Some Lessons Learned on the Impact of the Storage Conditions, Syringe Wash Solvent, and the Way of GC-MS Injection on the Reproducibility of Metabolomic Studies
Abstract
1. Introduction
2. Materials and Methods
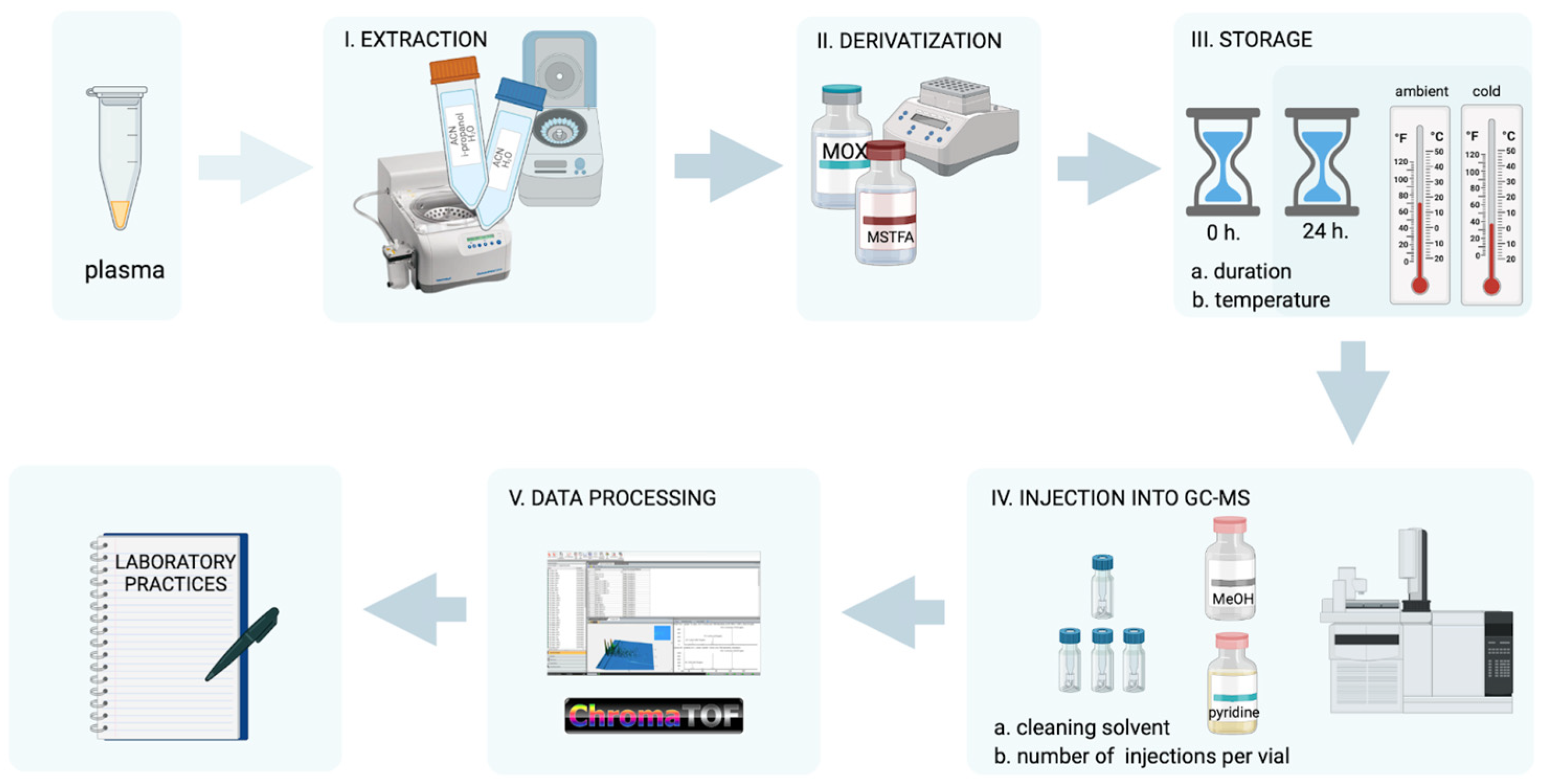
2.1. Design of the Experiment
2.2. Chemicals
2.3. Sample Preparation
2.4. GC×GC-MS Analysis
2.5. Data Processing
3. Results and Discussion
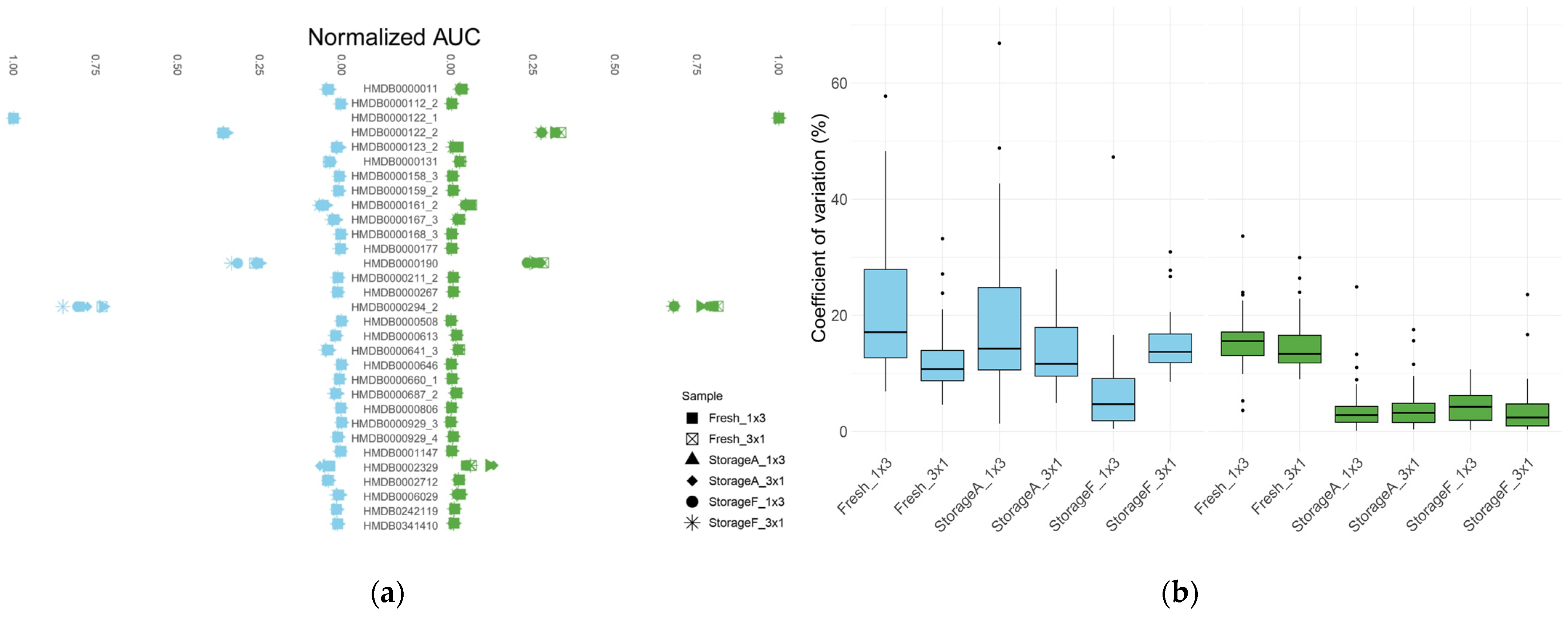
3.1. Effect of Storage
3.2. Effect of Solvent
3.3. Effect of Vial
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moldoveanu, S.C.; David, V. Derivatization Methods in GC and GC/MS. In Gas Chromatography—Derivatization, Sample Preparation, Application; Kusch, P., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83881-865-4. [Google Scholar]
- Kiseleva, O.; Kurbatov, I.; Ilgisonis, E.; Poverennaya, E. Defining Blood Plasma and Serum Metabolome by GC-MS. Metabolites 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Orata, F. Derivatization Reactions and Reagents for Gas Chromatography Analysis. In Advanced Gas Chromatography—Progress in Agricultural, Biomedical and Industrial Applications; Ali Mohd, M., Ed.; InTech: Rang-Du-Fliers, France, 2012; ISBN 978-953-51-0298-4. [Google Scholar]
- Kanani, H.H.; Klapa, M.I. Data Correction Strategy for Metabolomics Analysis Using Gas Chromatography–Mass Spectrometry. Metab. Eng. 2007, 9, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Jia, N. Formation of Multiple Trimethylsilyl Derivatives in the Derivatization of 17α-Ethinylestradiol with BSTFA or MSTFA Followed by Gas Chromatography-Mass Spectrometry Determination. J. Environ. Sci. 2007, 19, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of Recent Developments in GC–MS Approaches to Metabolomics-Based Research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography–Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 21.33.1–21.33.11. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass Spectral and Retention Index Libraries for Metabolomics Based on Quadrupole and Time-of-Flight Gas Chromatography/Mass Spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Smoluch, M.; Piechura, K. Basic Definitions. In Mass Spectrometry; Smoluch, M., Grasso, G., Suder, P., Silberring, J., Eds.; Wiley: New York, NY, USA, 2019; pp. 9–12. ISBN 978-1-119-37730-6. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 22 December 2022).
