Bioavailable Microbial Metabolites of Flavanols Demonstrate Highly Individualized Bioactivity on In Vitro β-Cell Functions Critical for Metabolic Health
Abstract
1. Introduction
2. Methods
2.1. Animals and Diets
2.2. Antibiotics (Abx) Treatment
2.3. Flavanol Standards and Extracts
2.4. Flavanol Metabolite Collection and Purification
2.5. Metabolite Profiling
2.6. Cell Culture
2.7. Glucose Stimulated Insulin Secretion (GSIS)
2.8. Statistical Analysis
3. Results
3.1. Flavanol Type and Abx Treatment Modify Bioavailable Flavanol and Metabolite Profiles
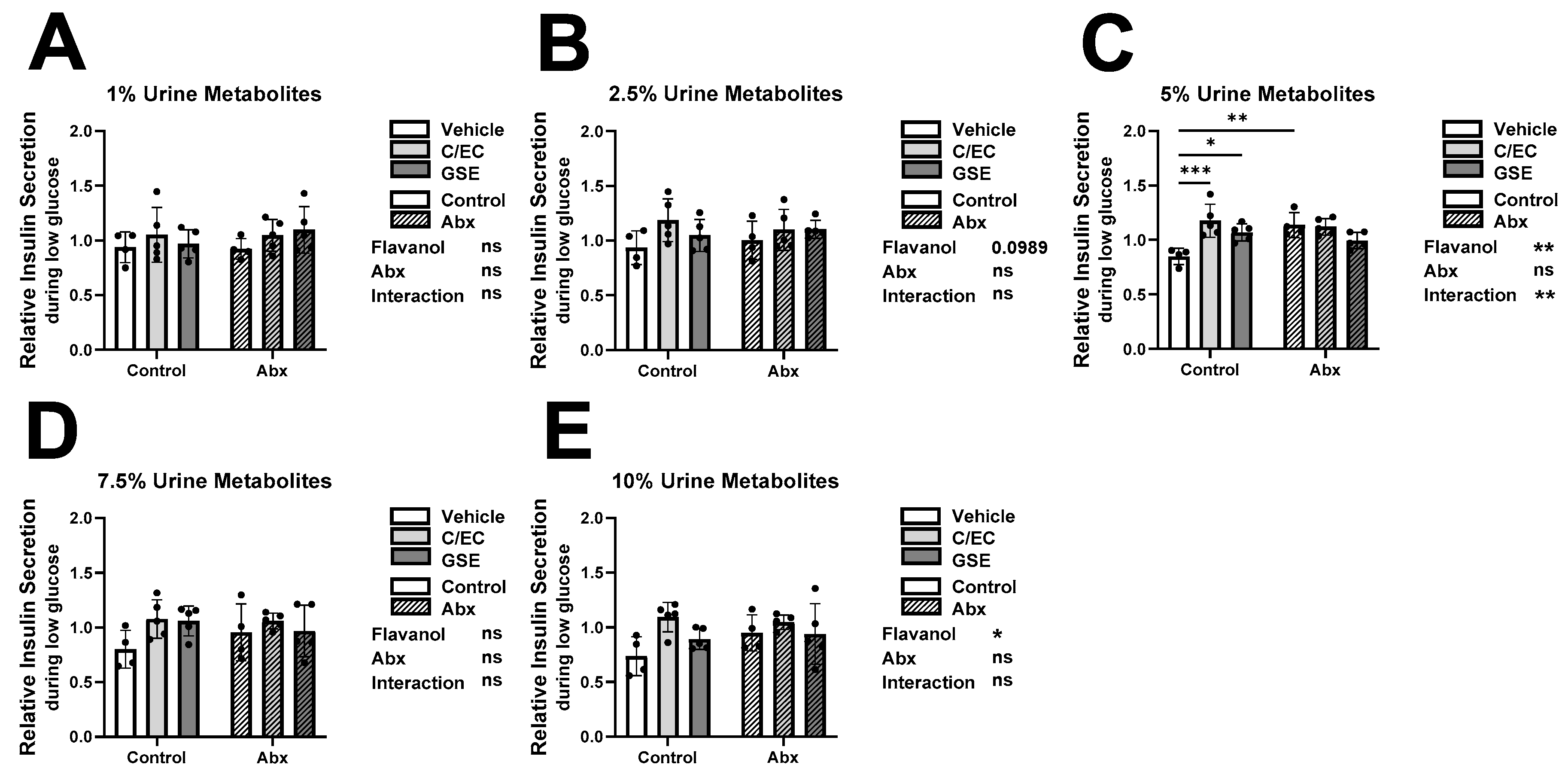
3.2. Treatment-Level Comparisons: Urine Metabolites Increase β-Cell Insulin Secretion during Low Glucose Condition
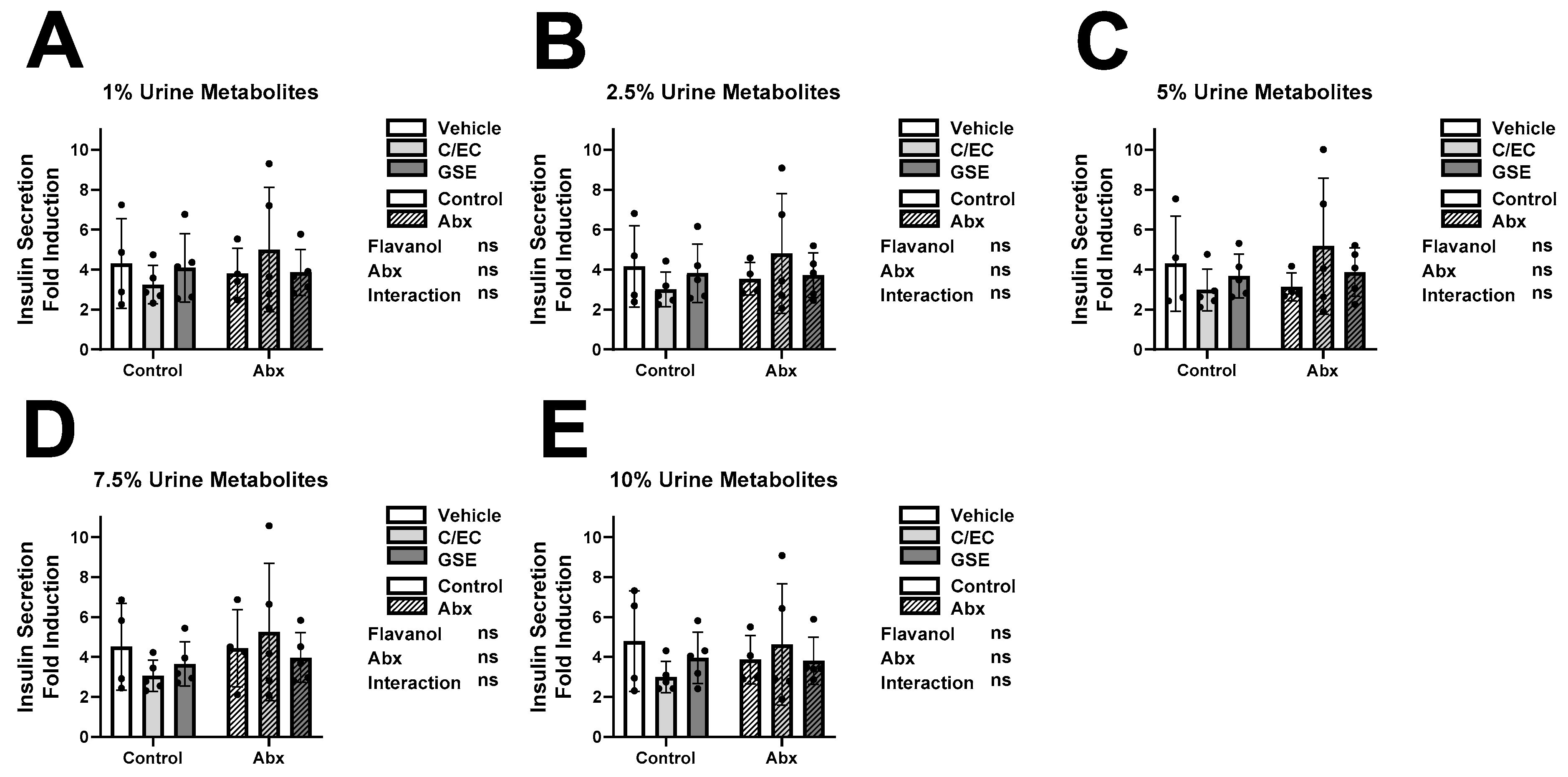
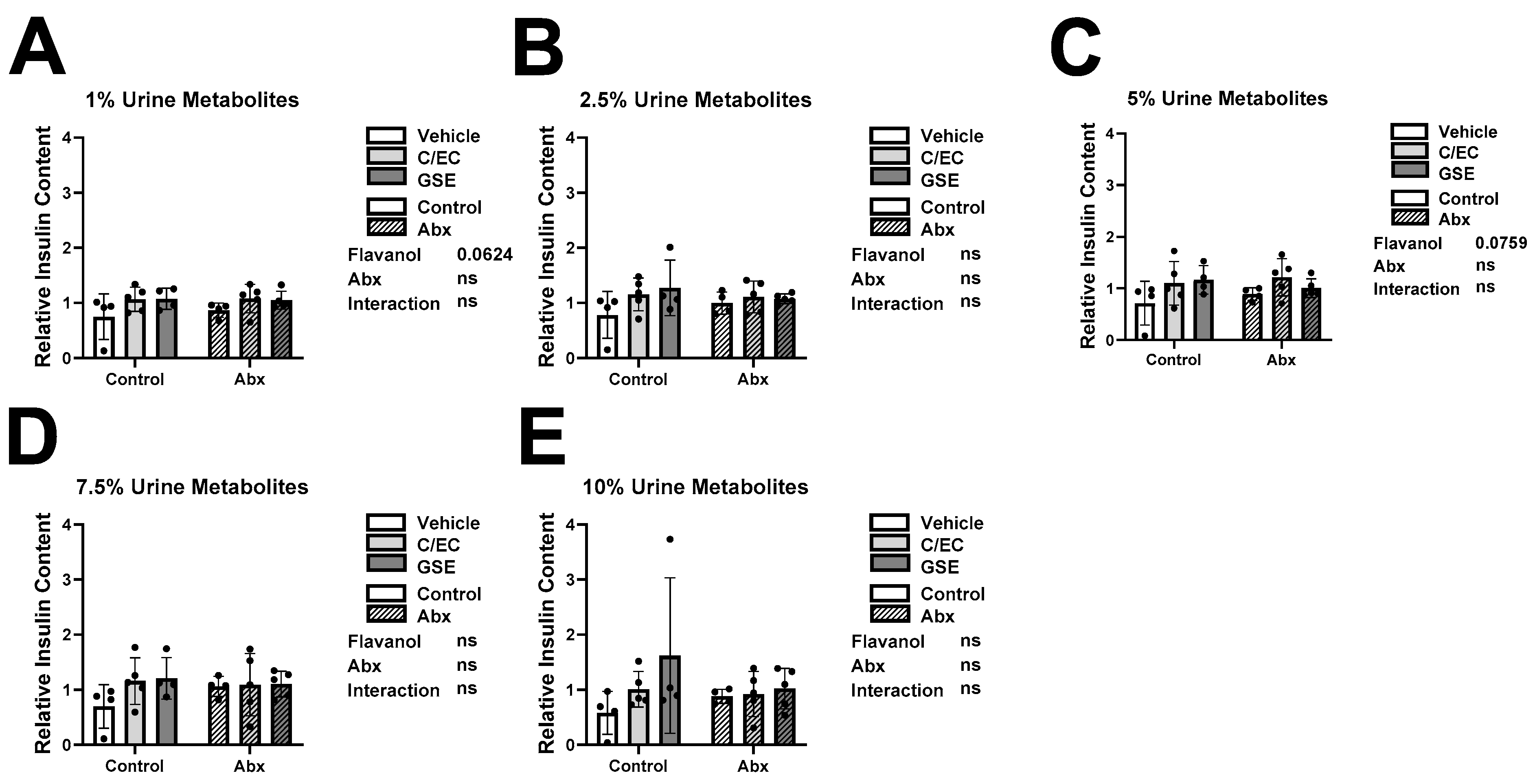
3.3. Metabolites Do Not Affect Insulin Secretion or Content during High Glucose Conditions
3.4. Individual-Level Effects on GSIS Regardless of Treatment
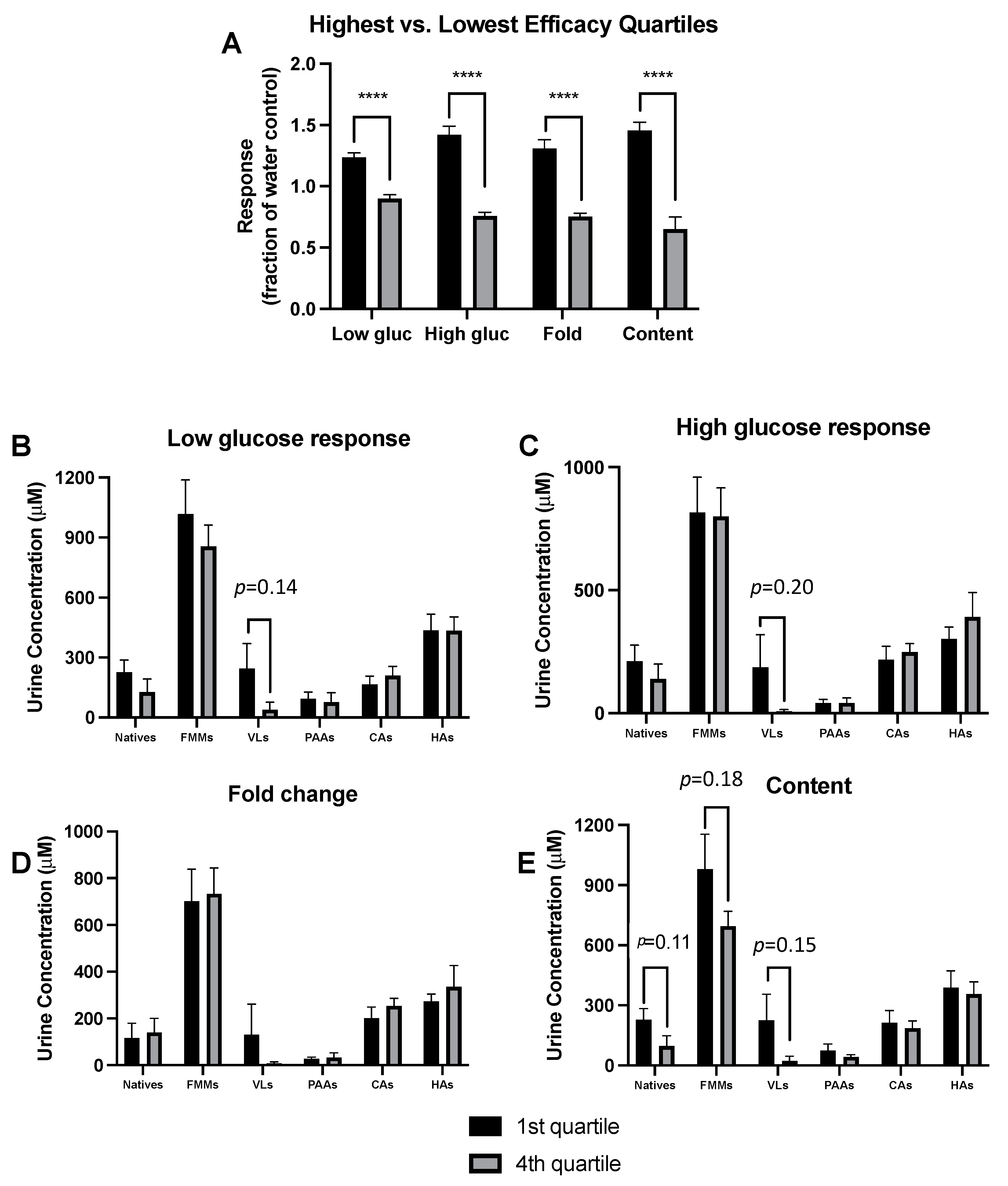
3.5. Responder Analyses Show Metabolite Profiles Predict GSIS Activity Independent of Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behr, C.; Kamp, H.; Fabian, E.; Krennrich, G.; Mellert, W.; Peter, E.; Strauss, V.; Walk, T.; Rietjens, I.; van Ravenzwaay, B. Gut microbiome-related metabolic changes in plasma of antibiotic-treated rats. Arch Toxicol. 2017, 91, 3439–3454. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomas-Barberan, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. The Chemistry of Gut Microbial Metabolism of Polyphenols. Phytochem. Rev. 2016, 15, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019, 10, 2138–2147. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S. Dietary Flavonoids and Insulin Signaling in Diabetes and Obesity. Cells 2021, 10, 1474. [Google Scholar] [CrossRef]
