Abstract
Saliva is a very complex fluid and it is essential to maintain several physiological processes and functions, including oral health, taste, digestion and immunological defenses. Saliva composition and the oral microbiome can be influenced by several factors, like diet and smoking habits, and their alteration can represent an important access point for pathogens and, thus, for systemic illness onset. In this review, we explore the potentiality of saliva as a new tool for the early detection of some pathological conditions, such as oral diseases, chronic degenerative non-communicable diseases, among these chronic kidney disease (CKD). We also examined the possible correlation between oral and systemic diseases and oral and gut microbiota dysbiosis. In particular, we deeply analyzed the relationship between oral diseases and CKD. In this context, some salivary parameters can represent a new device to detect either oral or systemic pathologies. Moreover, the positive modulation of oral and gut microbiota induced by prebiotics, postbiotics, or symbiotics could represent a new possible adjuvant therapy in the clinical management of oral diseases and CKD.
1. Introduction
The importance of saliva as a biological fluid is often neglected, but saliva is involved in several physiological processes, including the perception of oral taste, digestion and immunological defenses [1]. In fact, saliva is a very complex fluid and its composition is influenced by many factors related to the physiological status of the body. Thus, it represents a new challenge from a clinical point of view.
Saliva is the product of salivary gland secretion, which is regulated by both the sympathetic and parasympathetic systems and once poured into the oral cavity, saliva is mixed with liquids secreted by the buccal epithelial cells, cellular infiltrate and microorganisms [2]. It is rich in minerals, electrolytes (calcium, zinc and magnesium), hormones (adrenomedullin), enzymes (α-amylase), immunoglobulins, cytokines, antimicrobial peptides (AMP), glycoprotein (lactoferrin), mucins and oral tissue repairs (epidermal growth factor and histatins) [3]. Hence, the analysis of saliva and the study of the salivary metabolic profile can represent a new useful tool in the diagnosis and prognosis of chronic degenerative non-communicable diseases (NCDs), including those not related to oral health.
Several factors influence and induce modifications in saliva composition and the oral microbiome, which is one of the most balanced ecosystems after the gut microbiome. The oral cavity in a healthy individual can contain more than 300 species of microorganisms [4], including bacteria, fungi, viruses, archaea and protozoa, even if aerobes are mainly present [5].
Recent studies have shown that the oral microbiota can be influenced by various factors such as diet [6,7] and smoking, which can lead to the growth of one type of bacterial species rather than others. An imbalance in the microbial ecosystem is called dysbiosis. Dysbiosis can lead to the numerical expansion of some potentially pathogenic commensal bacterial species. Their consequent dominance in the niche causes an unhealthy status [8]. Dysbiosis of oral microbes is an impairment that can be observed not only in oral diseases, such as periodontitis, but also in systemic diseases, such as cardiovascular and renal diseases [8].
Furthermore, the oral cavity is an important access point for pathogens. Since the oral microbiome has direct access to respiratory and gastrointestinal systems, oral dysbiosis can be responsible for various pulmonary diseases including pneumonia, lung cancer, etc. [9], as well as inflammatory bowel diseases [10].
In general, saliva composition should be linked to several pathological conditions such as chronic degenerative NCDs [11]. The literature widely demonstrates a correlation between salivary proteomics and oral and systemic diseases. In particular, dysbiosis of the oral microbiome was found to be strictly related to chronic kidney disease (CKD) [12,13], pulmonary diseases, cardiovascular diseases, diabetes mellitus, Alzheimer’s disease, cancer and other metabolic diseases [1,8,14,15].
The aim of this review is to highlight how saliva biomarkers can be exploited as a new potential tool for the early detection of some pathological conditions, such as oral diseases and CKD. Moreover, we point out the close relationship between oral dysbiosis and CKD and how CKD itself can induce or worsen oral diseases, and vice versa.
2. Search Methods
An extensive literature search was performed for articles published up to 30 April 2023 using the Scopus, Web of Science and PubMed online databases. To screen for suitable articles, we included the following keywords, alone or in combination: “oral dysbiosis” with “chronic kidney disease” and/or “chronic degenerative non-communicable diseases” and/or “oral diseases” and/or “gut dysbiosis”, “saliva proteomics” and/or “non-communicable diseases”, and “saliva biomarker” and/or “clinical biochemistry” (Figure 1). All articles included in this review were in the English language and were manually selected by the authors.
Figure 1.
Search methods.
3. Potential Role of Salivary Biomarkers in the Diagnosis of Chronic Degenerative NCDs
Thanks to noninvasive and relatively easy sampling, saliva has become an increasingly important tool for researchers and clinicians. In fact, up to now, it has been used to detect some pathological conditions, such as infectious diseases, genetic disorders and oral cavity cancer. Moreover, in saliva, biomarkers, represented by different molecule classes including proteome, transcriptome, micro-RNA, metabolome, and microbiome [1], are used for screening purposes in epidemiological studies. The enzyme-linked immunosorbent assay (ELISA), immunofluorometric assay (IFMA), or Luminex assay can be applied to determine salivary concentrations of interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, lysozyme, matrix metalloproteinases (MMP)-8, and tissue inhibitors of metalloproteinases (TIMP)-1. These unspecific inflammatory biomarkers can be used to assess systemic inflammation [16] and the obtained results from an analysis of salivary creatinine and urea can be used as additional CKD diagnostic parameters. To date, recent studies demonstrated a significant correlation between salivary and serum creatinine [17,18,19]. These data helped to establish that saliva is a promising alternative matrix for CKD clinical evaluation. In fact, emerging studies support the diagnostic potential of salivary biomarkers, like urea and creatinine, as a non-invasive tool to estimate renal function.
Several studies in the literature evaluated the performance and transversal utility of salivary biomarkers, not only for CKD but also for a broad spectrum of other pathologies. Moreover, recent studies demonstrated that transcriptomic biomarkers play a role in the noninvasive detection of some types of tumors, as well as resectable pancreatic cancer [20] and breast cancer [21].
Furthermore, a saliva proteomic analysis, including oral squamous cell carcinoma (OSCG) patients, identified transferrin as a potential salivary biomarker for the diagnosis of early-stage OSCG. In particular, the authors noticed that salivary transferrin levels were strongly correlated with tumor size, demonstrating its predictive power [22].
In addition, quantitative assessment of salivary biomarkers can play a key role in the early diagnosis of other chronic degenerative NCDs. A further advantage of saliva analysis is that saliva can be easily and non-invasively collected, and for this reason, saliva could be an ideal biomarker to monitor health status and screen the risk of chronic degenerative NCDs onset. An example of this perspective is a study including Alzheimer’s disease (AD) patients, in which it was determined that the phosphorylated tau/tau ratio in saliva significantly increased compared to healthy controls [23]. The importance of this result is highlighted by noninvasive saliva collection compared to cerebrospinal fluid (CSF) sampling.
Finally, saliva testing can be used to evaluate the health of the oral cavity, including the presence of bacteria and other microorganisms, which can affect overall health or can identify oral diseases such as periodontitis and dental caries [24].
4. The Role of Current Salivary Biomarkers in Use and of Oral Microbiota
Nowadays, despite the great number of studies on saliva and the fact that saliva is easy to be collected, its analysis has been used in a limited number of diagnostic applications. Few methods are currently validated for saliva analysis. In fact, many of them are intended for research purposes, instead of diagnostic purposes. Moreover, the available data were collected in studies conducted on a small sample of patients [25,26].
The main applications of salivary biomarkers and the correlations with all their applications are summarized in Table 1.

Table 1.
Salivary biomarkers and tests currently in use.
Furthermore, oral microbiota analysis, with over 700 species of bacteria, plays a fundamental role in the study and in the determination of oral and systemic diseases. For the mouth, the most identifiable bacterial species is Streptococcus salivarius, but it also contains bacteria, fungi, viruses and protozoa.
Nowadays, the methods available to study the microbiome are various and numerous. In short, they include:
- -
- Next generation sequencing (NGS): gene marker analysis (the most used are 16S ribosomal RNA gene sequencing and internal transcribed spacer (ITS)), shogun metagenomics (using untargeted sequencing method), and metatrascriptomics;
- -
- Liquid chromatography–mass spectrometry (LC/CG-MS): metabolomics and metaproteomics.
5. Sample Collection for Saliva Proteomics
Saliva turns out to be an easily available matrix but, simultaneously, its collection requires specific considerations and analytical pre-treatment to obtain a suitable sample.
Saliva is a hypotonic fluid secreted by the salivary glands located in the oral cavity. Like all secretions, saliva is primarily composed of water (99%), while only 1% consists of inorganic and organic substances. Salivary secretion is performed by various glands: submandibular, parotid, sublingual and minor salivary glands.
The fluid secreted by the salivary glands does not always exhibit the same biochemical characteristics. For instance, the quantity of saliva produced by the parotid glands increases significantly after stimuli. Thus, several biochemical differences can be observed between stimulated and unstimulated saliva [70,71,72,73].
Some general differences include:
- Flow rate: stimulation, such as chewing, talking, or smelling food leads to an increased flow of saliva compared to the resting or unstimulated state. Flow rate affects the concentrations of various salivary components.
- Water content: unstimulated saliva has a lower water content compared to stimulated saliva, and stimulation triggers the release of larger volumes of watery saliva.
- Protein composition: the protein composition of saliva can differ between stimulated and unstimulated states. Stimulation typically leads to increased secretion of proteins, such as amylase, mucins and immunoglobulins. These proteins play roles in enzymatic digestion, lubrication and immune defense.
- Electrolytes: stimulation can affect the concentration of electrolytes in saliva. Stimulated saliva often contains higher levels of electrolytes like sodium, potassium, calcium and bicarbonate compared to unstimulated saliva. These electrolytes are important for maintaining oral pH balance and overall oral health.
- pH level: stimulation can result in changes in salivary pH. Unstimulated saliva generally has a slightly acidic pH, while stimulated saliva tends to be more neutral or slightly alkaline. The buffering capacity of stimulated saliva helps in neutralizing acids and maintaining a healthier oral environment.
- Enzymes: stimulation triggers the release of various enzymes in saliva. For example, stimulated saliva contains higher levels of alpha-amylase, which initiates the digestion of carbohydrates. Other enzymes, such as lingual lipase and lysozyme, may be more abundant in stimulated saliva.
- Immunological factors: the immune-related components in saliva, including immunoglobulins (e.g., IgA) and antimicrobial peptides, may be influenced by stimulation. Increased saliva flow during stimulation can enhance the presence of these immune factors, contributing to oral defense mechanisms.
It is also important to note that a variation exists among individuals and the aforementioned differences may not be universally applicable. Additionally, the specific differences in salivary composition between stimulated and unstimulated states can be further influenced by factors like oral health, general health status, drugs and nutritional habits.
Therefore, regardless of the method or type of saliva sample chosen, the procedures used to collect samples should be standardized as much as possible.
Saliva can be collected using four different methods: the passive drool method, the spit method, the suction method and the absorbent method [74,75].
The passive saliva collection method is considered the gold standard by many researchers [75] as it avoids any type of bias, such as reflex stimulation. Considering its ease of use, this method appears to be the most common for saliva collection in experimental studies.
Although there are no standardized procedures to reduce variability in saliva collection, there are several guidelines and considerations that ensure the least interference and greatest reproducibility of the collected specimens [76].
Saliva should be collected in the morning, 2 h after waking up to minimize the circadian rhythms’ influence, preferably after fasting or at least 2 h after eating and/or drinking [77]. Oral hygiene procedures should be performed at least 1 h before collection. Subjects should rinse their mouth with tap water for at least 30 s to remove desquamated epithelial cells, microorganisms and remnant food and drinks. Moreover, they should rest for 5 min before the collection to avoid sample dilution. Blood-contaminated samples must be rejected [77].
The total time required to collect saliva samples should be recorded in order to obtain the volume measured over time (production per unit of time, mL/min). Low flow rates are an indication of salivary gland pathological conditions or of specific drug use.
Saliva samples should be stored immediately after saliva collection at −20 °C or −80 °C, depending on the analyte being tested, until the analysis is performed [76].
At the time of saliva collection, several factors must also be considered that influence its composition and its flow rate, such as a high inter-individual and intra-individual variability or aging, which can induce hyposalivation [78]. In fact, the use of drugs and various pathological conditions, including diabetes mellitus, CKD, and liver and autoimmune diseases, can determine alterations in salivary components [79]. Other factors are body mass index, lifestyle, and, in particular, smoking or elevated use of caffeine, which appear to have strong effects on the salivary proteome pattern [76].
