Predicting Valproate-Induced Liver Injury Using Metabolomic Analysis of Ex Ovo Chick Embryo Allantoic Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Incubation of Fertilized Eggs and Ex Ovo Culture
2.2. Exposure of Models to VPA
2.3. Sample Preparation and Spectra Aquisition
2.4. Spectra Processing and Multivariate Data Analysis
2.5. Metabolic Signature Validation, Statistical Tests, and Heatmap
3. Results
3.1. Model Characterization
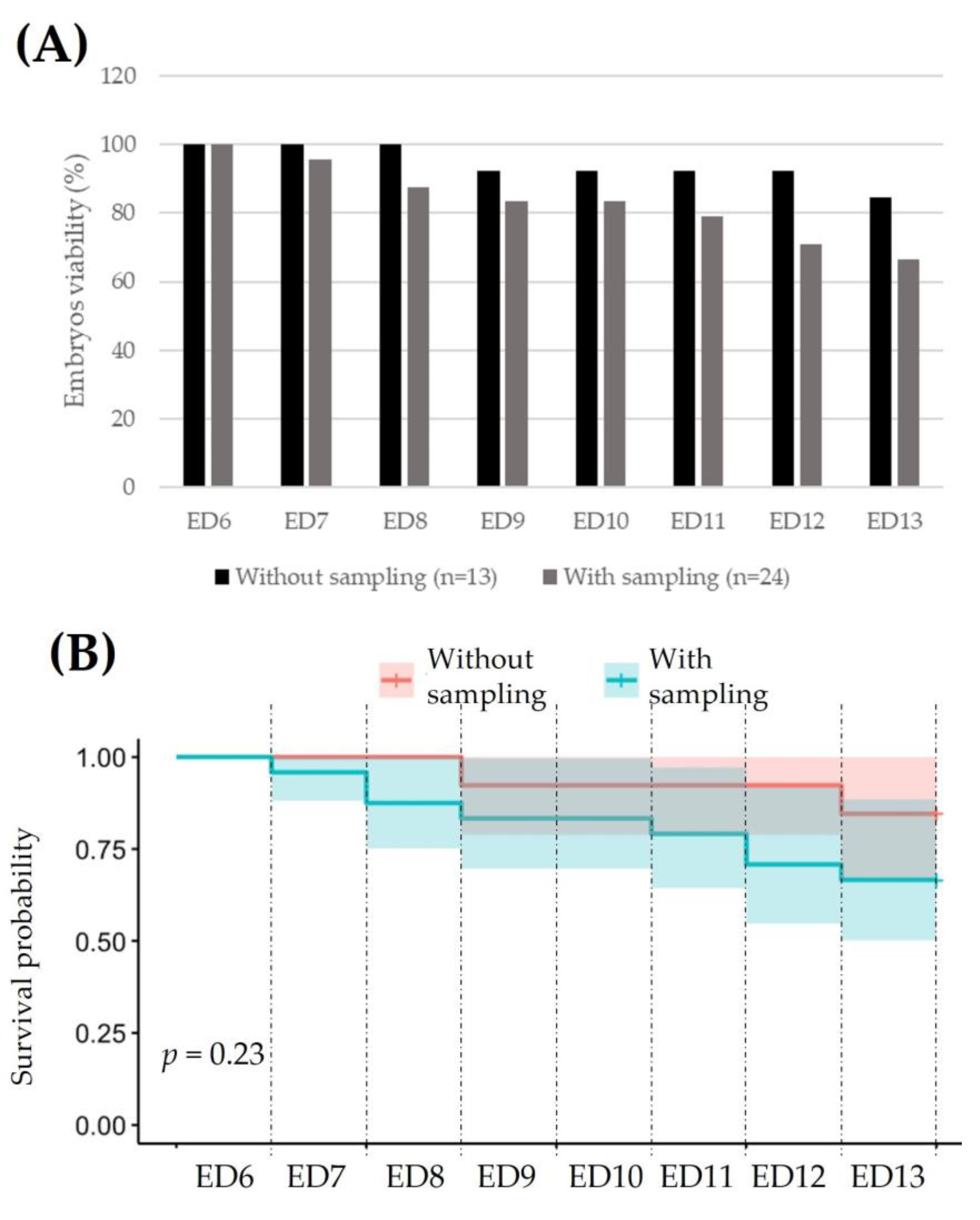
3.1.1. Fertilization and Viability of the Embryo in Ex Ovo Condition
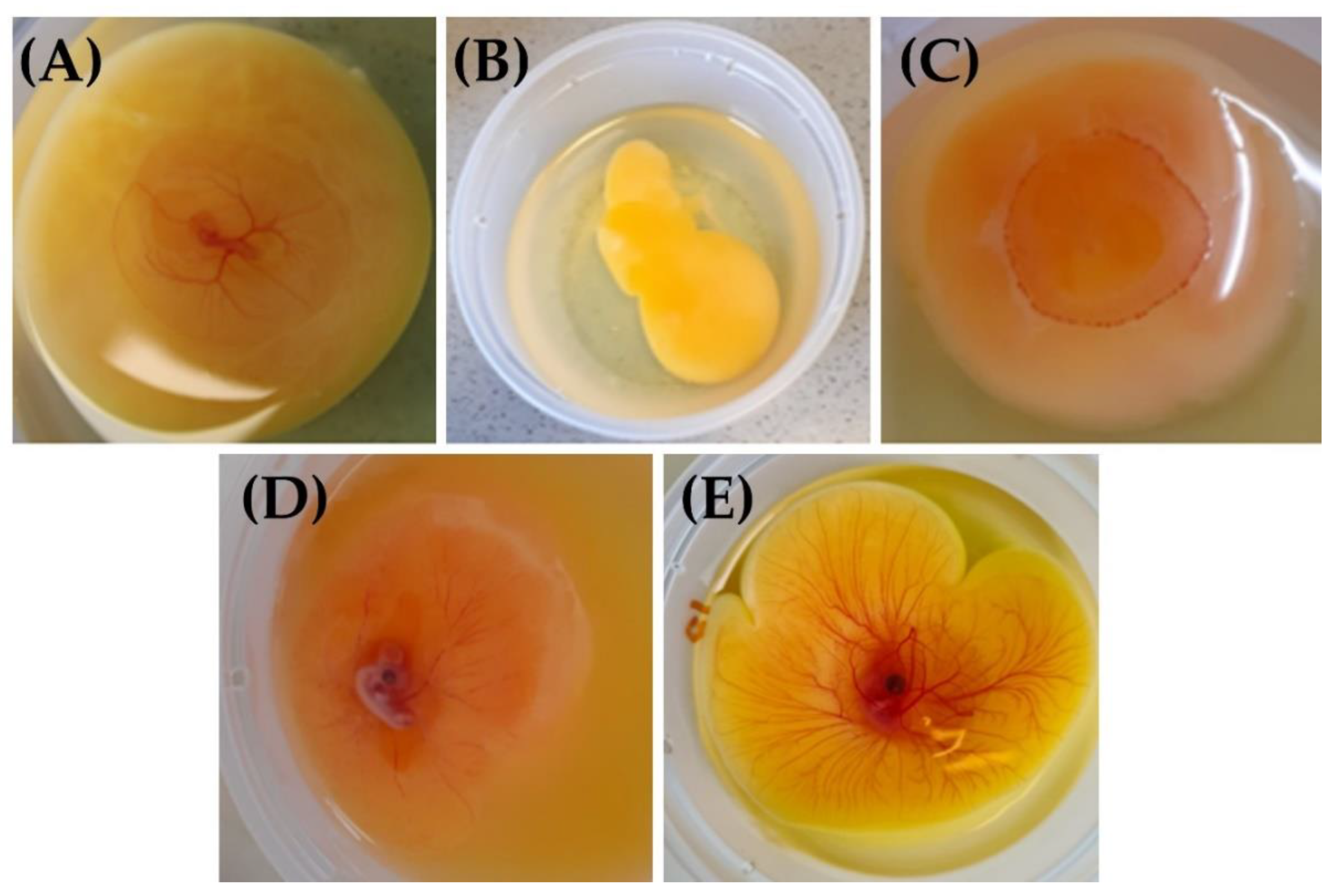
