Abstract
Recent advancements in omics technologies have generated a wealth of biological data. Integrating these data within mathematical models is essential to fully leverage their potential. Genome-scale metabolic models (GEMs) provide a robust framework for studying complex biological systems. GEMs have significantly contributed to our understanding of human metabolism, including the intrinsic relationship between the gut microbiome and the host metabolism. In this review, we highlight the contributions of GEMs and discuss the critical challenges that must be overcome to ensure their reproducibility and enhance their prediction accuracy, particularly in the context of precision medicine. We also explore the role of machine learning in addressing these challenges within GEMs. The integration of omics data with GEMs has the potential to lead to new insights, and to advance our understanding of molecular mechanisms in human health and disease.
1. Introduction
The integration of omics data such as genomics, transcriptomics, proteomics, and metabolomics has revolutionized our understanding of biological systems by providing a holistic view of the complex molecular processes associated with human health [1]. Genome-scale metabolic modeling (GSMM) is a constraint-based mathematical modeling technique that has been instrumental in the analysis of omics data [2,3]. Genome-scale metabolic models (GEMs) provide a robust framework that enables the integration of multiple omics datasets [4,5]. By harnessing the power of GEMs, researchers can delve into the complexities of biological pathways, enabling a comprehensive understanding of cellular metabolism and its underlying mechanisms [2,6,7,8,9]. Furthermore, incorporating biochemical and genetic information into GEMs has enabled researchers to understand the interplay between genes, proteins, and metabolites involved in cellular metabolism. This integrative approach effectively bridges the gap between genotypes and phenotypes [4,5,10].
GEMs serve as a valuable tool for predicting metabolic capabilities and identifying key regulatory nodes in biological systems, representing a paradigm shift in omics data analysis. GSMM has been used to study gut ecosystems, exploring the metabolic interactions between the host and the microbial communities in the gut [11,12,13,14]. Notably, the development of GEMs for catalogued human gut microbes [15,16] by uncovering their metabolic functions has been a major recent achievement.
In this review, we explore how GSMM has contributed to our understanding of human metabolism. Specifically, we focus on two important approaches: (a) tissue-specific modeling and (b) host-microbiome modeling. We address key challenges associated with model reconstruction and validation to improve the reproducibility and reliability of GEM-based predictions. In addition, we emphasize the wide-ranging scope and diverse applications of GEMs in the field of precision medicine.
2. Exploring Human Metabolism: The Evolution of Genome-Scale Metabolic Models
Throughout the past three decades, GEMs have undergone continuous evolution, deepening our understanding of metabolic processes and their implications in various biological and clinical studies [9,17]. GEMs have been widely used in metabolic engineering, where they have demonstrated their ability to predict cellular growth under different nutrient conditions [18,19]. Furthermore, they have been applied to design studies, investigating the essentiality of reactions/genes [20,21] and the relevance of metabolic pathways [22] and modeling phenotypes by manipulating these pathway(s) [23].
Over the past 15 years, there has been a focused and continuous effort by researchers to develop and enhance GEMs for human metabolism. Recon 1 was one of the first generic reconstructions of human metabolism, which aimed to integrate and analyze diverse biological datasets, providing a foundation for studying human metabolic pathways [24]. EHMN (Edinburgh Human Metabolic Network) was developed to capture the intricacies of human metabolism and facilitate comprehensive analyses of metabolic functions. EHMN served as a valuable resource for exploring metabolic interactions and pathways in humans [25]. Following that, Recon 2 was built upon Recon 1, leading to an expanded coverage of human metabolic pathways and thereby offering an enhanced understanding of metabolic processes in health and disease [26,27]. HMR (Human Metabolic Reaction) encompasses a comprehensive collection of metabolic reactions, enzymes, and associated genes. It serves as a valuable resource for studying metabolic networks [6,28]. Recently, Recon 3D, a three-dimensional reconstruction of human metabolism, was developed, which integrates spatial information to better represent the complexity of metabolic reactions within cellular compartments. Recon 3D provides a detailed and context-specific view of human metabolism, enabling deeper insights into cellular function than previously possible [3]. HMR and Recon reconstructions have been extensively utilized to study various diseases, such as type 2 diabetes (T2D), non-alcoholic fatty liver disease (NAFLD), cancer, and immunometabolism [6,29,30,31,32]. In addition, Robinson et al. developed Human1 [33], a unified Human-GEM, and a web portal, Metabolic Atlas (https://metabolicatlas.org/ (accessed on 23 July 2015)), by integrating and curating the Recon and HMR model’s lineages. The entire development process followed a systematic approach. Moreover, Human1 and Metabolic Atlas were demonstrated to be valuable tools in identifying metabolic vulnerabilities, for instance, in acute myeloid leukemia; predicting essential genes for specific metabolic functions; and estimating metabolic fluxes and growth rates. These advancements enhance the capacity of GEMs to model metabolic pathways associated with health and disease.
Taken together, genome-scale metabolic reconstructions have contributed to our understanding of metabolism, providing a foundation for studying metabolic pathways, predicting regulations, and exploring the impact of genetic variations and environmental factors on human health.
3. Integrating Omics Data into Genome-Scale Metabolic Models: Overcoming Challenges and Shaping Perspectives
Omics data have been extensively used to deepen our understanding of various biological systems, including cells, tissues, and organs. By incorporating individual- or condition-specific omics datasets, researchers can develop models that accurately represent the metabolic characteristics of an individual at a particular condition. This approach allows for a more precise investigation of metabolic pathways and therefore provides a foundation for precision medicine and the selection of optimal treatment strategies [34,35]. Furthermore, overlaying omics data with the metabolic networks has enabled researchers to infer metabolic regulations and identify patterns associated with metabolic states [7,8,28,36].
The integration of omics data poses several challenges, and addressing these challenges requires continuous advancements in data acquisition, standardization, computational methodologies, and model validation techniques to enhance the accuracy and applicability of integrated models in various biological contexts.
A vast amount of data makes their integration and harmonization a daunting task. Diverse data types, formats, and measurement scales require meticulous effort with respect to data integration and standardization. The heterogeneity of data sources, i.e., datasets generated from different studies, experiments, or platforms, introduces variations in the data. Managing these technical variations is crucial to ensure the consistency of the results. Moreover, quality control measures such as outlier removal, artifact correction, and noise filtering are undertaken to improve data quality [1,37,38,39]. Dealing with the missing values is a critical aspect of omics data that can undermine the accuracy and reliability of the outcomes, if not properly adjusted for. To mitigate this issue, imputation methods are commonly employed to estimate missing values and minimize the impact of data sparsity [40]. Lastly, normalization plays an important role in standardizing the scale and range of omics data across different samples or conditions [41,42]. The normalization of omics data involves the utilization of various tools, with each suited to specific omics data types and analytical requirements. Central tendency-based normalization, such as mean and median, is a simple yet effective method employed for normalizing proteomics and metabolomics data. It rescales the intensity values of individual samples to align with the mean or median intensity across all samples. Quantile normalization [43] is a widely used technique for normalizing gene expression data derived from microarrays. By aligning the empirical distributions of expression values across samples, it ensures comparability and robustness. For high-throughput genomic data, including microarrays or DNA methylation arrays, ComBat [44] is a specialized tool designed to address batch effects. By employing an empirical Bayes framework, ComBat effectively adjusts for batch-related variations, leading to more reliable results. ComBat-seq [45] is an advanced method that utilizes a negative binomial regression model to address batch effects in RNA-seq studies. RUVSeq (remove unwanted variation in RNA-seq) is a valuable tool dedicated to eliminating the unwanted sources of variations in RNA-seq data, such as batch effects or confounding factors [46]. Leveraging a factor analysis-based approach, RUVSeq estimates and removes these sources of variation, enhancing the accuracy of downstream analyses. Limma and Limma-Voom are versatile tools that are extensively used for normalizing gene expression microarray data and RNA-seq data, respectively. These tools utilize linear modeling and empirical Bayes methods to effectively handle technical variations and batch effects, ensuring robust and accurate analysis. DESeq2 [47] is a widely adopted tool that is specifically designed for normalizing RNA-seq data. By utilizing a negative binomial distribution model, DESeq2 effectively accounts for sequencing depth and sample-specific biases, enabling reliable differential expression analysis. In addition, edgeR [48] is another popular tool employed for RNA-seq data normalization. It utilizes a robust empirical Bayes approach to estimate normalization factors, considering library size and gene-specific biases. Next-generation sequencing (NGS) data can be normalized using multiple methods such as TMM (trimmed mean of M values), RPKM (reads per kilobase per million mapped reads), and CPM (counts per million), utilizing tools such as DESeq2, edgeR, and limma. Furthermore, NOMIS [49], a normalization method that is specifically developed for metabolomics data, employs the optimal selection of multiple internal standards to ensure accurate and reliable normalization. A thorough description of normalization methods applied to omics data is discussed elsewhere [41,50].
Furthermore, omics data may not capture the entire metabolic network or pathway, leading to incomplete coverage of the GEMs. Missing or unmeasured components can limit the model’s accuracy and predictive capabilities, particularly in scenarios where these components play critical roles.
Integrating omics data into GSMMs involves complex mathematical and computational algorithms. The process requires sophisticated tools and expertise to handle large datasets, perform data preprocessing, and apply appropriate integration methods, which can be computationally demanding.
