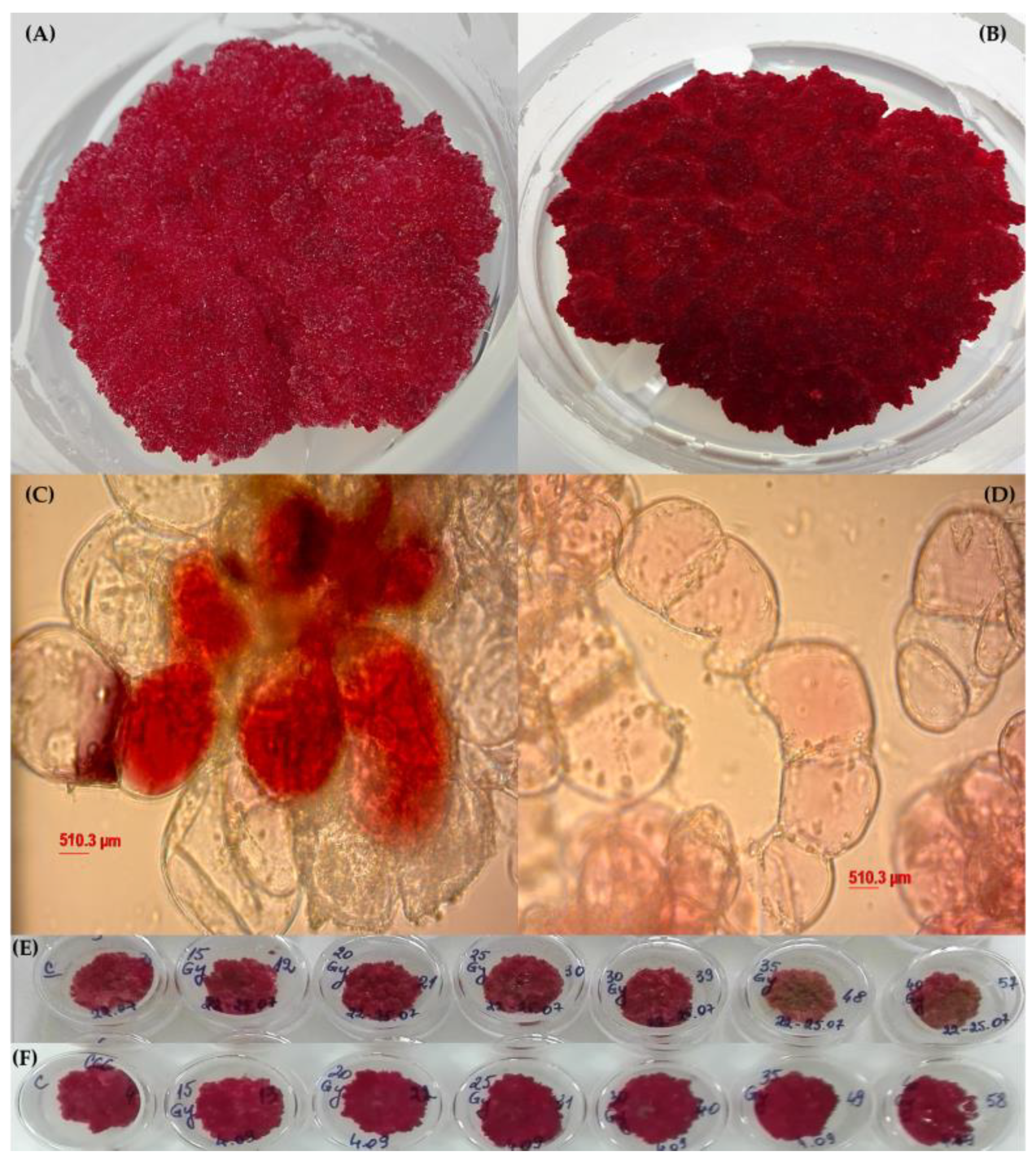
3.1. Callus Cultures Characterization
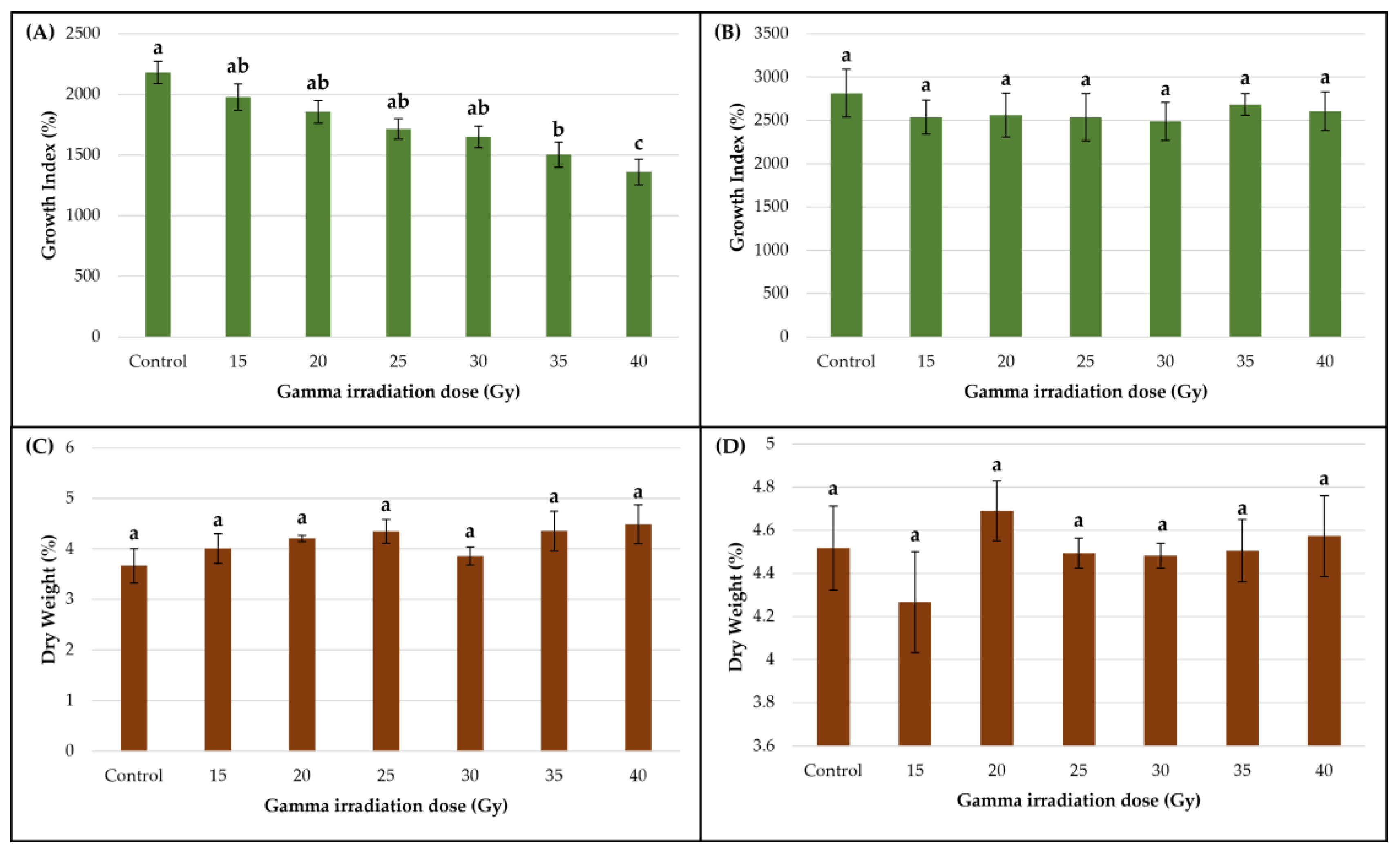
3.2. Impact of Gamma Irradiation on Callus Biomass
3.3. Impact of Gamma Irradiation on Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and Total Monomeric Anthocyanins’ Content (MAC)
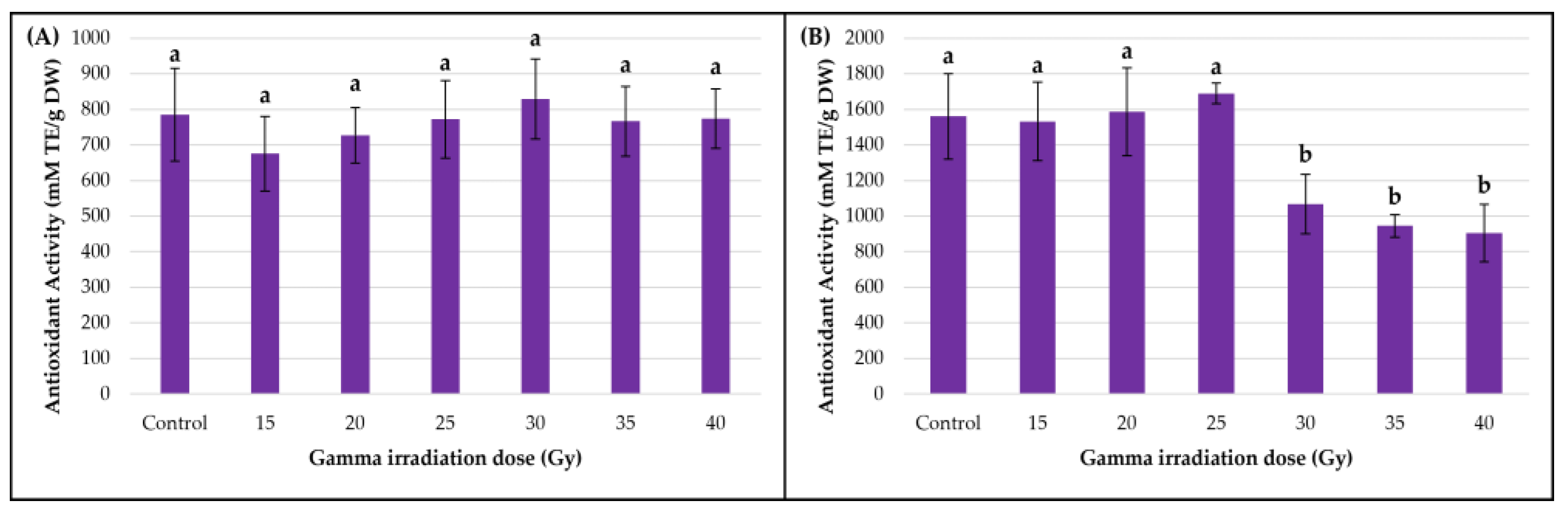
3.4. Impact of Gamma Irradiation on Antioxidant Activity (DPPH)
3.5. UPLC-HRMS Analysis of Secondary Metabolites Post Gamma Irradiation
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Xiong, K.; Wen, W.; Li, L.; Xu, D. Functional Endophytes Regulating Plant Secondary Metabolism: Current Status, Prospects and Applications. Int. J. Mol. Sci. 2023, 24, 1153. [Google Scholar] [CrossRef]
- Li, M.; Cai, S.; You, S.; Liu, Y. Multiomics techniques for plant secondary metabolism engineering: Pathways to shape the bioeconomy. In Translational and Applied Genomics, Genomics and the Global Bioeconomy, 1st ed.; Lopez-Correa, C., Suarez-Gonzalez, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 1, pp. 205–252. [Google Scholar] [CrossRef]
- Farkya, S.; Bisaria, V.S.; Srivastava, A.K. Biotechnological aspects of the production of the anticancer drug podophyllotoxin. Appl. Microbiol. Biotechnol. 2004, 65, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kültür, Ş. Medicinal plants used in Kırklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef]
- Antal, D.S.; Ardelean, F. “Cotinus coggygria Scop.”, in Romanian traditional medicine: Results of an ethnobotanical survey performed in the South-Western part of the country. In Natural Products as a Source of Compounds with Chemopreventive and Anti-inflammatory Activity. Abstracts of the International Workshop of the Romanian-French Bilateral Project, 1st ed.; Antal, D.S., Ollivier, E., Eds.; PN II-CT-789/30.06.2014; Victor Babes University: Timisoara, Romania, 2015; p. 22. ISBN 978-606-8456-67-6. [Google Scholar]