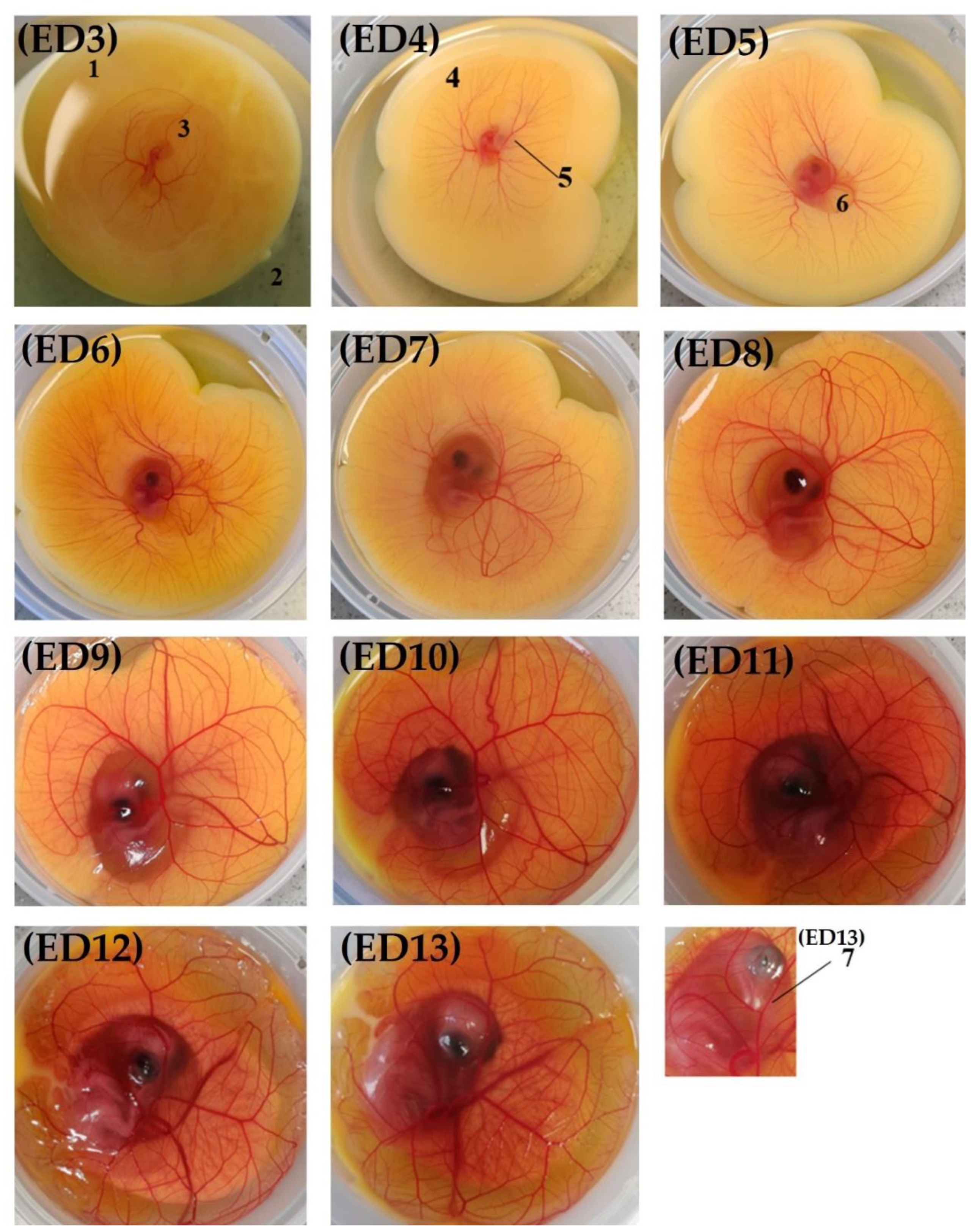
3.1.2. Macroscopic Characterization of the Model
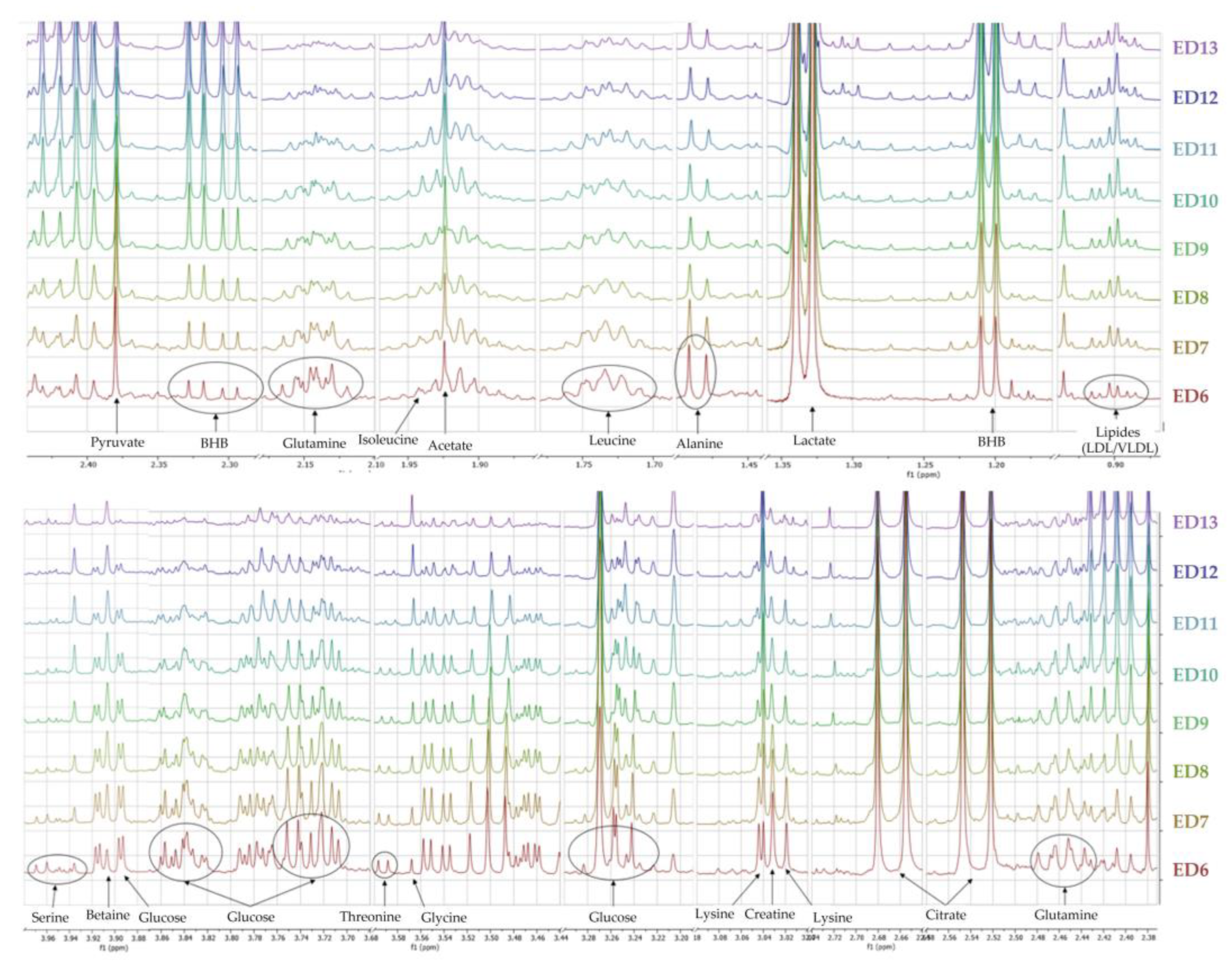
3.1.3. Metabolomic Characterization of Ex Ovo Embryos