Several standalone software suites, such as COBRA (constraint-based reconstruction and analysis) [51,52,53], Microbiome Modeling Toolbox [54], FastMM (a toolbox for personalized constraint-based metabolic modeling) [55], rBioNet [56], and RAVEN (reconstruction, analysis, and visualization of metabolic networks) [57,58], offer comprehensive functionalities for metabolic reconstructions, modeling, and the integration of omics data. A comprehensive list of the resources used for human metabolic pathway reconstructions is given in (Table 1).

Table 1.
Resources for microbial and human metabolic pathway reconstructions.
4. Modeling Tissue-Specific Interactions: Integrating Omics Data for Contextualization
Tissue-specific GEMs have emerged as powerful tools for investigating the complex metabolic processes that occur within specific cell or tissue types [6,8,80]. These models are reconstructed by integrating various types of omics data, including gene, transcript, protein, and metabolite data, allowing for a more nuanced and context-specific representation of the metabolic networks within a specific cell type. To leverage expression data, several algorithms have been developed. GIMME (gene inactivity moderated by metabolism and expression) utilizes gene expression or transcriptomics data to predict active reactions in a GEM [81,82], aiming to identify a consistent set of metabolic reactions that are active under the given conditions. This approach provides insights into the metabolic activity of specific cell-types. Another notable algorithm, iMAT (integrative metabolic analysis tool), integrates gene expression data to identify active metabolic reactions [83]. By combining transcriptomic data with GEMs, it formulates an optimization problem to determine the set of reactions that is likely to be active, enabling the identification of condition-specific metabolic activity and offering a valuable framework for understanding tissue-specific metabolism. The MADE (metabolic adjustment by differential expression) algorithm leverages gene expression data to predict metabolic adaptations in response to different conditions [84]. By considering changes in gene expression levels and utilizing a network-based approach, MADE identifies reactions that are differentially regulated between conditions, shedding light on how the metabolic network adjusts its activity in response to cellular or environmental changes. The E-flux algorithm incorporates gene expression data to estimate the metabolic flux distribution within a tissue-specific GEM [85]. By combining gene expression profiles with flux balance analysis (FBA), E-flux estimates the distribution of metabolic fluxes and predicts the activity of specific reactions, offering a valuable tool for understanding the flow of metabolites through metabolic pathways in specific tissues. The tINIT (transcriptional integration of tissue-specific GEMs) algorithm integrates gene expression data with a GEM to predict active metabolic reactions [80,86]. By considering gene expression levels and regulatory interactions, tINIT estimates the transcriptional activity of metabolic genes and identifies active reactions within the metabolic network, providing a deeper understanding of tissue-specific metabolic activity by incorporating transcriptional regulation. The METRADE (metabolic and transcriptomics adaptation estimator) framework offers a comprehensive approach for integrating gene and protein expression data, allowing the combination of both types of omics data and enabling a more holistic understanding of biological processes [87]. Similarly, the IOMA (integrative omics-metabolic analysis) platform provides an opportunity to integrate proteomic and metabolomic data [88]. Other algorithms include FASTCORE [89], FASTCORMICS [90], mCADRE [91], PRIME [92], RegrEX [93], and CORDA [94], and a detail description of these methods, including their implementation, characteristics, and applications, is reviewed elsewhere [4,95].
Automated methodologies have been utilized to develop tissue- and cell-specific models by combining human metabolic reconstructions, primarily from HMR and Recon lineages, along with omics data [80,91]. Semi-automated techniques for model reconstructions are included in software suites such as COBRA [51,52,53] and RAVEN [57,58], which offer advantages in terms of speed and throughput, enabling the construction of large-scale models within a reasonable time frame. However, it is important to note that the speed and throughput may vary depending on the specific methodologies employed and the complexity of the model being constructed. While semi-automated methods provide valuable efficiencies, they may also have some limitations. These include a reliance on manual curation and expert knowledge, which can introduce subjectivity and potential biases into the model development process [2,96]. Additionally, the need for manual intervention and decision making during the model’s construction phase may introduce human error. To facilitate the automated reconstruction of GEMs, web-servers like KBase [97] and ModelSEED [61] have been designed to incorporate large datasets. However, the transition from semi-automated to fully automated approaches face certain barriers. One significant barrier is the requirement for high-quality and comprehensive data, as well as accurate and reliable algorithms, to automate the model generation process effectively. Furthermore, the dynamic and context-specific nature of biological systems poses challenges in capturing the full complexity of metabolic networks in a fully automated manner. Overcoming these barriers requires refining computational techniques to handle large-scale data integration and model construction, ensuring the accuracy and reliability of automated approaches.
Notable examples of tissue-specific models include liver GEMs that are specifically tailored to mimic the metabolism of the human liver [6,8,98,99]. In a recent study, the GEMs of the human liver have been developed to examine patients with NAFLD at different stages of fibrosis [8]. The study revealed metabolic signatures related to vitamins (A and E), glycosphingolipids, and complex glycosaminoglycans in advanced fibrosis. Furthermore, the models identified metabolic patterns that are associated with three gene variants (PNPLA3, TM6SF2, and HSD17B13) linked to NAFLD, providing insights into metabolic dysregulation in the liver and its contribution towards NAFLD progression.
To unravel the contributions of various brain cells in neurodegenerative diseases, researchers have employed the brain-specific GEMs of neurons, astrocytes, and microglia, along with multi-omics data [100,101]. These models successfully replicate the metabolic interactions between these cell types in both healthy and pathological conditions. Moreover, these cell-type-specific models demonstrated the potential to reproduce observed physiological alterations, with simulations exhibiting strong agreement with experimental studies [100,101,102,103,104].
Adipocyte-GEM has been instrumental in studying the metabolic processes in adipocytes, which are the cells primarily responsible for storing and releasing energy as fat [28]. By integrating transcriptome and fluxome data with the adipocyte-GEM, the authors discovered specific metabolic alterations in obese subjects, including increased metabolic activity and decreased mitochondrial metabolism. Myocyte-GEM [29] was used to map transcriptional changes in T2D, uncovering significant transcriptional regulation related to pyruvate oxidation, branched-chain amino acid catabolism, and tetrahydrofolate metabolism. In another study, Zhao et al. reconstructed a comprehensive metabolic network specifically tailored to the human heart by incorporating transcriptome and proteome data [105]. By applying this heart-specific metabolic network, the authors identified novel biomarkers for cardiovascular disease (CVD) and predicted potential drug targets for different CVD subtypes. The findings from the study underscored the relevance of a heart-specific metabolic network in accurately representing the dynamic interplay between environmental factors and associated metabolic processes.
Furthermore, by incorporating gene expression data and lipidomic experiments into HMR2, cell-specific GEMs were developed to study the activation and differentiation of CD4+ T cell subsets. The study revealed specific metabolic changes during CD4+ T cell activation and differentiation. Importantly, the significance of ceramide and glycosphingolipid biosynthesis pathways in Th17 cell differentiation was highlighted. Moreover, model predictions were validated using gene knockdown experiments, demonstrating the role of serine palmitoyltransferase (SPT) in promoting the production of proinflammatory cytokines by Th17 cells [7].
In addition, a comprehensive metabolic reconstruction of human small intestinal epithelial cells (sIECs) was assembled and curated [106]. The sIEC reconstructions incorporate both experimentally validated and putatively identified transporters [3,106,107]. These models have been used in studying the physiological functions of the small intestine and in gaining insights into their role in tissue metabolism.
In a study conducted by Gustafsson et al., they introduced a novel approach for generating cell-type models by integrating single-cell RNA sequencing (scRNA-seq) with GEMs. By clustering single-cell RNA profiles, the authors demonstrated the effectiveness of combining scRNA-seq and GEMs in advancing the understanding of human metabolism. This method shows promising potential, particularly as single-cell datasets and GEMs become increasingly accessible [108]. A schematic workflow depicting the process of generating context-specific GEMs via the integration of multi-omics data is shown in Figure 1.
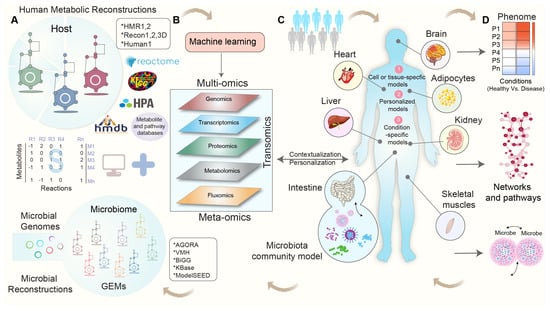
Figure 1.
A schematic workflow for context-specific genome-scale metabolic reconstructions using multi-omics data. (A) Human and microbial metabolic reconstructions and pathway databases serve as a scaffold for integrating omics data, with the stoichiometric matrix (S) representing the mathematical representation of the metabolic network and capturing the stoichiometric coefficients of metabolites (M) in each reaction (R). (B) Various types of multi-omics and/or meta-omics datasets are employed to contextualize human and microbial metabolic reconstructions. (C) Cell-, tissue-, and organ-specific-GEMs are developed using metabolic reconstructions and omics datasets. (D) These condition-specific GEMs enable the predictions of flux phenotypes, the regulation of metabolic pathways, the identification of reporter metabolites and pathways, and the study of microbe-microbe interactions and explore the intricate relationship among diet, host, and microbiota in healthy and disease states.