- Marčetić, M.; Božić, D.; Milenković, M.; Malešević, N.; Radulović, S.; Kovačević, N. Antimicrobial, antioxidant and anti-inflammatory activity of young shoots of the smoke tree, Cotinus coggygria Scop. Phytother. Res. 2013, 27, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Sen, A.; Sancar, M.; Sekerler, T.; Akakin, D.; Bitis, L.; Uras, F.; Kultur, S.; Izzettin, F.V. Ethanol extract of Cotinus coggygria leaves accelerates wound healing process in diabetic rats. Pharm. Biol. 2016, 54, 2732–2736. [Google Scholar] [CrossRef] [PubMed]
- Westenburg, H.E.; Lee, K.J.; Lee, S.K.; Fong, H.H.; van Breemen, R.B.; Pezzuto, J.M.; Kinghorn, A.D. Activity-Guided Isolation of Antioxidative Constituents of Cotinus coggygria. J. Nat. Prod. 2000, 63, 1696–1698. [Google Scholar] [CrossRef] [PubMed]
- Antal, D.S.; Schwaiger, S.; Ellmerer-Müller, E.P.; Stuppner, H. Cotinus coggygria wood: Novel flavanone dimer and development of an HPLC/UV/MS method for the simultaneous determination of fourteen phenolic constituents. Planta Med. 2010, 76, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Stanić, S.; Bogojević, D.; Vidaković, M.; Grdović, N.; Dinić, S.; Solujić, S.; Mladenović, M.; Stanković, N.; Mihailović, M. Methanol extract from the stem of Cotinus coggygria Scop., and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2013, 755, 81–89. [Google Scholar] [CrossRef]
- Antal, D.S.; Ardelean, F.; Jijie, R.; Pinzaru, I.; Soica, C.; Dehelean, C. Integrating Ethnobotany, Phytochemistry, and Pharmacology of Cotinus coggygria and Toxicodendron vernicifluum: What Predictions can be Made for the European Smoketree? Front. Pharmacol. 2021, 12, 662852. [Google Scholar] [CrossRef] [PubMed]
