A GC-MS Chemotaxonomic Study on Lipophilic Compounds in the Bark of S. aucuparia subsp. sibirica Trees from the Population Growing in Akademgorodok, Novosibirsk (Russia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents and Solvents
2.3. Metabolite Extraction, Derivatization, Detection by GC-MS, Data Processing and Annotation
2.4. Statistical Methods
3. Results and Discussion
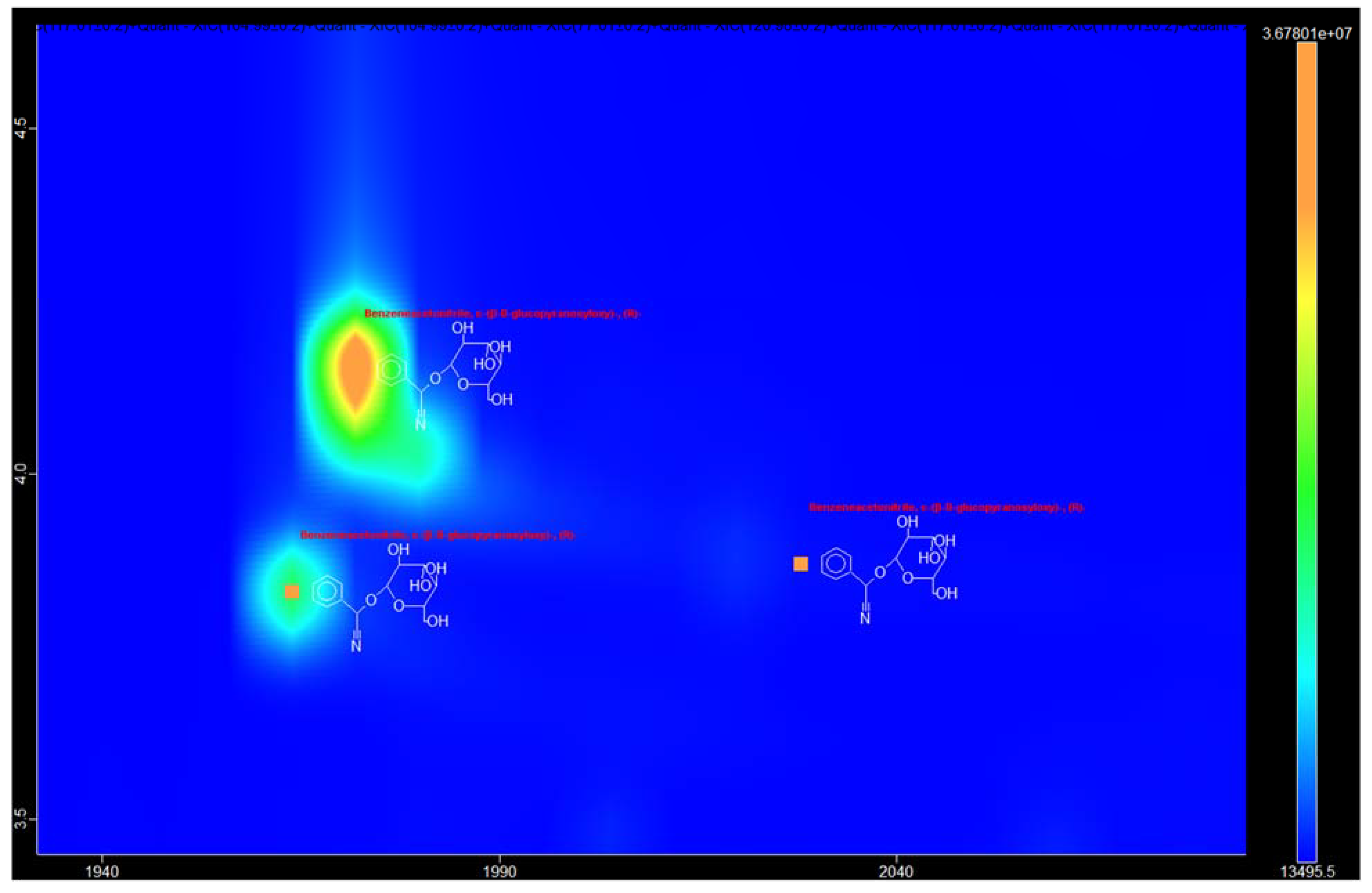
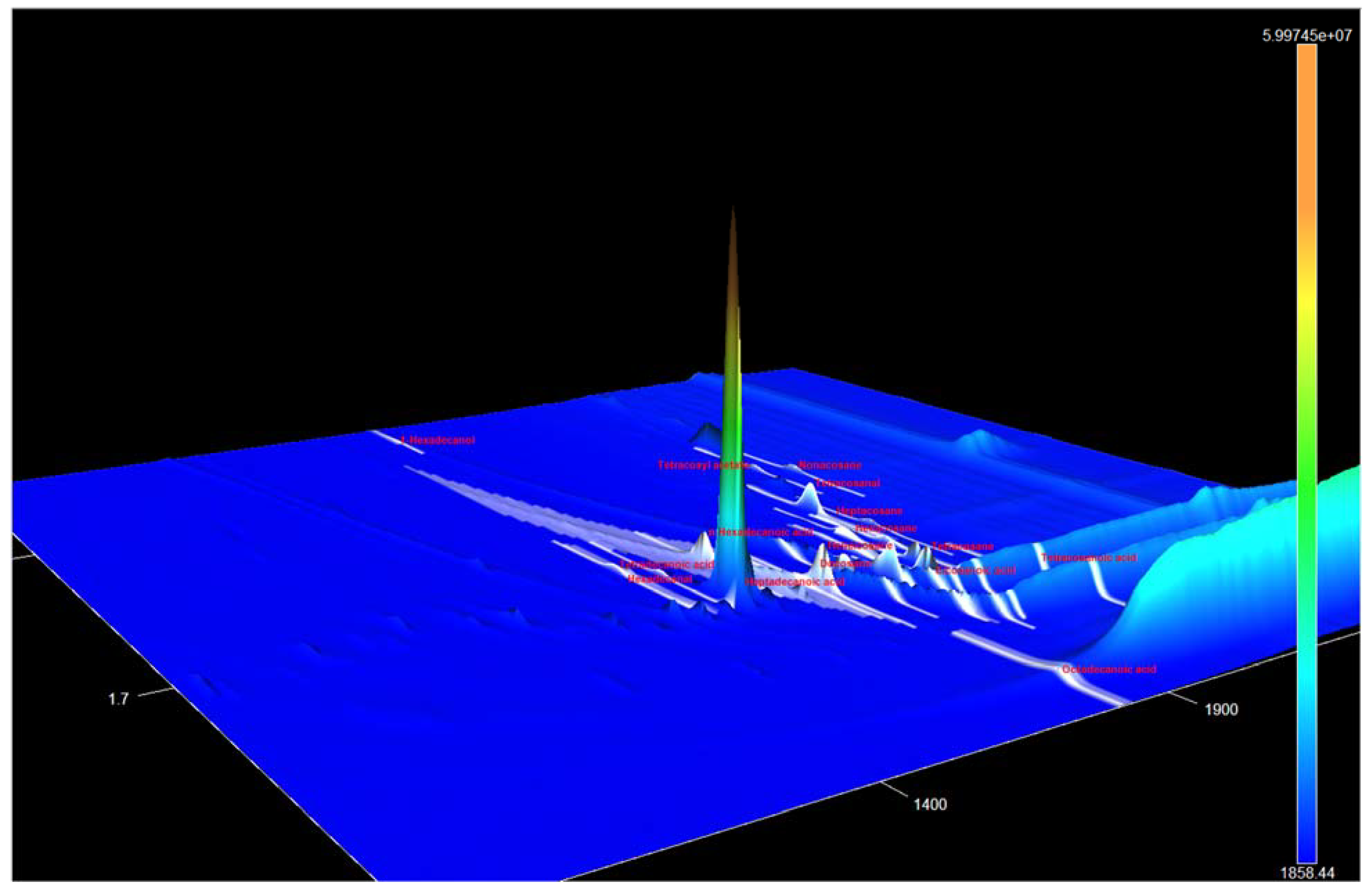
3.1. Identification of the Components of S. aucuparia subsp. sibirica Bark Extracts
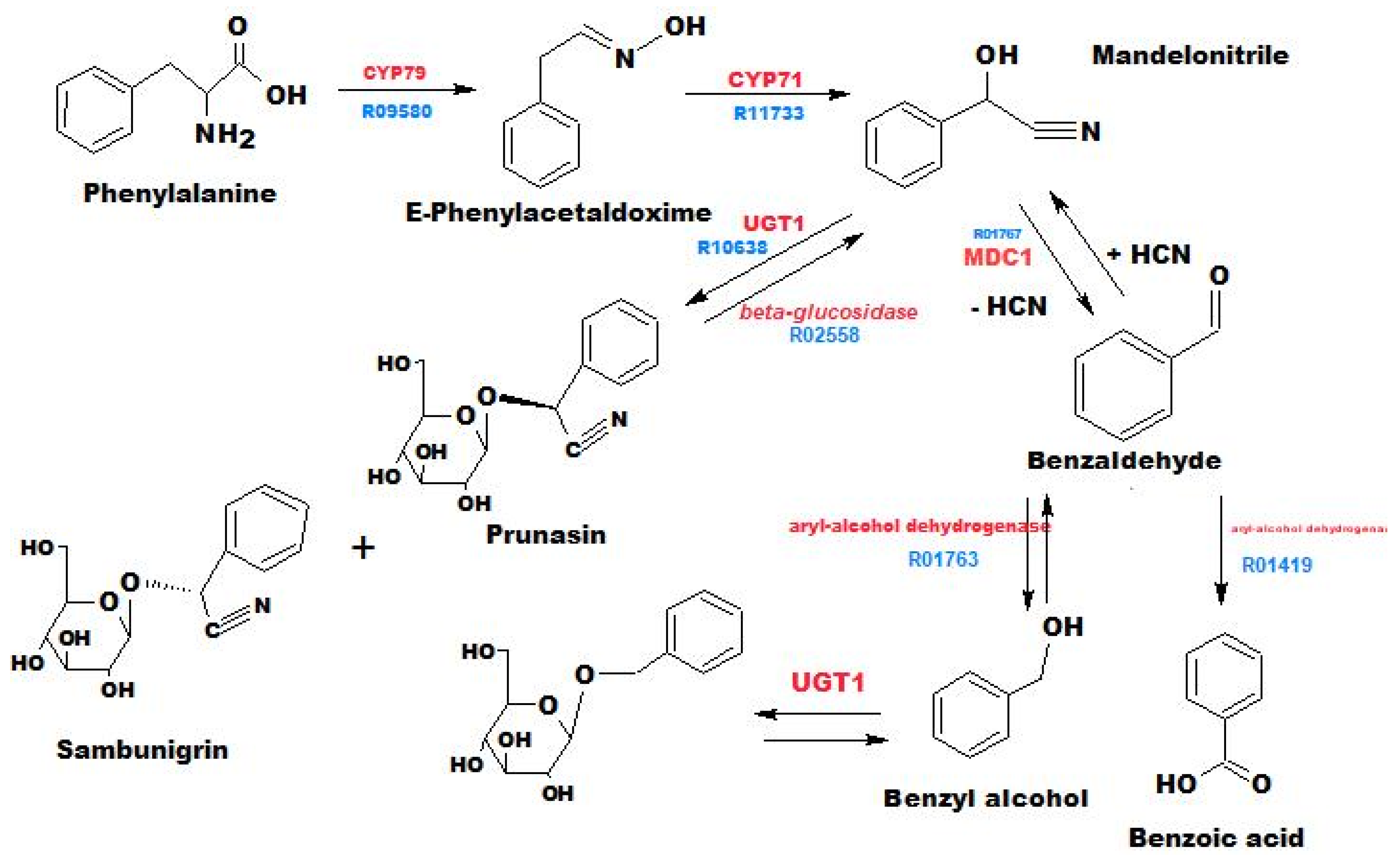
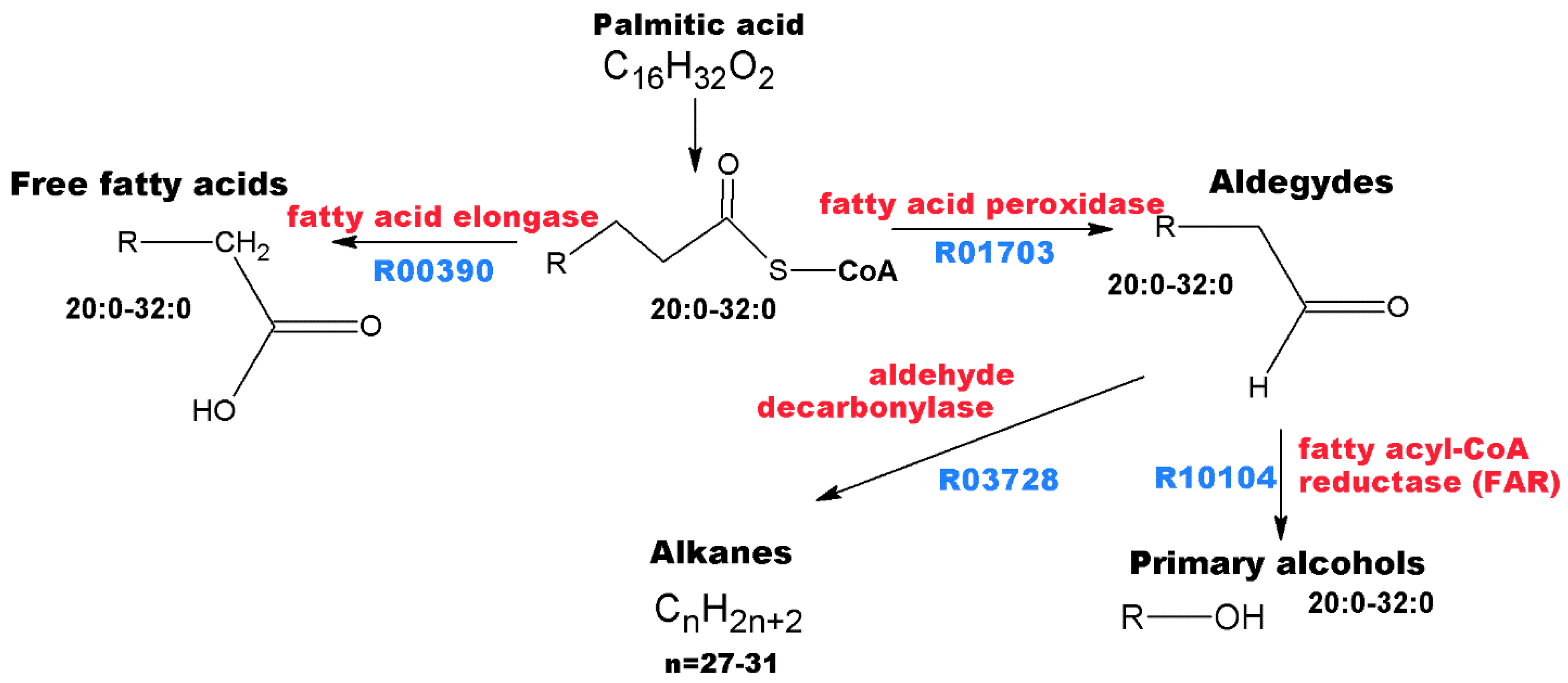
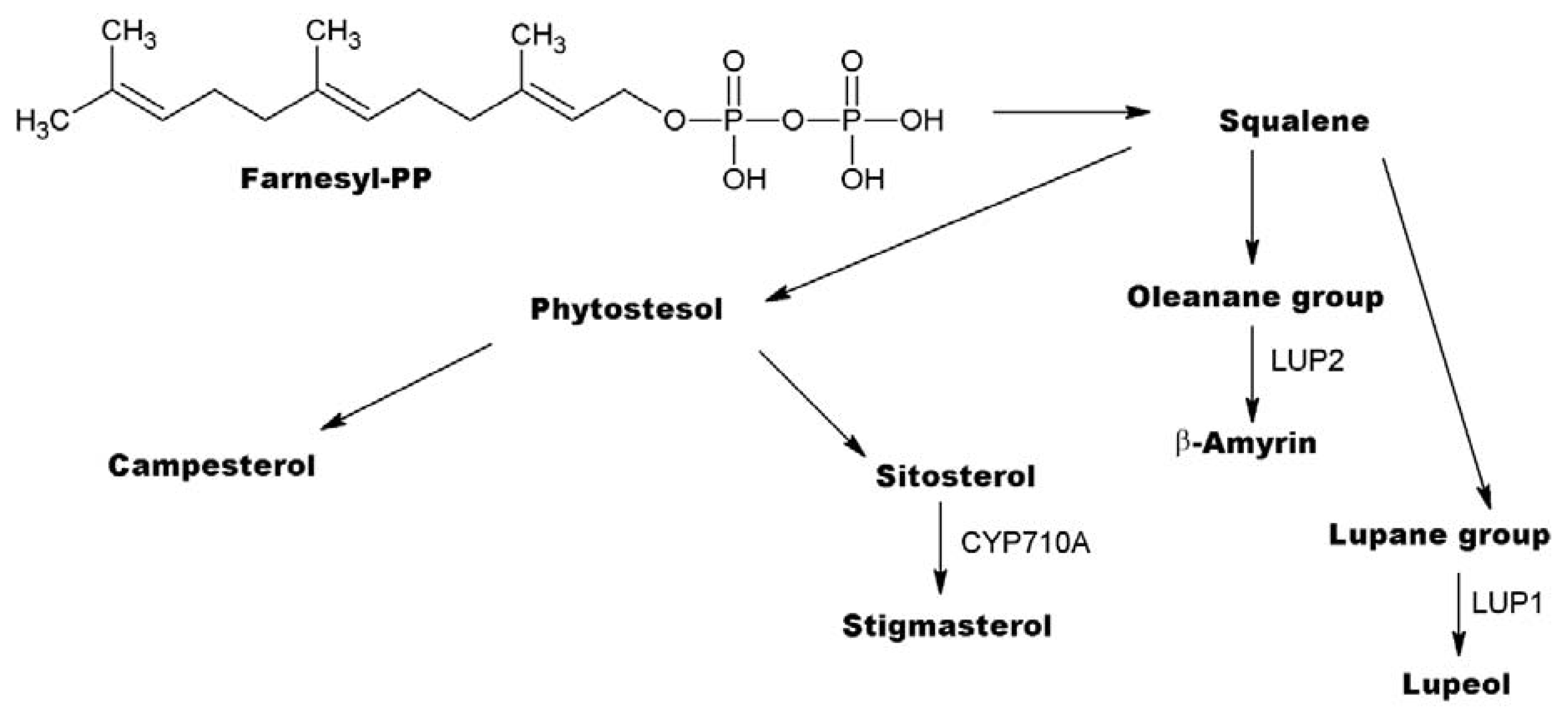
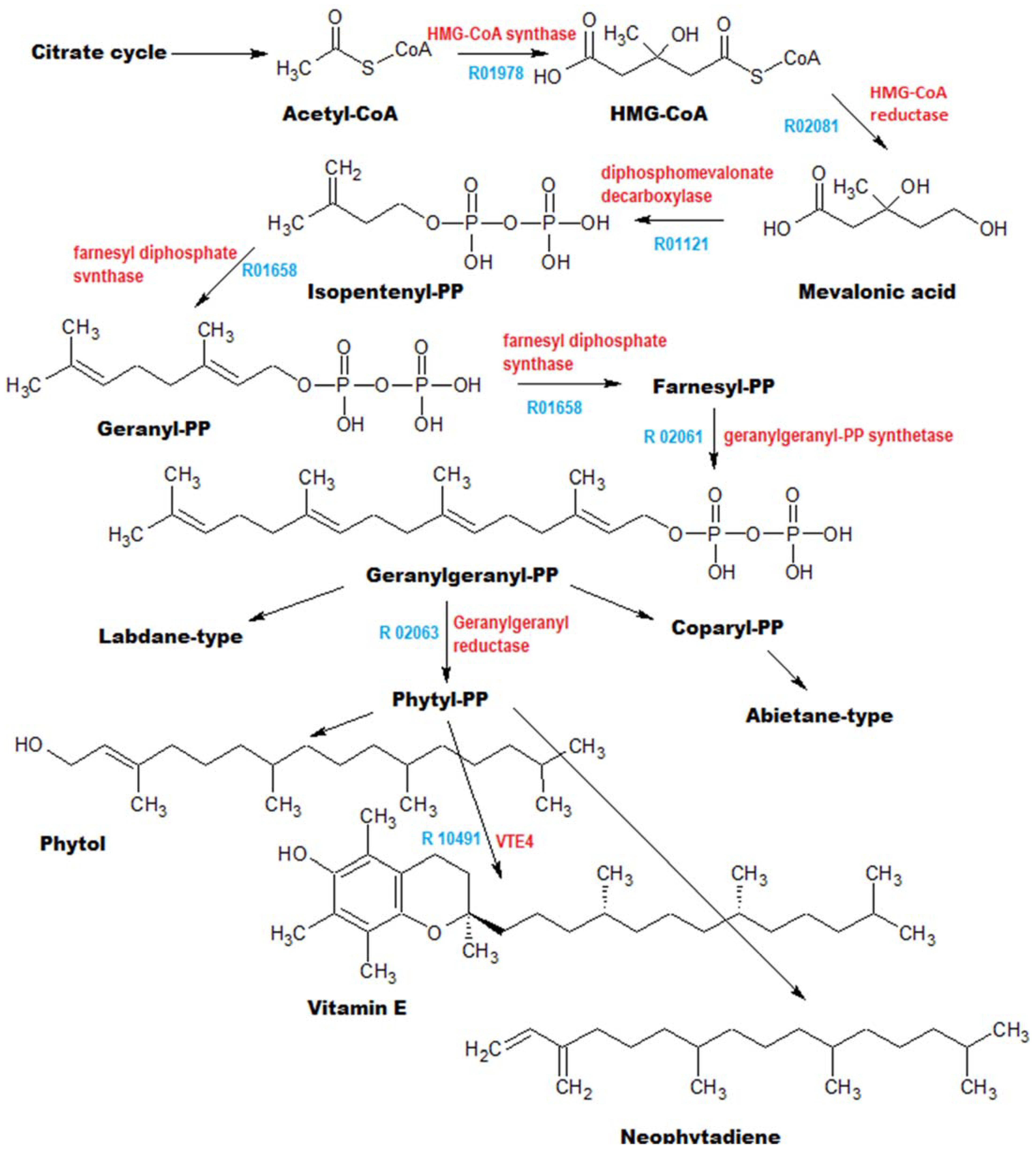
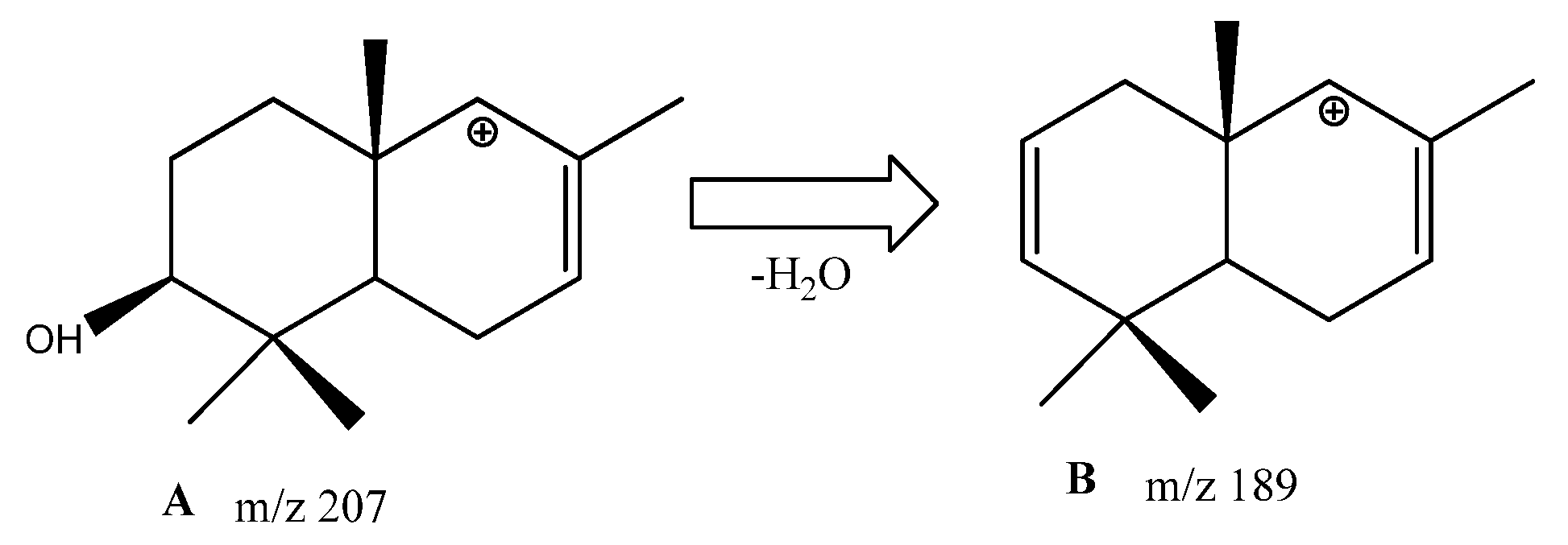
3.2. Annotation of Identified Metabolites
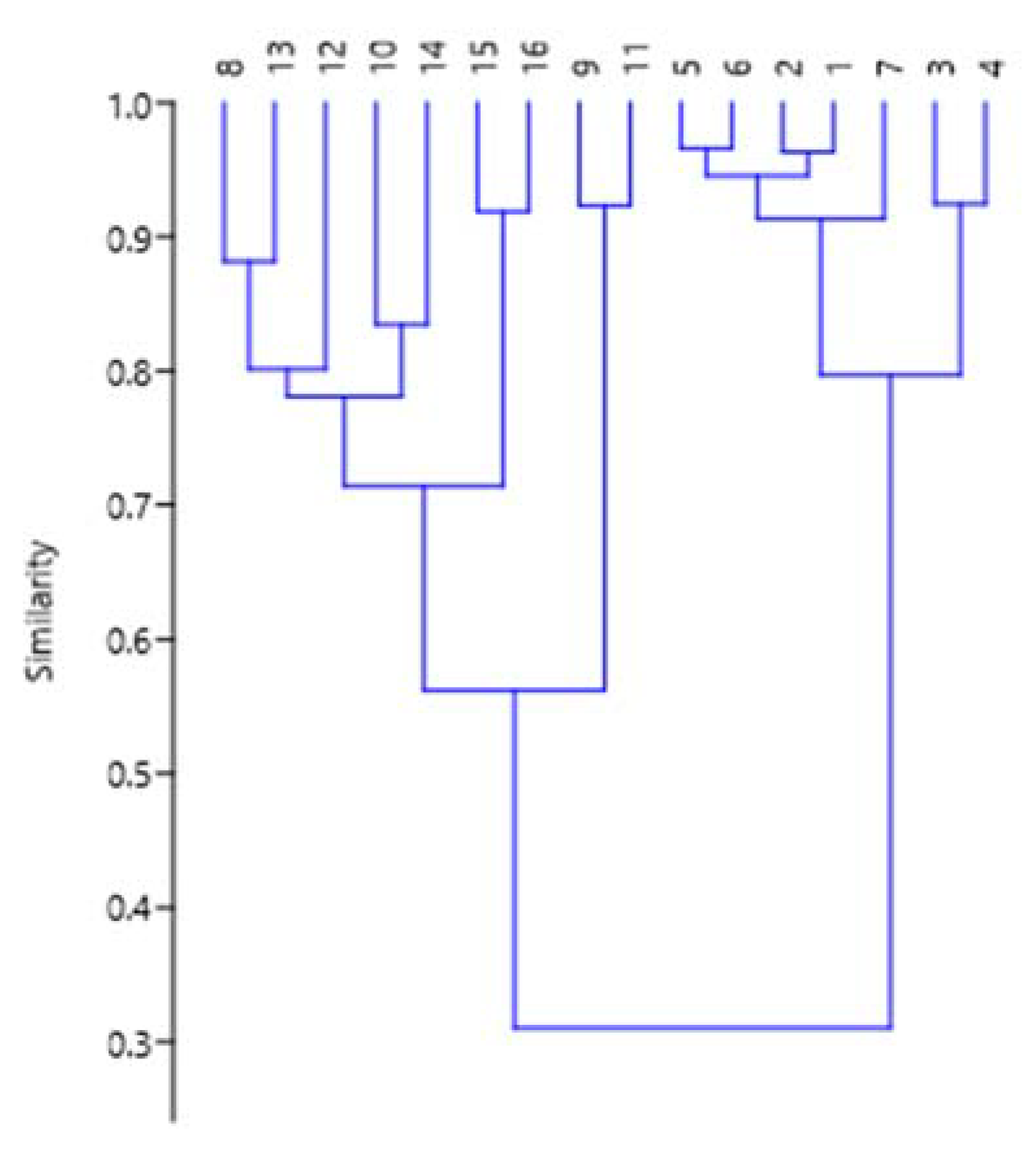
3.3. Statistical Analysis
3.4. Pharmacological Significance of Annotated Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polatoğlu, K. “Chemotypes”—A Fact that should not be Ignored in Natural Product Studies. Nat. Prod. J. 2013, 3, 10–14. [Google Scholar] [CrossRef]