Recently, multi-tissue modeling frameworks were developed to study the interactions between cell and tissue-specific models [109,110]. Bordbar et al. have developed a multi-tissue type genome-scale metabolic network for adipocytes, hepatocytes, and myocytes, allowing for the study of intercellular interactions [110]. By integrating omics data, differential metabolic activity between obese and T2D obese gastric bypass patients was examined in a whole-body context. In another study, Foguet et al. developed personalized organ-specific (multiple tissues) metabolic flux models for a large cohort; these models identified associations between the personalized flux profiles and concentrations of metabolites in the blood via a fluxome-wide association study (FWAS) [111]. Several metabolic fluxes were found to be associated with the risk of developing coronary artery disease (CAD), unravelling a mechanism. Additionally, incorporating omics data into GEM networks enables the identification of functional modules, gene regulatory networks, and metabolic interactions, contributing to a better understanding of complex metabolic pathways within cells [36,80,112]. A list of cell- and tissue-specific GEMs developed by the integration of omics datasets can be found in Table 2.

Table 2.
Cell or tissue-specific GEMs developed using omics data. Abbreviations G, T, P, M, and F denote gene expression (microarray), transcriptomics (NGS), proteomics, metabolomics, and fluxomics, data, respectively. CVD, NAFLD, AD, T2D, T1D, MTB, IEMs, PBMCs, and HEK denote cardiovascular disease, non-alcoholic fatty liver disease, Alzheimer’s disease, type 2 diabetes, type 1 diabetes, and Mycobacterium tuberculosis, inborn errors of metabolism, peripheral blood mononuclear cells, and human embryonic kidney, respectively.
5. Modeling the Interactions between Gut Microbial Communities and Host Metabolism
The emergence of high-throughput “meta”-omics technologies has significantly improved our understanding of the gut microbiome and its significance in human health and disease. Nevertheless, the vast amount of data generated by gut microbiome research demands the development of innovative computational tools and mathematical models, while approaches such as 16S rRNA amplicon sequencing and whole-genome shotgun metagenomics sequencing (WGS) have been utilized for microbiome profiling [121]; these genome-centric methods alone cannot offer mechanistic insights into individual taxa, their interactions with other gut flora, or their influence on host metabolism [13,122,123].
GSMM has emerged as a valuable approach for investigating the metabolic interactions between microbial communities and their host in gut ecosystems [12,15,112,124,125,126,127,128]. Recent advancements have led to the development of GEMs that are specifically tailored to catalogued human gut microbes, leveraging their metabolic functions [129,130]. One notable example is the AGORA (assembly of gut organisms through reconstruction and Analysis) project, which successfully conducted semi-automatic genome-scale metabolic reconstructions of 773 human gut bacteria, encompassing 205 genera and 605 species [15]. Recently, AGORA2, an expanded resource of human gut microbial metabolic reconstructions incorporating 7302 strains, was developed [16]. AGORA2 facilitated personalized modeling by predicting diverse drug conversion potential in the gut microbiomes of patients with colorectal cancer vs. controls. It outperformed other resources by predicting microbial drug transformations and enabling the personalized modeling of gut microbiomes in colorectal cancer patients. By using the AGORA framework [15,16], metabolic interactions among microbial species were modeled by considering their metabolic capabilities and nutrient availability. AGORA reconstructions are publicly accessible via the VMH (Virtual Metabolic Human) database [60].
In addition, BiGG models [59] and the Metabolic Atlas [33,62] serve as open-access knowledge bases for genome-scale metabolic reconstructions. To facilitate the automated reconstruction of microbial GEMs, web-servers like KBase [97] and ModelSEED [61] integrate genome sequences and metagenomics datasets. Other tools, including COMET [131], BacArena [132], dOptCom [133], MatNet [134], DyMMM [135], MCM [136], and CASINO [126], have been developed to investigate the interplay between diets, microbiomes, and hosts. CASINO, for instance, successfully predicted interactions along the diet-microbiota-host axis in obesity and overweight individuals [126]. A comprehensive review discussing the implementation, characteristics, and applications of these methods can be found in [127].
Of note, the development of personalized microbiota models tailored to individual subjects is an area of recent interest. Leveraging metagenomics, meta-transcriptomics, meta-proteomics, and metabolomics data, estimating the pathway activities within the gut becomes possible, offering an approximation of the metabolic functionality specific to the gut microbes under defined conditions [137,138]. Personalized microbiota models have already demonstrated their ability to investigate secondary bile acid (BA) metabolism facilitated by human gut microbiome [128,139]. A recent study has uncovered changes in systemic BAs and microbial secondary BA pathways in infants and children who later seroconverted to multiple islet autoantibodies (i.e., being at high risk for type 1 diabetes, T1D). These findings suggest a disrupted BA metabolism that could potentially contribute to the risk and development of T1D [128]. A description of GSMM of gut microbiome including their implementation, applications, and tools are reviewed elsewhere [11,12,13,122,124,125,127,140,141].
6. Towards Whole-Body Metabolic Reconstruction: Bridging Precision Medicine and Systems Biology
The integration of high-throughput omics data with GEMs enables a deeper understanding of the molecular mechanisms underlying disease and facilitates the development of personalized treatment strategies. By constructing patient-specific metabolic models based on individual genomic, proteomic, and transcriptomic data, GEMs can predict metabolic alterations that are associated with specific diseases and identify potential therapeutic targets. In a study by Lewis et al. [142], personalized GEMs were developed with the aim to understand redox metabolism in cancer patients with different types of tumors. The study identified that radiation-resistant tumors produce elevated levels of reduced redox cofactors. This intriguing phenomenon suggests a potential link between redox metabolism and the ability of tumors to withstand radiation treatment. Interestingly, these models have also unveiled the presence of redox-metabolic heterogeneity among tumors that share similar clinical phenotypes. The insights provided by the personalized GEMs pave the way for more targeted approaches to cancer treatment, offering the potential for personalized interventions that consider the unique metabolic characteristics of each patient’s tumor. In another study, Turanli et al. [143] developed prostate-cancer-specific GEMs to explore cancer metabolism and identify potential therapeutic agents for effective treatment. By integrating gene expression data and a database of over 1000 drugs, the authors have predicted drug-gene interactions and identified key reactions with altered fluxes in prostate cancer. By using in silico cell viability assays, they identified potential repurposed drugs for prostate cancer treatment. Furthermore, in vitro cell assays confirmed the inhibitory effect of ifenprodil on prostate cancer cells. Agren et al. [86] employed immunohistochemistry to evaluate the presence or absence of proteins encoded by genes in 27 hepatocellular carcinoma (HCC) patients, leveraging this information, along with the HMR 2.0 database and a task-driven model reconstruction algorithm (tINIT), to develop personalized GEMs for six HCC patients based on proteomics data. These personalized GEMs served as a foundation for identifying potential anticancer drugs using the concept of antimetabolites. To assess the toxicity of each antimetabolite, the authors examined the in silico functionality of 83 healthy cell-type-specific GEMs. The study predicted several antimetabolites that could effectively inhibit tumor growth in all HCC patients.
A personalized whole-body metabolic (WBM) reconstruction serves as a powerful tool that provides a comprehensive view of an individual’s metabolic profile by considering the intricate relationship between different biological systems. A WBM reconstruction enables a deeper understanding of how metabolism is influenced by factors like diet, lifestyle, and gut microbial composition; it facilitates the investigation of individual-specific responses to dietary interventions, drug treatments, and disease conditions [120]. In a groundbreaking study, Thiele et al. introduced novel WBM network reconstructions for males and females [120]. These reconstructions encompassed 26 organs, 6 blood cell types, and over 80,000 biochemical reactions, offering a comprehensive representation of whole-body organ-resolved metabolism. Notably, the WBM models successfully replicated inter-organ metabolic cycles and the patterns of energy utilization. When personalized with physiological data, they demonstrated superior predictive performance compared to existing models, particularly in estimating basal metabolic rates. Furthermore, the integration of microbiome data into these models allowed for the exploration of host-microbiome co-metabolism.
In summary, GEMs serve as valuable tools in precision medicine, providing a multifaceted approach to understanding disease mechanisms, facilitating biomarker discovery, and guiding personalized treatment strategies that are tailored to specific patient groups.
7. Enhancing the Reproducibility of Genome-Scale Metabolic Models by Addressing Key Challenges
GEMs have certain limitations that need to be considered. In the context of human metabolism, GEMs face the challenge of incomplete knowledge about metabolic pathways and regulatory mechanisms. GEMs rely on the available genomic and biochemical information, which may result in gaps in the model, leading to an incomplete representation of metabolic pathways [2,26]. When it comes to microbiome modeling, a significant limitation lies in the functional characterization of the microbial species (taxa) within the gut ecosystem. Our knowledge regarding the metabolic capabilities of some individual microbes in the microbiome is incomplete, which hinders the reliability of GEMs in capturing the intricacies of microbiomes and their metabolic functions [14,127,140]. By incorporating newly available and well-annotated datasets into the existing GEMs, we can effectively address the challenges associated with the completeness of the model. Furthermore, the incorporation of multi-omics data not only facilitates the refinement and evaluation of these models but also enhances their validation, thereby increasing our confidence in the predictions. An iterative process of feedback between model predictions and experimental validation plays a crucial role in identifying and rectifying any discrepancies, enabling the continuous improvement and evolution of GEMs. This iterative process of model refinement serves as a driving force for the advancement of the models, ensuring their ability to represent complex biological systems [2]. MEMOTE (metabolic model testing) is a valuable framework designed to standardize and streamline the testing of GEMs and thereby enhance their reproducibility and credibility [144]. MEMOTE provides comprehensive test metrics to assess various aspects of model performance, including stoichiometry, thermodynamics, and network connectivity. By applying MEMOTE, researchers can compare different models and ensure the accuracy and robustness of their metabolic models.