- Tanchev, S.S.; Timberlake, C.F. Anthocyanins in leaves of Cotinus coggygria. Phytochemistry 1969, 8, 2367–2369. [Google Scholar] [CrossRef]
- Iwashina, T. Detection and distribution of chrysanthemin and idaein in autumn leaves of plants by high performance liquid chromatography. Ann. Tsukuba Bot. Gard. 1996, 15, 1–18. Available online: https://cir.nii.ac.jp/crid/1520853835465945856 (accessed on 9 February 2023).
- Valianou, L.; Stathopoulou, K.; Karapanagiotis, I.; Magiatis, P.; Pavlidou, E.; Skaltsounis, A.-L.; Chryssoulakis, Y. Phytochemical analysis of young fustic (Cotinus coggygria heartwood) and identification of isolated colourants in historical textiles. Anal. Bioanal. Chem. 2009, 394, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Djordjevic, I.; Todorovic, N.; Trifunovic, S.; Andjelkovic, B.; Mandic, B.; Jadranin, M.; Vuckovic, I.; Vajs, V.; Milosavljevic, S.; et al. New aurone epoxide and auronolignan from the heartwood of Cotinus coggygria Scop. Nat. Prod. Res. 2018, 33, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Rendeková, K.; Fialová, S.; Jánošová, L.; Mučaji, P.; Slobodníková, L. The Activity of Cotinus coggygria Scop. Leaves on Staphylococcus aureus Strains in Planktonic and Biofilm Growth Forms. Molecules 2016, 21, 50. [Google Scholar]
- Demirci, B.; Demirci, F.; Başer, K.H.C. Composition of the essential oil of Cotinus coggygria Scop. from Turkey. Flavour Fragr. J. 2003, 18, 43–44. [Google Scholar] [CrossRef]
- Bahadirli, N.P. Essential oil content and composition of Cotinus coggygria Scop. from Hatay, Turkey. Int. J. Agric. For. Life Sci. 2020, 4, 111–114. Available online: http://dergipark.gov.tr/ijafls (accessed on 20 May 2023).