- Martucci, M.E.P.; Vos, R.C.H.D.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a Potential Chemotaxonomical Tool: Application in the Genus Vernonia Schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef] [PubMed]
- Aldasoro, J.J.; Aedo, C.; Navarro, C.; Garmendia, F.M. The genus Sorbus (Maloideae, Rosaceae) in Europe and in North Africa: Morphological analysis and systematics. Syst. Bot. 1998, 23, 189–212. [Google Scholar] [CrossRef]
- Gabrielian, E.T. Ryabiny (Sorbus L.) Zapadnoi Azii i Gimalayev [Rowans (Sorbus L.) of Western Asia and Himalayas]; Izvestiya Akademii Nauk Armyanskoi SSR: Yerevan, Armenia, 1978; 264p. [Google Scholar]
- McAllister, H. The Genus Sorbus: Mountain Ash and Other Rowans; Royal Botanic Gardens, Kew: Richmond, UK, 2005; 252p. [Google Scholar]
- Robertson, A.; Rich, T.C.G.; Allen, A.M.; Houston, L.; Roberts, C.; Bridle, J.R.; Harris, S.A.; Hiscock, S.J. Hybridization and polyploidy as drivers of continuing evolution and speciation in Sorbus. Mol. Ecol. 2010, 19, 1675–1690. [Google Scholar] [CrossRef]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Polozhij, A.V.; Malyschev, L.I. Rosaceae. In Flora of Siberia; Science Publishers Inc.: Enfield, NH, USA, 2004; Volume 8, 208p. [Google Scholar]