Another limitation is the static nature of GEMs since they primarily represent steady-state conditions and may not fully capture the dynamic and temporal changes in cellular metabolism. This means that they might not accurately model changes in metabolite concentrations and reaction rates over time [10]. To overcome this limitation, dynamic modeling approaches, such as dynamic flux balance analysis (dFBA) [145] or kinetic modeling, can be employed to capture the temporal behavior and regulatory mechanisms of metabolic networks [146,147,148].
Estimating parameter values in GEMs is subject to uncertainty [149]; the absence of crucial information, such as metabolite concentrations and enzyme kinetics combined with the high degrees of freedom in GEMs, presents challenges in effectively constraining these models. However, researchers have employed a range of parameter estimation and sensitivity analysis techniques to address these limitations and enhance the reliability of GEMs [149,150,151,152,153]. Notably, the GECKO method has been developed to construct GEMs with enzymatic constraints by incorporating kinetic and omics data [154]. This is achieved by expanding the stoichiometric matrix of the GEM to include additional information representing enzymes and their usage in metabolic reactions. Enzyme kinetics, represented by pseudo stoichiometric coefficients in the matrix, is used to model the Kcat values. By constraining protein abundance in this manner, GECKO effectively reduces flux variability and enhances the accuracy of predictions.
A common limitation of GEMs is their lack of tissue and cell-type specificity. When generic metabolic networks are reconstructed to represent specific tissues, they may not fully capture the unique metabolic characteristics of each tissue or cell-type. To address this issue and improve the contextualization of GEMs for specific conditions, the integration of multiple omics datasets becomes crucial. While algorithms have been developed to generate tissue-specific models, most rely on a single omics dataset that may not provide a comprehensive representation of cell/tissue metabolism. Therefore, there is a need for new tools and methods that effectively integrate multi-omics data to create more accurate models. Recently, a promising approach using scRNA-seq data was introduced, which provided insights into the heterogeneity and interactions within a specific cell type [108].
To ensure the biological relevance of GEMs, it is important to validate their predictions by comparing them against experimental data and existing knowledge. By conducting targeted experiments, researchers can assess the accuracy and reliability of the model’s predictions and gain further insights into the underlying biological processes. The experimental validation of GSMMs involves various techniques, such as in vitro assays, cell culture experiments, and animal studies. These experiments aim to measure and compare metabolic fluxes, metabolite concentrations, enzyme activities, and other relevant parameters predicted by the model against experimental observations. Using these validations, researchers can evaluate the model’s ability to capture the complex dynamics of cellular metabolism and assess its predictive power. Furthermore, the experimental validation of GSMMs also involves testing specific hypotheses generated by the model. Researchers can design experiments to manipulate specific genes, enzymes, or metabolites within the metabolic network to confirm the model’s predictions and understand the functional consequences of these perturbations.
8. Leveraging Machine Learning for Genome-Scale Metabolic Modeling: An Advancing Solution to Address the Key Challenges
Machine learning (ML) techniques have demonstrated their ability to identify metabolic pathways from chemical compounds; various ML methods have also been employed for metabolic pathway prediction, analysis, and modeling [155,156,157,158,159]. For instance, Li et al. developed the mummichog framework, which enables the direct prediction of pathway activity using spectral features from metabolomics data [160]. Another tool, Lilikoi, developed by AlAkwaa et al., specializes in personalized pathway-based classification modeling using metabolomics data [157]. Unlike conventional pathway tools, Lilikoi incorporates metabolomic profiles to generate personalized pathway profiles and identify pathways significantly associated with specific disease phenotypes.
There is a growing interest in combining ML techniques with GSMM [8,155,156,158,161,162]. ML approaches can refine input constraints for GEMs and compare the results from simulations using experimental data [163,164]. ML methods, including linear regression (LR), decision trees, and naïve Bayes, have been used for gap-filling in draft GEMs [165,166]. Moreover, it can complement constraint-based modeling methods like FBA by incorporating additional constraints derived from omics data, such as gene expression profiles, protein abundances, and metabolite concentrations. In a recent study by Zelezniak et al. [167], the integration of ML and metabolic control analysis (MCA) was instrumental in mapping regulatory patterns and predicting cell metabolomes. The study unveiled global enzymatic changes, leading to extensive shifts in metabolic control across different sets of enzymes.
Furthermore, GSMM and ML methods have been applied to determine metabolite secretion, flux quantification, protein turnover rate estimation, gene essentiality, metabolic gene prediction, and the assessment of a medication effect [155,156,162,163]. ML coupled with prospective GSMM has been instrumental in investigating antibiotic efficacy, sensitivity, and lethality mechanisms [168]. Guo et al. developed DeepMetabolism, a knowledge-based deep learning (DL) system that predicts cellular phenotypes from transcriptomics data using a combination of unsupervised and supervised approaches [169]. The framework incorporates an autoencoder with biologically guided connections and leverages FBA to evaluate the connectivity between proteomic and phenomic layers. The methodology and applications of ML for the integration of omics data into constraint-based metabolic modelling have been reviewed in detail elsewhere [159,161,170].
To summarize, ML techniques together with GSMM can unravel the complexity of biological data. ML enables the consolidation of large omics datasets, extracting key features that are linked to the desired outcomes. In contrast, GSMM offers a mechanistic comprehension, generating testable hypotheses regarding the specific metabolic processes taking place in cells, tissues, organs, or microbial communities via the incorporation of omics data. The integration of ML and GSMM holds immense promise in achieving a comprehensive understanding of human metabolism in healthy and diseased states.
9. Conclusions and Future Perspectives
Today, the field of GSMM has witnessed widespread application across various domains, owing to the abundance of biological data, advancements in automatic reconstruction tools, and the introduction of novel mathematical modeling techniques. As GEMs continue to evolve, they will encompass a broader range of biological pathways and gene-protein-reaction (GPR) associations. To further enhance their capabilities, incorporating additional biochemical information into GEMs becomes necessary, i.e., going beyond metabolism. This includes aspects such as protein allocation, cellular macromolecular composition, and detailed structural information about the proteins. Notably, certain molecular processes such as enzyme-substrate interactions, protein-protein complex structures, and post-translational modifications require careful consideration.
The integration of omics data into GEMs has revolutionized our understanding of biological systems. However, there are still challenges that need to be addressed to improve the reliability and reproducibility of GEM-based predictions. Efforts should be directed towards standardizing and harmonizing omics data, developing automated pipelines and tools for data integration and normalization, and incorporating ML algorithms to enhance accuracy and robustness. ML algorithms can play a crucial role in automating the reconstruction of GEMs by leveraging omics data and existing metabolic network reconstructions. Furthermore, they can be utilized to validate and refine GEMs using experimental data, ensuring an accurate representation of metabolic characteristics. ML algorithms can enable the integration of diverse omics data, allowing for the accurate prediction of active metabolic reactions under specific conditions. This is particularly important for developing high-quality models that are specific to cell types or tissues. Additionally, the incorporation of single-cell omics data enhances the accuracy of GEMs, enabling the construction of cell-type-specific models and expanding our knowledge of cellular metabolism.
In conclusion, the integration of GEMs with multi-omics data, powered by the incorporation of ML approaches, has the potential to significantly enhance our understanding of biological systems and their underlying mechanisms. These advancements pave the way for precision medicine and the development of targeted interventions for human health and disease.
