Role of Gamma-Aminobutyric Acid in Plant Defense Response
Abstract
1. Introduction
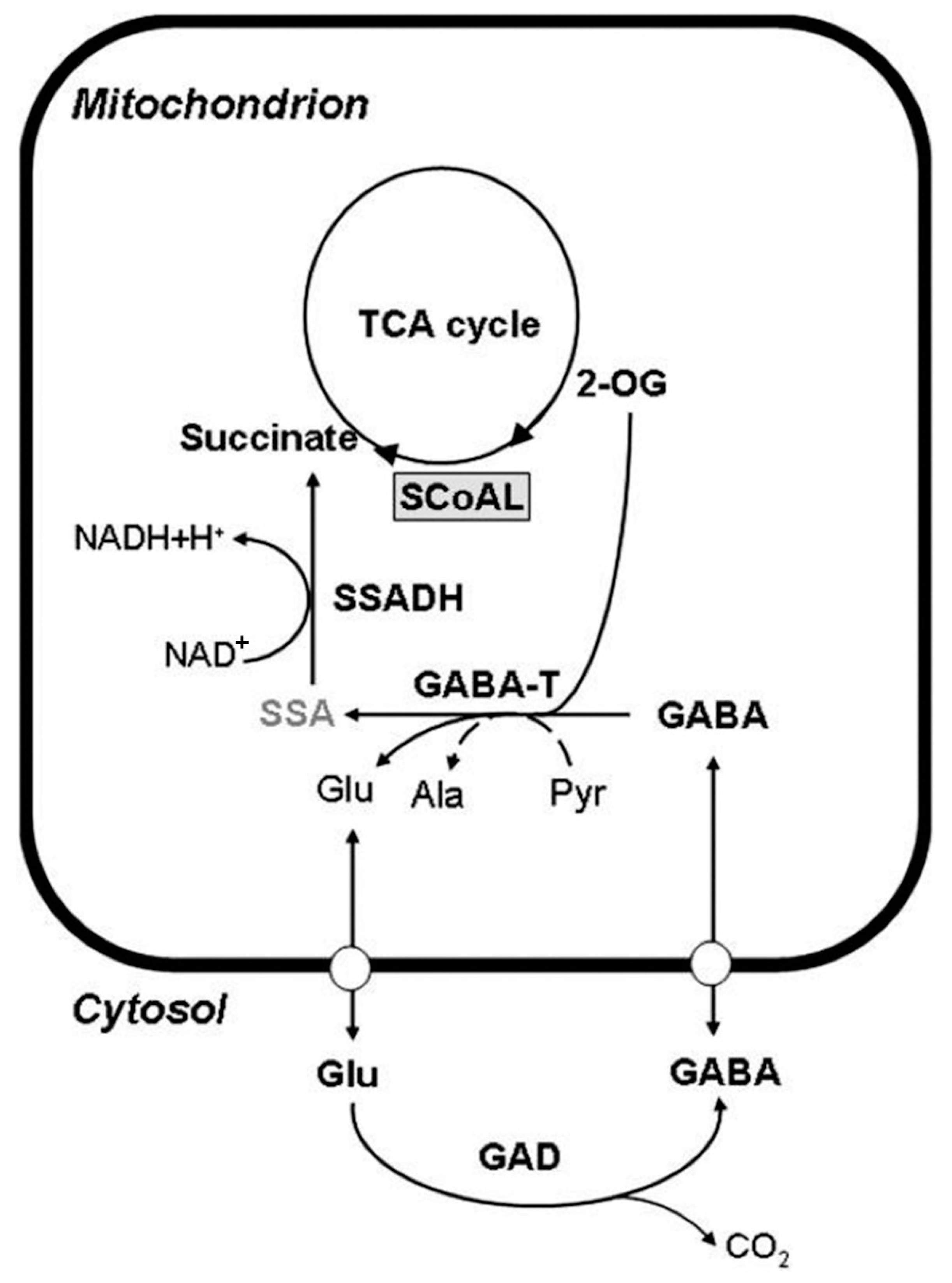
2. Synthesis and Metabolism of GABA
3. GABA Is Involved in Plant Metabolism
4. GABA Regulates Intracellular pH
5. GABA Is Involved in the Antioxidant System
6. GABA and Ca2+
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wali, M.; Gunse, B.; Llugany, M.; Corrales, I.; Abdelly, C.; Poschenrieder, C.; Ghnaya, T. High salinity helps the halophyte Sesuvium portulacastrum in defense against Cd toxicity by maintaining redox balance and photosynthesis. Planta 2016, 244, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, L.P.; Signorelli, S.; Hofte, M. gamma-Aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, Y.; Huang, B. Alteration of Transcripts of Stress-Protective Genes and Transcriptional Factors by gamma-Aminobutyric Acid (GABA) Associated with Improved Heat and Drought Tolerance in Creeping Bentgrass (Agrostis stolonifera). Int. J. Mol. Sci. 2018, 19, 61623. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Yuan, P.; Tanaka, K.; Poovaiah, B.W. Calcium/Calmodulin-Mediated Defense Signaling: What Is Looming on the Horizon for AtSR1/CAMTA3-Mediated Signaling in Plant Immunity. Front. Plant Sci. 2021, 12, 795353. [Google Scholar] [CrossRef]
- Yadav, M.; Pandey, J.; Chakraborty, A.; Hassan, M.I.; Kundu, J.K.; Roy, A.; Singh, I.K.; Singh, A. A Comprehensive Analysis of Calmodulin-Like Proteins of Glycine max Indicates Their Role in Calcium Signaling and Plant Defense Against Insect Attack. Front. Plant Sci. 2022, 13, 817950. [Google Scholar] [CrossRef]
- Li, K.X.; He, M.; Ye, W.; Simms, J.; Gill, M.; Xiang, X.; Jan, Y.N.; Jan, L.Y. TMEM16B regulates anxiety-related behavior and GABAergic neuronal signaling in the central lateral amygdala. eLife 2019, 8. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse role of gamma-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA signalling modulates stomatal opening to enhance plant water use efficiency and drought resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. gamma-Aminobutyric acid (GABA) signalling in plants. Cell Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Fromm, H. GABA signaling in plants: Targeting the missing pieces of the puzzle. J. Exp. Bot. 2020, 71, 6238–6245. [Google Scholar] [CrossRef] [PubMed]
- Studart-Guimaraes, C.; Fait, A.; Nunes-Nesi, A.; Carrari, F.; Usadel, B.; Fernie, A.R. Reduced expression of succinyl-coenzyme A ligase can be compensated for by up-regulation of the gamma-aminobutyrate shunt in illuminated tomato leaves. Plant Physiol. 2007, 145, 626–639. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 1945. [Google Scholar] [CrossRef]
- Wuddineh, W.; Minocha, R.; Minocha, S.C. Polyamines in the Context of Metabolic Networks. Methods Mol. Biol. 2018, 1694, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Podlesakova, K.; Ugena, L.; Spichal, L.; Dolezal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. N. Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in plant growth, development and senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Gut, H.; Dominici, P.; Pilati, S.; Astegno, A.; Petoukhov, M.V.; Svergun, D.I.; Grutter, M.G.; Capitani, G. A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase. J. Mol. Biol. 2009, 392, 334–351. [Google Scholar] [CrossRef]