- Shaulo, D.N.; Drachev, N.S.; Kuzmin, I.V. Introgressive hybridization of Sorbus (Rosaceae) in Tyumen region boreal forests. Vestnik Tyumenskogo Gosudarstvennogo Universiteta Ekologiya Prirodopol’zovanie 2009, 3, 209–215. Available online: https://www.elibrary.ru/item.asp?id=12907189 (accessed on 23 April 2023). (In Russian).
- Asbaganov, S.V. Leaf morphological variation among populations of mountain ash across Russia. In Proceedings of the Problemy Izucheniya Rastitel’nogo Pokrova Sibiri, Materialy V International Scientific Conference, Tomsk, Russia, 20–22 October 2015; pp. 56–59. Available online: https://www.elibrary.ru/item.asp?id=32342646 (accessed on 23 April 2023). (In Russian).
- Rengarten, G.A.; Sorokopudov, V.N. Selection of rows as a decorative culture in Russia and in European countries. Vestnik KrasGAU Agron. 2019, 6, 9–15. [Google Scholar]
- Goncharov, N.P.; Savel’ev, N.I. On the 160th anniversary of Ivan V. Michurin’s birth. Vavilovskii Zhurnal Genetiki Selektsii Vavilov J. Genet. Breed. 2015, 19, 339–358. [Google Scholar] [CrossRef]