Author Contributions
The manuscript was drafted by P.S. and M.O. provided critical comments and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Council of Finland (grant no. 333981 to M.O.) and by the “Inflammation in human early life: targeting impacts on life-course health” (INITIALISE) consortium funded by the Horizon Europe Program of the European Union under Grant Agreement 101094099.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef] [PubMed]
- Thiele, I.; Palsson, B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Brunk, E.; Sahoo, S.; Zielinski, D.C.; Altunkaya, A.; Dräger, A.; Mih, N.; Gatto, F.; Nilsson, A.; Gonzalez, G.A.P.; Aurich, M.K. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 2018, 36, 272. [Google Scholar] [CrossRef]
- Blazier, A.S.; Papin, J.A. Integration of expression data in genome-scale metabolic network reconstructions. Front. Physiol. 2012, 3, 299. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.S.; DeJongh, M.; Best, A.A.; Frybarger, P.M.; Linsay, B.; Stevens, R.L. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat. Biotechnol. 2010, 28, 977–982. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Agren, R.; Kampf, C.; Asplund, A.; Uhlen, M.; Nielsen, J. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat. Commun. 2014, 5, 3083. [Google Scholar] [CrossRef]
- Sen, P.; Andrabi, S.B.A.; Buchacher, T.; Khan, M.M.; Kalim, U.U.; Lindeman, T.M.; Alves, M.A.; Hinkkanen, V.; Kemppainen, E.; Dickens, A.M.; et al. Quantitative genome-scale metabolic modeling of human CD4(+) T cell differentiation reveals subset-specific regulation of glycosphingolipid pathways. Cell Rep. 2021, 37, 109973. [Google Scholar] [CrossRef]
- Sen, P.; Govaere, O.; Sinioja, T.; McGlinchey, A.; Geng, D.; Ratziu, V.; Bugianesi, E.; Schattenberg, J.M.; Vidal-Puig, A.; Allison, M.; et al. Quantitative modeling of human liver reveals dysregulation of glycosphingolipid pathways in nonalcoholic fatty liver disease. iScience 2022, 25, 104949. [Google Scholar] [CrossRef]
- Cook, D.J.; Nielsen, J. Genome-scale metabolic models applied to human health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1393. [Google Scholar] [CrossRef]
- Lewis, N.E.; Nagarajan, H.; Palsson, B.O. Constraining the metabolic genotype-phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 2012, 10, 291–305. [Google Scholar] [CrossRef]
- Esvap, E.; Ulgen, K.O. Advances in Genome-Scale Metabolic Modeling toward Microbial Community Analysis of the Human Microbiome. ACS Synth. Biol. 2021, 10, 2121–2137. [Google Scholar] [CrossRef]
- Heinken, A.; Basile, A.; Hertel, J.; Thinnes, C.; Thiele, I. Genome-Scale Metabolic Modeling of the Human Microbiome in the Era of Personalized Medicine. Annu. Rev. Microbiol. 2021, 75, 199–222. [Google Scholar] [CrossRef]
- Shoaie, S.; Nielsen, J. Elucidating the interactions between the human gut microbiota and its host through metabolic modeling. Front. Genet. 2014, 5, 86. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Monk, J.M.; Palsson, B.O. Using genome-scale models to predict biological capabilities. Cell 2015, 161, 971–987. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Heinken, A.; Kutt, L.; Ravcheev, D.A.; Bauer, E.; Noronha, A.; Greenhalgh, K.; Jäger, C.; Baginska, J.; Wilmes, P. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol. 2017, 35, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Hertel, J.; Acharya, G.; Ravcheev, D.A.; Nyga, M.; Okpala, O.E.; Hogan, M.; Magnusdottir, S.; Martinelli, F.; Nap, B.; et al. Genome-scale metabolic reconstruction of 7302 human microorganisms for personalized medicine. Nat. Biotechnol. 2023, 1–12. [Google Scholar] [CrossRef]
- Gu, C.; Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.J.; Lerman, J.A.; Chang, R.L.; Hyduke, D.R.; Palsson, B.O. Genome-scale models of metabolism and gene expression extend and refine growth phenotype prediction. Mol. Syst. Biol. 2013, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Förster, J.; Famili, I.; Fu, P.; Palsson, B.Ø.; Nielsen, J. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Res. 2003, 13, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Rocha, I.; Förster, J.; Nielsen, J. Evolutionary programming as a platform for in silico metabolic engineering. BMC Bioinform. 2005, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Suthers, P.F.; Zomorrodi, A.; Maranas, C.D. Genome-scale gene/reaction essentiality and synthetic lethality analysis. Mol. Syst. Biol. 2009, 5, 301. [Google Scholar] [CrossRef] [PubMed]
- Pharkya, P.; Burgard, A.P.; Maranas, C.D. OptStrain: A computational framework for redesign of microbial production systems. Genome Res. 2004, 14, 2367–2376. [Google Scholar] [CrossRef]
- Pharkya, P.; Maranas, C.D. An optimization framework for identifying reaction activation/inhibition or elimination candidates for overproduction in microbial systems. Metab. Eng. 2006, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Duarte, N.C.; Becker, S.A.; Jamshidi, N.; Thiele, I.; Mo, M.L.; Vo, T.D.; Srivas, R.; Palsson, B.O. Global reconstruction of the human metabolic network based on genomic and bibliomic data. Proc. Natl. Acad. Sci. USA 2007, 104, 1777–1782. [Google Scholar] [CrossRef]
- Ma, H.; Sorokin, A.; Mazein, A.; Selkov, A.; Selkov, E.; Demin, O.; Goryanin, I. The Edinburgh human metabolic network reconstruction and its functional analysis. Mol. Syst. Biol. 2007, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Thiele, I.; Swainston, N.; Fleming, R.M.; Hoppe, A.; Sahoo, S.; Aurich, M.K.; Haraldsdottir, H.; Mo, M.L.; Rolfsson, O.; Stobbe, M.D.; et al. A community-driven global reconstruction of human metabolism. Nat. Biotechnol. 2013, 31, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Swainston, N.; Smallbone, K.; Hefzi, H.; Dobson, P.D.; Brewer, J.; Hanscho, M.; Zielinski, D.C.; Ang, K.S.; Gardiner, N.J.; Gutierrez, J.M. Recon 2.2: From reconstruction to model of human metabolism. Metabolomics 2016, 12, 109. [Google Scholar] [CrossRef]
- Mardinoglu, A.; Agren, R.; Kampf, C.; Asplund, A.; Nookaew, I.; Jacobson, P.; Walley, A.J.; Froguel, P.; Carlsson, L.M.; Uhlen, M.; et al. Integration of clinical data with a genome-scale metabolic model of the human adipocyte. Mol. Syst. Biol. 2013, 9, 649. [Google Scholar] [CrossRef]
- Väremo, L.; Scheele, C.; Broholm, C.; Mardinoglu, A.; Kampf, C.; Asplund, A.; Nookaew, I.; Uhlén, M.; Pedersen, B.K.; Nielsen, J. Proteome-and Transcriptome-Driven Reconstruction of the Human Myocyte Metabolic Network and Its Use for Identification of Markers for Diabetes. Cell Rep. 2016, 14, 1567. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, C.; Liu, Z.; Klevstig, M.; Mukhopadhyay, B.; Bergentall, M.; Cinar, R.; Stahlman, M.; Sikanic, N.; Park, J.K.; et al. Network analyses identify liver-specific targets for treating liver diseases. Mol. Syst. Biol. 2017, 13, 938. [Google Scholar] [CrossRef]
- Bidkhori, G.; Benfeitas, R.; Klevstig, M.; Zhang, C.; Nielsen, J.; Uhlen, M.; Boren, J.; Mardinoglu, A. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc. Natl. Acad. Sci. USA 2018, 115, E11874–E11883. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Wang, C.; Fessler, J.; DeTomaso, D.; Avila-Pacheco, J.; Kaminski, J.; Zaghouani, S.; Christian, E.; Thakore, P.; Schellhaass, B.; et al. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell 2021, 184, 4168–4185. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Kocabas, P.; Wang, H.; Cholley, P.E.; Cook, D.; Nilsson, A.; Anton, M.; Ferreira, R.; Domenzain, I.; Billa, V.; et al. An atlas of human metabolism. Sci. Signal. 2020, 13, eaaz1482. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.; Kelly, L. Bringing microbiome-drug interaction research into the clinic. eBioMedicine 2019, 44, 708–715. [Google Scholar] [CrossRef]
- Sahoo, S.; Haraldsdottir, H.S.; Fleming, R.M.; Thiele, I. Modeling the effects of commonly used drugs on human metabolism. FEBS J. 2015, 282, 297–317. [Google Scholar] [CrossRef]
- Patil, K.R.; Nielsen, J. Uncovering transcriptional regulation of metabolism by using metabolic network topology. Proc. Natl. Acad. Sci. USA 2005, 102, 2685–2689. [Google Scholar] [CrossRef]
- Picard, M.; Scott-Boyer, M.P.; Bodein, A.; Perin, O.; Droit, A. Integration strategies of multi-omics data for machine learning analysis. Comput. Struct. Biotechnol. J. 2021, 19, 3735–3746. [Google Scholar] [CrossRef]
- Gomez-Cabrero, D.; Abugessaisa, I.; Maier, D.; Teschendorff, A.; Merkenschlager, M.; Gisel, A.; Ballestar, E.; Bongcam-Rudloff, E.; Conesa, A.; Tegner, J. Data integration in the era of omics: Current and future challenges. BMC Syst. Biol. 2014, 8 (Suppl. S2), I1. [Google Scholar] [CrossRef]
- Misra, B.B.; Langefeld, C.D.; Olivier, M.; Cox, L.A. Integrated Omics: Tools, Advances, and Future Approaches. J. Mol. Endocrinol. 2018, 62, R21–R45. [Google Scholar] [CrossRef]