- Astegno, A.; Capitani, G.; Dominici, P. Functional roles of the hexamer organization of plant glutamate decarboxylase. Biochim. Biophys. Acta 2015, 1854, 1229–1237. [Google Scholar] [CrossRef]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lu, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na(+) uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A Brief Review on the Non-protein Amino Acid, Gamma-amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Molina-Rueda, J.J.; Pascual, M.B.; Canovas, F.M.; Gallardo, F. Characterization and developmental expression of a glutamate decarboxylase from maritime pine. Planta 2010, 232, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Kilian, J.; Whitehead, D.; Horak, J.; Wanke, D.; Weinl, S.; Batistic, O.; D’Angelo, C.; Bornberg-Bauer, E.; Kudla, J.R.; Harter, K.J.P.J. The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2010, 50, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zheng, L.; Yue, J.; Yao, X.; Chang, E.; Xie, T.; Deng, N.; Chen, L.; Huang, Y.; Jiang, Z.; et al. Identification of two CiGADs from Caragana intermedia and their transcriptional responses to abiotic stresses and exogenous abscisic acid. PeerJ 2017, 5, e3439. [Google Scholar] [CrossRef]
- Narsai, R.; Howell, K.A.; Carroll, A.; Ivanova, A.; Millar, A.H.; Whelan, J. Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Physiol. 2009, 151, 306–322. [Google Scholar] [CrossRef]
- Huang, H.; He, Y.; Cui, A.; Sun, L.; Han, M.; Wang, J.; Rui, C.; Lei, Y.; Liu, X.; Xu, N.; et al. Genome-wide identification of GAD family genes suggests GhGAD6 functionally respond to Cd(2+) stress in cotton. Front. Genet. 2022, 13, 965058. [Google Scholar] [CrossRef]
- Clark, S.M.; Di, L.R.; Van, C.O.R.; Mullen, R.T.; Shelp, B.J. Subcellular localization and expression of multiple tomato γ-aminobutyrate transaminases that utilize both pyruvate and glyoxylate. J. Exp. Bot. 2009, 60, 3255–3267. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
- Akihiro, T.; Koike, S.; Tani, R.; Tominaga, T.; Watanabe, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Matsukura, C.; et al. Biochemical mechanism on GABA accumulation during fruit development in tomato. Plant Cell Physiol. 2008, 49, 1378–1389. [Google Scholar] [CrossRef]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef]
- Deleu, C. GABA Accumulation Causes Cell Elongation Defects and a Decrease in Expression of Genes Encoding Secreted and Cell Wall-Related Proteins in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 894–908. [Google Scholar]
- Ji, J.; Shi, Z.; Xie, T.; Zhang, X.; Chen, W.; Du, C.; Sun, J.; Yue, J.; Zhao, X.; Jiang, Z.; et al. Responses of GABA shunt coupled with carbon and nitrogen metabolism in poplar under NaCl and CdCl(2) stresses. Ecotoxicol. Environ. Saf. 2020, 193, 110322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Z.; Fan, Y.; Liu, C.; Wang, H.; Li, Y.; Xin, Y.; Gai, Y.; Ji, X. Characterization of GABA-Transaminase Gene from Mulberry (Morus multicaulis) and Its Role in Salt Stress Tolerance. Genes 2022, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S.; et al. GABA operates upstream of H+-ATPase and improves salinity tolerance in Arabidopsis by enabling cytosolic K+ retention and Na+ exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef]