- Bitner, B.F.; Ray, J.D.; Kener, K.B.; Herring, J.A.; Tueller, J.A.; Johnson, D.K.; Tellez Freitas, C.M.; Fausnacht, D.W.; Allen, M.E.; Thomson, A.H.; et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in beta-cells and skeletal muscle cells. J. Nutr. Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox. Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Goya, L.; Martin, M.A.; Sarria, B.; Ramos, S.; Mateos, R.; Bravo, L. Effect of Cocoa and Its Flavonoids on Biomarkers of Inflammation: Studies of Cell Culture, Animals and Humans. Nutrients 2016, 8, 212. [Google Scholar] [CrossRef]
- Marquez Campos, E.; Stehle, P.; Simon, M.C. Microbial Metabolites of Flavan-3-Ols and Their Biological Activity. Nutrients 2019, 11, 2260. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Improving the estimation of flavonoid intake for study of health outcomes. Nutr. Rev. 2015, 73, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hemon, B.; Moskal, A.; Overvad, K.; Tjonneland, A.; Kyro, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Strat, K.M.; Rowley, T.J.t.; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilson, A.P. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Richelle, M.; Tavazzi, I.; Enslen, M.; Offord, E.A. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr. 1999, 53, 22–26. [Google Scholar] [CrossRef]
- Fernandez-Millan, E.; Ramos, S.; Alvarez, C.; Bravo, L.; Goya, L.; Martin, M.A. Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against tert-butyl hydroperoxide-induced toxicity via ERKs and PKC pathways. Food. Chem. Toxicol. 2014, 66, 245–253. [Google Scholar] [CrossRef]