- Flores, J.E.; Claborne, D.M.; Weller, Z.D.; Webb-Robertson, B.M.; Waters, K.M.; Bramer, L.M. Missing data in multi-omics integration: Recent advances through artificial intelligence. Front. Artif. Intell. 2023, 6, 1098308. [Google Scholar] [CrossRef]
- Valikangas, T.; Suomi, T.; Elo, L.L. A systematic evaluation of normalization methods in quantitative label-free proteomics. Brief. Bioinform. 2018, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Walach, J.; Filzmoser, P.; Hron, K. Chapter Seven—Data Normalization and Scaling: Consequences for the Analysis in Omics Sciences. In Comprehensive Analytical Chemistry; Jaumot, J., Bedia, C., Tauler, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 82, pp. 165–196. [Google Scholar]
- Bolstad, B.M.; Irizarry, R.A.; Astrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Parmigiani, G.; Johnson, W.E. ComBat-seq: Batch effect adjustment for RNA-seq count data. NAR Genom. Bioinform. 2020, 2, lqaa078. [Google Scholar] [CrossRef]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Sysi-Aho, M.; Katajamaa, M.; Yetukuri, L.; Oresic, M. Normalization method for metabolomics data using optimal selection of multiple internal standards. BMC Bioinform. 2007, 8, 93. [Google Scholar] [CrossRef]
- Ejigu, B.A.; Valkenborg, D.; Baggerman, G.; Vanaerschot, M.; Witters, E.; Dujardin, J.C.; Burzykowski, T.; Berg, M. Evaluation of normalization methods to pave the way towards large-scale LC-MS-based metabolomics profiling experiments. OMICS A J. Integr. Biol. 2013, 17, 473–485. [Google Scholar] [CrossRef]
- Becker, S.A.; Feist, A.M.; Mo, M.L.; Hannum, G.; Palsson, B.O.; Herrgard, M.J. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox. Nat. Protoc. 2007, 2, 727–738. [Google Scholar] [CrossRef]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdottir, H.S.; Keating, S.M.; Vlasov, V.; Wachowiak, J. Creation and analysis of biochemical constraint-based models: The COBRA Toolbox v3.0. arXiv 2017, arXiv:1710.04038. [Google Scholar] [CrossRef] [PubMed]
- Schellenberger, J.; Que, R.; Fleming, R.M.; Thiele, I.; Orth, J.D.; Feist, A.M.; Zielinski, D.C.; Bordbar, A.; Lewis, N.E.; Rahmanian, S.; et al. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox v2.0. Nat. Protoc. 2011, 6, 1290–1307. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Heinken, A.; Heirendt, L.; Magnusdottir, S.; Fleming, R.M.T.; Thiele, I. The Microbiome Modeling Toolbox: From microbial interactions to personalized microbial communities. Bioinformatics 2019, 35, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Dai, S.; Han, F.; Li, W.; Huang, J.; Xiao, W. FastMM: An efficient toolbox for personalized constraint-based metabolic modeling. BMC Bioinform. 2020, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, S.G.; Thiele, I. rBioNet: A COBRA toolbox extension for reconstructing high-quality biochemical networks. Bioinformatics 2011, 27, 2009–2010. [Google Scholar] [CrossRef]
- Agren, R.; Liu, L.; Shoaie, S.; Vongsangnak, W.; Nookaew, I.; Nielsen, J. The RAVEN toolbox and its use for generating a genome-scale metabolic model for Penicillium chrysogenum. PLoS Comput. Biol. 2013, 9, e1002980. [Google Scholar] [CrossRef]
- Wang, H.; Marcisauskas, S.; Sanchez, B.J.; Domenzain, I.; Hermansson, D.; Agren, R.; Nielsen, J.; Kerkhoven, E.J. RAVEN 2.0: A versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLoS Comput. Biol. 2018, 14, e1006541. [Google Scholar] [CrossRef]
- King, Z.A.; Lu, J.; Dräger, A.; Miller, P.; Federowicz, S.; Lerman, J.A.; Ebrahim, A.; Palsson, B.O.; Lewis, N.E. BiGG Models: A platform for integrating, standardizing and sharing genome-scale models. Nucleic Acids Res. 2015, 44, D515–D522. [Google Scholar] [CrossRef]
- Noronha, A.; Modamio, J.; Jarosz, Y.; Guerard, E.; Sompairac, N.; Preciat, G.; Danielsdottir, A.D.; Krecke, M.; Merten, D.; Haraldsdottir, H.S.; et al. The Virtual Metabolic Human database: Integrating human and gut microbiome metabolism with nutrition and disease. Nucleic Acids Res. 2019, 47, D614–D624. [Google Scholar] [CrossRef]
- Seaver, S.M.D.; Liu, F.; Zhang, Q.; Jeffryes, J.; Faria, J.P.; Edirisinghe, J.N.; Mundy, M.; Chia, N.; Noor, E.; Beber, M.E.; et al. The ModelSEED Biochemistry Database for the integration of metabolic annotations and the reconstruction, comparison and analysis of metabolic models for plants, fungi and microbes. Nucleic Acids Res. 2021, 49, D575–D588. [Google Scholar] [CrossRef]
- Pornputtapong, N.; Nookaew, I.; Nielsen, J. Human metabolic atlas: An online resource for human metabolism. Database 2015, 2015, bav068. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2013, 42, D199–D205. [Google Scholar] [CrossRef]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, W606–W612. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, I.; Jeske, L.; Ulbrich, M.; Placzek, S.; Chang, A.; Schomburg, D. The BRENDA enzyme information system—From a database to an expert system. J. Biotechnol. 2017, 261, 194–206. [Google Scholar] [CrossRef]
- D’Eustachio, P. Reactome knowledgebase of human biological pathways and processes. Methods Mol. Biol. 2011, 694, 49–61. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Samaras, P.; Schmidt, T.; Frejno, M.; Gessulat, S.; Reinecke, M.; Jarzab, A.; Zecha, J.; Mergner, J.; Giansanti, P.; Ehrlich, H.C.; et al. ProteomicsDB: A multi-omics and multi-organism resource for life science research. Nucleic Acids Res. 2020, 48, D1153–D1163. [Google Scholar] [CrossRef]
- Maglott, D.; Ostell, J.; Pruitt, K.D.; Tatusova, T. Entrez Gene: Gene-centered information at NCBI. Nucleic Acids Res. 2011, 39, D52–D57. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, H.; Kapushesky, M.; Shojatalab, M.; Abeygunawardena, N.; Coulson, R.; Farne, A.; Holloway, E.; Kolesnykov, N.; Lilja, P.; Lukk, M.; et al. ArrayExpress—A public database of microarray experiments and gene expression profiles. Nucleic Acids Res. 2007, 35, D747–D750. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, I.; Almeida-King, J.; Kumanduri, V.; Senf, A.; Spalding, J.D.; Ur-Rehman, S.; Saunders, G.; Kandasamy, J.; Caccamo, M.; Leinonen, R.; et al. The European Genome-phenome Archive of human data consented for biomedical research. Nat. Genet. 2015, 47, 692–695. [Google Scholar] [CrossRef]
- GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef]
- Franzen, O.; Ermel, R.; Cohain, A.; Akers, N.K.; Di Narzo, A.; Talukdar, H.A.; Foroughi-Asl, H.; Giambartolomei, C.; Fullard, J.F.; Sukhavasi, K.; et al. Cardiometabolic risk loci share downstream cis- and trans-gene regulation across tissues and diseases. Science 2016, 353, 827–830. [Google Scholar] [CrossRef]
- Li, C.; Donizelli, M.; Rodriguez, N.; Dharuri, H.; Endler, L.; Chelliah, V.; Li, L.; He, E.; Henry, A.; Stefan, M.I.; et al. BioModels Database: An enhanced, curated and annotated resource for published quantitative kinetic models. BMC Syst. Biol. 2010, 4, 92. [Google Scholar] [CrossRef]
- Agren, R.; Bordel, S.; Mardinoglu, A.; Pornputtapong, N.; Nookaew, I.; Nielsen, J. Reconstruction of genome-scale active metabolic networks for 69 human cell types and 16 cancer types using INIT. PLoS Comput. Biol. 2012, 8, e1002518. [Google Scholar] [CrossRef]
- Becker, S.A.; Palsson, B.O. Context-specific metabolic networks are consistent with experiments. PLoS Comput. Biol. 2008, 4, e1000082. [Google Scholar] [CrossRef]
- Bordbar, A.; Mo, M.L.; Nakayasu, E.S.; Schrimpe-Rutledge, A.C.; Kim, Y.M.; Metz, T.O.; Jones, M.B.; Frank, B.C.; Smith, R.D.; Peterson, S.N.; et al. Model-driven multi-omic data analysis elucidates metabolic immunomodulators of macrophage activation. Mol. Syst. Biol. 2012, 8, 558. [Google Scholar] [CrossRef] [PubMed]
- Zur, H.; Ruppin, E.; Shlomi, T. iMAT: An integrative metabolic analysis tool. Bioinformatics 2010, 26, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.A.; Papin, J.A. Functional integration of a metabolic network model and expression data without arbitrary thresholding. Bioinformatics 2011, 27, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Colijn, C.; Brandes, A.; Zucker, J.; Lun, D.S.; Weiner, B.; Farhat, M.R.; Cheng, T.Y.; Moody, D.B.; Murray, M.; Galagan, J.E. Interpreting expression data with metabolic flux models: Predicting Mycobacterium tuberculosis mycolic acid production. PLoS Comput. Biol. 2009, 5, e1000489. [Google Scholar] [CrossRef]
- Agren, R.; Mardinoglu, A.; Asplund, A.; Kampf, C.; Uhlen, M.; Nielsen, J. Identification of anticancer drugs for hepatocellular carcinoma through personalized genome-scale metabolic modeling. Mol. Syst. Biol. 2014, 10, 721. [Google Scholar] [CrossRef]
- Angione, C.; Lio, P. Predictive analytics of environmental adaptability in multi-omic network models. Sci. Rep. 2015, 5, 15147. [Google Scholar] [CrossRef]