- Ji, J.; Yue, J.; Xie, T.; Chen, W.; Du, C.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Roles of gamma-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: Involving in abscisic acid and ethylene-signalling pathways. Planta 2018, 248, 675–690. [Google Scholar] [CrossRef]
- Bandehagh, A.; Taylor, N.L. Can Alternative Metabolic Pathways and Shunts Overcome Salinity Induced Inhibition of Central Carbon Metabolism in Crops? Front. Plant Sci. 2020, 11, 1072. [Google Scholar] [CrossRef]
- Elisabetta, M.; Alfredo, T.; Luigi, C.; Giuseppe, F. Metabolism of γ-aminobutyric acid during cold acclimation and freezing and its relationship to frost tolerance in barley and wheat. J. Exp. Bot. 2006, 57, 3755. [Google Scholar]
- Fedorin, D.N.; Eprintsev, A.T.; Florez Caro, O.J.; Igamberdiev, A.U. Effect of Salt Stress on the Activity, Expression, and Promoter Methylation of Succinate Dehydrogenase and Succinic Semialdehyde Dehydrogenase in Maize (Zea mays L.) Leaves. Plants 2022, 12, 68. [Google Scholar] [CrossRef]
- Andreasson, C.; Ott, M.; Buttner, S. Mitochondria orchestrate proteostatic and metabolic stress responses. EMBO Rep. 2019, 20, e47865. [Google Scholar] [CrossRef]
- Nehela, Y.; Killiny, N. Not Just a Cycle: Three gab Genes Enable the Non-Cyclic Flux Toward Succinate via GABA Shunt in 'Candidatus Liberibacter asiaticus'-Infected Citrus. Mol. Plant-Microbe Interact. MPMI 2022, 35, 200–214. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Lemaitre, T.; Urbanczyk-Wochniak, E.; Flesch, V.; Bismuth, E.; Hodges, F.M. NAD-Dependent Isocitrate Dehydrogenase Mutants of Arabidopsis Suggest the Enzyme Is Not Limiting for Nitrogen Assimilation. Plant Physiol. 2007, 144, 1546–1558. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Sweetlove, L.J.; Fernie, A.R. Operation and function of the tricarboxylic acid cycle in the illuminated leaf. Physiol. Plant. 2007, 129, 45–56. [Google Scholar] [CrossRef]
- Nunes-Nesi, F.C.A.; Lytovchenko, A.; Anna, M.O.; Smith, M.; Ehlers Loureiro, R.; Ratcliffe, G.; Lee, J.; Sweetlove; Fernie, A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005, 137, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Claudia Studart-Guimares, A.F. Adriano Nunes-Nesi, Fernando Carrari, Bjrn Usadel,; Fernie, A.R. Reduced Expression of Succinyl-Coenzyme A Ligase Can Be Compensated for by Up-Regulation of the γ-Aminobutyrate Shunt in Illuminated Tomato Leaves. Plant Physiology 2007, 145, 626–639. [Google Scholar] [CrossRef]
- Hijaz, F.; Killiny, N. Exogenous GABA is quickly metabolized to succinic acid and fed into the plant TCA cycle. Plant Signal Behav. 2019, 14, e1573096. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef]
- Zheng, Z.L. Carbon and nitrogen nutrient balance signaling in plants. Plant Signal Behav. 2009, 4, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ruiz, R.; Martinez, F.; Knauf-Beiter, G.; Tejada Moral, M. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Turano, F.J.; Fang, T.K. Characterization of two glutamate decarboxylase cDNA clones from Arabidopsis. Plant Physiol. 