- State Register for Selection Achievements Admitted for Usage (National List). “Plant Varieties” (Official Publication); FGBNU “Rosinformagrotekh”: Moscow, Russia, 2021; Volume 1, p. 719. Available online: https://gossortrf.ru/ (accessed on 19 December 2021).
- Kadam, S.T.; Kim, S.S. Catalyst-free silylation of alcohols and phenols by promoting HMDS in CH3NO2 as solvent. Green Chem. 2010, 12, 94–98. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://webbook.nist.gov/chemistry/gc-ri/ (accessed on 23 April 2023).
- Zhong, L.; Wang, Y.; Peng, W.; Liu, Y.; Wan, J.; Yang, S.; Li, L.; Wu, C.; Zhou, X. Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometric Analysis of Volatile Components of Raw and Stir-Fried Fruit of C. pinnatifida (FCP). Trop. J. Pharm. Res. 2015, 14, 891. [Google Scholar] [CrossRef]
- Hammer, Ø.H.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 23 April 2023).
- Buhrmester, R.A.; Ebinger, J.E.; Seigler, D.S. Sambunigrin and cyanogenic variability in populations of Sambucus canadensis L. (Caprifoliaceae). Biochem. Syst. Ecol. 2000, 28, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.M.; Vecchies, A.C.; Woodrow, I.E. Cyanogenic Eucalyptus nobilis is polymorphic for both prunasin and specific β-glucosidases. Phytochemistry 2003, 63, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.S.; Ali, L.; Shaheen, F. Phytochemical Investigations on Sorbus Cashmiriana: Isolation, Structure Elucidation and Biological Evaluation of the Chemical Constituents; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2013; p. 84. [Google Scholar]
- Pastor, K.; Ačanski, M.; Vujić, Đ.; Jovanović, Đ.; Wienkoop, S. Authentication of Cereal Flours by Multivariate Analysis of GC–MS Data. Chromatographia 2016, 79, 1387–1393. [Google Scholar] [CrossRef]
- He, X.; Wang, S.; Shi, J.; Sun, Z.; Lei, Z.; Yin, Z.; Qian, Z.; Tang, H.; Xie, H. Genotypic and Environmental Effects on the Volatile Chemotype of Valeriana jatamansi Jones. Front. Plant Sci. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M. Chemotaxonomic significance of leaf wax alkanes in the gramineae. Biochem. Syst. Ecol. 1996, 24, 53–64. [Google Scholar] [CrossRef]
- Kegg Pathway Database. Available online: https://www.kegg.jp/kegg/pathway.html (accessed on 23 April 2023).