- Jones, O.A.H. Illuminating the Dark Metabolome to Advance the Molecular Characterisation of Biological Systems. Metabolomics 2018, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- Almstetter, M.F.; Appel, I.J.; Gruber, M.A.; Lottaz, C.; Timischl, B.; Spang, R.; Dettmer, K.; Oefner, P.J. Integrative Normalization and Comparative Analysis for Metabolic Fingerprinting by Comprehensive Two-Dimensional Gas Chromatography−Time-of-Flight Mass Spectrometry. Anal. Chem. 2009, 81, 5731–5739. [Google Scholar] [CrossRef] [PubMed]
- Almstetter, M.F.; Appel, I.J.; Dettmer, K.; Gruber, M.A.; Oefner, P.J. Comparison of Two Algorithmic Data Processing Strategies for Metabolic Fingerprinting by Comprehensive Two-Dimensional Gas Chromatography–Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2011, 1218, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, K.; Almstetter, M.F.; Appel, I.J.; Nürnberger, N.; Schlamberger, G.; Gronwald, W.; Meyer, H.H.D.; Oefner, P.J. Comparison of Serum versus Plasma Collection in Gas Chromatography—Mass Spectrometry-Based Metabolomics. Electrophoresis 2010, 31, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
| Compound | Modifications | HMDB ID | Subclass | Retention Time, s | Actual Masses (XIC), m/z | |
|---|---|---|---|---|---|---|
| 1D | 2D | |||||
| 3-Hydroxybutyric acid | 2TMS | HMDB0000011 | Beta hydroxy acids and derivatives | 497.99 | 1.95 | 73 |
| 4-Aminobutanoic acid | 2TMS | HMDB0000112 | Amino acids, peptides, and analogues | 509.99 | 1.91 | 130 |
| D-Glucose | MOX. 5TMS | HMDB0000122 | Carbohydrates and carbohydrate conjugates | 1053.95 | 1.79 | 73 |
| D-Glucose | MOX, 5TMS | HMDB0000122 | Carbohydrates and carbohydrate conjugates | 1065.95 | 1.80 | 73 |
| Glycine | 2TMS | HMDB0000123 | Amino acids, peptides, and analogues | 461.99 | 1.99 | 102 |
| Glycerol | 3TMS | HMDB0000131 | Carbohydrates and carbohydrate conjugates | 601.98 | 1.79 | 73 |
| L-Tyrosine | 3TMS | HMDB0000158 | Amino acids, peptides, and analogues | 1069.95 | 2.19 | 73 |
| L-Phenylalanine | 2TMS | HMDB0000159 | Amino acids, peptides, and analogues | 869.97 | 2.30 | 73 |
| L-Alanine | 2TMS | HMDB0000161 | Amino acids, peptides, and analogues | 449.99 | 1.89 | 116 |
| L-Threonine | 3TMS | HMDB0000167 | Amino acids, peptides, and analogues | 693.98 | 1.87 | 73 |
| L-Asparagine | 3TMS | HMDB0000168 | Amino acids, peptides, and analogues | 901.97 | 2.18 | 73 |
| L-Histidine | 3TMS | HMDB0000177 | Amino acids, peptides, and analogues | 1061.95 | 2.46 | 73 |
| Lactic acid | 2TMS | HMDB0000190 | Alpha hydroxy acids and derivatives | 410.00 | 1.90 | 73 |
| Inositol | 6TMS | HMDB0000211 | Alcohols and polyols | 1165.95 | 1.78 | 73 |
| Pidolic acid | 2TMS | HMDB0000267 | Amino acids, peptides, and analogues | 793.97 | 2.57 | 73 |
| Urea | 2TMS | HMDB0000294 | Ureas | 561.99 | 2.43 | 171 |