1998, 117, 1411–1421. [Google Scholar] [CrossRef]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Fait, A.; Zik, M.; Fromm, H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant. Mol. Biol. 2004, 55, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat mitochondrial respiration shifts from the tricarboxylic acid cycle to the GABA shunt under salt stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Stepansky, A.; Less, H.; Angelovici, R.; Aharon, R.; Zhu, X.; Galili, G. Lysine catabolism, an effective versatile regulator of lysine level in plants. Amino Acids 2006, 30, 121–125. [Google Scholar] [CrossRef]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Grillich, N.; Yellin, A.; Bar, D.; Khan, M.; Fernie, A.R.; Turano, F.J.; et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Bor, M.; Turkan, I. The involvement of gamma-aminobutyric acid shunt in the endoplasmic reticulum stress response of Arabidopsis thaliana. J. Plant Physiol. 2020, 253, 153250. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, N.; Liu, C.; Wang, H.; Li, Y.; Xie, Y.; Ma, F.; Liang, J.; Li, C. Exogenous GABA improves the resistance of apple seedlings to long-term drought stress by enhancing GABA shunt and secondary cell wall biosynthesis. Tree Physiol. 2022, 42, 2563–2577. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Al-Share, A.T. Characterization of the γ-aminobutyric acid shunt pathway and oxidative damage in Arabidopsis thaliana pop 2 mutants under various abiotic stresses. Biol. Plant. 2016, 60, 132–138. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ahmad, I.; Ansari, M.I. Functional loss of GABA transaminase (GABA-T) expressed early leaf senescence under various stress conditions in Arabidopsis thaliana. Curr. Plant Biol. 2017, 9–10, 11–22. [Google Scholar] [CrossRef]
- Uzma Jalil, S.; Khan, M.I.R.; Ansari, M.I. Role of GABA transaminase in the regulation of development and senescence in Arabidopsis thaliana. Curr. Plant Biol. 2019, 19, 100119. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Li, C.; Niu, M.; Dong, H.; Zhao, S.; Jia, C.; Xu, Y. Accelerated Accumulation of gamma-Aminobutyric Acid and Modifications on Its Metabolic Pathways in Black Rice Grains by Germination under Cold Stress. Foods 2023, 12, 1290. [Google Scholar] [CrossRef]
- Al-Quraan, N.A.; Al-Ajlouni, Z.I.; Obedat, D.I. The GABA shunt pathway in germinating seeds of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) under salt stress. Seed Sci. Res. 2019, 29, 250–260. [Google Scholar] [CrossRef]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against abiotic stress tolerance in legumes: A review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Ansari, M.I.; Jalil, S.U.; Ansari, S.A.; Hasanuzzaman, M. GABA shunt: A key-player in mitigation of ROS during stress. Plant Growth Regul. 2021, 94, 131–149. [Google Scholar] [CrossRef]
- Zhu, X.; Liao, J.; Xia, X.; Xiong, F.; Li, Y.; Shen, J.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W. Physiological and iTRAQ-based proteomic analyses reveal the function of exogenous gamma-aminobutyric acid (GABA) in improving tea plant (Camellia sinensis L.) tolerance at cold temperature. BMC Plant Biol. 2019, 19, 43. [Google Scholar] [CrossRef]