6. Impact of Oral Diseases on Oral Microbiota Composition
Dysbiosis of microbial communities in the gut and in the mouth precedes many oral and systemic diseases and can induce the breakdown of innate barriers and immune dysregulation. Moreover, this impairment of gut and oral microbiota can stimulate pro-inflammatory signaling.
The oral and maxillofacial regions present various anatomical structures that act as ecological niches for bacterial colonization [80]. The latter is influenced by nutrients availability, host immune system, oxygen content and temperature [24].
The oral microbiota has multiple stages of maturation, acquiring Streptococcus as pioneer colonizers before being populated by other organisms (Table 2) [81]. Although there is a wide inter-individuality in colonizing species, a ‘core taxa’ of oral microbes has been identified and it includes Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Fusobacteria [82].
In general, Streptococcus mitis is found in the buccal mucosa, Streptococcus salivarius is found in the saliva and on the dorsal tongue and Streptococcus sanguinis is found on the tooth surface. These bacteria modify the environment through pH modulation, nutrients availability, and other factors, creating conditions for subsequent microbial colonization. Therefore, more complex bacterial communities are developed and a homeostatic balance of microorganisms within their respective niches is established. Although the genus Streptococcus dominates several oral surfaces, there are other bacterial species implicated in microbial homeostasis: Neisseria, Haemophilus, Corynebacterium, Rothia, Actinomyces, Prevotella, Capnocytophaga, Porphyromonas, and Fusobacterium phylotypes [83]. The bacterial genus mostly associated with oral cavity infections is Streptococcus spp. The oral cavity of a newborn is rapidly and primarily colonized by bacteria such as Streptococcus salivarius. During the first year of life, with the eruption of teeth, the colonization continues with Streptococcus mutans and Streptococcus sanguinis. Later, the gingival area becomes the ideal habitat for anaerobic bacteria belonging to Bacteroides and Spirochetes, which are generally associated with periodontal diseases. In addition to microbial infections that cause halitosis, gingivitis and periodontitis, other viral infections (herpesvirus) and oral fungal infections can frequently occur [84].
Among oral diseases, the most common are dental caries and periodontitis. Dental caries represents a prevalent chronic illness, characterized by the degradation of hard dental tissues from bacterial acids, which results in decay and loss of tooth structure. Its main etiological agent is Streptococcus mutans, which has the ability to resist higher levels of oxidative stress, thus changing the homeostatic balance of the oral microbiota [85]. However, recent studies examined other bacteria (present also in the gut) that can be involved in the pathogenesis of carious lesions [86]. Carious lesions initially caused by Streptococcus mutans tend to be fed by microbes belonging to Lactobacillus, Propionibacterium, the genus Atopobium and Scardovia wiggsiae. The latter has been strongly associated with severe early childhood caries (S-ECC) [87].
Periodontitis, while sharing the same dysbiotic organization of caries, follows different pathways and mechanisms of etiopathogenesis and progression [88].
Socranksy and co-workers described the so-called “red complex”, which includes Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola [88], and they claimed that periodontitis is best represented by complexes of bacteria rather than by a single etiologic agent [89]. Upon primary infection of the dental pulp, in addition to the mentioned species, a significant level of Enterococcus faecalis was also observed. However, with root canal treatment and retreatment, elevated levels of Enterococcus faecalis, Filifactor alocis, Pseudoramibacter alactolyticus, Parvimonas micra, Propionibacterium propionicus, Streptococcus constellatus and Streptococcus anginosus have been detected [90].
Several studies have investigated whether the correlation between microbial dysbiosis in the oral cavity and in the gastrointestinal tract can trigger systemic diseases. When oral changes occur, the microbial balance is altered, raising the levels of bacterial species that favor the pathogenesis of various oral diseases, including dental caries, periodontitis and endodontic infections.
In periodontitis disease, Porphyromonas gingivalis [91] may represent one of the clearest connections between oral and gastrointestinal dysbiosis. In fact, the oral administration of Porphyromonas gingivalis significantly increases endotoxemia and reduces the mRNA expression of occludin tight junction proteins (ZO-1) in the small intestine [92,93]. Furthermore, even a single oral administration of Porphyromonas gingivalis can increase the prevalence of Bacteroidetes while, in the meantime, it can decrease the abundance of Firmicutes [93]. In the GI system, the effect of Porphyromonas gingivalis is also magnified, despite its low abundance, changing the expression of tight junction proteins [93]. Moreover, its presence in the gut microbiota has been linked with inflammatory systemic diseases [12,13,94,95]. Therefore, the oral cavity and the gastrointestinal system have a close connection, which is also reflected in their specific microbiomes. This natural interconnection suggests potential routes for bacterial transfer.
Two hypotheses have emerged for the transmission of oral bacteria to the gut:
- (i)
- the haematogenic route, whereby oral bacteria systematically circulate until they colonize the gastrointestinal mucosa;
- (ii)
- the enteral route, in which bacteria from the oral cavity, via the stomach, reach the intestine.
The human body possesses several defense mechanisms and barriers against microbes, including their neutralization through gastric acidity. However, some mechanisms can impair these barriers (for example, the use of antibiotics alters the gut microbiota composition). Evidence for this comes from inflammatory bowel diseases (IBDs), which are known to encode antibiotic-resistant genes [96], thus paving the way for the colonization of the gastrointestinal tract. Furthermore, patients with achlorhydria, commonly associated with the long-term use of proton pump inhibitors, present high levels of oral bacteria in their gastrointestinal system [97]. Among these, there is also Porphyromonas gingivalis, which is known to be acid-resistant [98].
Regardless of the route of transmission, evidence suggests that more than half of the bacterial species present in the gastrointestinal system undergo an oro-intestinal translocation, even in the absence of pathology. Among oral bacteria that can be found in the gut of patients with gastrointestinal diseases, there are members of the genera Staphylococcus, Porphyromonas, Veillonella, Fusobacterium, Actinomyces and Parvimonas [97].
Gut dysbiosis has been observed in nephropathic patients [99] and many studies have also suggested an implication of the oral microbiome [100] in CKD pathogenesis. In fact, the oral microbiome, particularly the salivary microbiome, is altered in CKD patients and it is characterized by an increase in Lautropia, Pseudomonas and Neisseria and a decrease in Actinomyces, Prevotella 7, Veillonella, Haemophilus and Trichococcus [101]. Moreover, the last two have a negative association with estimated GFR (e-GFR) [101]. In addition, periodontal pathogens are increased in CKD patients undergoing hemodialysis [102]. Concurrently, the potential impact of the salivary microbiome on the onset and progression of diabetes mellitus and arterial hypertension was evaluated and it was found that these two diseases are correlated with CKD. Patients with concomitant diabetes mellitus show a decrease in the bacterial diversity of the gut and salivary microbiome [102,103,104,105] and higher levels of Porphyromonas gingivalis, Tannerella forsythia, and Filifactor alocis [106]. In contrast, patients with arterial hypertension have a higher concentration of certain pathogenic oral species in the oral plaque, such as Actinobacillus actinomycetemcomitans [107].
The kidneys are a frequent target of systemic immune diseases [108] and the human microbiome is responsible for the induction, development and modulation of immune responses [109]. Focusing on a few altered bacterial taxa, the phylum Actinobacteria and its genus Actinomyces are drastically decreased, as well as Prevotella 7, which plays an important role in CKD pathogenesis because it is a proteolytic bacterium that can break down proteins and peptides into amino acids [110], reducing the production of short-chain fatty acids (SCFAs).
It was discovered that immunoglobulin G (IgG) levels are negatively associated with Pseudomonas abundance [111]. IgG is an important factor for humoral immunity; lower serum levels of IgG are associated with a higher percentage of CKD, lower e-GFR and poor renal outcome [13,112]. This negative association between IgG and Pseudomonas indicates the involvement of the salivary microbiome in the regulation of immunity in CKD patients. In contrast, the taxa Lautropia is increased in CKD patients. The enrichment of salivary Lautropia could indicate an unhealthy state in the human oral cavity, as previous studies have shown their increase in various pathological conditions. For example, patients with erosive lesions due to oral lichen planus have a higher level of Lautropia compared to those without erosive lesions and healthy subjects [113]. In fact, Lautropia can be used as a diagnostic biomarker for patients with Barrett’s esophagus [114] and hepatitis B [115]. On the other hand, the decrease in Trichococcus in CKD patients could be associated with an unhealthy state of the oral cavity. A previous study demonstrated a decrease in Trichococcus in pediatric patients with obstructive sleep apnea [116]. However, considering the therapeutic front, further multicenter studies must be performed to correlate the salivary microbiome with the gut microbiome using intestinal permeability markers, inflammatory markers, epigenetic factors and biomarkers for renal function. Therefore, future clinical trials are necessary to better understand the salivary microbiome as a potential diagnostic biomarker and to investigate its diagnostic and therapeutic value in CKD patients. It was hypothesized that salivary microbiome transplantation could replace the therapeutic method of fecal microbiome transplantation in CKD [116].
Lactoferrin (Lf) is one of the components of saliva, along with hormones, peptides, organic and inorganic compounds (Fe3+, Mg2+, Na+, K+, Ca2+, Cl−, HPO42−, and HCO3−), and enzymes [117]. It is a glycoprotein capable of chelating two iron atoms per molecule and has an anti-inflammatory and antibacterial function together with lysozyme, mucins (MG1 and MG2), IgA, IgM, IgG, alpha-amylase and organic compounds [117,118,119,120,121]. Saliva contains an Lf concentration of approximately 20 µg/mL and this value is altered in subjects with oral diseases [122,123]. As in the intestine, a free iron excess in the oral cavity stimulates microbial multiplication, synthesis of reactive oxygen species (ROS), inflammatory processes, pigment formation and the occurrence of black stains [121,124,125]. Iron is the most important element for the development of all living cells and for microbial virulence [121,126,127]. It was also reported that an overload of free and available iron in saliva is critical for the transition of bacteria from the planktonic into the sessile state in biofilms, which characterizes ineradicable oral infections [124]. Some bacteria, such as Streptococcus mutans and Prevotella intermedia, take advantage of free iron increase for their multiplication, amplifying the severity of gingivitis and periodontal diseases.
All these mechanisms contribute to the onset of destructive inflammatory processes caused primarily by Lf deficiency, but these processes are also caused by an excess of available iron and an increased bacterial colonization [128,129].

Table 2.
Changes in the oral microbiota according to different physiological and pathological conditions. (A) Impact of age on the oral microbiota. (B) Oral microbiota changes in oral diseases compared to healthy subjects. (C) Oral microbiota changes in NCDs. Abbreviation: A, Actinomyces; NCDS, chronic non-communicable diseases; S, Streptococcus.
Table 2.
Changes in the oral microbiota according to different physiological and pathological conditions. (A) Impact of age on the oral microbiota. (B) Oral microbiota changes in oral diseases compared to healthy subjects. (C) Oral microbiota changes in NCDs. Abbreviation: A, Actinomyces; NCDS, chronic non-communicable diseases; S, Streptococcus.
| A | Phase of life | Age-related changes in Oral Microbiota | Reference |
| Newborn | Streptococcus (S. salivarius is the pioneer and then S. sanguinis, S. peroris, S. lactarius), Actinomyces | [82] | |
| Child (after 1 year of life) | Streptococcus (S. mutans), Granulicatella, Actinomyces (A. odontolyticus), Fusobacterium, Abiotrophia | [82] | |
| Adult | Streptococcus, Lactobacillus, Bifidobacterium, Neisseria, Haemophilus, Corynebacterium, Rothia, Actinomyces, Prevotella, Capnocytophaga, Porphyromonas | [83] | |
| Elderly | Increase in Prevotella, Veillonella, Streptococcus, Candida | [82] | |
| B | Presence of healthy oral cavity or oral diseases | Changes in Oral Microbiota related to oral cavity health | |
| Healthy subjects | Streptococcus, Lactobacillus, Bifidobacterium, Neisseria, Haemophilus, Corynebacterium, Rothia, Actinomyces, Prevotella, Capnocytophaga, Porphyromonas | [83] | |
| Periodontal Diseases | Increase in Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola | [84] [85] | |
| Caries | Increase in Streptococcus mutans, Lactobacillus, Propionibacterium, Atopobium genera, Scardovia wiggsiae | [86] [87] | |
| Root infections | Increase in Enterococcus faecalis, Filifactor alocis, Pseudoramibacter alactolyticus, Parvimonas micra, Propionibacterium propionicus, Streptococcus constellatus, Streptococcus anginosus | [90] | |
| C | NCDs | Oral microbiota changes in NCDs | |
| CKD | Increase in Lautropia, Pseudomonas, Neisseria and decrease in Actinomyces, Prevotella, Veillonella, Haemophilus, Trichococcus | [92] | |
| Gastrointestinal diseases | Increase in Staphylococcus, Porphyromonas, Veillonella, Fusobacterium, Actinomyces, Parvimonas | [93] | |
| Diabetes mellitus | Increase in Porphyromonas gingivalis, Tannerella forsythia, Filifactor alocis | [13] | |
| Arterial hypertension | Increase in Actinobacillus actinomycetemcomitans | [13] |
7. Pharmacological Treatment of Oral Dysbiosis
In order to avoid specific dysbiosis conditions of the oral cavity, physicians try to make limited use of broad-spectrum antibiotics, which can alter the entire microbial flora, incentivizing the use of a targeted therapy [130].