- Sánchez-Pérez, R.; Jørgensen, K.; Olsen, C.E.; Dicenta, F.; Møller, B.L. Bitterness in almonds. Plant Physiol. 2008, 146, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Biała, W.; Jasiński, M. The Phenylpropanoid Case—It Is Transport That Matters. Front. Plant Sci. 2018, 9, 1610. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, W.; McLean, J.; Taylor, G.R. Triterpenoids in the bark of mountain ash (Sorbus aucuparia L.). J. Chem. Soc. 1960, 4303–4308. [Google Scholar] [CrossRef]
- Radulović, N.S.; Đorđević, N.D. Steroids from poison hemlock (Conium maculatum L.): A GC–MS analysis. J. Serb. Chem. Soc. 2011, 76, 1471–1483. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Kuo, Y.-H. Two novel α-tocopheroids from the aerial roots of Ficus microcarpa. Tetrahedron Lett. 2003, 44, 5125–5128. [Google Scholar] [CrossRef]
- De Carvalho, T.C.; Polizeli, A.M.; Turatti, I.C.C.; Severiano, M.E.; de Carvalho, C.E.; Ambrósio, S.R.; Crotti, A.E.M.; de Figueiredo, U.S.; Vieira, P.C.; Furtado, N.A.J.C. Screening of Filamentous Fungi to Identify Biocatalysts for Lupeol Biotransformation. Molecules 2010, 15, 6140–6151. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press; Taylor and Francis Group: Boca Raton, FL, USA, 2008; 336p, Available online: https://www.mn-net.com/tabid/10234/default.aspx (accessed on 23 April 2023). [CrossRef]
- Challice, J.S. Phenolic Compounds of The Subfamily Pomoideae: A Chemotaxonomic Survey. Phytochemistry 1973, 12, 1095–1101. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Podolak, I. Ethnopharmacologically important but underestimated genus Sorbus: A comprehensive review. Phytochem. Rev. 2020, 19, 491–526. [Google Scholar] [CrossRef]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Biol. 2014, 52, 855–866. [Google Scholar] [CrossRef]
- Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants 2020, 9, 813. [Google Scholar] [CrossRef]
- Santos, C.C.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; De Almeida, A.A.C.; De Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; De Freitas, R.M.; De Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. J. Neurosci. 2013, 2013, 949452. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Kim, B.Y.; Osada, H.; Ahn, J.H. Inhibition of protein tyrosine phosphatase 1B by lupeol and lupenone isolated from Sorbus commixta. J. Enzym. Inhib. Med. Chem. 2009, 24, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Melo, C.M.; Morais, T.C.; Tomé, A.R. Anti-inflammatory effect of α,β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. Inflamm. Res. 2011, 60, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; An, H.-J. β-sitosteryl-3-O-β-glucopyranoside isolated from the bark of Sorbus commixta ameliorates pro-inflammatory mediators in RAW 264.7 macrophages. Immunopharmacol. Immunotoxicol. 2014, 36, 70–77. [Google Scholar] [CrossRef]