- Carillo, P. GABA Shunt in Durum Wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Chen, W.; Meng, C.; Ji, J.; Li, M.H.; Zhang, X.; Wu, Y.; Xie, T.; Du, C.; Sun, J.; Jiang, Z.; et al. Exogenous GABA promotes adaptation and growth by altering the carbon and nitrogen metabolic flux in poplar seedlings under low nitrogen conditions. Tree Physiol. 2020, 40, 1744–1761. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, M.I. Physiological Role of Gamma-Aminobutyric Acid in Salt Stress Tolerance. In Salt and Drought Stress Tolerance in Plants; Signaling and Communication in Plants; Springer: Cham, Switzerland, 2020; pp. 337–350. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Geilfus, C.M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Kesten, C.; Gamez-Arjona, F.M.; Menna, A.; Scholl, S.; Dora, S.; Huerta, A.I.; Huang, H.Y.; Tintor, N.; Kinoshita, T.; Rep, M.; et al. Pathogen-induced pH changes regulate the growth-defense balance in plants. EMBO J. 2019, 38, e101822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Hao, D.L.; Yang, G.Z. Regulation of Cytosolic pH: The Contributions of Plant Plasma Membrane H(+)-ATPases and Multiple Transporters. Int. J. Mol. Sci. 2021, 22, 12998. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Schmidt, W. The enigma of environmental pH sensing in plants. Nat. Plants 2021, 7, 106–115. [Google Scholar] [CrossRef]
- Pittman, J.K. Multiple Transport Pathways for Mediating Intracellular pH Homeostasis: The Contribution of H+/ion Exchangers. Front. Plant Sci. 2012, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Sze, H.; Chanroj, S. Plant Endomembrane Dynamics: Studies of K(+)/H(+) Antiporters Provide Insights on the Effects of pH and Ion Homeostasis. Plant Physiol. 2018, 177, 875–895. [Google Scholar] [CrossRef]
- Wegner, L.H.; Shabala, S. Biochemical pH clamp: The forgotten resource in membrane bioenergetics. New Phytol. 2020, 225, 37–47. [Google Scholar] [CrossRef]
- Zhan, X.; Yi, X.; Yue, L.; Fan, X.; Xu, G.; Xing, B. Cytoplasmic pH-Stat during Phenanthrene Uptake by Wheat Roots: A Mechanistic Consideration. Environ. Sci. Technol. 2015, 49, 6037–6044. [Google Scholar] [CrossRef]
- Sakano, K. Metabolic regulation of pH in plant cells: Role of cytoplasmic pH in defense reaction and secondary metabolism. J. Int. Rev. Cytol. 2001, 206, 1–44. [Google Scholar]
- Kamran, M.; Ramesh, S.A.; Gilliham, M.; Tyerman, S.D.; Bose, J. Role of TaALMT1 malate-GABA transporter in alkaline pH tolerance of wheat. Plant Cell Environ. 2020, 43, 2443–2459. [Google Scholar] [CrossRef]
- Thwaites, D.T.; Basterfield, L.; Mccleave, P.M.J.; Carter, S.M.; Simmons, N.L. Gamma-aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br. J. Pharmacol. 2000, 129, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.A.; Bown, A.W.; Breitkreuz, K.E.; Guinel, F.C. The Synthesis of [gamma]-Aminobutyric Acid in Response to Treatments Reducing Cytosolic pH. Plant Physiol. 1994, 104, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.D.; Fox, G.G.; Laurie, S.; Phillips, R.; Ratcliffe, R.G.; Stewart, G.R. Ammonium Assimilation and the Role of [gamma]-Aminobutyric Acid in pH Homeostasis in Carrot Cell Suspensions. Plant Physiol. 1994, 106, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, H.; Song, Y.; Gu, Z. Effects of soaking and aeration treatment on γ-aminobutyric acid accumulation in germinated soybean (Glycine max L.). Eur. Food Res. Technol. 2011, 232, 787–795. [Google Scholar] [CrossRef]