Broad-spectrum antimicrobial mouthwashes such as chlorhexidine are often used to control dysbiosis. However, a new decapeptide called KSL (KKVVFKVKFK-NH2) demonstrated significant antimicrobial effects through the inhibition of biofilm formation. This peptide has also shown antimycotic properties against Candida albicans.
Antimicrobial peptides may therefore prove to be an effective approach in restoring oral health [131].
Using saliva sampling, it has been shown an individual predisposition to caries development. Subjects that presented increased levels of lipid breakdown products, decreased salivary pH and low salivary microbial diversity with a prevalence of saccharolytic microbes were more prone to develop caries. In contrast, individuals with increased salivary pH, reduced lysozyme activity and a prevalence of proteolytic microorganisms were predisposed to periodontal disease and gingival inflammation [132].
Streptococcus mutans has two key virulence factors: the surface adhesin protein PAc (Antigen I/II, P1) and glucosyltransferases (GTF) used to generate glucans from sucrose [133]. An attempt was made to develop a targeted therapy against these virulence factors in oral Streptococcus mutans using a monoclonal antibody, and this study showed promising results [134]. The rate of caries was reduced after administration of polyclonal IgG antibodies against GTF and glucan binding protein (GBP) [135].
Individuals with a low salivary pH and a cariogenic ecotype may benefit from treatment with Streptococcus dentisani [136]. This novel strain is cultured from the dentition of caries-free individuals, and it seems to increase the pH in the oral environment through the breakdown of arginine with subsequent ammonia production [137]. Furthermore, it was discovered that Streptococcus dentisani-derived supernatants inhibit the growth of many oral pathogenic microorganisms, including Streptococcus mutans, Streptococcus sobrinus, Fusobacteriun nucleatum, and Prevotella intermedia, showing structural changes in the cell wall [138].
8. The Possible Link between Oral Dysbiosis and Gut Dysbiosis and Its Influence on CKD Onset and Progression
Several studies showed the presence of alterations in saliva composition and oral microbiota in CKD patients. Saliva is a unique biological exocrine excretion, which is composed of hundreds of different proteins and thousands of peptide sequences [116]. The composition and the correct amount of saliva are crucial to preserve the health of the oral cavity [139]. The total amount of saliva produced per day is about 500–1500 mL [140]. There are similar functions between salivary glands and renal tubular epithelial cells: in fact, the saliva composition is more similar to urine than blood plasma. Furthermore, a study showed that the saliva composition in CKD patients changed in association with an increase in urea, creatinine, calcium, sodium, potassium, phosphorus, bicarbonate and phosphate blood levels compared to the control group [141,142,143,144]. These modifications showed a direct association between blood and salivary alterations [144]. Therefore, a mutual correlation between CKD and oral diseases is evident. In fact, a low-grade chronic inflammatory status, a low salivary flow rate and its impaired composition, and oral microflora dysbiosis, contribute to inducing or worsening CKD. At the same time, the presence of CKD is a possible cause of oral disease onset (Figure 2).
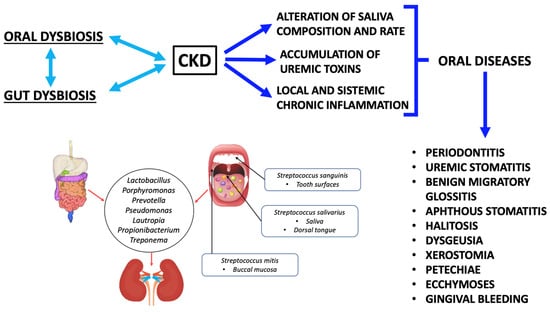
Figure 2.
The link between oral dysbiosis, gut dysbiosis, and chronic kidney disease. Abbreviations: chronic kidney disease, CKD.
In this regard, a study conducted by Trzcionka underlined a correlation between CKD and oral diseases, especially caused by saliva deficiency [145]. Moreover, in end-stage renal disease (ESRD), oral alterations such as benign migratory glossitis, aphthous stomatitis, ecchymoses, xerostomia, petechiae and gingival bleeding can be detected [145,146,147,148,149,150,151]. CKD patients often have halitosis [152], dysgeusia and an increased risk of periodontal disease [153]. Halitosis can be caused by urea increase in saliva secretions. In fact, urea is converted into ammonia (NH3) by oral enzymes [142]. Regarding the buffer capacity of saliva, a study conducted by Trzcionka et al. observed that CKD patients under conservative therapy had a low buffer capacity compared to hemodialysis (HD) patients [145].
Previous studies demonstrated that both CKD patients under conservative therapy and patients on renal replacement therapy (RRT) have impaired saliva composition, and they never restore physiological metabolite levels [154]. Despite that, HD can help CKD patients by enhancing the salivary flow rate. There are different hypothesizes that explain this phenomenon:
- (i)
- HD can restore the intravascular volume because of an ultrafiltration mechanism. The latter causes a high gland perfusion able to stimulate saliva production [155].
- (ii)
- HD treatment corrects blood concentrations of electrolytes and bicarbonate and reduces serum creatinine and urea levels. This correction induces a higher production of saliva, and it may also happen after the first hemodialysis session [156].
- (iii)
- HD session reduces arterial blood pressure. This phenomenon could favor the sympathetic activity of salivary glands and, therefore, the production of saliva [142].
Regarding peritoneal dialysis (PD), a study conducted on pediatric PD patients by Freitas-Fernandes demonstrated an increase in salivary creatinine in PD patients compared to subjects with normal renal function (control group) matched for age. The authors proved that PD could not reestablish adequate levels of salivary creatinine [154].
Another study showed that children with CKD had higher levels of dental calculus and lower levels of dental caries, probably due to an increase in salivary urea concentration and a change in pH [157,158].
A case-control study monitored the salivary concentration of calcium and phosphorus in adult CKD patients [142]. The authors demonstrated that there were higher salivary concentrations of calcium, phosphorus and potassium in CKD patients compared to the control group with normal renal function. These data are probably due to alterations in calcium–phosphorus metabolism induced by CKD.
The most efficient system to assess the GFR is the creatinine clearance [159]. Furthermore, the urea blood concentration represents an indirect biomarker for the monitoring of renal function [160].
In order to monitor the disease severity, CKD patients need to undergo blood tests repeatedly. To avoid this invasive procedure [161], a possible alternative is to analyze salivary compounds. Currently, they seem to be effective biomarkers that can help monitor the stage of several diseases [162,163]. Their advantages are low cost, easy collection and non-invasiveness [164].
Other factors that influence oral microflora composition are uremic toxins (UTs). UTs are directly related to the CKD stage and severity. Their increase can impact on the oral environment, generating a link between oral dysbiosis and CKD [146]. UTs accumulation is a consequence of decline in renal function.
Some UTs are gut-derived metabolites [165] and the main ones include:
(i) phenols, comprising phenyl sulfate, p-cresol, p-cresol sulfate (PCS), phenylacetic acid, sulfate, p-cresyl (PC) and p-cresyl glucuronide (PCG). These compounds are mostly generated by the tobacco consumption and the ingestion and catabolism of tyrosine and phenylalanine through intestinal bacteria. Moreover, PCS is the main circulating metabolite of p-cresol [166].
(ii) Indoles, including indoleacetic acid (IAA) and indoxyl sulfate (IS). These compounds are derived from tryptophan degradation by gut bacteria. IAA is consequently sulfated in the liver into IS [167].
(iii) Amines and polyamines. These compounds are also derived from gut microbial metabolism. The most important amine is trimethylamine N-oxide (TMAO), produced by quaternary amine metabolism, such as betaine, choline/phosphatidylcholine, and L-carnitine [168]. Polyamines include spermidine, spermine, putrescine and cadaverine [169].
Recent studies by Noce et al. demonstrated that CKD progression is also related to oral dysbiosis [12,170,171]. In particular, three oral pathological conditions (oral infections, periodontitis and uremic stomatitis) may induce and exacerbate systemic chronic inflammation [172]. In turn, the latter causes cell-mediated immunity suppression. This phenomenon partly explains the susceptibility of CKD patients to infections [146]. In fact, the oral microbiome of CKD patients could be colonized by enterobacteria in the periodontal pockets [173], thus favoring the systemic inflammation. Moreover, a lot of these pathogenic bacteria (like Enterobacteriaceae), have an antibiotic-resistant phenotype. For example, these bacteria have chromosomal genes that codify different proteins, like antibiotic-inactivating enzymes and proteins involved in non-enzymatic pathways (such as mechanisms that regulate cell permeability, efflux pumps and target molecule modifications) [174].
Oral dysbiosis in CKD patients provokes oral bacterial translocation into the bloodstream during different daily activities, such as tooth brushing, and during invasive dental procedures. Therefore, oral bacteria may become opportunistic infectious agents in different body sites, like the peritoneum [175].
At the same time, CKD seems to interfere with the gut and oral microbiota composition, generating a harmful dysbiosis either in the gut or oral cavity [176].
In 2019, Olsen and Yamazaki suggested that oral dysbiosis can affect the gut microflora, causing systemic dysfunctions [177].
Moreover, an in vivo study showed that the administration of 109 CFU of live Porphyromonas gingivalis (strain W83), a relevant periodontal pathogen, to C57BL/6 mice, twice a week, for 5 weeks, influenced not only the gut microbiota but also the mucosal permeability, gut physiological functions and bacterial-derived toxin concentrations in the bloodstream [92]. Regarding the gut microflora, a decrease in the proportion of the phylum Firmicutes and RoRγ t gene expression and an increase in the proportion of the phylum Bacteroides were observed [93].
Lately, other studies demonstrated that oral bacteria can cause an imbalance in the gut microbiota and the immune system; these bacteria include Streptococcus, Fusobacterium and Staphylococcus [178]. Moreover, the M1/M2 macrophage ratio in the small gut seems to increase because of the alteration in oral microflora. On the contrary, it was shown that Lactobacillus and other probiotic bacteria can suppress the M1/M2 macrophage ratio, inducing an anti-inflammatory action [179].
As previously described, there seems to be a mutual relationship between CKD and gut dysbiosis. In fact, gut dysbiosis increases the risk of developing CKD and its comorbidities (like cardiovascular diseases, arterial hypertension, diabetes mellitus, etc.), especially in elderly people [180]. This link is closely related to the consequences of gut dysbiosis, characterized by alterations in several metabolic pathways and the immune system [181]. The first scientific evidence for this connection was found in the study conducted by Simenhoff et al. [182]. The authors showed that gut dysbiosis should increase the pre-existent renal damage through several mechanisms including:
- (i)
- gut bacteria produce metabolites, such as TMAO, IS, PCS and phenylacetylglutamine (PAG), with a toxic action against the kidneys and the cardiovascular system [99].
- (ii)
- In CKD patients, there are alterations in the gut microflora characterized by an increase in pathogenic species. In these patients, alterations in gut permeability can be observed. These permeability alterations allow the translocation of endotoxins into the bloodstream. This phenomenon worsens the systemic low-grade inflammatory state, accelerating the CKD progression [183].
On the other hand, CKD itself impacts on gut microbiota composition [180]. In particular, CKD nutritional and pharmacological treatments should increase gut dysbiosis [184]. One concern is the negative effect caused by the use of drugs. In particular, antibiotics can alter the gut microflora, while other medicines, such as ion exchange resins, phosphorus binders and iron supplements, can slow down the physiological intestinal transit [185,186,187,188,189].
In fact, one of the most common complications of CKD is iron deficiency anemia (IDA). To treat IDA, oral iron therapy is often administered to pre-dialysis patients. The gut is a key modulator of iron homeostasis and iron oral supplementation is an effective option to replenish iron stores. However, adverse effects on the gut microbiota have been reported, like an increased risk of gut inflammation [190,191].