- Alvarez-Cilleros, D.; Martin, M.A.; Ramos, S. (−)-Epicatechin and the Colonic 2,3-Dihydroxybenzoic Acid Metabolite Regulate Glucose Uptake, Glucose Production, and Improve Insulin Signaling in Renal NRK-52E Cells. Mol. Nutr. Food Res. 2018, 62, 1700470. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Griffin, L.E.; Kohrt, S.E.; Rathore, A.; Kay, C.D.; Grabowska, M.M.; Neilson, A.P. Microbial Metabolites of Flavanols in Urine are Associated with Enhanced Anti-Proliferative Activity in Bladder Cancer Cells In Vitro. Nutr. Cancer 2022, 74, 194–210. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patan, F.; Llorach, R.; Garrido, I.; Gomez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortes-Martin, A.; Avila-Galvez, M.A.; Gimenez-Bastida, J.A.; Selma, M.V.; Gonzalez-Sarrias, A.; Espin, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef]
- Morand, C.; Tomas-Barberan, F.A. Interindividual Variability in Absorption, Distribution, Metabolism, and Excretion of Food Phytochemicals Should Be Reported. J. Agric. Food Chem. 2019, 67, 3843–3844. [Google Scholar] [CrossRef]
- Milenkovic, D.; Morand, C.; Cassidy, A.; Konic-Ristic, A.; Tomas-Barberan, F.; Ordovas, J.M.; Kroon, P.; De Caterina, R.; Rodriguez-Mateos, A. Interindividual Variability in Biomarkers of Cardiometabolic Health after Consumption of Major Plant-Food Bioactive Compounds and the Determinants Involved. Adv. Nutr. 2017, 8, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Van de Wiele, T.; Possemiers, S. Gut metabotypes govern health effects of dietary polyphenols. Curr. Opin. Biotechnol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Morand, C.; Tomas-Barberan, F.A. Contribution of plant food bioactives in promoting health effects of plant foods: Why look at interindividual variability? Eur. J. Nutr. 2019, 58, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andres-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Takei, M.; Ishii, H.; Sato, Y. Glucose-stimulated insulin secretion: A newer perspective. J. Diabetes Investig. 