| Ribitol | 5TMS | HMDB0000508 | Carbohydrates and carbohydrate conjugates | 941.96 | 1.71 | 73 |
| Erythronic acid | TMS | HMDB0000613 | Carbohydrates and carbohydrate conjugates | 825.97 | 1.84 | 73 |
| L-Glutamine | 3TMS | HMDB0000641 | Amino acids, peptides, and analogues | 965.96 | 2.22 | 73 |
| β-L-Arabinose | MOX, 4TMS | HMDB0000646 | Carbohydrates and carbohydrate conjugates | 901.97 | 1.80 | 73 |
| D-Fructose | MOX, 5TMS | HMDB0000660 | Carbohydrates and carbohydrate conjugates | 1037.96 | 1.78 | 73 |
| L-Leucine | 2TMS | HMDB0000687 | Amino acids, peptides, and analogues | 597.98 | 1.90 | 158 |
| Tetradecanoic acid | TMS | HMDB0000806 | Fatty acids and conjugates | 1005.96 | 2.15 | 73 |
| L-Tryptophan | 2TMS | HMDB0000929 | Indolyl carboxylic acids and derivatives | 1221.94 | 3.14 | 73 |
| L-Tryptophan | 3TMS | HMDB0000929 | Indolyl carboxylic acids and derivatives | 1229.94 | 2.57 | 73 |
| Aminomalonic acid | MOX, TMS | HMDB0001147 | Amino acids, peptides, and analogues | 757.97 | 2.11 | 73 |
| Oxalic acid | 2TMS | HMDB0002329 | Dicarboxylic acids and derivatives | 473.99 | 2.21 | 73 |
| 1,5-Anhydrosorbitol | 4TMS | HMDB0002712 | Carbohydrates and carbohydrate conjugates | 1021.96 | 1.95 | 73 |
| N2-Acetyl-L-glutamine | 4TMS | HMDB0006029 | Amino acids, peptides, and analogues | 941.96 | 1.84 | 73 |
| D-Mannonic acid | 4TMS | HMDB0242119 | Medium-chain hydroxy acids and derivatives | 1085.95 | 1.99 | 73 |
| 2-Hydroxybutanoic acid | 2TMS | HMDB0341410 | Alpha hydroxy acids and derivatives | 469.99 | 1.94 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurbatov, I.; Kiseleva, O.; Arzumanian, V.; Dolgalev, G.; Poverennaya, E. Some Lessons Learned on the Impact of the Storage Conditions, Syringe Wash Solvent, and the Way of GC-MS Injection on the Reproducibility of Metabolomic Studies. Metabolites 2023, 13, 75. https://doi.org/10.3390/metabo13010075
Kurbatov I, Kiseleva O, Arzumanian V, Dolgalev G, Poverennaya E. Some Lessons Learned on the Impact of the Storage Conditions, Syringe Wash Solvent, and the Way of GC-MS Injection on the Reproducibility of Metabolomic Studies. Metabolites. 2023; 13(1):75. https://doi.org/10.3390/metabo13010075
Chicago/Turabian StyleKurbatov, Ilya, Olga Kiseleva, Viktoriia Arzumanian, Georgii Dolgalev, and Ekaterina Poverennaya. 2023. "Some Lessons Learned on the Impact of the Storage Conditions, Syringe Wash Solvent, and the Way of GC-MS Injection on the Reproducibility of Metabolomic Studies" Metabolites 13, no. 1: 75. https://doi.org/10.3390/metabo13010075
APA StyleKurbatov, I., Kiseleva, O., Arzumanian, V., Dolgalev, G., & Poverennaya, E. (2023). Some Lessons Learned on the Impact of the Storage Conditions, Syringe Wash Solvent, and the Way of GC-MS Injection on the Reproducibility of Metabolomic Studies. Metabolites, 13(1), 75. https://doi.org/10.3390/metabo13010075