3.2. Metabolomic Evaluation of VPA-Induced Hepatotoxicity
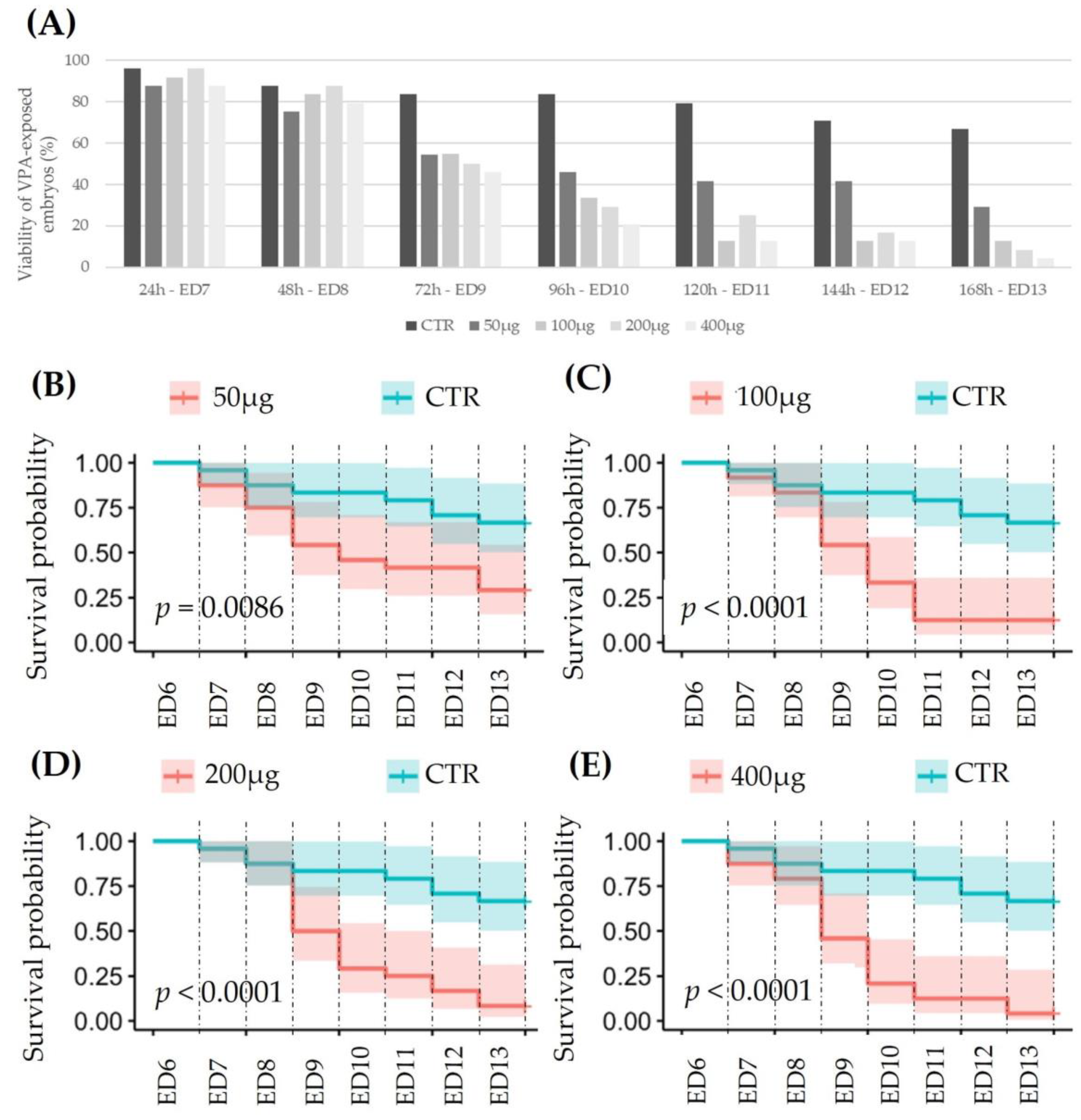
3.2.1. Viability of VPA-Exposed Ex Ovo Embryos