2013, 4, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.A.; Cordero-Herrera, I.; Bravo, L.; Ramos, S.; Goya, L. Cocoa flavanols show beneficial effects in cultured pancreatic beta cells and liver cells to prevent the onset of type 2 diabetes. Food Res. Int. 2014, 63, 400–408. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Park, J.Y.; Harvatine, K.; Lambert, J.D. Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. J. Nutr. Biochem. 2014, 25, 439–445. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef]
- Inagaki, N.; Gonoi, T.; Seino, S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett. 1997, 409, 232–236. [Google Scholar] [CrossRef]
- Dean, P.M.; Matthews, E.K. Electrical activity in pancreatic islet cells. Nature 1968, 219, 389–390. [Google Scholar] [CrossRef]
- Cedo, L.; Castell-Auvi, A.; Pallares, V.; Blay, M.; Ardevol, A.; Arola, L.; Pinent, M. Grape seed procyanidin extract modulates proliferation and apoptosis of pancreatic beta-cells. Food Chem. 2013, 138, 524–530. [Google Scholar] [CrossRef]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)-Epigallocatechin-3-Gallate Protect Murine MIN6 Pancreatic Beta-Cells Against Iron Toxicity and Erastin-Induced Ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef]
- Martin, M.A.; Fernandez-Millan, E.; Ramos, S.; Bravo, L.; Goya, L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol. Nutr. Food Res. 2014, 58, 447–456. [Google Scholar] [CrossRef]