Recently, it was shown [120] that circulating iron deficiency is not associated with a true lack of iron in the body but with its delocalization (Figure 3). In physiological conditions, iron is absorbed daily by the duodenal enterocytes (1–2 mg/day) and modulated by ferritin which, after sequestering iron, releases it back to the cell via ferroportin (Fpn), the only protein capable of exporting iron from the cells to the circulation. This mechanism also occurs in other cells, including epithelia in the oral mucosa. In the absence of Fpn, iron cannot be exported and remains accumulated within the cell. Furthermore, the synthesis of Fpn is under the control of IL-6 (a pro-inflammatory cytokine). An increased concentration of IL-6 inhibits the synthesis of Fpn, inducing the accumulation of iron in the tissues and, at the same time, its deficiency in the bloodstream [118,120]. In other words, iron supplementation results in a lack of the element’s absorption, which then reaches the colon, where it is potentially available for the gut microbiota. Free iron can stimulate the virulence of pathogenic bacteria residing in the gut and it can contribute to the development of a proinflammatory oxidative environment [190], which can directly affect intestinal epithelial integrity [192]. Consequently, an impairment of the gut barrier could lead to an increased exposure of the host to the endotoxins. These mechanisms can induce systemic microinflammation in CKD patients and local renal immune cell responses, accelerating cardiovascular comorbidities and the progression of renal failure [99,193,194].
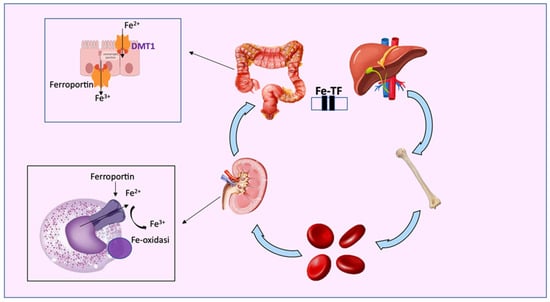
Figure 3.
Iron metabolism: transferrin (TF) transports free iron, both the ferrous (Fe2+) and ferric forms (Fe3+), to tissues and organs, such as the bone marrow and liver. Free iron is active after dissociation from TF, while excess intracellular iron is exported from ferroportin (FPN) or stored as Fe3+ in ferritin. Iron is also released by metal divalent conveyor 1 (DMT1).
Oral iron administration causes a reduction in the number of species, like Lactobacillaceae and Bifidobacteriaceae, which are generally beneficial. Therefore, the simultaneous intake of these families of probiotic and/or prebiotic bacteria can counteract the side effects of iron administration and can contribute to the maintenance of these beneficial strains in the colon. Prebiotic fibers can increase the number of Bifidobacteriaceae and decrease the pH in the colon [195]. Similarly, natural forms of iron, such as Lf, could be good candidates to replace the current oral iron supplements [187,196]. When the Lf physiological concentration in the saliva is restored with one or more daily administrations, IL-6 synthesis decreases and, subsequently, Fpn function is resumed. In other words, Lf prevents and treats inflammation, the main cause of iron overload, and infection in the mucous membranes, thus restoring Fpn physiological synthesis and reversing iron homeostasis disorders [197,198].
Therefore, pharmacological treatments contribute to altering the intestinal surface, in relation to a reduced production of SCFAs (derived by saccharolytic fermentation). In fact, SCFAs physiologically protect the gut mucosa against damages [165].
Increased mucosal permeability generates an access point for bacterial products of intestinal origin (i.e., DNA fragments of intestinal aerobic and anaerobic pathogens). The presence of these products in the bloodstream activates innate immunity and inflammatory pathways and increases cardiovascular risk [185,186,188,190,199,200,201,202].
An elevated UTs concentration in the blood increases the gut permeability. This impairment of the intestinal barrier induces gut colonization of bacteria, some of which can express ureases and uricase enzymes, which convert urea into ammonia. Ammonia raises the gut pH, influencing the growth of pathogenic bacteria that can subsequently cause dysbiosis [203]. The presence of UTs in combination with an impaired gut permeability generates a worsening of inflammation and oxidative stress [183].
A study conducted by Vaziri et al. revealed that CKD patients from several ethnicities, differed of 190 bacterial operational taxonomic units (OTUs), in particular, Pseudomonadaceae, Actinobacteria, Firmicutes (mainly Clostridia) and Proteobacteria families were changed compared with the control group. Another analysis confirmed this finding. In fact, different subjects showed an increment of pathogenic bacteria in the gut, especially new micro-florae that express enzymes involved in the conversion of aromatic amino acids and in the production of IS or PC [204,205].
In another study conducted on 24 CKD patients from different ethnicities, it was demonstrated that CKD patients express:
- (i)
- twelve of the nineteen bacteria families with urease activity, including Clostridiaceae, Dermabacteraceae, Halomonadaceae, Methylococcaceae, Alteromonadaceae, Cellulomonadaceae, Pseudomonadaceae, Xanthomonadaceae, Enterobacteriaceae, Moraxellaceae, Polyangiaceae, and Micrococcaceae;
- (ii)
- three families with tryptophanase activity, including Verrucomicrobiaceae, Clostridiaceae, and Enterobacteriaceae;
- (iii)
- five families with uricase activity, including Dermabacteraceae, Micrococcaceae, Cellulomonadaceae, Xanthomonadaceae, and Polyangiaceae.
These patients also registered a reduction in the Prevotellaceae and Lactobacillaceae families, which are involved in the protective processes of the gut mucosa [206].
Furthermore, CKD patients show a relevant susceptibility to oral diseases, especially due to the Enterobacteriaceae family, which also represents etiological agents of dialysis-associated and nosocomial infections [175]. Enterobacteriaceae species are particularly pathogenic in ESRD patients; in fact, they are responsible from 10 to 12 percent of all peritoneal dialysis-associated peritonitis [207]. A study that involved PD patients showed that they presented more microbial counts and Enterobacteriaceae compared to the controls. The authors also observed a high diversity of Enterobacteriaceae species. In fact, the controls presented only three species, while the PD patients presented eight species. In particular, Raoultella ornithinolytica, a histamine-producing aquatic-commensal enterobacteria, was significantly present in the oral cavity of PD patients, but it was absent in the oral cavity of the controls. In detail, the colonization of this bacteria depended on the age, sex and ethnicity of the participants. Raoultella ornithinolytica can rarely survive in human saliva and several studies demonstrated [175] that this species is responsible for primary peritonitis in humans [175,208].
In conclusion, the presence of UTs, the alterations of metabolic pathways, high levels of ammonia and urea and high pH, are involved in the alteration of the oral environment as well as of the gut environment [146,209].
9. Pharmacological and Nutritional Treatment of Gut Dysbiosis in CKD
As previously described, there is a strict correlation between gut dysbiosis and CKD. Therefore, an amelioration of one of them seems to influence both positively. In fact, a study conducted by Ramezani et al. showed that a change in the gut microbiota can influence CKD pathogenesis [201].
Several studies were conducted with the aim of finding a new possible therapy for CKD gut dysbiosis. One study was based on the administration of specific prebiotics [210]. A prebiotic is a compound used against host microorganisms and is characterized by healthy properties [211]. These substances should be inulin-type fructans (oligofructose, fructo-oligosaccharides and inulin), galactans (galacto-oligosaccharides), polyunsaturated fatty acids (PUFAs), polyphenols and conjugated linoleic acids [210,212]. Among them, an element that can have a beneficial role in CKD patients affected by gut dysbiosis is the resistant starch (RS), a α-linked glucose polymer that is not hydrolysable in the human small gut [213]. Two studies conducted on healthy and CKD animal models, fed with a RS-supplemented diet, highlighted a reduced plasma urea concentration [210,214]. Vaziri et al. demonstrated that, in mice models with adenine-induced CKD, there was an improvement in creatinine clearance, serum creatinine, interstitial fibrosis and renal inflammation, after a diet containing 59% high-amylose maize starch [99]. To date, the benefits of RS have not been confirmed in human studies on CKD patients.
Therefore, dietary fibers, including RS, are capable to positively modify the gut microbiota because they represent a nutritional substrate for saccharolytic bacteria [210]. On the contrary, there are some substances that can decrease the gut microbial balance, such as antibiotics (like amoxicillin) or alcohol [215,216].
Other oral food supplements used for gut dysbiosis are probiotics, that are, “live microorganism that show beneficial effects on the health of the host” [217]. Among the different species, Saccharomyces cerevisiae is the most used. There are also other types like Lactobacillus (L. john-sonii, L. sporogens, L. casei, L. plantarum, L. bulgaricus, L. delbrueckii, L. salivarius, L. rhamnosus, L. reuteri, and L. acidophilus), Bifidobacterium (B. longum, B. bifi-dum, B. breve (Yakult), B. lactis, B. bifidus, and B. infantis), Streptococcus (S. thermophilus and acidophilus), Enterococcus SF68, Lactococcus lactis, and Escherichia coli Nissle (serotype O6:K5:H1) [218].
Symbiotics are sometimes used to improve host conditions, not only in CKD patients but also in other pathological conditions. They are dietary supplements or food ingredients, composed of probiotics and prebiotics that work together for the health promotion. FOS/Lactobacillus sporogens and OAT fiber/Lactobacillus plantarum are actually used in clinical practice [219].
In conclusion, probiotics and prebiotics induce several beneficial effects, including the competitive exclusion of pathological bacteria in gut colonization, integrity and homeostasis of the gut, metabolism of primary to secondary bile salts, production of vitamins and SCFAs, regulation of gastrointestinal transit and neutralization of carcinogens or xenobiotics [220]. These positive changes could represent a good start for the gut dysbiosis treatment in CKD patients and a possible solution to ameliorate the host’s health.
10. Conclusions
Saliva composition monitoring could be a new, cheap, non-invasive and easy tool to diagnose and clinically evaluate oral and systemic diseases. From this perspective, it would be useful to standardize the saliva analysis method in order to apply it on a large scale.
Moreover, recent studies demonstrated a correlation between oral and systemic diseases and this connection is represented by gut and oral microbiota dysbiosis. Chronic degenerative NCDs, in particular, CKD, are characterized by gut dysbiosis and recent studies also highlighted the presence of oral dysbiosis in these pathological conditions.
In this context, it is important to promote the positive modulation of oral and gut microbiota in order to counteract dysbiosis with the administration of specific prebiotics, postbiotics and synbiotics.