- Thapa, P.; Prakash, O.; Rawat, A.; Kumar, R.; Srivastava, R.M.; Rawat, D.S.; Pant, A.K. Essential Oil Composition, Antioxidant, Anti-inflammatory, Insect Antifeedant and Sprout Suppressant Activity in Essential Oil from Aerial Parts of Cotinus coggygria Scop. J. Essent. Oil Bear. Plants 2020, 23, 65–76. [Google Scholar] [CrossRef]
- Hethelyi, I.; Domokos, J.; Lemberkovic, E.; Verzar-Petri, G. Analysis of the essential oil of Cotinus coggygria by means of mass spectrometry. Herba Hun 1986, 25, 135–148. [Google Scholar]
- Tsankova, E.T.; Dyulgerov, A.S.; Milenkov, B.K. Chemical composition of the Bulgarian sumac oil. J. Essent. Oil Res. 1993, 5, 205–207. [Google Scholar] [CrossRef]
- Tzakou, O.; Bazos, I.; Yannitsaros, A. Essential oils of leaves, inflorescences and infructescences of spontaneous Cotinus coggygria Scop. from Greece. Flavour Fragr. J. 2005, 20, 531–533. [Google Scholar] [CrossRef]
- Novaković, M.; Vučković, I.; Janaćković, P.; Soković, M.; Filipović, A.; Tešević, V.; Milosavljević, S. Chemical composition, antibacterial and antifungal activity of the essential oils of Cotinus coggygria from Serbia. J. Serb. Chem. Soc. 2007, 72, 1045–1051. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D. Chemical composition and antimicrobial activity of the essential oil of Cotinus coggygria Scoop. from Italy. J. Essent. Oil Bear. Plants 2014, 17, 366–370. [Google Scholar] [CrossRef]
- da Silva, J.A.T.; Pacholczak, A.; Ilczuk, A. Smoke tree (Cotinus coggygria Scop.) propagation and biotechnology: A mini-review. South Afr. J. Bot. 2018, 114, 232–240. [Google Scholar] [CrossRef]
- Almeida, J.R.; D’Amico, E.; Preuss, A.; Carbone, F.; de Vos, C.R.; Deiml, B.; Mourgues, F.; Perrotta, G.; Fischer, T.C.; Bovy, A.G.; et al. Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria × ananassa). Arch. Biochem. Biophys. 2007, 465, 61–71. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, H.; Huang, T.; Shen, X.; Xia, J.; Pang, F.; Wang, J.; Zhao, M. The complexity of the Fragaria × ananassa (octoploid) transcriptome by single-molecule long-read sequencing. Hortic. Res. 2019, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Mazzoni, L.; Romandini, S.; Bompadre, S.; Diamanti, J.; Capocasa, F.; Mezzetti, B.; Quiles, J.L.; Ferreiro, M.S. The potential impact of strawberry on human health. Nat. Prod. Res. 2013, 27, 448–455. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Weaver, J.; Briscoe, T.; Hou, M.; Goodman, C.; Kata, S.; Ross, H.; McDougall, G.; Stewart, D.; Riches, A. Strawberry polyphenols are equally cytotoxic to tumourigenic and normal human breast and prostate cell lines. Int. J. Oncol. 2009, 34, 777–786. [Google Scholar] [CrossRef]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, A.-G.; Tecuceanu, V.; Enache-Preoteasa, C.; Mitoi, E.M.; Helepciuc, F.E.; Dimov, T.V.; Simon-Gruita, A.; Cogălniceanu, G.C. Phenological and Environmental Factors’ Impact on Secondary Metabolites in Medicinal Plant Cotinus coggygria Scop. Plants 2023, 12, 1762. [Google Scholar] [CrossRef] [PubMed]
- Mitoi, M.E.; Ciocan, A.G.; Maximilian, C.R.; Helepciuc, F.E.; Cogălniceanu, G.C. Biomass and Valuable Metabolites Dynamic Accumulation In Strawberry Callus Cultures. AgroLife Sci. J. 2022, 11, 100–1009, ISSN ONLINE 2286-0126. [Google Scholar] [CrossRef]
- Katiyar, P.; Pandey, N.; Keshavkant, S. Gamma radiation: A potential tool for abiotic stress mitigation and management of agroecosystem. Plant Stress 2022, 5, 100089. [Google Scholar] [CrossRef]
- Chung, B.Y.; Lee, Y.B.; Baek, M.H.; Kim, J.H.; Wi, S.G.; Kim, J.S. Effects of low-dose gamma-irradiation on production of shikonin derivatives in callus cultures of Lithospermum erythrorhizon S. Radiat. Phys. Chem. 2006, 75, 1018–1023. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat. Phys. Chem. 2011, 80, 968–976. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Bae, T.W.; Boo, K.H.; Sun, H.J.; Song, I.J.; Pham, C.H.; Ganesan, M.; Yang, D.H.; Kang, H.G.; Ko, S.M.; et al. Ginsenoside production and morphological characterization of wild ginseng (Panax ginseng Meyer) mutant lines induced by γ-irradiation (60Co) of adventitious roots. J. Ginseng Res. 2011, 35, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Song, M.; Kim, S.H.; Jang, D.S.; Kim, J.B.; Ha, B.K.; Kim, S.H.; Lee, K.J.; Kang, S.Y.; Jeong, I.Y. The improvement of ginsenoside accumulation in Panax ginseng as a result of γ-irradiation. J. Ginseng Res. 2013, 37, 332–340. [Google Scholar] [CrossRef]