- Fernandez-Millan, E.; Cordero-Herrera, I.; Ramos, S.; Escriva, F.; Alvarez, C.; Goya, L.; Martin, M.A. Cocoa-rich diet attenuates beta cell mass loss and function in young Zucker diabetic fatty rats by preventing oxidative stress and beta cell apoptosis. Mol. Nutr. Food Res. 2015, 59, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Andujar, I.; Recio, M.C.; Giner, R.M.; Rios, J.L. Cocoa Polyphenols and Their Potential Benefits for Human Health. Oxid. Med. Cell Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef]
- Ramos, S.; Martin, M.A.; Goya, L. Effects of Cocoa Antioxidants in Type 2 Diabetes Mellitus. Antioxidants 2017, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Hohmeier, H.E.; Mulder, H.; Chen, G.; Henkel-Rieger, R.; Prentki, M.; Newgard, C.B. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 2000, 49, 424–430. [Google Scholar] [CrossRef]
- Krueger, E.S.; Beales, J.L.; Russon, K.B.; Elison, W.S.; Davis, J.R.; Hansen, J.M.; Neilson, A.P.; Hansen, J.M.; Tessem, J.S. Gut Metabolite Trimethylamine N-Oxide Protects INS-1 beta-Cell and Rat Islet Function under Diabetic Glucolipotoxic Conditions. Biomolecules 2021, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Rowley, T.J.t.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance beta-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef]
- Reynolds, M.S.; Hancock, C.R.; Ray, J.D.; Kener, K.B.; Draney, C.; Garland, K.; Hardman, J.; Bikman, B.T.; Tessem, J.S. beta-Cell deletion of Nr4a1 and Nr4a3 nuclear receptors impedes mitochondrial respiration and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E186–E201. [Google Scholar] [CrossRef]
- Ray, J.D.; Kener, K.B.; Bitner, B.F.; Wright, B.J.; Ballard, M.S.; Barrett, E.J.; Hill, J.T.; Moss, L.G.; Tessem, J.S. Nkx6.1-mediated insulin secretion and beta-cell proliferation is dependent on upregulation of c-Fos. FEBS Lett. 2016, 590, 1791–1803. [Google Scholar] [CrossRef]