Author Contributions
Conceptualization, P.B., S.B. and A.N.; writing—original draft preparation, M.B., E.N., M.D.L., V.P., F.T. and I.V.; writing—review and editing, M.P., G.M., E.N. and A.N.; visualization, V.P., F.T. and I.V.; supervision, P.B., S.B. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Gabriella Venafro for the English language revision of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva Diagnostics—Current Views and Directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B. The Physiology of Salivary Secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Farnaud, S.J.C.; Kosti, O.; Getting, S.J.; Renshaw, D. Saliva: Physiology and Diagnostic Potential in Health and Disease. Sci. World J. 2010, 10, 434–456. [Google Scholar] [CrossRef] [PubMed]
- Pflughoeft, K.J.; Versalovic, J. Human Microbiome in Health and Disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef]
- Deo, P.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef]
- Canale, M.P.; Noce, A.; Di Lauro, M.; Marrone, G.; Cantelmo, M.; Cardillo, C.; Federici, M.; Di Daniele, N.; Tesauro, M. Gut Dysbiosis and Western Diet in the Pathogenesis of Essential Arterial Hypertension: A Narrative Review. Nutrients 2021, 13, 1162. [Google Scholar] [CrossRef]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. 2018, 6, 10. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The Role of Oral Microbiome in Respiratory Health and Diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Hatting, M.; Bott, A.; Dahlhausen, A.; Keller, D.; Trautwein, C.; Conrads, G. The Oral-Gut Axis: Salivary and Fecal Microbiome Dysbiosis in Patients with Inflammatory Bowel Disease. Front. Cell Infect. Microbiol. 2022, 12, 1010853. [Google Scholar] [CrossRef]
- Malamud, D. Saliva as a Diagnostic Fluid. Dent. Clin. N. Am. 2011, 55, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marchetti, M.; Marrone, G.; Di Renzo, L.; Di Lauro, M.; Di Daniele, F.; Albanese, M.; Di Daniele, N.; De Lorenzo, A. Link Between Gut Microbiota Dysbiosis and Chronic Kidney Disease. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 2057–2074. [Google Scholar] [PubMed]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidi, M.; Allsop, D.; Karim, S.; Martin, F.L.; Crean, S. Diagnostic Biomarkers for Alzheimer’s Disease Using Non-Invasive Specimens. J. Clin. Med. 2020, 9, 1673. [Google Scholar] [CrossRef]
- Sobczak-Jaskow, H.; Kochańska, B.; Drogoszewska, B. Composition and Properties of Saliva in Patients with Osteoporosis Taking Antiresorptive Drugs. Int. J. Environ. Res. Public Health 2023, 20, 4294. [Google Scholar] [CrossRef]
- Rathnayake, N.; Åkerman, S.; Klinge, B.; Lundegren, N.; Jansson, H.; Tryselius, Y.; Sorsa, T.; Gustafsson, A. Salivary Biomarkers for Detection of Systemic Diseases. PLoS ONE 2013, 8, e61356. [Google Scholar] [CrossRef]
- Peng, C.; Xia, Y.; Wu, Y.; Zhou, Z.; Cheng, P.; Xiao, P. Influencing Factors for Saliva Urea and Its Application in Chronic Kidney Disease. Clin. Biochem. 2013, 46, 275–277. [Google Scholar] [CrossRef]
- Suresh, G.; Ravi Kiran, A.; Samata, Y.; Purnachandrarao Naik, N.; Vijay Kumar, A. Analysis of Blood and Salivary Urea Levels in Patients Undergoing Haemodialysis and Kidney Transplant. J. Clin. Diagn. Res. 2014, 8, ZC18–ZC20. [Google Scholar] [CrossRef]
- Temilola, D.O.; Bezuidenhout, K.; Erasmus, R.T.; Stephen, L.; Davids, M.R.; Holmes, H. Salivary Creatinine as a Diagnostic Tool for Evaluating Patients with Chronic Kidney Disease. BMC Nephrol. 2019, 20, 387. [Google Scholar] [CrossRef]
- Zhang, L.; Farrell, J.J.; Zhou, H.; Elashoff, D.; Akin, D.; Park, N.; Chia, D.; Wong, D.T. Salivary Transcriptomic Biomarkers for Detection of Resectable Pancreatic Cancer. Gastroenterology 2010, 138, 949–957.e7. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Karlan, S.; Zhou, H.; Gross, J.; Elashoff, D.; Akin, D.; Yan, X.; Chia, D.; Karlan, B.; et al. Discovery and Preclinical Validation of Salivary Transcriptomic and Proteomic Biomarkers for the Non-Invasive Detection of Breast Cancer. PLoS ONE 2010, 5, e15573. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.-J.; Lin, C.-D.; Lai, C.-H.; Chen, C.-H.; Kao, J.-Y.; Chen, S.-Y.; Tsai, M.-H.; Huang, S.-H.; Lin, C.-W. Proteomic Identification of Salivary Transferrin as a Biomarker for Early Detection of Oral Cancer. Anal. Chim. Acta 2010, 681, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sui, Y.-T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary Tau Species Are Potential Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 27, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Adam, E.K.; Kumari, M. Assessing Salivary Cortisol in Large-Scale, Epidemiological Research. Psychoneuroendocrinology 2009, 34, 1423–1436. [Google Scholar] [CrossRef]
- Köksal, B. Is Correlation between Plasma and Salivary Cortisol Levels an Important Indicator of Stress?: A Meta-Analysis Study. Acta Fac. Medicae Naissensis 2021, 38, 351–359. [Google Scholar] [CrossRef]
- An, K.; Salyer, J.; Brown, R.E.; Kao, H.-F.S.; Starkweather, A.; Shim, I. Salivary Biomarkers of Chronic Psychosocial Stress and CVD Risks. Biol. Res. Nurs. 2016, 18, 241–263. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Zhu, J.; Liao, Z.; Wang, S.; Liu, W. Correlations of Salivary and Blood Glucose Levels among Six Saliva Collection Methods. Int. J. Environ. Res. Public Health 2022, 19, 4122. [Google Scholar] [CrossRef]
- Malathi, N.; Mythili, S.; Vasanthi, H.R. Salivary Diagnostics: A Brief Review. ISRN Dent. 2014, 2014, 158786. [Google Scholar] [CrossRef]
- Rutherfurd-Markwick, K.; Starck, C.; Dulson, D.K.; Ali, A. Salivary Diagnostic Markers in Males and Females During Rest and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 27. [Google Scholar] [CrossRef]
- Fadel, H.T.; Pliaki, A.; Gronowitz, E.; Mårild, S.; Ramberg, P.; Dahlèn, G.; Yucel-Lindberg, T.; Heijl, L.; Birkhed, D. Clinical and Biological Indicators of Dental Caries and Periodontal Disease in Adolescents with or without Obesity. Clin. Oral Investig. 2014, 18, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Kheirmand Parizi, M.; Akbari, H.; Malek-Mohamadi, M.; Kheirmand Parizi, M.; Kakoei, S. Association of Salivary Levels of Immunoglobulin-a and Amylase with Oral-Dental Manifestations in Patients with Controlled and Non-Controlled Type 2 Diabetes. BMC Oral Health 2019, 19, 175. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Fasanmade, A.A. Comparative Analysis of Salivary Glucose and Electrolytes in Diabetic Individuals with Periodontitis. Ann. Ib. Postgrad. Med. 2012, 10, 25–30. [Google Scholar]
- Mohammadnejad, P.; Asl, S.S.; Aminzadeh, S.; Haghbeen, K. A New Sensitive Spectrophotometric Method for Determination of Saliva and Blood Glucose. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 229, 117897. [Google Scholar] [CrossRef]
- Pérez-Ros, P.; Navarro-Flores, E.; Julián-Rochina, I.; Martínez-Arnau, F.M.; Cauli, O. Changes in Salivary Amylase and Glucose in Diabetes: A Scoping Review. Diagnostics 2021, 11, 453. [Google Scholar] [CrossRef]
- Andrusishina, I.N. Diagnostic Values of Calcium and Magnesium Forms Determined in Human Serum and Saliva. J. Elem.-UWN 2010, 15, 425–433. [Google Scholar]
- Jawed, M.; Shahid, S.M.; Qader, S.A.; Azhar, A. Dental Caries in Diabetes Mellitus: Role of Salivary Flow Rate and Minerals. J. Diabetes Complicat. 2011, 25, 183–186. [Google Scholar] [CrossRef]
- Machado, A.; Maneiras, R.; Bordalo, A.A.; Mesquita, R.B.R. Monitoring Glucose, Calcium, and Magnesium Levels in Saliva as a Non-Invasive Analysis by Sequential Injection Multi-Parametric Determination. Talanta 2018, 186, 192–199. [Google Scholar] [CrossRef]
- Rajesh, K.S.; Zareena; Hegde, S.; Arun Kumar, M.S. Assessment of Salivary Calcium, Phosphate, Magnesium, PH, and Flow Rate in Healthy Subjects, Periodontitis, and Dental Caries. Contemp. Clin. Dent. 2015, 6, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Celec, P.; Ostatnikova, D.; Putz, Z.; Kudela, M. The Circalunar Cycle of Salivary Testosterone and the Visual-Spatial Performance. Bratisl. Lek. Listy 2002, 103, 59–69. [Google Scholar]
- Diago-Galmés, A.; Guillamón-Escudero, C.; Tenías-Burillo, J.M.; Soriano, J.M.; Fernández-Garrido, J. Salivary Testosterone and Cortisol as Biomarkers for the Diagnosis of Sarcopenia and Sarcopenic Obesity in Community-Dwelling Older Adults. Biology 2021, 10, 93. [Google Scholar] [CrossRef]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current Methods for Stress Marker Detection in Saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Enter, D.; Zitman, F.G.; Penninx, B.W.J.H.; van Pelt, J.; Spinhoven, P.; Roelofs, K. Salivary Testosterone: Associations with Depression, Anxiety Disorders, and Antidepressant Use in a Large Cohort Study. J. Psychosom. Res. 2012, 72, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kellesarian, S.V.; Malmstrom, H.; Abduljabbar, T.; Vohra, F.; Kellesarian, T.V.; Javed, F.; Romanos, G.E. Low Testosterone Levels in Body Fluids Are Associated with Chronic Periodontitis. Am. J. Mens. Health 2017, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Escribano, A.; Maroto-García, J.; Ruiz-Galdón, M.; Barrios-Rodríguez, R.; Álvarez-Millán, J.J.; Cabezas-Sánchez, P.; Plaza-Andrades, I.; Molina-Vega, M.; Tinahones, F.J.; Queipo-Ortuño, M.I.; et al. Measurement of Serum Testosterone in Nondiabetic Young Obese Men: Comparison of Direct Immunoassay to Liquid Chromatography-Tandem Mass Spectrometry. Biomolecules 2020, 10, 1697. [Google Scholar] [CrossRef]
- Sahun, Y.A.; Cheetham, T.; Boot, C.; Straub, V.; Wood, C.L. Measurement of Salivary Testosterone in Adolescents and Young Men with Duchenne Muscular Dystrophy. BMC Endocr. Disord. 2021, 21, 63. [Google Scholar] [CrossRef]
- Babaei, M.; Rezaei, S.; Saghafi Khadem, S.; Shirinbak, I.; Basir Shabestari, S. The Role of Salivary C-Reactive Protein in Systemic and Oral Disorders: A Systematic Review. Med. J. Islam. Repub. Iran 2022, 36, 138. [Google Scholar] [CrossRef]
- Hadžić, Z.; Puhar, I.C. Reactive Protein in Saliva of Non-Smoking Patients with Periodontitis (a Pilot Study). J. Health Sci. 2021, 11, 98–101. [Google Scholar] [CrossRef]
- McGeer, P.L.; Lee, M.; Kennedy, K.; McGeer, E.G. Saliva Diagnosis as a Disease Predictor. J. Clin. Med. 2020, 9, 377. [Google Scholar] [CrossRef]
- Riis, J.L.; Bryce, C.I.; Matin, M.J.; Stebbins, J.L.; Kornienko, O.; van Huisstede, L.; Granger, D.A. The Validity, Stability, and Utility of Measuring Uric Acid in Saliva. Biomark. Med. 2018, 12, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, D.; Orzechowska, Z.; Rutkowski, R.; Prymas, A.; Mrall-Wechta, M.; Bednarek-Hatlińska, D.; Roszak, M.; Surdacka, A.; Samborski, W.; Witowski, J. Diagnostic Value of Salivary CRP and IL-6 in Patients Undergoing Anti-TNF-Alpha Therapy for Rheumatic Disease. Inflammopharmacology 2018, 26, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Bäcklund, N.; Brattsand, G.; Israelsson, M.; Ragnarsson, O.; Burman, P.; Edén Engström, B.; Høybye, C.; Berinder, K.; Wahlberg, J.; Olsson, T.; et al. Reference Intervals of Salivary Cortisol and Cortisone and Their Diagnostic Accuracy in Cushing’s Syndrome. Eur. J. Endocrinol. 2020, 182, 569–582. [Google Scholar] [CrossRef]