- Yin, Y.; Cheng, C.; Fang, W. Effects of the inhibitor of glutamate decarboxylase on the development and GABA accumulation in germinating fava beans under hypoxia-NaCl stress. RSC Adv. 2018, 8, 20456–20461. [Google Scholar] [CrossRef]
- Mekonnen, D.W.; Flugge, U.I.; Ludewig, F. Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016, 245, 25–34. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress || An Introduction to Antioxidants and Their Roles in Plant Stress Tolerance; Springer: Singapore, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidans 2019, 8, 384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidans 2020, 9, 681. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, N.; Zhu, X.; Li, S.; Si, H. Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Sci. Hortic. 2020, 261, 108949. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidans 2021, 10, 277. [Google Scholar] [CrossRef]
- AL-Quraan; Nisreen, A. GABA shunt deficiencies and accumulation of reactive oxygen species under UV treatments: Insight from Arabidopsis thaliana calmodulin mutants. Acta Physiol. Plant 2015, 37, 86. [Google Scholar] [CrossRef]
- Seifi, K.M.; Aliniaeifard, S.; Mehdi, S.; Javadi, A.E.; Fran?Oise, B.; Batool, H. Enhanced salt tolerance and photosynthetic performance: Implication of -amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157. [Google Scholar]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Qu, F.; Jin, X.; Huang, N.; Wang, J.; Hu, X. Nitric oxide mediates γ-aminobutyric acid-enhanced muskmelon tolerance to salinity–alkalinity stress conditions. Sci. Hortic. 2021, 286, 110229. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Jannatizadeh, A.; Sarcheshmeh, M.A.A.; Babalar, M. Enhancement of postharvest chilling tolerance of anthurium cut flowers by γ-aminobutyric acid (GABA) treatments. Sci. Hortic. 2016, 198, 52–60. [Google Scholar] [CrossRef]
- Song, H.; Xu, X.; Wang, H.; Wang, H.; Tao, Y. Exogenous gamma-aminobutyric acid alleviates oxidative damage caused by aluminium and proton stresses on barley seedlings. J. Sci. Food Agric. 2010, 90, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011, 129, 1619–1622. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce gamma-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef]
- Xing, S.G.; Jun, Y.B.; Hau, Z.W.; Liang, L.Y. Higher accumulation of gamma-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. roots. Plant Physiol. Biochem. 2007, 45, 560–566. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendía, A.M.; Bose, J.; Zepeda-Jazo, I.; Shabala, S.; Dobrovinskaya, O. Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasma membrane: Implications for plant adaptive responses. J. Exp. Bot. 2014, 2014, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Pirayesh, N.; Giridhar, M.; Ben Khedher, A.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef]
- Kim, N.H.; Jacob, P.; Dangl, J.L. Con-Ca(2+) -tenating plant immune responses via calcium-permeable cation channels. New Phytol. 2022, 234, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ding, P. Calcium signaling in plant immunity: A spatiotemporally controlled symphony. Trends Plant Sci. 2023, 28, 74–89. [Google Scholar] [CrossRef]