- Yizhak, K.; Benyamini, T.; Liebermeister, W.; Ruppin, E.; Shlomi, T. Integrating quantitative proteomics and metabolomics with a genome-scale metabolic network model. Bioinformatics 2010, 26, i255–i260. [Google Scholar] [CrossRef]
- Vlassis, N.; Pacheco, M.P.; Sauter, T. Fast reconstruction of compact context-specific metabolic network models. PLoS Comput. Biol. 2014, 10, e1003424. [Google Scholar] [CrossRef]
- Pacheco, M.P.; Bintener, T.; Ternes, D.; Kulms, D.; Haan, S.; Letellier, E.; Sauter, T. Identifying and targeting cancer-specific metabolism with network-based drug target prediction. eBioMedicine 2019, 43, 98–106. [Google Scholar] [CrossRef]
- Wang, Y.; Eddy, J.A.; Price, N.D. Reconstruction of genome-scale metabolic models for 126 human tissues using mCADRE. BMC Syst. Biol. 2012, 6, 153. [Google Scholar] [CrossRef]
- Yizhak, K.; Gaude, E.; Le Devedec, S.; Waldman, Y.Y.; Stein, G.Y.; van de Water, B.; Frezza, C.; Ruppin, E. Phenotype-based cell-specific metabolic modeling reveals metabolic liabilities of cancer. eLife 2014, 3, e03641. [Google Scholar] [CrossRef] [PubMed]
- Robaina Estevez, S.; Nikoloski, Z. Context-Specific Metabolic Model Extraction Based on Regularized Least Squares Optimization. PLoS ONE 2015, 10, e0131875. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Qutub, A.A. Reconstruction of Tissue-Specific Metabolic Networks Using CORDA. PLoS Comput. Biol. 2016, 12, e1004808. [Google Scholar] [CrossRef] [PubMed]
- Moskon, M.; Rezen, T. Context-Specific Genome-Scale Metabolic Modelling and Its Application to the Analysis of COVID-19 Metabolic Signatures. Metabolites 2023, 13, 126. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Arkin, A.P.; Stevens, R.L.; Cottingham, R.W.; Maslov, S.; Henry, C.S.; Dehal, P.; Ware, D.; Perez, F.; Harris, N.L.; Canon, S. The DOE Systems Biology Knowledgebase (KBase). bioRxiv 2016, 096354. [Google Scholar] [CrossRef]
- Hyotylainen, T.; Jerby, L.; Petaja, E.M.; Mattila, I.; Jantti, S.; Auvinen, P.; Gastaldelli, A.; Yki-Jarvinen, H.; Ruppin, E.; Oresic, M. Genome-scale study reveals reduced metabolic adaptability in patients with non-alcoholic fatty liver disease. Nat. Commun. 2016, 7, 8994. [Google Scholar] [CrossRef]
- Jerby, L.; Shlomi, T.; Ruppin, E. Computational reconstruction of tissue-specific metabolic models: Application to human liver metabolism. Mol. Syst. Biol. 2010, 6, 401. [Google Scholar] [CrossRef]
- Baloni, P.; Funk, C.C.; Readhead, B.; Price, N.D. Systems modeling of metabolic dysregulation in neurodegenerative diseases. Curr. Opin. Pharmacol. 2021, 60, 59–65. [Google Scholar] [CrossRef]
- Lewis, N.E.; Schramm, G.; Bordbar, A.; Schellenberger, J.; Andersen, M.P.; Cheng, J.K.; Patel, N.; Yee, A.; Lewis, R.A.; Eils, R. Large-scale in silico modeling of metabolic interactions between cell types in the human brain. Nat. Biotechnol. 2010, 28, 1279. [Google Scholar] [CrossRef]
- Kishk, A.; Pacheco, M.P.; Heurtaux, T.; Sinkkonen, L.; Pang, J.; Fritah, S.; Niclou, S.P.; Sauter, T. Review of Current Human Genome-Scale Metabolic Models for Brain Cancer and Neurodegenerative Diseases. Cells 2022, 11, 2486. [Google Scholar] [CrossRef] [PubMed]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Mahmoudiandehkordi, S.; Louie, G.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef] [PubMed]
- Baloni, P.; Arnold, M.; Buitrago, L.; Nho, K.; Moreno, H.; Huynh, K.; Brauner, B.; Louie, G.; Kueider-Paisley, A.; Suhre, K.; et al. Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer’s disease. Commun. Biol. 2022, 5, 1074. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, J. Reconstruction and analysis of human heart-specific metabolic network based on transcriptome and proteome data. Biochem. Biophys. Res. Commun. 2011, 415, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Thiele, I. Predicting the impact of diet and enzymopathies on human small intestinal epithelial cells. Human. Mol. Genet. 2013, 22, 2705–2722. [Google Scholar] [CrossRef]
- Sahoo, S.; Aurich, M.K.; Jonsson, J.J.; Thiele, I. Membrane transporters in a human genome-scale metabolic knowledgebase and their implications for disease. Front. Physiol. 2014, 5, 91. [Google Scholar] [CrossRef]
- Gustafsson, J.; Anton, M.; Roshanzamir, F.; Jornsten, R.; Kerkhoven, E.J.; Robinson, J.L.; Nielsen, J. Generation and analysis of context-specific genome-scale metabolic models derived from single-cell RNA-Seq data. Proc. Natl. Acad. Sci. USA 2023, 120, e2217868120. [Google Scholar] [CrossRef]
- Do Rosario Martins Conde, P.; Sauter, T.; Pfau, T. Constraint Based Modeling Going Multicellular. Front. Mol. Biosci. 2016, 3, 3. [Google Scholar] [CrossRef]
- Bordbar, A.; Feist, A.M.; Usaite-Black, R.; Woodcock, J.; Palsson, B.O.; Famili, I. A multi-tissue type genome-scale metabolic network for analysis of whole-body systems physiology. BMC Syst. Biol. 2011, 5, 180. [Google Scholar] [CrossRef]
- Foguet, C.; Xu, Y.; Ritchie, S.C.; Lambert, S.A.; Persyn, E.; Nath, A.P.; Davenport, E.E.; Roberts, D.J.; Paul, D.S.; Di Angelantonio, E.; et al. Genetically personalised organ-specific metabolic models in health and disease. Nat. Commun. 2022, 13, 7356. [Google Scholar] [CrossRef]
- Pascal Andreu, V.; Augustijn, H.E.; Chen, L.; Zhernakova, A.; Fu, J.; Fischbach, M.A.; Dodd, D.; Medema, M.H. gutSMASH predicts specialized primary metabolic pathways from the human gut microbiota. Nat. Biotechnol. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gille, C.; Bolling, C.; Hoppe, A.; Bulik, S.; Hoffmann, S.; Hubner, K.; Karlstadt, A.; Ganeshan, R.; Konig, M.; Rother, K.; et al. HepatoNet1: A comprehensive metabolic reconstruction of the human hepatocyte for the analysis of liver physiology. Mol. Syst. Biol. 2010, 6, 411. [Google Scholar] [CrossRef] [PubMed]
- Karlstadt, A.; Fliegner, D.; Kararigas, G.; Ruderisch, H.S.; Regitz-Zagrosek, V.; Holzhutter, H.G. CardioNet: A human metabolic network suited for the study of cardiomyocyte metabolism. BMC Syst. Biol. 2012, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Martin-Jimenez, C.A.; Salazar-Barreto, D.; Barreto, G.E.; Gonzalez, J. Genome-Scale Reconstruction of the Human Astrocyte Metabolic Network. Front. Aging Neurosci. 2017, 9, 23. [Google Scholar] [CrossRef]
- Sohrabi-Jahromi, S.; Marashi, S.A.; Kalantari, S. A kidney-specific genome-scale metabolic network model for analyzing focal segmental glomerulosclerosis. Mamm. Genome 2016, 27, 158–167. [Google Scholar] [CrossRef]
- Quek, L.E.; Dietmair, S.; Hanscho, M.; Martinez, V.S.; Borth, N.; Nielsen, L.K. Reducing Recon 2 for steady-state flux analysis of HEK cell culture. J. Biotechnol. 2014, 184, 172–178. [Google Scholar] [CrossRef]
- Bordbar, A.; Lewis, N.E.; Schellenberger, J.; Palsson, B.O.; Jamshidi, N. Insight into human alveolar macrophage and M. tuberculosis interactions via metabolic reconstructions. Mol. Syst. Biol. 2010, 6, 422. [Google Scholar] [CrossRef]
- Sen, P.; Dickens, A.M.; Lopez-Bascon, M.A.; Lindeman, T.; Kemppainen, E.; Lamichhane, S.; Ronkko, T.; Ilonen, J.; Toppari, J.; Veijola, R.; et al. Metabolic alterations in immune cells associate with progression to type 1 diabetes. Diabetologia 2020, 63, 1017–1031. [Google Scholar] [CrossRef]
- Thiele, I.; Sahoo, S.; Heinken, A.; Hertel, J.; Heirendt, L.; Aurich, M.K.; Fleming, R.M. Personalized whole-body models integrate metabolism, physiology, and the gut microbiome. Mol. Syst. Biol. 2020, 16, e8982. [Google Scholar] [CrossRef]
- Hamady, M.; Knight, R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009, 19, 1141–1152. [Google Scholar] [CrossRef]
- Ji, B.; Nielsen, J. New insight into the gut microbiome through metagenomics. Adv. Genom. Genet. 2015, 5, 77–91. [Google Scholar]
- Bauer, E.; Thiele, I. From Network Analysis to Functional Metabolic Modeling of the Human Gut Microbiota. mSystems 2018, 3, 157–197. [Google Scholar] [CrossRef] [PubMed]
- Shoaie, S.; Karlsson, F.; Mardinoglu, A.; Nookaew, I.; Bordel, S.; Nielsen, J. Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci. Rep. 2013, 3, 2532. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ji, B.; Babaei, P.; Das, P.; Lappa, D.; Ramakrishnan, G.; Fox, T.E.; Haque, R.; Petri, W.A., Jr.; Bäckhed, F. Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: Lessons from genome-scale metabolic modeling. Metab. Eng. 2018, 49, 128–142. [Google Scholar] [CrossRef]