- Fulzele, D.P.; Satdive, R.; Kamble, S.; Singh, S.; Singh, S. Improvement of Anticancer Drug Camptothecin Production by Gamma Irradiation on Callus Cultures of Nothapodytes foetida. Int. J. Pharm. Res. Allied Sci. 2015, 4, 19–27, ISSN 2277-3657. [Google Scholar]
- Khalil, S.A.; Ahmad, N.; Zamir, R. Gamma radiation induced variation in growth characteristics and production of bioactive compounds during callogenesis in Stevia rebaudiana (Bert.). New Negat. Plant Sci. 2015, 1–2, 1–5. [Google Scholar] [CrossRef]
- Azeez, H.; Ibrahim, K.; Pop, R.; Pamfil, D.; Hârţa, M.; Bobiș, O. Changes induced by gamma ray irradiation on biomass production and secondary metabolites accumulation in Hypericum triquetrifolium Turra callus cultures. Ind. Crops Prod. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Patil, A.; Suryavanshi, P.; Fulzele, D. In vitro regeneration of gamma irradiated callus of Artemisia annua and evaluation of increase artemisinin content by HPLC analysis. J. Anal. Pharm. Res. 2018, 7, 569–573. [Google Scholar] [CrossRef]
- Khalifa, A.M.; Abd-ElShafy, E.; Abu-Khudir, R.; Gaafar, R.M. Influence of gamma radiation and phenylalanine on secondary metabolites in callus cultures of milk thistle (Silybum marianum L.). J. Genet. Eng. Biotechnol. 2022, 20, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, A.G.; Mitoi, E.M.; Helepciuc, F.E.; Neguţ, D.; Moldovan, R.C.; Petrache, A.M.; Iuga, C.A.; Holobiuc, I.M.; Maximilian, C.R.; Cogălniceanu, G.C. Is acute low-dose gamma irradiation an effective elicitor for secondary metabolism in Leontopodium alpinum (Cass.) callus culture? Ind. Crops Prod. 2023, 197, 116547. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Cogălniceanu, G.C.; Mitoi, E.M.; Ciocan, A.G.; Holobiuc, M.I.; Maximilian, R.C.; Helepciuc, F.E.; Morosanu, A.M. Biotechnological Procedure for Initiating and Obtaining High Proliferative Cell Mass Producing Bioactive Compounds in Cotinus coggygria Scop. (Smoketree) and the Crude Extract. OSIM Romania Patent Application A/00837/17.12.2020; (Patent pending),
- Cogălniceanu, G.C.; Mitoi, E.M.; Ciocan, A.G.; Holobiuc, M.I.; Maximilian, R.C.; Helepciuc, F.E.; Morosanu, A.M. Biotechnological Procedure for Initiating and Obtaining High Proliferative Cell Mass Producing Bioactive Compounds in Fragaria x ananassa Duch. (Strawberry) and the Crude Extract. OSIM Romania Patent Application A/00808/04.12.2020; (Patent pending),
- Stankovic, M.S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp. polium. Acta Soc. Bot. Pol. 2012, 81, 117–122. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterisation of the flavonoids from Opuntia milpa alta skin. Czech J. Food Sci. 2010, 28, 108–116. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–13. [Google Scholar] [CrossRef]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH Radical Oxidation Caused by Methanolic Extracts of Some Microalgal Species by Linear Regression Analysis of Spectrophotometric Measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef]
- Hawkins, C.; Ginzburg, D.; Zhao, K.; Dwyer, W.; Xue, B.; Xu, A.; Rice, S.; Cole, B.; Paley, S.; Karp, P.; et al. Plant Metabolic Network 15: A resource of genome-wide metabolism databases for 126 plants and algae. J. Integr. Plant Biol. 2021, 63, 1888–1905. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS Profile, Gastrointestinal and Gut Microbiota Stability and Antioxidant Activity of Rhodiola rosea Herb Metabolites: A Comparative Study with Subterranean Organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Stanić, S.; Mihailović, M.; Bogojević, D. Cotinus coggygria Scop.: An overview of its chemical constituents, pharmacological and toxicological potential. Saudi J. Biol. Sci. 2016, 23, 452–461. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 13 April 2023).