- Draney, C.; Hobson, A.E.; Grover, S.G.; Jack, B.O.; Tessem, J.S. Cdk5r1 Overexpression Induces Primary beta-Cell Proliferation. J. Diabetes Res. 2016, 2016, 6375804. [Google Scholar] [CrossRef]
- Hobson, A.; Draney, C.; Stratford, A.; Becker, T.C.; Lu, D.; Arlotto, M.; Tessem, J.S. Aurora Kinase A is critical for the Nkx6.1 mediated beta-cell proliferation pathway. Islets 2015, 7, e1027854. [Google Scholar] [CrossRef]
- Truchan, N.A.; Brar, H.K.; Gallagher, S.J.; Neuman, J.C.; Kimple, M.E. A single-islet microplate assay to measure mouse and human islet insulin secretion. Islets 2015, 7, e1076607. [Google Scholar] [CrossRef] [PubMed]
- Cantley, J.; Ashcroft, F.M. Q&A: Insulin secretion and type 2 diabetes: Why do beta-cells fail? BMC Biol. 2015, 13, 33. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Racine, K.C.; Iglesias-Carres, L.; Herring, J.A.; Ferruzzi, M.G.; Kay, C.D.; Tessem, J.S.; Neilson, A.P. Cocoa extract exerts sex-specific anti-diabetic effects in an aggressive type-2 diabetes model: A pilot study. Biochem Biophys Res Commun 2022, 626, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Bharat, D.; Wankhade, U.D.; Kim, J.S.; Cutler, B.R.; Denetso, C.; Gholami, S.; Nelson, S.; Bigley, J.; Johnson, A.; et al. Dietary Blueberry Ameliorates Vascular Complications in Diabetic Mice Possibly through NOX4 and Modulates Composition and Functional Diversity of Gut Microbes. Mol. Nutr. Food Res. 2022, 66, e2100784. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Bharat, D.; Cutler, B.R.; Gholami, S.; Denetso, C.; Mueller, J.E.; Cho, J.M.; Kim, J.S.; Symons, J.D.; Anandh Babu, P.V. Circulating metabolites of strawberry mediate reductions in vascular inflammation and endothelial dysfunction in db/db mice. Int. J. Cardiol. 2018, 263, 111–117. [Google Scholar] [CrossRef]