- Shoaie, S.; Ghaffari, P.; Kovatcheva-Datchary, P.; Mardinoglu, A.; Sen, P.; Pujos-Guillot, E.; de Wouters, T.; Juste, C.; Rizkalla, S.; Chilloux, J.; et al. Quantifying Diet-Induced Metabolic Changes of the Human Gut Microbiome. Cell Metab. 2015, 22, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Oresic, M. Metabolic Modeling of Human Gut Microbiota on a Genome Scale: An Overview. Metabolites 2019, 9, 22. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Alves, M.A.; Harkonen, T.; Honkanen, J.; Vatanen, T.; Xavier, R.J.; Hyotylainen, T.; Knip, M.; et al. Dysregulation of secondary bile acid metabolism precedes islet autoimmunity and type 1 diabetes. Cell Rep. Med. 2022, 3, 100762. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalog established by metagenomic sequencing. Nature 2010, 464, 59. [Google Scholar] [CrossRef]
- Harcombe, W.R.; Riehl, W.J.; Dukovski, I.; Granger, B.R.; Betts, A.; Lang, A.H.; Bonilla, G.; Kar, A.; Leiby, N.; Mehta, P.; et al. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 2014, 7, 1104–1115. [Google Scholar] [CrossRef]
- Bauer, E.; Zimmermann, J.; Baldini, F.; Thiele, I.; Kaleta, C. BacArena: Individual-based metabolic modeling of heterogeneous microbes in complex communities. PLoS Comput. Biol. 2017, 13, e1005544. [Google Scholar] [CrossRef] [PubMed]
- Zomorrodi, A.R.; Islam, M.M.; Maranas, C.D. d-OptCom: Dynamic multi-level and multi-objective metabolic modeling of microbial communities. ACS Synth. Biol. 2014, 3, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Biggs, M.B.; Papin, J.A. Novel multiscale modeling tool applied to Pseudomonas aeruginosa biofilm formation. PLoS ONE 2013, 8, e78011. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Izallalen, M.; Mouser, P.; Richter, H.; Risso, C.; Mahadevan, R.; Lovley, D.R. Genome-scale dynamic modeling of the competition between Rhodoferax and Geobacter in anoxic subsurface environments. ISME J. 2011, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Doebeli, M. Calibration and analysis of genome-based models for microbial ecology. eLife 2015, 4, e08208. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef]
- Segata, N.; Boernigen, D.; Tickle, T.L.; Morgan, X.C.; Garrett, W.S.; Huttenhower, C. Computational meta’omics for microbial community studies. Mol. Syst. Biol. 2013, 9, 666. [Google Scholar] [CrossRef]
- Heinken, A.; Ravcheev, D.A.; Baldini, F.; Heirendt, L.; Fleming, R.; Thiele, I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 2019, 7, 75. [Google Scholar] [CrossRef]
- Heinken, A.; Sahoo, S.; Fleming, R.M.; Thiele, I. Systems-level characterization of a host-microbe metabolic symbiosis in the mammalian gut. Gut Microbes 2013, 4, 28–40. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Dickens, A.M.; Oresic, M.; Bertram, H.C. Gut metabolome meets microbiome: A methodological perspective to understand the relationship between host and microbe. Methods 2018, 149, 3–12. [Google Scholar] [CrossRef]
- Lewis, J.E.; Forshaw, T.E.; Boothman, D.A.; Furdui, C.M.; Kemp, M.L. Personalized Genome-Scale Metabolic Models Identify Targets of Redox Metabolism in Radiation-Resistant Tumors. Cell Syst. 2021, 12, 68–81.E11. [Google Scholar] [CrossRef] [PubMed]
- Turanli, B.; Zhang, C.; Kim, W.; Benfeitas, R.; Uhlen, M.; Arga, K.Y.; Mardinoglu, A. Discovery of therapeutic agents for prostate cancer using genome-scale metabolic modeling and drug repositioning. eBioMedicine 2019, 42, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Lieven, C.; Beber, M.E.; Olivier, B.G.; Bergmann, F.T.; Ataman, M.; Babaei, P.; Bartell, J.A.; Blank, L.M.; Chauhan, S.; Correia, K.; et al. MEMOTE for standardized genome-scale metabolic model testing. Nat. Biotechnol. 2020, 38, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, R.; Edwards, J.S.; Doyle, F.J., III. Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys. J. 2002, 83, 1331–1340. [Google Scholar] [CrossRef]
- Bordbar, A.; Monk, J.M.; King, Z.A.; Palsson, B.O. Constraint-based models predict metabolic and associated cellular functions. Nat. Rev. Genet. 2014, 15, 107–120. [Google Scholar] [CrossRef]
- Sen, P.; Vial, H.J.; Radulescu, O. Kinetic modelling of phospholipid synthesis in Plasmodium knowlesi unravels crucial steps and relative importance of multiple pathways. BMC Syst. Biol. 2013, 7, 123. [Google Scholar] [CrossRef]
- Chan, S.H.J.; Friedman, E.S.; Wu, G.D.; Maranas, C.D. Predicting the Longitudinally and Radially Varying Gut Microbiota Composition using Multi-Scale Microbial Metabolic Modeling. Processes 2019, 7, 394. [Google Scholar] [CrossRef]
- Dinh, H.V.; Sarkar, D.; Maranas, C.D. Quantifying the propagation of parametric uncertainty on flux balance analysis. Metab. Eng. 2022, 69, 26–39. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Price, N.D. Probabilistic integrative modeling of genome-scale metabolic and regulatory networks in Escherichia coli and Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2010, 107, 17845–17850. [Google Scholar] [CrossRef]
- Reznik, E.; Mehta, P.; Segre, D. Flux imbalance analysis and the sensitivity of cellular growth to changes in metabolite pools. PLoS Comput. Biol. 2013, 9, e1003195. [Google Scholar] [CrossRef]
- Tervo, C.J.; Reed, J.L. Expanding Metabolic Engineering Algorithms Using Feasible Space and Shadow Price Constraint Modules. Metab. Eng. Commun. 2014, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, R.; Schilling, C.H. The effects of alternate optimal solutions in constraint-based genome-scale metabolic models. Metab. Eng. 2003, 5, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.J.; Zhang, C.; Nilsson, A.; Lahtvee, P.J.; Kerkhoven, E.J.; Nielsen, J. Improving the phenotype predictions of a yeast genome-scale metabolic model by incorporating enzymatic constraints. Mol. Syst. Biol. 2017, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic-Culf, M. Machine Learning Methods for Analysis of Metabolic Data and Metabolic Pathway Modeling. Metabolites 2018, 8, 4. [Google Scholar] [CrossRef]
- Angione, C. Human Systems Biology and Metabolic Modelling: A Review-From Disease Metabolism to Precision Medicine. Biomed. Res. Int. 2019, 2019, 8304260. [Google Scholar] [CrossRef]
- Alakwaa, F.M.; Chaudhary, K.; Garmire, L.X. Deep Learning Accurately Predicts Estrogen Receptor Status in Breast Cancer Metabolomics Data. J. Proteome Res. 2018, 17, 337–347. [Google Scholar] [CrossRef]
- Lewis, J.E.; Kemp, M.L. Integration of machine learning and genome-scale metabolic modeling identifies multi-omics biomarkers for radiation resistance. Nat. Commun. 2021, 12, 2700. [Google Scholar] [CrossRef]
- Sen, P.; Lamichhane, S.; Mathema, V.B.; McGlinchey, A.; Dickens, A.M.; Khoomrung, S.; Oresic, M. Deep learning meets metabolomics: A methodological perspective. Brief. Bioinform. 2021, 22, 1531–1542. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef]
- Zampieri, G.; Vijayakumar, S.; Yaneske, E.; Angione, C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput. Biol. 2019, 15, e1007084. [Google Scholar] [CrossRef]
- Pearcy, N.; Hu, Y.; Baker, M.; Maciel-Guerra, A.; Xue, N.; Wang, W.; Kaler, J.; Peng, Z.; Li, F.; Dottorini, T. Genome-Scale Metabolic Models and Machine Learning Reveal Genetic Determinants of Antibiotic Resistance in Escherichia coli and Unravel the Underlying Metabolic Adaptation Mechanisms. mSystems 2021, 6, e0091320. [Google Scholar] [CrossRef]
- Rana, P.; Berry, C.; Ghosh, P.; Fong, S.S. Recent advances on constraint-based models by integrating machine learning. Curr. Opin. Biotechnol. 2019, 64, 85–91. [Google Scholar] [CrossRef]
- Medlock, G.L.; Papin, J.A. Guiding the Refinement of Biochemical Knowledgebases with Ensembles of Metabolic Networks and Machine Learning. Cell Syst. 2020, 10, 109–119.E3. [Google Scholar] [CrossRef]
- Kotera, M.; Tabei, Y.; Yamanishi, Y.; Tokimatsu, T.; Goto, S. Supervised de novo reconstruction of metabolic pathways from metabolome-scale compound sets. Bioinformatics 2013, 29, i135–i144. [Google Scholar] [CrossRef] [PubMed]
- Liberal, R.; Pinney, J.W. Simple topological properties predict functional misannotations in a metabolic network. Bioinformatics 2013, 29, i154–i161. [Google Scholar] [CrossRef] [PubMed]
- Zelezniak, A.; Vowinckel, J.; Capuano, F.; Messner, C.B.; Demichev, V.; Polowsky, N.; Mulleder, M.; Kamrad, S.; Klaus, B.; Keller, M.A.; et al. Machine Learning Predicts the Yeast Metabolome from the Quantitative Proteome of Kinase Knockouts. Cell Syst. 2018, 7, 269–283.e6. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wright, S.N.; Hamblin, M.; McCloskey, D.; Alcantar, M.A.; Schrubbers, L.; Lopatkin, A.J.; Satish, S.; Nili, A.; Palsson, B.O.; et al. A White-Box Machine Learning Approach for Revealing Antibiotic Mechanisms of Action. Cell 2019, 177, 1649–1661. [Google Scholar] [CrossRef]
- Guo, W.; Xu, Y.; Feng, X. DeepMetabolism: A deep learning system to predict phenotype from genome sequencing. arXiv 2017, arXiv:1705.03094. [Google Scholar] [CrossRef]
- Antonakoudis, A.; Barbosa, R.; Kotidis, P.; Kontoravdi, C. The era of big data: Genome-scale modelling meets machine learning. Comput. Struct. Biotechnol. J. 2020, 18, 3287–3300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).