- Alexander, J.; Autrup, H.; Bard, D.; Benford, D.; Carere, A.; Costa, L.G.; Verger, P. Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to cyanogenic compounds as undesirable substances in animal feed. EFSA J. 2007, 434, 1–67. [Google Scholar] [CrossRef]
- Cheeke, P.R. Toxicants of Plant Origin: Glycosides; CRC Press: Boca Raton, FL, USA, 1989; Volume 2, p. 137. [Google Scholar]
- Patton, C.A.; Ranney, T.G.; Burton, J.B. Natural pest resistance of Prunus taxa to feeding by adult Japanese beetles: Role of endogenous allelochemicals in host resistance. J. Am. Hortic. Sci. 1997, 122, 668–672. [Google Scholar] [CrossRef]

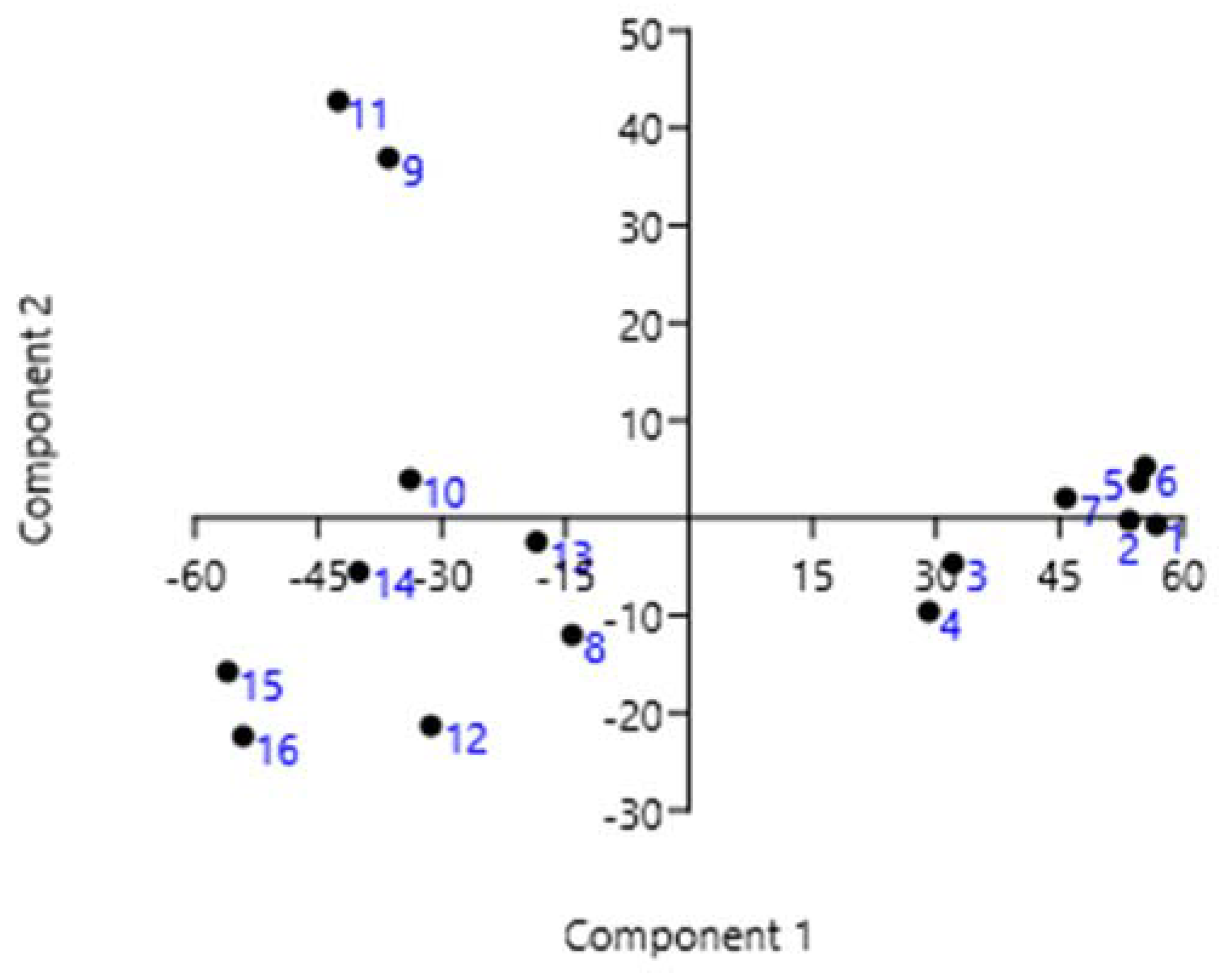

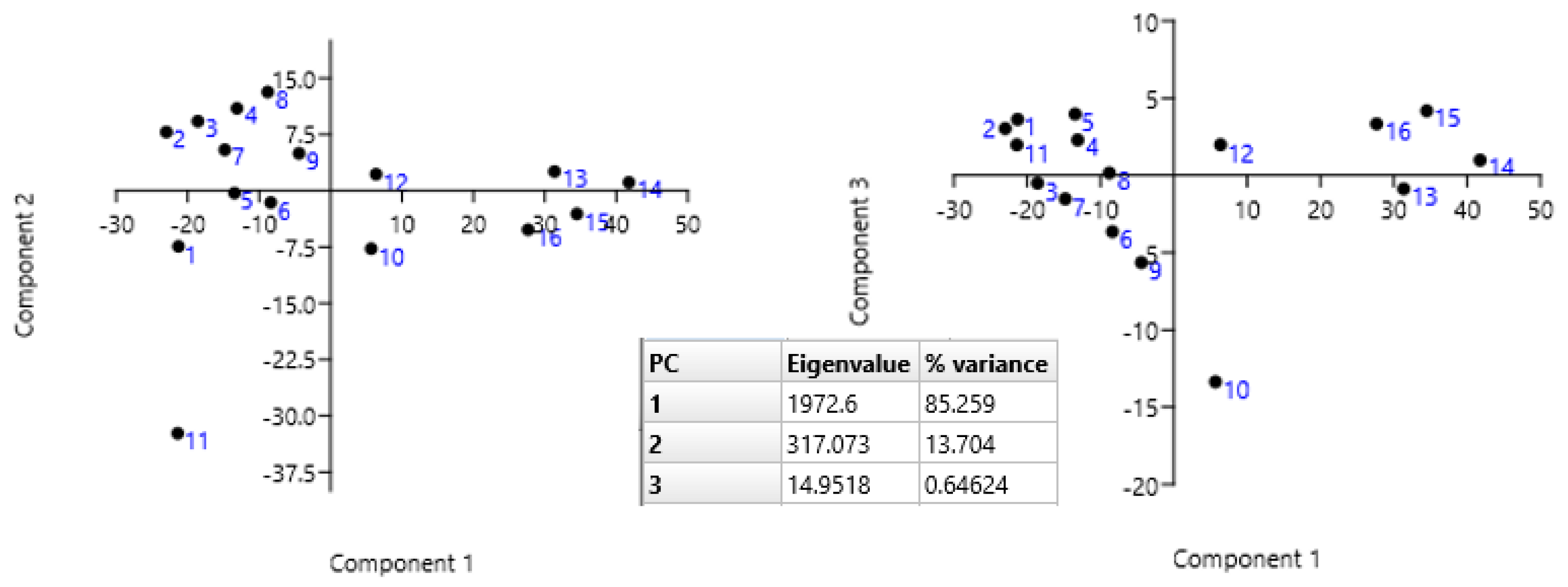
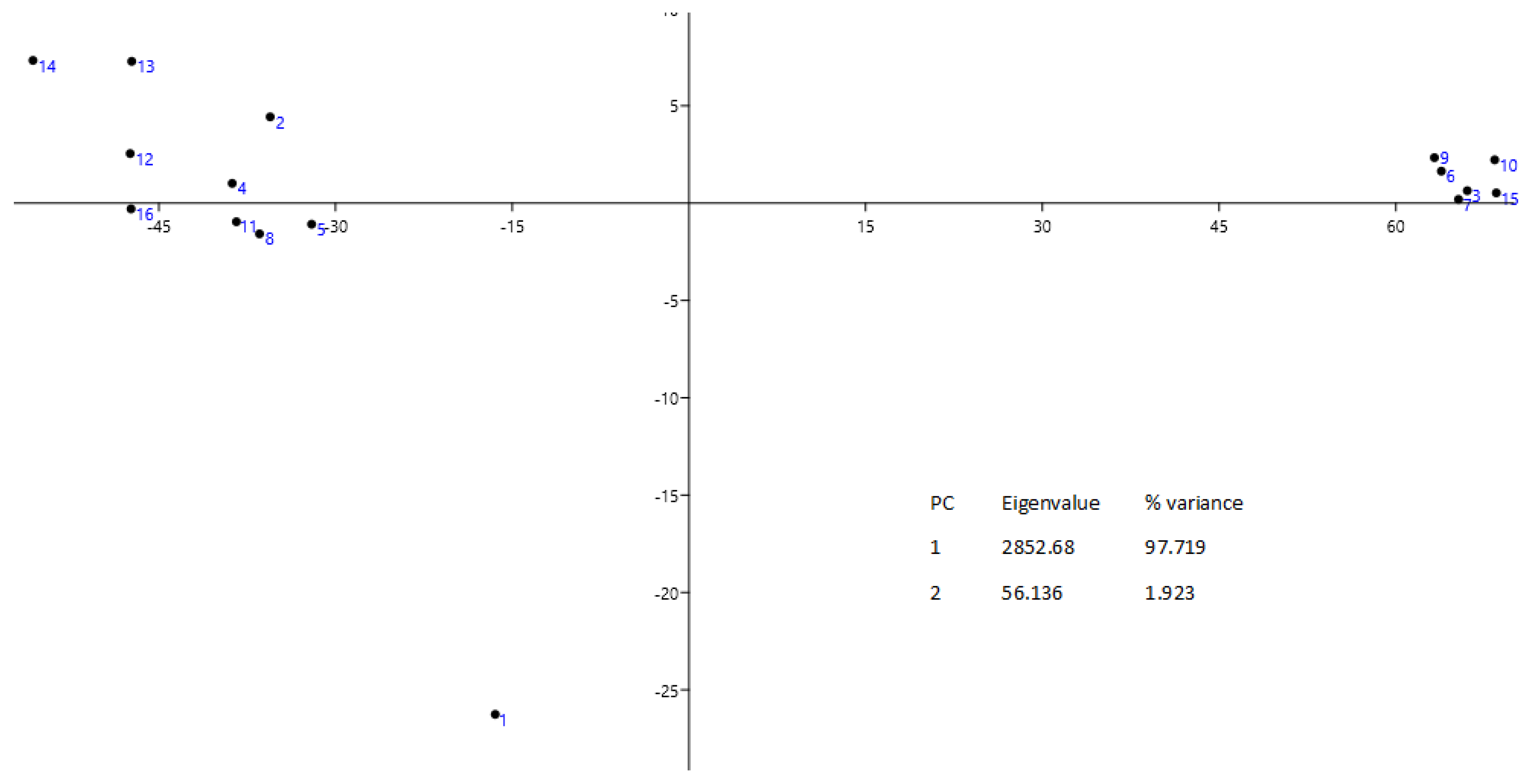
| CC | PhPC | PhC | PhSC | UFAC | AC | |
|---|---|---|---|---|---|---|
| 1 | 29.99 | 2.02 | 10.28 | 29.97 | 6.96 | 15.04 |
| 2 | 42.91 | 1.46 | 7.55 | 35.88 | 0.56 | 10.19 |
| 3 | 41 | 3.3 | 5.83 | 39.7 | 2.15 | 6.68 |
| 4 | 39.32 | 1.51 | 4.15 | 45.17 | 0.34 | 9.38 |
| 5 | 31.69 | 1.8 | 10.17 | 40.22 | 0.86 | 12.96 |
| 6 | 27.88 | 7.29 | 12.38 | 43.63 | 1.06 | 5.92 |
| 7 | 35.58 | 5.5 | 4.06 | 40.86 | 2.64 | 10.03 |
| 8 | 38.05 | 2.09 | 2.64 | 49.23 | 0.66 | 6.26 |
| 9 | 28.9 | 7.78 | 4.53 | 48.54 | 0.68 | 5.17 |
| 10 | 13.29 | 16.52 | 5.84 | 50.84 | 3.33 | 6.48 |
| 11 | 13.53 | 7.56 | 26.15 | 20.18 | 7.67 | 18.84 |
| 12 | 21.5 | 1.85 | 5.27 | 56.95 | 2.23 | 10.39 |
| 13 | 7.61 | 1.84 | 6.57 | 77.24 | 0.15 | 3.04 |
| 14 | 0.78 | 0.27 | 6.42 | 85.26 | 0.06 | 4.18 |
| 15 | 2.19 | 0.22 | 7.08 | 77.99 | 0.04 | 11.29 |
| 16 | 4.21 | 1.59 | 7.41 | 71.21 | 0.04 | 12.54 |
| CC | PhPC | PhC | PhSC | UFAC | AC | |
|---|---|---|---|---|---|---|
| 1 | 85.87 | 1.72 | 3.27 | 4.35 | 0.45 | 3.71 |
| 2 | 84.13 | 0.94 | 5.35 | 6.98 | 0.34 | 3.73 |
| 3 | 69.21 | 1.53 | 8.12 | 22.95 | 0.09 | 2.94 |
| 4 | 64.52 | 1.23 | 1.7 | 24.09 | 0.4 | 0.85 |
| 5 | 84.3 | 1.79 | 8.17 | 4.04 | 0.62 | 3.12 |
| 6 | 83.7 | 3.45 | 8.18 | 1.69 | 0.54 | 0.96 |
| 7 | 77.76 | 2.72 | 9.09 | 10.13 | 0.66 | 2.95 |
| 8 | 31.52 | 1.68 | 11.17 | 50.69 | 0.72 | 4.76 |
| 9 | 5.38 | 2.38 | 55.26 | 34.8 | 0.05 | 1.31 |
| 10 | 13.95 | 1.89 | 29.13 | 53.4 | 0.04 | 0.88 |
| 11 | 0.12 | 0.01 | 61.93 | 35.43 | 0.04 | 1.07 |
| 12 | 21.07 | 1.2 | 9.1 | 67.54 | 0.06 | 0.57 |
| 13 | 28.41 | 9.38 | 21.54 | 49.84 | 0.42 | 1.93 |
| 14 | 2.49 | 0 | 15.26 | 53.65 | 0 | 1.97 |
| 15 | 0.19 | 0.03 | 19.18 | 77.7 | 0 | 2.98 |
| 16 | 0.12 | 0 | 11.19 | 77.31 | 0 | 10.71 |
| RI | Mol. Peak (m/z) | Main Fragments Observed, m/z (Relative Abundance, %) |