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted metabolomics strategies—Challenges and emerging directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Hasbullah, N.A.; Taha, R.M.; Saleh, A.; Mahmad, N. Irradiation effect on in vitro organogenesis, callus growth and plantlet development of Gerbera jamesonii. Hortic. Bras. 2012, 30, 252–257. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Jaafar, H.; Ibrahim, R.; Rahmat, A.; Aziz, M.A.; Philip, E. Effects of acute gamma irradiation on physiological traits and flavonoid accumulation of Centella asiatica. Molecules 2011, 6, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Ashouri Sheikhi, A.; Hassanpour, H.; Jonoubi, P.; Ghorbani Nohooji, M.; Nadimifar, M.S. The effect of gamma irradiation on in vitro total phenolic content and antioxidant activity of Ferula gummosa Bioss. J. Med. Plants 2016, 3, 122–131. [Google Scholar]
- Ghosh, J.; Sil, P.C. Arjunolic acid: A new multifunctional therapeutic promise of alternative medicine. Biochimie 2013, 95, 1098–1109. [Google Scholar] [CrossRef]
- Manna, P.; Sinha, M.; Sil, P.C. Protection of arsenic-induced hepatic disorder by arjunolic acid. Basic Clin. Pharmacol. Toxicol. 2007, 101, 333–338. [Google Scholar] [CrossRef]
- Manna, P.; Sinha, M.; Sil, P.C. Arsenic-induced oxidative myocardial injury: Protective role of arjunolic acid. Arch. Toxicol. 2008, 82, 137–149. [Google Scholar] [CrossRef]
- Sinha, M.; Manna, P.; Sil, P. Arjunolic acid attenuates arsenic-induced nephrotoxicity. Pathophysiology 2008, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Sinha, M.; Sil, P.C. Protection of arsenic-induced testicular oxidative stress by arjunolic acid. Redox Rep. 2008, 13, 67–77. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, Q.; Lu, Z.; Luan, X.; Fan, L.; Wang, Z.; Luo, D. Maslinic acid attenuates UVB-induced oxidative damage in HFF-1 cells. J. Cosmet. Dermatol. 2023, 22, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Liu, C.; Xu, M.; Li, T.; Wang, Y.; Feng, J.; Yin, X.; Du, X.; Lu, C. Maslinic Acid Ameliorates Myocardial Ischemia Reperfusion Injury-Induced Oxidative Stress via Activating Nrf2 and Inhibiting NF-κ B Pathways. Am. J. Chin. Med. 2023, 51, 929–951. [Google Scholar] [CrossRef]
- Lahlou, E.H.; Hirai, N.; Kamo, T.; Tsuda, M.; Ohigashi, H. Actinidic acid, a new triterpene phytoalexin from unripe kiwi fruit. Biosci. Biotechnol. Biochem. 2001, 65, 480–483. [Google Scholar] [CrossRef]
- Ma, J.T.; Li, D.W.; Liu, J.K.; He, J. Advances in research on chemical constituents and their biological activities of the genus Actinidia. Nat. Prod. Bioprospect. 2021, 11, 573–609. [Google Scholar] [CrossRef]
- Jang, D.S.; Lee, G.Y.; Kim, J.; Lee, Y.M.; Kim, J.M.; Kim, Y.S.; Kim, J.S. A new pancreatic lipase inhibitor isolated from the roots of Actinidia arguta. Arch. Pharmacal Res. 2008, 31, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Essafi, H.; Trabelsi, N.; Benincasa, C.; Tamaalli, A.; Perri, E.; Zarrouk, M. Phytochemical profile, antioxidant and antiproliferative activities of olive leaf extracts from autochthonous Tunisian cultivars. Acta Aliment. 2019, 48, 384–390. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Yao, Z.-M.; Sun, X.-R.; Tong, X.-H.; Hu, M.; Zhang, Y.; Dong, S.-Y. Procyanidins Alleviated Cerebral Ischemia/Reperfusion Injury by Inhibiting Ferroptosis via the Nrf2/HO-1 Signaling Pathway. Molecules 2023, 28, 3582. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Oh, A.; Hou, L.; Lee, E.; Aguilar, J.; Li, A.; Mack, W.J. Flavonoid quercetin and its glucuronide and sulfate conjugates bind to 67-kDa laminin receptor and prevent neuronal cell death induced by serum starvation. Biochem. Biophys. Res. Commun. 2023, 3, 116–123. [Google Scholar] [CrossRef]
- Yu, P.R.; Hsu, J.Y.; Tseng, C.Y.; Chen, J.H.; Lin, H.H. The inhibitory effect of quercetin-3-glucuronide on pulmonary injury in vitro and in vivo. J. Food Drug Anal. 2023, 31, 254–277. [Google Scholar] [CrossRef]