- Shirtcliff, E.A.; Allison, A.L.; Armstrong, J.M.; Slattery, M.J.; Kalin, N.H.; Essex, M.J. Longitudinal Stability and Developmental Properties of Salivary Cortisol Levels and Circadian Rhythms from Childhood to Adolescence. Dev. Psychobiol. 2012, 54, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Aita, A.; Basso, D.; Cattelan, A.M.; Fioretto, P.; Navaglia, F.; Barbaro, F.; Stoppa, A.; Coccorullo, E.; Farella, A.; Socal, A.; et al. SARS-CoV-2 Identification and IgA Antibodies in Saliva: One Sample Two Tests Approach for Diagnosis. Clin. Chim. Acta 2020, 510, 717–722. [Google Scholar] [CrossRef]
- Costantini, V.P.; Nguyen, K.; Lyski, Z.; Novosad, S.; Bardossy, A.C.; Lyons, A.K.; Gable, P.; Kutty, P.K.; Lutgring, J.D.; Brunton, A.; et al. Development and Validation of an Enzyme Immunoassay for Detection and Quantification of SARS-CoV-2 Salivary IgA and IgG. medRxiv 2021. [Google Scholar] [CrossRef]
- Varadhachary, A.; Chatterjee, D.; Garza, J.; Garr, R.P.; Foley, C.; Letkeman, A.F.; Dean, J.; Haug, D.; Breeze, J.; Traylor, R.; et al. Salivary Anti-SARS-CoV-2 IgA as an Accessible Biomarker of Mucosal Immunity against COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Azzi, L.; Baj, A.; Alberio, T.; Lualdi, M.; Veronesi, G.; Carcano, G.; Ageno, W.; Gambarini, C.; Maffioli, L.; Saverio, S.D.; et al. Rapid Salivary Test Suitable for a Mass Screening Program to Detect SARS-CoV-2: A Diagnostic Accuracy Study. J. Infect. 2020, 81, e75–e78. [Google Scholar] [CrossRef]
- Ren, A.; Sohaei, D.; Ulndreaj, A.; Pons-Belda, O.D.; Fernandez-Uriarte, A.; Zacharioudakis, I.; Sigal, G.B.; Stengelin, M.; Mathew, A.; Campbell, C.; et al. Ultrasensitive Assay for Saliva-Based SARS-CoV-2 Antigen Detection. Clin. Chem. Lab. Med. 2022, 60, 771–777. [Google Scholar] [CrossRef]
- Dewhurst, R.E.; Heinrich, T.; Watt, P.; Ostergaard, P.; Marimon, J.M.; Moreira, M.; Houldsworth, P.E.; Rudrum, J.D.; Wood, D.; Kõks, S. Validation of a Rapid, Saliva-Based, and Ultra-Sensitive SARS-CoV-2 Screening System for Pandemic-Scale Infection Surveillance. Sci. Rep. 2022, 12, 5936. [Google Scholar] [CrossRef]
- dos Santos, C.; de Oliveira, K.; Mendes, G.; Silva, L.; de Souza, M., Jr.; Estrela, P.F.; Guimarães, R.; Silveira-Lacerda, E.; Duarte, G. Detection of SARS-CoV-2 in Saliva by RT-LAMP During a Screening of Workers in Brazil, Including Pre-Symptomatic Carriers. J. Braz. Chem. Soc. 2021, 32, 2071–2077. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Brito, A.F.; Wyllie, A.L.; Fauver, J.R.; Ott, I.M.; Kalinich, C.C.; Petrone, M.E.; Casanovas-Massana, A.; Catherine Muenker, M.; Moore, A.J.; et al. Analytical Sensitivity and Efficiency Comparisons of SARS-CoV-2 RT-QPCR Primer-Probe Sets. Nat. Microbiol. 2020, 5, 1299–1305. [Google Scholar] [CrossRef]
- Koizumi, T.; Shetty, V.; Yamaguchi, M. Salivary Cytokine Panel Indicative of Non-Small Cell Lung Cancer. J. Int. Med. Res. 2018, 46, 3570–3582. [Google Scholar] [CrossRef]
- Lee, L.T.; Wong, Y.K.; Hsiao, H.Y.; Wang, Y.W.; Chan, M.Y.; Chang, K.W. Evaluation of Saliva and Plasma Cytokine Biomarkers in Patients with Oral Squamous Cell Carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef]
- Morales-Rojas, T.; Viera, N.; Morón-Medina, A.; Alvarez, C.J.; Alvarez, A. Proinflammatory Cytokines during the Initial Phase of Oral Mucositis in Patients with Acute Lymphoblastic Leukaemia. Int. J. Paediatr. Dent. 2012, 22, 191–196. [Google Scholar] [CrossRef]
- Resende, R.G.; Abreu, M.H.N.G.; de Souza, L.N.; Silva, M.E.S.; Gomez, R.S.; Correia-Silva, J.d.F. Association between IL1B (+3954) Polymorphisms and IL-1β Levels in Blood and Saliva, Together with Acute Graft-versus-Host Disease. J. Interferon Cytokine Res. 2013, 33, 392–397. [Google Scholar] [CrossRef]
- Spear, G.T.; Alves, M.E.A.F.; Cohen, M.H.; Bremer, J.; Landay, A.L. Relationship of HIV RNA and Cytokines in Saliva from HIV-Infected Individuals. FEMS Immunol. Med. Microbiol. 2005, 45, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Vastardis, S.; Leigh, J.E.; Wozniak, K.; Yukna, R.; Fidel, P.L. Influence of Periodontal Disease on Th1/Th2-Type Cytokines in Saliva of HIV-Positive Individuals. Oral Microbiol. Immunol. 2003, 18, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, G.; Nandan, S.R.K.; Kulkarni, P.G. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2087–2093. [Google Scholar] [CrossRef]
- Jacobs, R.; Tshehla, E.; Malherbe, S.; Kriel, M.; Loxton, A.G.; Stanley, K.; van der Spuy, G.; Walzl, G.; Chegou, N.N. Host Biomarkers Detected in Saliva Show Promise as Markers for the Diagnosis of Pulmonary Tuberculosis Disease and Monitoring of the Response to Tuberculosis Treatment. Cytokine 2016, 81, 50–56. [Google Scholar] [CrossRef]
- Anuradha, B.R.; Katta, S.; Kode, V.S.; Praveena, C.; Sathe, N.; Sandeep, N.; Penumarty, S. Oral and Salivary Changes in Patients with Chronic Kidney Disease: A Clinical and Biochemical Study. J. Indian Soc. Periodontol. 2015, 19, 297–301. [Google Scholar] [CrossRef]
- Gomar-Vercher, S.; Simón-Soro, A.; Montiel-Company, J.M.; Almerich-Silla, J.M.; Mira, A. Stimulated and Unstimulated Saliva Samples Have Significantly Different Bacterial Profiles. PLOS ONE 2018, 13, e0198021. [Google Scholar] [CrossRef]
- Jo, R.; Nishimoto, Y.; Umezawa, K.; Yama, K.; Aita, Y.; Ichiba, Y.; Murakami, S.; Kakizawa, Y.; Kumagai, T.; Yamada, T.; et al. Comparison of Oral Microbiome Profiles in Stimulated and Unstimulated Saliva, Tongue, and Mouth-rinsed Water. Sci. Rep. 2019, 9, 16124. [Google Scholar] [CrossRef]
- Salvolini, E.; Martarelli, D.; Di Giorgio, R.; Mazzanti, L.; Procaccini, M.; Curatola, G. Age-related modifications in human unstimulated whole saliva: A Biochemical Study. Aging Clin. Exp. Res. 2000, 12, 445–448. [Google Scholar] [CrossRef]
- Gröschl, M.; Rauh, M. Influence of Commercial Collection Devices for Saliva on the Reliability of Salivary Steroids Analysis. Steroids 2006, 71, 1097–1100. [Google Scholar] [CrossRef]
- Michishige, F.; Kanno, K.; Yoshinaga, S.; Hinode, D.; Takehisa, Y.; Yasuoka, S. Effect of Saliva Collection Method on the Concentration of Protein Components in Saliva. J. Med. Investig. 2006, 53, 140–146. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Kim, H.-R.; Chae, H.-J. Compliance with Saliva Collection Protocol in Healthy Volunteers: Strategies for Managing Risk and Errors. Int. J. Med. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef]
- Rosa, N.; Marques, J.; Esteves, E.; Fernandes, M.; Mendes, V.M.; Afonso, Â.; Dias, S.; Pereira, J.P.; Manadas, B.; Correia, M.J.; et al. Protein Quality Assessment on Saliva Samples for Biobanking Purposes. Biopreserv. Biobank. 2016, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Murr, A.; Pink, C.; Hammer, E.; Michalik, S.; Dhople, V.M.; Holtfreter, B.; Völker, U.; Kocher, T.; Gesell Salazar, M. Cross-Sectional Association of Salivary Proteins with Age, Sex, Body Mass Index, Smoking, and Education. J. Proteome Res. 2017, 16, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, H.P.; Graamans, K.; Horrée, W.A. Diagnosis of Salivary Gland Diseases. Ned. Tijdschr. Geneeskd. 1987, 131, 1754–1759. [Google Scholar]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A Practical Guide to the Oral Microbiome and Its Relation to Health and Disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Jiang, W.-X.; Hu, Y.-J.; Gao, L.; He, Z.-Y.; Zhu, C.-L.; Ma, R.; Huang, Z.-W. The Impact of Various Time Intervals on the Supragingival Plaque Dynamic Core Microbiome. PLoS ONE 2015, 10, e0124631. [Google Scholar] [CrossRef][Green Version]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the Healthy “Core Microbiome” of Oral Microbial Communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Berezow, A.B.; Darveau, R.P. Microbial Shift and Periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- Chimenos-Küstner, E.; Giovannoni, M.L.; Schemel-Suárez, M. Dysbiosis as a Determinant Factor of Systemic and Oral Pathology: Importance of Microbiome. Med. Clin. (Barc.) 2017, 149, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Mathney, J.M.J.; Kent, R.L.; Chalmers, N.I.; Hughes, C.V.; Loo, C.Y.; Pradhan, N.; Kanasi, E.; Hwang, J.; Dahlan, M.A.; et al. Cultivable Anaerobic Microbiota of Severe Early Childhood Caries. J. Clin. Microbiol. 2011, 49, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Manji, F.; Dahlen, G.; Fejerskov, O. Caries and Periodontitis: Contesting the Conventional Wisdom on Their Aetiology. Caries Res. 2018, 52, 548–564. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Diversity of Endodontic Microbiota Revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The Oral Microbiota in Colorectal Cancer Is Distinctive and Predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral Pathobiont Induces Systemic Inflammation and Metabolic Changes Associated with Alteration of Gut Microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. Gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Rovella, V.; Rodia, G.; Di Daniele, F.; Cardillo, C.; Campia, U.; Noce, A.; Candi, E.; Della-Morte, D.; Tesauro, M. Association of Gut Hormones and Microbiota with Vascular Dysfunction in Obesity. Nutrients 2021, 13, 613. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic Colonization of Oral Bacteria in the Intestine Drives TH1 Cell Induction and Inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of Oral and Gut Microbiome in Nitric Oxide-Mediated Colon Motility. Nitric Oxide 2018, 73, 81–88. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M. V Altered Intestinal Microbial Flora and Impaired Epithelial Barrier Structure and Function in CKD: The Nature, Mechanisms, Consequences and Potential Treatment. Nephrol. Dial. Transpl. 2016, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D. The Salivary Microbiota in Health and Disease. J. Oral Microbiol. 2020, 12, 1723975. [Google Scholar] [CrossRef]
- Hu, J.; Iragavarapu, S.; Nadkarni, G.N.; Huang, R.; Erazo, M.; Bao, X.; Verghese, D.; Coca, S.; Ahmed, M.K.; Peter, I. Location-Specific Oral Microbiome Possesses Features Associated with CKD. Kidney Int. Rep. 2018, 3, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chen, X.; Gupta, M.; Seriwatanachai, D.; Xue, H.; Xiong, Q.; Xu, T.; Li, D.; Mo, A.; Tang, X.; et al. Salivary Microbiome in Patients Undergoing Hemodialysis and Its Associations with the Duration of the Dialysis. BMC Nephrol. 2020, 21, 414. [Google Scholar] [CrossRef]
- Gogeneni, H.; Buduneli, N.; Ceyhan-Öztürk, B.; Gümüş, P.; Akcali, A.; Zeller, I.; Renaud, D.E.; Scott, D.A.; Özçaka, Ö. Increased Infection with Key Periodontal Pathogens during Gestational Diabetes Mellitus. J. Clin. Periodontol. 2015, 42, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Polidori, I.; Marullo, L.; Ialongo, C.; Tomassetti, F.; Colombo, R.; di Gaudio, F.; Calugi, G.; Marrone, G.; Noce, A.; Bernardini, S.; et al. Characterization of Gut Microbiota Composition in Type 2 Diabetes Patients: A Population-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 5913. [Google Scholar] [CrossRef]
- Sabharwal, A.; Ganley, K.; Miecznikowski, J.C.; Haase, E.M.; Barnes, V.; Scannapieco, F.A. The Salivary Microbiome of Diabetic and Non-Diabetic Adults with Periodontal Disease. J. Periodontol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Janem, W.F.; Scannapieco, F.A.; Sabharwal, A.; Tsompana, M.; Berman, H.A.; Haase, E.M.; Miecznikowski, J.C.; Mastrandrea, L.D. Salivary Inflammatory Markers and Microbiome in Normoglycemic Lean and Obese Children Compared to Obese Children with Type 2 Diabetes. PLoS ONE 2017, 12, e0172647. [Google Scholar] [CrossRef]