- Trobacher, C.P.; Zarei, A.; Liu, J.; Clark, S.M.; Bozzo, G.G.; Shelp, B.J. Calmodulin-dependent and calmodulin-independent glutamate decarboxylases in apple fruit. BMC Plant Biol. 2013, 13, 144. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Lv, Y.G.; Zhang, H. Purification of calmodulin from rice bran and activation of glutamate decarboxylase by Ca2+/calmodulin. J. Sci. Food Agric. 2010, 90, 669–675. [Google Scholar] [CrossRef]
- Wei, Q.; Xie, K.; Wang, H.; Shao, X.; Wei, Y.; Chen, Y.; Jiang, S.; Cao, M.; Chen, J.; Xu, F. Calcium Involved in the Enrichment of gamma-Aminobutyric Acid (GABA) in Broccoli Sprouts under Fructose Treatment. Plants 2023, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, F.; Cao, S.; Wang, H.; Wei, Y.; Shao, X.; Zhou, W.; Zheng, Y. Effects of exogenous calcium chloride (CaCl2) and ascorbic acid (AsA) on the γ-aminobutyric acid (GABA) metabolism in shredded carrots. Postharvest Biol. Technol. 2019, 152, 111–117. [Google Scholar] [CrossRef]
- Chi, Z.; Dai, Y.; Cao, S.; Wei, Y.; Wang, H. Exogenous calcium chloride (CaCl2) promotes γ-aminobutyric acid (GABA) accumulation in fresh-cut pears. Postharvest Biol. Technol. 2021, 174, 111446. [Google Scholar] [CrossRef]
- Haugh-Scheidt, L.; Malchow, R.P.; Ripps, H. GABA transport and calcium dynamics in horizontal cells from the skate retina. J. Physiol. 1995, 488 Pt 3, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.A.; Bordey, A. GABA increases Ca2+ in cerebellar granule cell precursors via depolarization: Implications for proliferation. IUBMB Life 2009, 61, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Kar, D.; Pradhan, A.A.; Datta, S. The role of solute transporters in aluminum toxicity and tolerance. Physiol. Plant 2021, 171, 638–652. [Google Scholar] [CrossRef]
- Luu, K.; Rajagopalan, N.; Ching, J.C.H.; Loewen, M.C.; Loewen, M.E. The malate-activated ALMT12 anion channel in the grass Brachypodium distachyon is co-activated by Ca(2+)/calmodulin. J. Biol. Chem. 2019, 294, 6142–6156. [Google Scholar] [CrossRef]
- Felle, H.H.; Zimmermann, M.R. Systemic signalling in barley through action potentials. Planta 2007, 226, 203–214. [Google Scholar] [CrossRef]
- Jacob, P.F.; Vaz, S.H.; Ribeiro, J.A.; Sebastiao, A.M. P2Y1 receptor inhibits GABA transport through a calcium signalling-dependent mechanism in rat cortical astrocytes. Glia 2014, 62, 1211–1226. [Google Scholar] [CrossRef]
- Gutermuth, T.; Herbell, S.; Lassig, R.; Brosche, M.; Romeis, T.; Feijo, J.A.; Hedrich, R.; Konrad, K.R. Tip-localized Ca(2+) -permeable channels control pollen tube growth via kinase-dependent R- and S-type anion channel regulation. New Phytol. 2018, 218, 1089–1105. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of Gamma-Aminobutyric Acid in Plant Defense Response. Metabolites 2023, 13, 741. https://doi.org/10.3390/metabo13060741
Guo Z, Gong J, Luo S, Zuo Y, Shen Y. Role of Gamma-Aminobutyric Acid in Plant Defense Response. Metabolites. 2023; 13(6):741. https://doi.org/10.3390/metabo13060741
Chicago/Turabian StyleGuo, Zhujuan, Junqing Gong, Shuitian Luo, Yixin Zuo, and Yingbai Shen. 2023. "Role of Gamma-Aminobutyric Acid in Plant Defense Response" Metabolites 13, no. 6: 741. https://doi.org/10.3390/metabo13060741
APA StyleGuo, Z., Gong, J., Luo, S., Zuo, Y., & Shen, Y. (2023). Role of Gamma-Aminobutyric Acid in Plant Defense Response. Metabolites, 13(6), 741. https://doi.org/10.3390/metabo13060741