- Banerjee, S.; Bose, S.; Mandal, S.C.; Dawn, S.; Sahoo, U.A.; Ramadan, M.; Mandal, S.K. Pharmacological Property of Pentacyclic Triterpenoids. Egypt. J. Chem. 2019, 62, 13–35. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R. Oleanolic acid and related triterpenoids from olives on vascular function: Molecular mechanisms and therapeutic perspectives. Curr. Med. Chem. 2015, 22, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, M.R.J.; Rao, V.C. Triterpenoids for cancer prevention and treatment: Current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef] [PubMed]
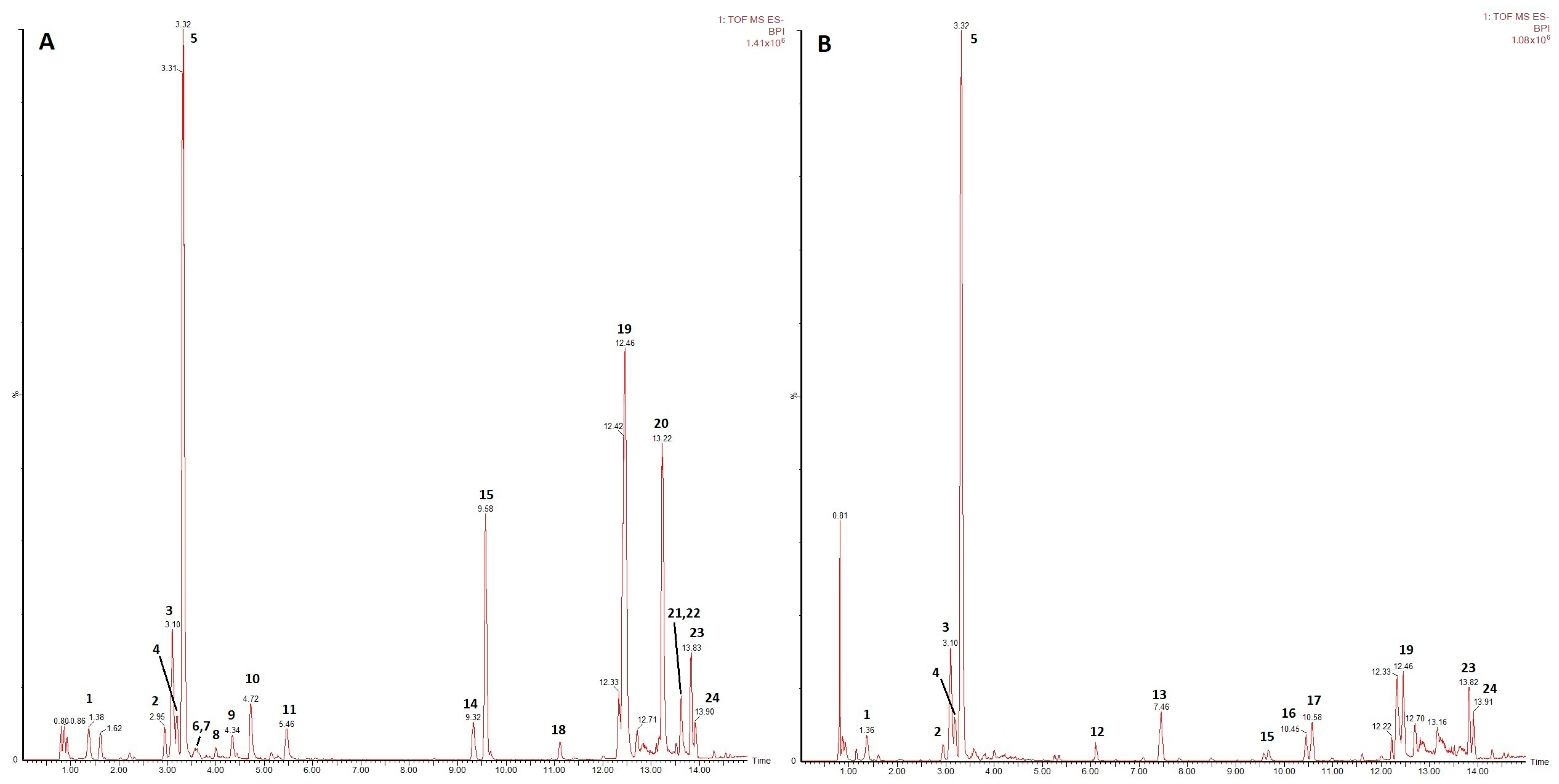


| Peak | Rt (min) | m/z Measured (Da) | m/z Calculated (Da) | ΔmDa | Formula | Major Fragments | Tentative Identification | Found in | Ident. Level * | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cotinus | Fragaria | ||||||||||
| 1 | 1.37 | 331.0666 | 331.0665 | 0.1 | C13H15O10 | 211.0233, 169.0083, 125.0199 | Galloylglucose | Yes | Yes | 2 | [54,55] |
| 2 | 2.95 | 577.1352 | 577.1346 | 0.6 | C30H25O12 | 407.0744, 289.0702, 245.0776, 161.0192 | Procyanidin dimer | Yes | Yes | 2 | [54,55,56] |
| 3 | 3.10 | 577.1354 | 577.1346 | 0.8 | C30H25O12 | 407.0746, 289.0703, 245.0786, 161.0245 | Procyanidin dimer | Yes | Yes | 2 | [54,55,56] |
| 4 | 3.19 | 865.1979 | 865.1979 | 0.0 | C45H37O18 | 695.1358, 577.1317, 407.0739, 289.0695, 243.0277, 161.0209 | Procyanidin trimer | Yes | Yes | 2 | [54,55,56] |
| 5 | 3.32 | 289.0717 | 289.0712 | 0.5 | C15H13O6 | 245.0803, 203.0696, 123.0434 | (+)-Catechin ** | Yes | Yes | 1 | [54,55] |
| 6 | 3.59 | 447.0933 | 447.0927 | 0.6 | C21H19O11 | 285.0372 | Luteolin-4-glucoside | Yes | No | 2 | [54,55,57] |
| 7 | 3.6 | 1153.2602 | 1153.2614 | −1.2 | C60H49O24 | 865.1873, 575.1165, 447.0907, 407.0699, | Procyanidin tetramer | Yes | No | 2 | [54,55] |
| 8 | 4.14 | 1153.2583 | 1153.2614 | −3.1 | C60H49O24 | 865.1932, 720.1556, 583.1995, 576.1232, 289.0698 | Procyanidin tetramer | Yes | No | 2 | [54,55] |
| 9 | 4.35 | 935.0794 | 935.0790 | 0.4 | C41H27O26 | 633.0715, 300.9980, 169.0133 | Ellagitannin (Casuarinin) | Yes | No | 2 | [54,55] |
| 10 | 4.72 | 937.0946 | 937.0948 | −0.1 | C41H29O26 | 468.0424, 300.9981, 169.0134 | Ellagitannin (Tellimagrandin II) | Yes | No | 2 | [54,55] |
| 11 | 5.46 | 939.1104 | 939.1103 | 0.1 | C41H31O26 | 769.0873, 617.0767, 169.0133 | Gallotannin (Pentagalloyl glucose) | Yes | No | 2 | [54,55,56] |
| 12 | 6.11 | 477.1033 | 477.1033 | 0.0 | C22H21O12 | 314.0402, 300.0200, 285.0390, 271.0211, 243.0244 | Quercetin-3-glucuronide | No | Yes | 2 | [54,55] |
| 13 | 7.45 | 711.3965 | - | - | - | 503.3334 | Unidentified | No | Yes | 5 | [54,55] |
| 14 | 9.32 | 695.4013 | - | - | - | 487.3402 | Unidentified | Yes | No | 5 | [54,55] |
| 15 | 9.57 | 695.4014 | - | - | - | 487.3407 | Unidentified | Yes | Yes | 5 | [54,55] |
| 16 | 10.45 | 503.3374 | 503.3372 | 0.2 | C30H47O6 | 485.3228, 453.3029 | Unidentified | No | Yes | 4 | [54,55] |
| 17 | 10.58 | 503.3374 | 503.3372 | 0.2 | C30H47O6 | 485.3263, 453.2953, 441.3333, 421.3064, 409.3079 | Unidentified | No | Yes | 4 | [54,55] |
| 18 | 11.11 | 693.3853 | 693.3850 | 0.3 | C37H57O12 | 485.3254 | Triterpenoid (Phytolaccoside) | Yes | No | 3 | [54,55] |
| 19 | 12.45 | 487.3430 | 487.3423 | 0.7 | C30H47O5 | 469.3312, 423.3254, 407.3299 | Pentacyclic triterpenoid (Arjunolic acid) | Yes | Yes | 3 | [54,55] |
| 20 | 13.23 | 485.327 | 485.3267 | −0.3 | C30H45O5 | 467.1826 | Pentacyclic triterpenoid (actinidic acid) | Yes | No | 3 | [54,55] |
| 21 | 13.54 | 633.3794 | 633.3791 | 0.3 | C39H53O7 | - | Unidentified | Yes | No | 4 | [54,55] |
| 22 | 13.62 | 471.3477 | 471.3474 | 0.3 | C30H47O4 | 381.2289 | Pentacyclic triterpene (Maslinic acid) | Yes | No | 3 | [54,55] |
| 23 | 13.82 | 471.3477 | 471.3474 | 0.3 | C30H47O4 | - | Pentacyclic triterpene | Yes | Yes | 3 | [54,55] |
| 24 | 13.91 | 471.3477 | 471.3474 | 0.3 | C30H47O4 | - | Pentacyclic triterpene | Yes | Yes | 3 | [54,55] |
| Peak | Compound | Gamma Irradiation Dose | |||||
|---|---|---|---|---|---|---|---|
| 15 Gy | 20 Gy | 25 Gy | 30 Gy | 35 Gy | 40 Gy | ||
| 1 | Galloyl glucose | 0.91 | 0.90 | 0.83 | 0.74 | 0.81 | 0.76 |
| 2 | Procyanidin dimer | 0.85 | 0.91 | 0.74 | 0.77 | 0.74 | 0.67 |