- Barbadoro, P.; Ponzio, E.; Coccia, E.; Prospero, E.; Santarelli, A.; Rappelli, G.G.L.; D’Errico, M.M. Association between Hypertension, Oral Microbiome and Salivary Nitric Oxide: A Case-Control Study. Nitric Oxide 2021, 106, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Panzer, U.; Anders, H.-J.; Rees, A.J. The Immune System and Kidney Disease: Basic Concepts and Clinical Implications. Nat. Rev. Immunol. 2013, 13, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Neish, A.S. Mucosal Immunity and the Microbiome. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 1), S28–S32. [Google Scholar] [CrossRef]
- Takahashi, N. Oral Microbiome Metabolism: From “Who Are They?” to “What Are They Doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Mizdrak, M.; Kumrić, M.; Kurir, T.T.; Božić, J. Emerging Biomarkers for Early Detection of Chronic Kidney Disease. J. Pers. Med. 2022, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cheng, Y.; Hou, Y.; Wu, H. Lower Serum and Higher Urine Immunoglobulin G Are Associated with an Increased Severity of Idiopathic Membranous Nephropathy. Ann. Clin. Lab. Sci. 2019, 49, 777–784. [Google Scholar] [PubMed]
- Yu, F.Y.; Wang, Q.Q.; Li, M.; Cheng, Y.-H.; Cheng, Y.-S.L.; Zhou, Y.; Yang, X.; Zhang, F.; Ge, X.; Zhao, B.; et al. Dysbiosis of Saliva Microbiome in Patients with Oral Lichen Planus. BMC Microbiol. 2020, 20, 75. [Google Scholar] [CrossRef]
- Snider, E.J.; Compres, G.; Freedberg, D.E.; Giddins, M.J.; Khiabanian, H.; Lightdale, C.J.; Nobel, Y.R.; Toussaint, N.C.; Uhlemann, A.-C.; Abrams, J.A. Barrett’s Esophagus Is Associated with a Distinct Oral Microbiome. Clin. Transl. Gastroenterol. 2018, 9, 135. [Google Scholar] [CrossRef]
- Li, D.; Xi, W.; Zhang, Z.; Ren, L.; Deng, C.; Chen, J.; Sun, C.; Zhang, N.; Xu, J. Oral Microbial Community Analysis of the Patients in the Progression of Liver Cancer. Microb. Pathog. 2020, 149, 104479. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sheng, J.; Hu, L.; Zhang, B.; Guo, W.; Wang, Y.; Gu, Y.; Jiang, P.; Lin, H.; Lydia, B.; et al. Salivary Microbiome in Chronic Kidney Disease: What Is Its Connection to Diabetes, Hypertension, and Immunity? J. Transl. Med. 2022, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Chicharro, J.L.; Lucía, A.; Pérez, M.; Vaquero, A.F.; Ureña, R. Saliva Composition and Exercise. Sports Med. 1998, 26, 17–27. [Google Scholar] [CrossRef]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and Oral Diseases: Current Status and Perspective in Periodontitis. Ann. Stomatol. (Roma) 2011, 2, 10–18. [Google Scholar]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin Efficacy versus Ferrous Sulfate in Curing Iron Disorders in Pregnant and Non-Pregnant Women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef]
- Paesano, R.; Natalizi, T.; Berlutti, F.; Valenti, P. Body Iron Delocalization: The Serious Drawback in Iron Disorders in Both Developing and Developed Countries. Pathog. Glob. Health 2012, 106, 200–216. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An Important Host Defence against Microbial and Viral Attack. Cell Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Groenink, J.; Walgreen-Weterings, E.; Nazmi, K.; Bolscher, J.G.; Veerman, E.C.; van Winkelhoff, A.J.; Nieuw Amerongen, A.V. Salivary Lactoferrin and Low-Mr Mucin MG2 in Actinobacillus Actinomycetemcomitans-Associated Periodontitis. J. Clin. Periodontol. 1999, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.d.M.; Zenóbio, E.G.; Van Dyke, T.; Silva, K.S.; Costa, F.O.; Soares, R.V. Differential Expression of Salivary Glycoproteins in Aggressive and Chronic Periodontitis. J. Appl. Oral Sci. 2012, 20, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Ajello, M.; Bosso, P.; Morea, C.; Petrucca, A.; Antonini, G.; Valenti, P. Both Lactoferrin and Iron Influence Aggregation and Biofilm Formation in Streptococcus Mutans. Biometals 2004, 17, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Żyła, T.; Kawala, B.; Antoszewska-Smith, J.; Kawala, M. Black Stain and Dental Caries: A Review of the Literature. Biomed. Res. Int. 2015, 2015, 469392. [Google Scholar] [CrossRef] [PubMed]
- Elass-Rochard, E.; Legrand, D.; Salmon, V.; Roseanu, A.; Trif, M.; Tobias, P.S.; Mazurier, J.; Spik, G. Lactoferrin Inhibits the Endotoxin Interaction with CD14 by Competition with the Lipopolysaccharide-Binding Protein. Infect. Immun. 1998, 66, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.; Rekdal, O.; Svendsen, J.S.; Gutteberg, T.J. Lactoferricin of Bovine Origin Is more Active than Lactoferricins of Human, Murine and Caprine Origin. Scand. J. Infect. Dis. 1998, 30, 513–517. [Google Scholar] [CrossRef]
- Alugupalli, K.R.; Kalfas, S. Degradation of Lactoferrin by Periodontitis-Associated Bacteria. FEMS Microbiol. Lett. 1996, 145, 209–214. [Google Scholar] [CrossRef]
- Komine, K.-I.; Kuroishi, T.; Ozawa, A.; Komine, Y.; Minami, T.; Shimauchi, H.; Sugawara, S. Cleaved Inflammatory Lactoferrin Peptides in Parotid Saliva of Periodontitis Patients. Mol. Immunol. 2007, 44, 1498–1508. [Google Scholar] [CrossRef]
- Zaura, E.; Brandt, B.W.; Prodan, A.; Teixeira de Mattos, M.J.; Imangaliyev, S.; Kool, J.; Buijs, M.J.; Jagers, F.L.; Hennequin-Hoenderdos, N.L.; Slot, D.E.; et al. On the Ecosystemic Network of Saliva in Healthy Young Adults. ISME J. 2017, 11, 1218–1231. [Google Scholar] [CrossRef]
- Weistroffer, P.L.; Joly, S.; Srikantha, R.; Tack, B.F.; Brogden, K.A.; Guthmiller, J.M. SMAP29 Congeners Demonstrate Activity Against Oral Bacteria and Reduced Toxicity Against Oral Keratinocytes. Oral Microbiol. Immunol. 2008, 23, 89–95. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the Oral Microbiome in Personalized Dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wen, S.; Yang, L.; Zheng, G. Mucosal Anti-Caries DNA Vaccine: A New Approach to Induce Protective Immunity against Streptococcus Mutans. Int. J. Exp. Pathol. 2017, 10, 853–857. [Google Scholar]
- Robinette, R.A.; Oli, M.W.; McArthur, W.P.; Brady, L.J. A Therapeutic Anti-Streptococcus Mutans Monoclonal Antibody Used in Human Passive Protection Trials Influences the Adaptive Immune Response. Vaccine 2011, 29, 6292–6300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smith, D.J. Dental Caries Vaccines: Prospects and Concerns. Expert Rev. Vaccines 2010, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Camelo-Castillo, A.; Ferrer, M.D.; Simon-Soro, Á.; Mira, A. Health-Associated Niche Inhabitants as Oral Probiotics: The Case of Streptococcus Dentisani. Front. Microbiol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Nascimento, M.; Burne, R.A. Progress toward Understanding the Contribution of Alkali Generation in Dental Biofilms to Inhibition of Dental Caries. Int. J. Oral Sci. 2012, 4, 135–140. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhou, X.; Hu, S.; Zhang, S.; Wu, H. Effect of the Antimicrobial Decapeptide KSL on the Growth of Oral Pathogens and Streptococcus Mutans Biofilm. Int. J. Antimicrob. Agents 2011, 37, 33–38. [Google Scholar] [CrossRef]
- Manley, K.J. Saliva Composition and Upper Gastrointestinal Symptoms in Chronic Kidney Disease. J. Ren. Care 2014, 40, 172–179. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A Review of Saliva: Normal Composition, Flow, and Function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Bagalad, B.; Mohankumar, K.; Madhushankari, G.; Donoghue, M.; Kuberappa, P. Diagnostic Accuracy of Salivary Creatinine, Urea, and Potassium Levels to Assess Dialysis Need in Renal Failure Patients. Dent. Res. J. (Isfahan) 2017, 14, 13. [Google Scholar] [CrossRef]
- Rodrigues, V.P.; Franco, M.M.; Marques, C.P.C.; de Carvalho, R.C.C.; Leite, S.A.M.; Pereira, A.L.A.; Benatti, B.B. Salivary Levels of Calcium, Phosphorus, Potassium, Albumin and Correlation with Serum Biomarkers in Hemodialysis Patients. Arch. Oral Biol. 2016, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Furuhashi, M.; Sesoko, S.; Kosuge, K.; Maeda, T.; Todoroki, K.; Inoue, K.; Min, J.Z.; Toyo’oka, T. Determination of Creatinine-Related Molecules in Saliva by Reversed-Phase Liquid Chromatography with Tandem Mass Spectrometry and the Evaluation of Hemodialysis in Chronic Kidney Disease Patients. Anal. Chim. Acta 2016, 911, 92–99. [Google Scholar] [CrossRef]
- Venkatapathy, R.; Govindarajan, V.; Oza, N.; Parameswaran, S.; Pennagaram Dhanasekaran, B.; Prashad, K.V. Salivary Creatinine Estimation as an Alternative to Serum Creatinine in Chronic Kidney Disease Patients. Int. J. Nephrol. 2014, 2014, 742724. [Google Scholar] [CrossRef]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Korkosz, R.; Tanasiewicz, M. Oral Mucosa Status and Saliva Parameters of Multimorbid Adult Patients Diagnosed with End-Stage Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2021, 18, 2515. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, M. Abhishek Oral Conditions in Renal Disorders and Treatment Considerations—A Review for Pediatric Dentist. Saudi Dent. J. 2015, 27, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Oyetola, E.O.; Owotade, F.J.; Agbelusi, G.A.; Fatusi, O.A.; Sanusi, A.A. Oral Findings in Chronic Kidney Disease: Implications for Management in Developing Countries. BMC Oral Health 2015, 15, 24. [Google Scholar] [CrossRef]
- Patil, S.; Khaandelwal, S.; Doni, B.; Rahuman, F.; Kaswan, S. Oral Manifestations in Chronic Renal Failure Patients Attending Two Hospitals in North Karnataka, India. Oral Health Dent. Manag. 2012, 11, 100–106. [Google Scholar]
- Proctor, R.; Kumar, N.; Stein, A.; Moles, D.; Porter, S. Oral and Dental Aspects of Chronic Renal Failure. J. Dent. Res. 2005, 84, 199–208. [Google Scholar] [CrossRef]
- Seraj, B.; Ahmadi, R.; Ramezani, N.; Mashayekhi, A.; Ahmadi, M. Oro-Dental Health Status and Salivary Characteristics in Children with Chronic Renal Failure. J. Dent. (Tehran) 2011, 8, 146–151. [Google Scholar]
- Yadav, A.; Deepak, U.; Misra, N.; Kumar, G.; Kaur, A. Oral Manifestations in Renal Failure Patients Undergoing Dialysis. Int. J. Med. Sci. Public Health 2015, 4, 1015. [Google Scholar] [CrossRef]
- Akar, H.; Akar, G.C.; Carrero, J.J.; Stenvinkel, P.; Lindholm, B. Systemic Consequences of Poor Oral Health in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Swapna, L.A. Oral Health Status in Haemodialysis Patients. J. Clin. Diagn. Res. 2013, 7, 2047. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Fernandes, L.B.; Fidalgo, T.K.S.; de Almeida, P.A.; Souza, I.P.R.; Valente, A.P. Salivary Metabolome of Children and Adolescents under Peritoneal Dialysis. Clin. Oral Investig. 2021, 25, 2345–2351. [Google Scholar] [CrossRef]
- Yu, I.-C.; Liu, C.-Y.; Fang, J.-T. Effects of Hemodialysis Treatment on Saliva Flow Rate and Saliva Composition during In-Center Maintenance Dialysis: A Cross-Sectional Study. Ren. Fail. 2021, 43, 71–78. [Google Scholar] [CrossRef]
- Hemodialysis Adequacy 2006 Work Group. Clinical Practice Guidelines for Hemodialysis Adequacy, Update 2006. Am. J. Kidney Dis. 2006, 48 (Suppl. 1), S2–S90. [Google Scholar] [CrossRef]