- Virdee, M.S.; Saini, N.; Kay, C.D.; Neilson, A.P.; Kwan, S.T.C.; Helfrich, K.K.; Mooney, S.M.; Smith, S.M. An enriched biosignature of gut microbiota-dependent metabolites characterizes maternal plasma in a mouse model of fetal alcohol spectrum disorder. Sci. Rep. 2021, 11, 248. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krueger, E.S.; Griffin, L.E.; Beales, J.L.; Lloyd, T.S.; Brown, N.J.; Elison, W.S.; Kay, C.D.; Neilson, A.P.; Tessem, J.S. Bioavailable Microbial Metabolites of Flavanols Demonstrate Highly Individualized Bioactivity on In Vitro β-Cell Functions Critical for Metabolic Health. Metabolites 2023, 13, 801. https://doi.org/10.3390/metabo13070801
Krueger ES, Griffin LE, Beales JL, Lloyd TS, Brown NJ, Elison WS, Kay CD, Neilson AP, Tessem JS. Bioavailable Microbial Metabolites of Flavanols Demonstrate Highly Individualized Bioactivity on In Vitro β-Cell Functions Critical for Metabolic Health. Metabolites. 2023; 13(7):801. https://doi.org/10.3390/metabo13070801
Chicago/Turabian StyleKrueger, Emily S., Laura E. Griffin, Joseph L. Beales, Trevor S. Lloyd, Nathan J. Brown, Weston S. Elison, Colin D. Kay, Andrew P. Neilson, and Jeffery S. Tessem. 2023. "Bioavailable Microbial Metabolites of Flavanols Demonstrate Highly Individualized Bioactivity on In Vitro β-Cell Functions Critical for Metabolic Health" Metabolites 13, no. 7: 801. https://doi.org/10.3390/metabo13070801
APA StyleKrueger, E. S., Griffin, L. E., Beales, J. L., Lloyd, T. S., Brown, N. J., Elison, W. S., Kay, C. D., Neilson, A. P., & Tessem, J. S. (2023). Bioavailable Microbial Metabolites of Flavanols Demonstrate Highly Individualized Bioactivity on In Vitro β-Cell Functions Critical for Metabolic Health. Metabolites, 13(7), 801. https://doi.org/10.3390/metabo13070801