|---|---|---|
| 2604 | 318 | 318 (36), 207 (100), 190 (43), 189 (79), 135 (49), 121 (35), 109 (34), 108 (51), 95 (62), 81 (36) |
| 2787 | 334 | 248 (45), 247 (54), 207 (100), 189 (36), 135 (47), 121 (44), 107 (42), 95 (43), 81 (40), 55 (34) |
| 2801 | 332 | 332 (22), 314 (17), 299 (22), 207 (100), 206 (32), 192 (15), 189 (18), 135 (20), 124 (35), 97 (68) |
| 2804 | 334 | 208 (45), 207 (100), 190 (40), 189 (45), 135 (32), 111 (22), 108 (51), 107 (24), 97 (76), 55 (24) |
| 2831 | 332 | 207 (100), 206 (28), 191 (25), 189 (26), 135 (28), 124 (28), 123 (23), 121 (22), 107 (22), 97 (92) |
| 2914 | 358 | 317 (74), 299 (100), 207 (72), 191 (51), 189 (52), 135 (37), 109 (38), 95 (57), 81 (33), 43 (46) |
| PC 1 | PC 2 | PC 3 | PC 4 | PC 5 | PC 6 | |
|---|---|---|---|---|---|---|
| CC | 805.84 | −162.3 | 545.64 | 118.75 | −111.62 | −1.4901 |
| PhPrC | 8.7344 | 7.693 | 267.77 | −515.57 | 812.86 | −40.083 |
| PHC | −225.62 | 803.7 | 522.41 | 155.08 | −78.748 | 3.1565 |
| PStC | −547.35 | −569.45 | 593.17 | 93.287 | −124.76 | 3.9015 |
| UAC | 4.3085 | −1.1393 | 6.4272 | −8.6889 | 41.601 | 999.07 |
| AC | −6.1579 | −58.249 | −76.042 | 829.01 | 550.73 | −15.273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilieva, A.R.; Slynko, N.M.; Tatarova, L.E.; Efimov, V.M.; Kuibida, L.V.; Asbaganov, S.V.; Peltek, S.E. A GC-MS Chemotaxonomic Study on Lipophilic Compounds in the Bark of S. aucuparia subsp. sibirica Trees from the Population Growing in Akademgorodok, Novosibirsk (Russia). Metabolites 2023, 13, 768. https://doi.org/10.3390/metabo13060768
Vasilieva AR, Slynko NM, Tatarova LE, Efimov VM, Kuibida LV, Asbaganov SV, Peltek SE. A GC-MS Chemotaxonomic Study on Lipophilic Compounds in the Bark of S. aucuparia subsp. sibirica Trees from the Population Growing in Akademgorodok, Novosibirsk (Russia). Metabolites. 2023; 13(6):768. https://doi.org/10.3390/metabo13060768
Chicago/Turabian StyleVasilieva, Asya R., Nikolay M. Slynko, Ljudmila E. Tatarova, Vadim M. Efimov, Leonid V. Kuibida, Sergey V. Asbaganov, and Sergey E. Peltek. 2023. "A GC-MS Chemotaxonomic Study on Lipophilic Compounds in the Bark of S. aucuparia subsp. sibirica Trees from the Population Growing in Akademgorodok, Novosibirsk (Russia)" Metabolites 13, no. 6: 768. https://doi.org/10.3390/metabo13060768
APA StyleVasilieva, A. R., Slynko, N. M., Tatarova, L. E., Efimov, V. M., Kuibida, L. V., Asbaganov, S. V., & Peltek, S. E. (2023). A GC-MS Chemotaxonomic Study on Lipophilic Compounds in the Bark of S. aucuparia subsp. sibirica Trees from the Population Growing in Akademgorodok, Novosibirsk (Russia). Metabolites, 13(6), 768. https://doi.org/10.3390/metabo13060768