| 3 | Procyanidin dimer | 0.91 | 0.97 | 0.88 | 0.90 | 0.92 | 0.89 |
| 4 | Procyanidin trimer | 0.90 | 0.98 | 0.86 | 0.90 | 0.91 | 0.87 |
| 5 | (+)-Catechin | 0.90 | 0.93 | 0.84 | 0.84 | 0.86 | 0.83 |
| 6 | Luteolin-4-glucoside | 1.10 | 1.22 | 1.26 | 1.12 | 1.11 | 1.10 |
| 7 | Procyanidin tetramer | 0.93 | 1.06 | 0.95 | 1.02 | 1.08 | 1.08 |
| 8 | Procyanidin tetramer | 0.94 | 1.14 | 1.00 | 1.07 | 1.08 | 1.08 |
| 9 | Ellagitannin (Casuarinin) | 0.87 | 0.64 | 0.66 | 0.39 | 0.52 | 0.58 |
| 10 | Ellagitannin (Tellimagrandin II) | 0.82 | 0.76 | 0.78 | 0.56 | 0.65 | 0.65 |
| 11 | Gallotannin (Pentagalloyl glucose) | 0.82 | 0.86 | 0.81 | 0.70 | 0.72 | 0.66 |
| 14 | Unidentified | 0.88 | 1.05 | 0.98 | 0.82 | 0.76 | 0.67 |
| 15 | Unidentified | 0.86 | 0.99 | 0.86 | 0.77 | 0.74 | 0.64 |
| 18 | Triterpenoid (Phytolaccoside) | 0.91 | 0.97 | 0.94 | 0.80 | 0.87 | 0.72 |
| 19 | Pentacyclic triterpenoid (Arjunolic acid) | 1.05 | 1.09 | 1.06 | 1.07 | 1.09 | 1.12 |
| 20 | Pentacyclic triterpenoid (Actinidic acid) | 1.05 | 1.09 | 1.11 | 1.16 | 1.29 | 1.34 |
| 21 | Unidentified | 0.88 | 1.07 | 0.85 | 1.10 | 1.09 | 1.11 |
| 22 | Pentacyclic triterpene (Maslinic acid) | 1.08 | 1.19 | 1.20 | 1.27 | 1.37 | 1.51 |
| 23 | Pentacyclic triterpene | 0.97 | 0.98 | 0.84 | 0.82 | 0.78 | 0.77 |
| 24 | Pentacyclic triterpene | 0.97 | 0.93 | 0.78 | 0.74 | 0.65 | 0.62 |
| Peak | Compound | Gamma Irradiation Dose | |||||
|---|---|---|---|---|---|---|---|
| 15 Gy | 20 Gy | 25 Gy | 30 Gy | 35 Gy | 40 Gy | ||
| 1 | Galloyl glucose | 1.03 | 0.93 | 0.98 | 0.91 | 0.75 | 0.59 |
| 2 | Procyanidin dimer | 0.96 | 0.98 | 0.99 | 0.96 | 1.04 | 1.02 |
| 3 | Procyanidin dimer | 0.94 | 0.99 | 1.01 | 0.98 | 1.03 | 1.02 |
| 4 | Procyanidin trimer | 0.93 | 0.98 | 0.98 | 0.94 | 1.05 | 1.05 |
| 5 | Catechin | 0.97 | 1.00 | 1.01 | 0.99 | 1.04 | 1.03 |
| 12 | Quercetin-3-glucuronide | 1.03 | 1.35 | 1.22 | 1.08 | 1.13 | 1.27 |
| 13 | Unidentified | 1.01 | 1.08 | 1.07 | 0.98 | 0.89 | 0.94 |
| 15 | Unidentified | 0.85 | 0.60 | 0.62 | 0.75 | 0.44 | 0.40 |
| 16 | Unidentified | 0.98 | 1.24 | 1.26 | 1.23 | 1.37 | 1.71 |
| 17 | Unidentified | 1.22 | 1.36 | 1.64 | 1.99 | 1.48 | 1.61 |
| 19 | Pentacyclic triterpenoid (Arjunolic acid) | 0.98 | 0.78 | 0.89 | 1.12 | 0.88 | 0.98 |
| 23 | Pentacyclic triterpene | 1.05 | 0.77 | 0.97 | 1.30 | 0.99 | 1.07 |
| 24 | Pentacyclic triterpene | 0.99 | 0.81 | 0.89 | 1.10 | 1.15 | 1.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciocan, A.-G.; Maximilian, C.; Mitoi, E.M.; Moldovan, R.-C.; Neguț, D.; Iuga, C.-A.; Helepciuc, F.E.; Holobiuc, I.; Radu, M.; Vassu Dimov, T.; et al. The Impact of Acute Low-Dose Gamma Irradiation on Biomass Accumulation and Secondary Metabolites Production in Cotinus coggygria Scop. and Fragaria × ananassa Duch. Red Callus Cultures. Metabolites 2023, 13, 894. https://doi.org/10.3390/metabo13080894
Ciocan A-G, Maximilian C, Mitoi EM, Moldovan R-C, Neguț D, Iuga C-A, Helepciuc FE, Holobiuc I, Radu M, Vassu Dimov T, et al. The Impact of Acute Low-Dose Gamma Irradiation on Biomass Accumulation and Secondary Metabolites Production in Cotinus coggygria Scop. and Fragaria × ananassa Duch. Red Callus Cultures. Metabolites. 2023; 13(8):894. https://doi.org/10.3390/metabo13080894
Chicago/Turabian StyleCiocan, Alexandra-Gabriela, Carmen Maximilian, Elena Monica Mitoi, Radu-Cristian Moldovan, Daniel Neguț, Cristina-Adela Iuga, Florența Elena Helepciuc, Irina Holobiuc, Mihai Radu, Tatiana Vassu Dimov, and et al. 2023. "The Impact of Acute Low-Dose Gamma Irradiation on Biomass Accumulation and Secondary Metabolites Production in Cotinus coggygria Scop. and Fragaria × ananassa Duch. Red Callus Cultures" Metabolites 13, no. 8: 894. https://doi.org/10.3390/metabo13080894
APA StyleCiocan, A.-G., Maximilian, C., Mitoi, E. M., Moldovan, R.-C., Neguț, D., Iuga, C.-A., Helepciuc, F. E., Holobiuc, I., Radu, M., Vassu Dimov, T., & Cogălniceanu, G. (2023). The Impact of Acute Low-Dose Gamma Irradiation on Biomass Accumulation and Secondary Metabolites Production in Cotinus coggygria Scop. and Fragaria × ananassa Duch. Red Callus Cultures. Metabolites, 13(8), 894. https://doi.org/10.3390/metabo13080894