- Almeida, P.A.; Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; Almeida, F.C.L.; Souza, I.P.R.; Valente, A.P. Salivary Metabolic Profile of Children and Adolescents after Hemodialysis. Metabolomics 2017, 13, 141. [Google Scholar] [CrossRef]
- Martins, C.; Siqueira, W.L.; Oliveira, E.; Nicolau, J.; Primo, L.G. Dental Calculus Formation in Children and Adolescents Undergoing Hemodialysis. Pediatr. Nephrol. 2012, 27, 1961–1966. [Google Scholar] [CrossRef]
- Pham, T.A.V. Validation of the Salivary Urea and Creatinine Tests as Screening Methods of Chronic Kidney Disease in Vietnamese Patients. Acta Odontol. Scand. 2017, 75, 551–556. [Google Scholar] [CrossRef]
- Nandan, R.K.; Sivapathasundharam, B.; Sivakumar, G. Oral Manifestations and Analysis of Salivary and Blood Urea Levels of Patients under Going Haemo Dialysis and Kidney Transplant. Indian J. Dent. Res. 2005, 16, 77–82. [Google Scholar]
- George, C.; Matsha, T.E.; Erasmus, R.T.; Kengne, A.P. Haematological Profile of Chronic Kidney Disease in a Mixed-Ancestry South African Population: A Cross-Sectional Study. BMJ Open 2018, 8, e025694. [Google Scholar] [CrossRef]
- Du, X.; Wang, F.; Hu, Z.; Wu, J.; Wang, Z.; Yan, C.; Zhang, C.; Tang, J. The Diagnostic Value of Pepsin Detection in Saliva for Gastro-Esophageal Reflux Disease: A Preliminary Study from China. BMC Gastroenterol. 2017, 17, 107. [Google Scholar] [CrossRef]
- Satish, B.N.V.S.; Srikala, P.; Maharudrappa, B.; Awanti, S.M.; Kumar, P.; Hugar, D. Saliva: A Tool in Assessing Glucose Levels in Diabetes Mellitus. J. Int. Oral Health 2014, 6, 114–117. [Google Scholar]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T.W. Salivary Biomarkers: Toward Future Clinical and Diagnostic Utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef]
- Plata, C.; Cruz, C.; Cervantes, L.G.; Ramírez, V. The Gut Microbiota and Its Relationship with Chronic Kidney Disease. Int. Urol. Nephrol. 2019, 51, 2209–2226. [Google Scholar] [CrossRef]
- Martinez, A.W.; Recht, N.S.; Hostetter, T.H.; Meyer, T.W. Removal of P-Cresol Sulfate by Hemodialysis. J. Am. Soc. Nephrol. 2005, 16, 3430–3436. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Vanholder, R.; Brunet, P. Protein-Bound Toxins—Update 2009. Semin. Dial. 2009, 22, 334–339. [Google Scholar] [CrossRef]
- Praveenraj, S.S.; Sonali, S.; Anand, N.; Tousif, H.A.; Vichitra, C.; Kalyan, M.; Kanna, P.V.; Chandana, K.A.; Shasthara, P.; Mahalakshmi, A.M.; et al. The Role of a Gut Microbial-Derived Metabolite, Trimethylamine N-Oxide (TMAO), in Neurological Disorders. Mol. Neurobiol. 2022, 59, 6684–6700. [Google Scholar] [CrossRef]
- Saito, A.; Takagi, T.; Chung, T.G.; Ohta, K. Serum Levels of Polyamines in Patients with Chronic Renal Failure. Kidney Int. Suppl. 1983, 16, S234–S237. [Google Scholar]
- Basilicata, M.; Di Lauro, M.; Campolattano, V.; Marrone, G.; Celotto, R.; Mitterhofer, A.P.; Bollero, P.; Di Daniele, N.; Noce, A. Natural Bioactive Compounds in the Management of Oral Diseases in Nephropathic Patients. Int. J. Environ. Res. Public Health 2022, 19, 1665. [Google Scholar] [CrossRef]
- Costacurta, M.; Basilicata, M.; Marrone, G.; Di Lauro, M.; Campolattano, V.; Bollero, P.; Docimo, R.; Di Daniele, N.; Noce, A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients 2022, 14, 2002. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic Diseases Caused by Oral Infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef]
- Gonçalves, M.O.; Coutinho-Filho, W.P.; Pimenta, F.P.; Pereira, G.A.; Pereira, J.A.A.; Mattos-Guaraldi, A.L.; Hirata, R. Periodontal Disease as Reservoir for Multi-Resistant and Hydrolytic Enterobacterial Species. Lett. Appl. Microbiol. 2007, 44, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Ruppé, É.; Woerther, P.-L.; Barbier, F. Mechanisms of Antimicrobial Resistance in Gram-Negative Bacilli. Ann. Intensive Care 2015, 5, 61. [Google Scholar] [CrossRef]
- Costa, C.F.F.A.; Merino-Ribas, A.; Ferreira, C.; Campos, C.; Silva, N.; Pereira, L.; Garcia, A.; Azevedo, Á.; Mesquita, R.B.R.; Rangel, A.O.S.S.; et al. Characterization of Oral Enterobacteriaceae Prevalence and Resistance Profile in Chronic Kidney Disease Patients Undergoing Peritoneal Dialysis. Front. Microbiol. 2021, 12, 736685. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Lu, W.; Zhu, L.; Zhou, W.; Song, Z. Characterization of the Subgingival Microbiota in the Peritoneal Dialysis Patients with Periodontitis. Arch. Oral Biol. 2020, 115, 104742. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can Oral Bacteria Affect the Microbiome of the Gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Dinakaran, V.; Mandape, S.N.; Shuba, K.; Pratap, S.; Sakhare, S.S.; Tabatabai, M.A.; Smoot, D.T.; Farmer-Dixon, C.M.; Kesavalu, L.N.; Adunyah, S.E.; et al. Identification of Specific Oral and Gut Pathogens in Full Thickness Colon of Colitis Patients: Implications for Colon Motility. Front. Microbiol. 2018, 9, 3220. [Google Scholar] [CrossRef]
- Ding, Y.-H.; Qian, L.-Y.; Pang, J.; Lin, J.-Y.; Xu, Q.; Wang, L.-H.; Huang, D.-S.; Zou, H. The Regulation of Immune Cells by Lactobacilli: A Potential Therapeutic Target for Anti-Atherosclerosis Therapy. Oncotarget 2017, 8, 59915–59928. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, T.; Dong, S.; Jiang, H.; Zhang, J.; Raza, H.K.; Lei, G. Association between Gut Dysbiosis and Chronic Kidney Disease: A Narrative Review of the Literature. J. Int. Med. Res. 2021, 49, 3000605211053276. [Google Scholar] [CrossRef]
- Meijers, B.; Farré, R.; Dejongh, S.; Vicario, M.; Evenepoel, P. Intestinal Barrier Function in Chronic Kidney Disease. Toxins 2018, 10, 298. [Google Scholar] [CrossRef]
- Simenhoff, M.L.; Saukkonen, J.J.; Burke, J.F.; Wesson, L.G.; Schaedler, R.W.; Gordon, S.J. Bacterial Populations of the Small Intestine in Uremia. Nephron 1978, 22, 63–68. [Google Scholar] [CrossRef]
- Cigarran Guldris, S.; González Parra, E.; Cases Amenós, A. Gut Microbiota in Chronic Kidney Disease. Nefrologia 2017, 37, 9–19. [Google Scholar] [CrossRef]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The Impact of Gut Microbiota on Kidney Function and Pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Reijnders, D.; Swinkels, D.W. Oral Iron Supplementation: Potential Implications for the Gut Microbiome and Metabolome in Patients with CKD. Hemodial. Int. 2017, 21, S28–S36. [Google Scholar] [CrossRef]
- Weiss, G. Dietary Iron Supplementation: A Proinflammatory Attack on the Intestine? Gut 2015, 64, 696–697. [Google Scholar] [CrossRef]
- Wu, M.-J.; Chang, C.-S.; Cheng, C.-H.; Chen, C.-H.; Lee, W.-C.; Hsu, Y.-H.; Shu, K.-H.; Tang, M.-J. Colonic Transit Time in Long-Term Dialysis Patients. Am. J. Kidney Dis. 2004, 44, 322–327. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Raffatellu, M.; Swinkels, D.W.; Tjalsma, H. Nutritional Iron Turned inside out: Intestinal Stress from a Gut Microbial Perspective. FEMS Microbiol. Rev. 2014, 38, 1202–1234. [Google Scholar] [CrossRef]
- Chaplin, S.; Bhandari, S. Oral Iron: Properties and Current Place in the Treatment of Anaemia. Prescriber 2012, 23, 12–18. [Google Scholar] [CrossRef]
- Lund, E.K.; Wharf, S.G.; Fairweather-Tait, S.J.; Johnson, I.T. Oral Ferrous Sulfate Supplements Increase the Free Radical-Generating Capacity of Feces from Healthy Volunteers. Am. J. Clin. Nutr. 1999, 69, 250–255. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The Gut-Kidney Axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Leemans, J.C.; Kors, L.; Anders, H.-J.; Florquin, S. Pattern Recognition Receptors and the Inflammasome in Kidney Disease. Nat. Rev. Nephrol. 2014, 10, 398–414. [Google Scholar] [CrossRef]
- Vieira, A.T.; Teixeira, M.M.; Martins, F.S. The Role of Probiotics and Prebiotics in Inducing Gut Immunity. Front. Immunol. 2013, 4, 445. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional Roles of Lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef]
- Cutone, A.; Frioni, A.; Berlutti, F.; Valenti, P.; Musci, G.; Bonaccorsi di Patti, M.C. Lactoferrin Prevents LPS-Induced Decrease of the Iron Exporter Ferroportin in Human Monocytes/Macrophages. Biometals 2014, 27, 807–813. [Google Scholar] [CrossRef]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.B.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin Differently Modulates the Inflammatory Response in Epithelial Models Mimicking Human Inflammatory and Infectious Diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef]
- Mafra, D.; Fouque, D. Gut Microbiota and Inflammation in Chronic Kidney Disease Patients. Clin. Kidney J. 2015, 8, 332–334. [Google Scholar] [CrossRef]
- McIntyre, C.W.; Harrison, L.E.A.; Eldehni, M.T.; Jefferies, H.J.; Szeto, C.-C.; John, S.G.; Sigrist, M.K.; Burton, J.O.; Hothi, D.; Korsheed, S.; et al. Circulating Endotoxemia: A Novel Factor in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 133–141. [Google Scholar] [CrossRef]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef]
- Vanholder, R.; Glorieux, G. The Intestine and the Kidneys: A Bad Marriage Can Be Hazardous. Clin. Kidney J. 2015, 8, 168–179. [Google Scholar] [CrossRef]
- Meijers, B.; Evenepoel, P.; Anders, H.-J. Intestinal Microbiome and Fitness in Kidney Disease. Nat. Rev. Nephrol. 2019, 15, 531–545. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Liu, S.-M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J. High Amylose Resistant Starch Diet Ameliorates Oxidative Stress, Inflammation, and Progression of Chronic Kidney Disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Jain, A.K.; Blake, P.G. Non-Pseudomonas Gram-Negative Peritonitis. Kidney Int. 2006, 69, 1107–1109. [Google Scholar] [CrossRef]
- Sibanda, M. Primary Peritonitis Caused by Raoultella Ornithinolytica in a 53-year-old Man. JMM Case Rep. 2014, 1, e002634. [Google Scholar] [CrossRef]
- Simões-Silva, L.; Silva, S.; Santos-Araujo, C.; Sousa, J.; Pestana, M.; Araujo, R.; Soares-Silva, I.; Sampaio-Maia, B. Oral Yeast Colonization and Fungal Infections in Peritoneal Dialysis Patients: A Pilot Study. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 4846363. [Google Scholar] [CrossRef]
- Snelson, M.; Kellow, N.J.; Coughlan, M.T. Modulation of the Gut Microbiota by Resistant Starch as a Treatment of Chronic Kidney Diseases: Evidence of Efficacy and Mechanistic Insights. Adv. Nutr. 2019, 10, 303–320. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Kalmokoff, M.; Zwicker, B.; O’Hara, M.; Matias, F.; Green, J.; Shastri, P.; Green-Johnson, J.; Brooks, S.P.J. Temporal Change in the Gut Community of Rats Fed High Amylose Cornstarch Is Driven by Endogenous Urea Rather than Strictly on Carbohydrate Availability. J. Appl. Microbiol. 2013, 114, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar]
- Matei-Lațiu, M.-C.; Gal, A.-F.; Rus, V.; Buza, V.; Martonos, C.; Lațiu, C.; Ștefănuț, L.-C. Intestinal Dysbiosis in Rats: Interaction between Amoxicillin and Probiotics, a Histological and Immunohistochemical Evaluation. Nutrients 2023, 15, 1105. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, Prebiotics and Amelioration of Diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B. V Probiotics, Prebiotics and Synbiotics—A Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of Short-Chain Fatty Acids and Their Receptors in Inflammation and Carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).