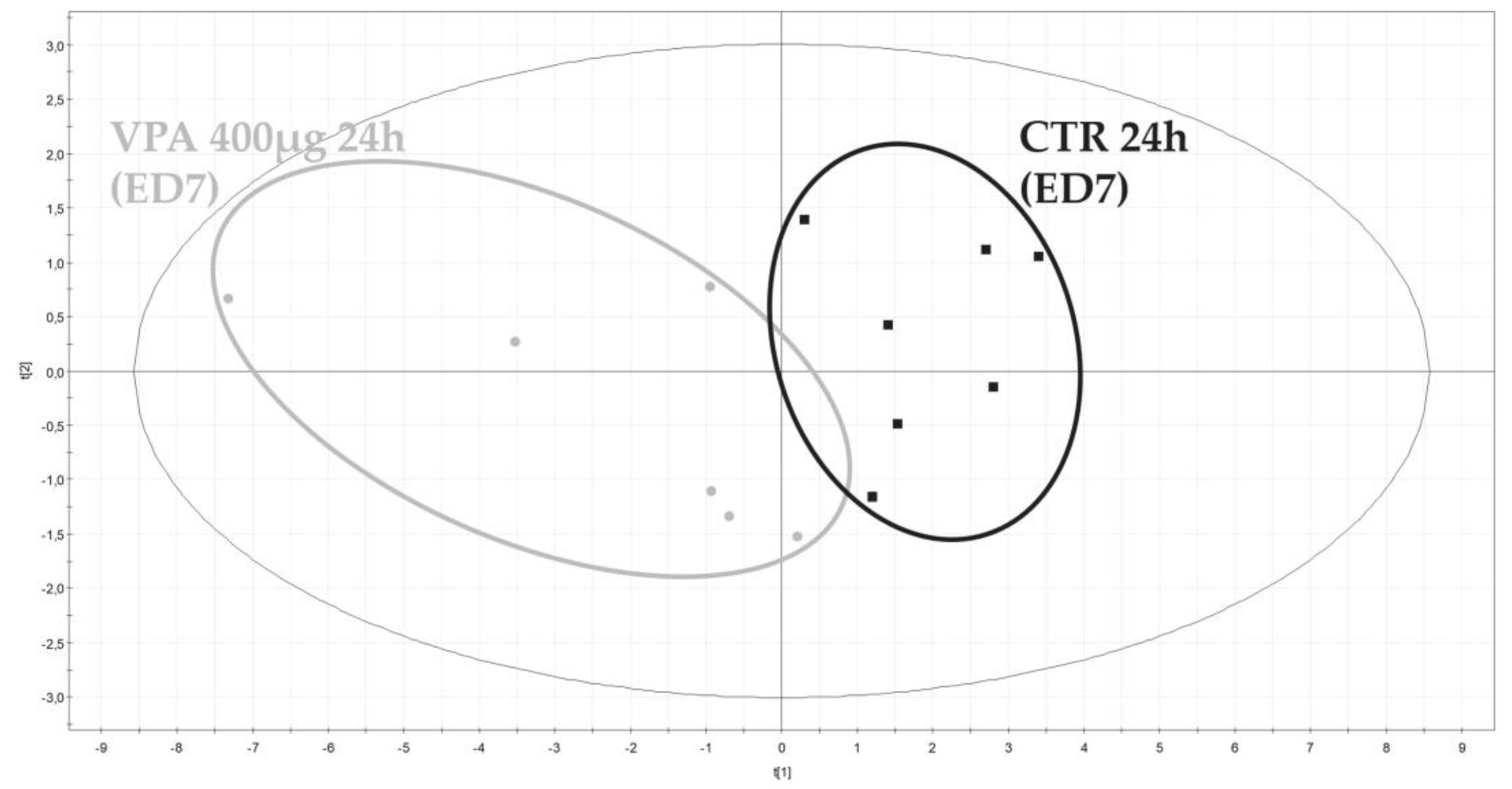
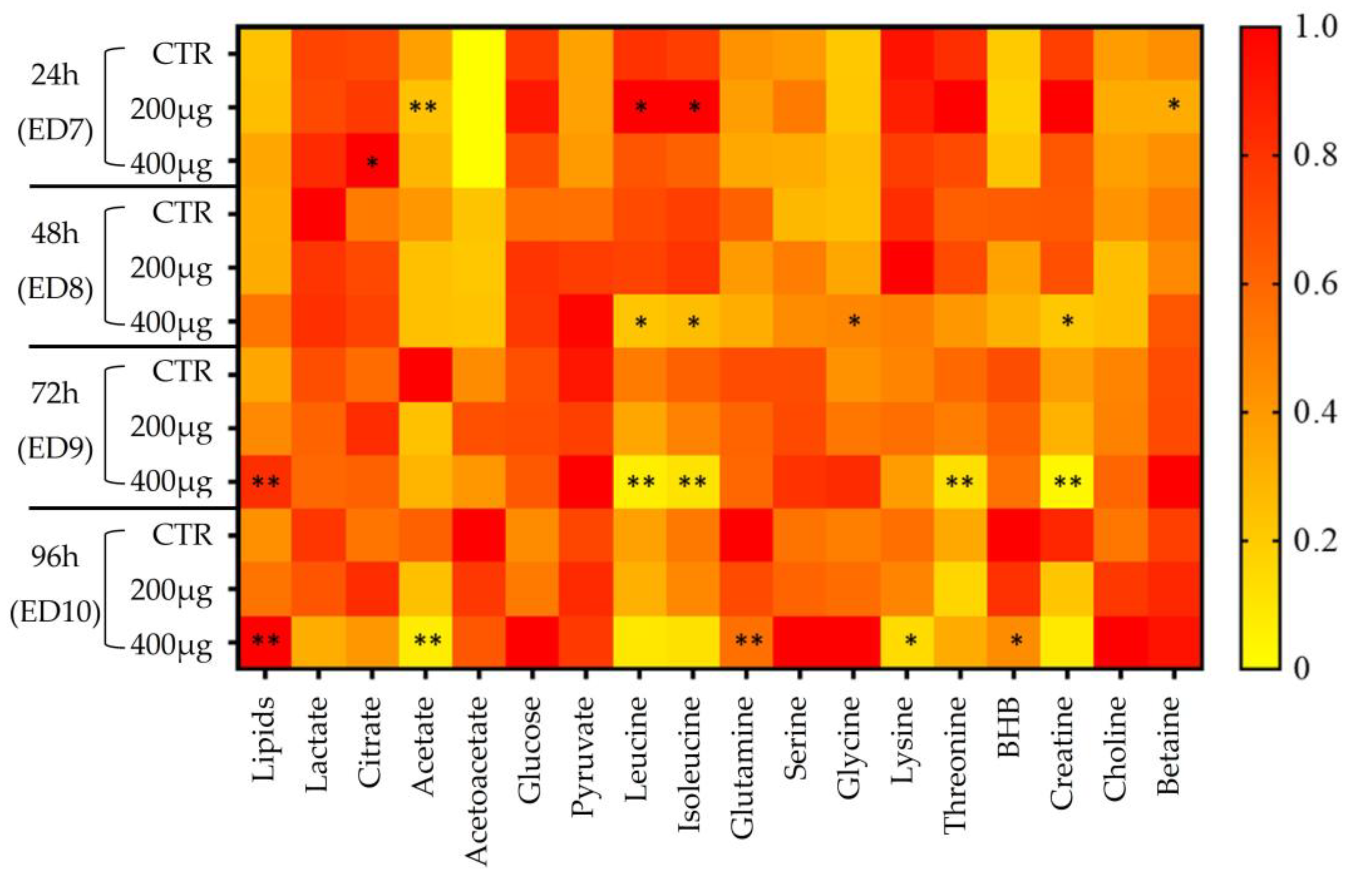
3.2.2. Metabolomic Evaluation of VPA-Exposed Ex Ovo Embryos
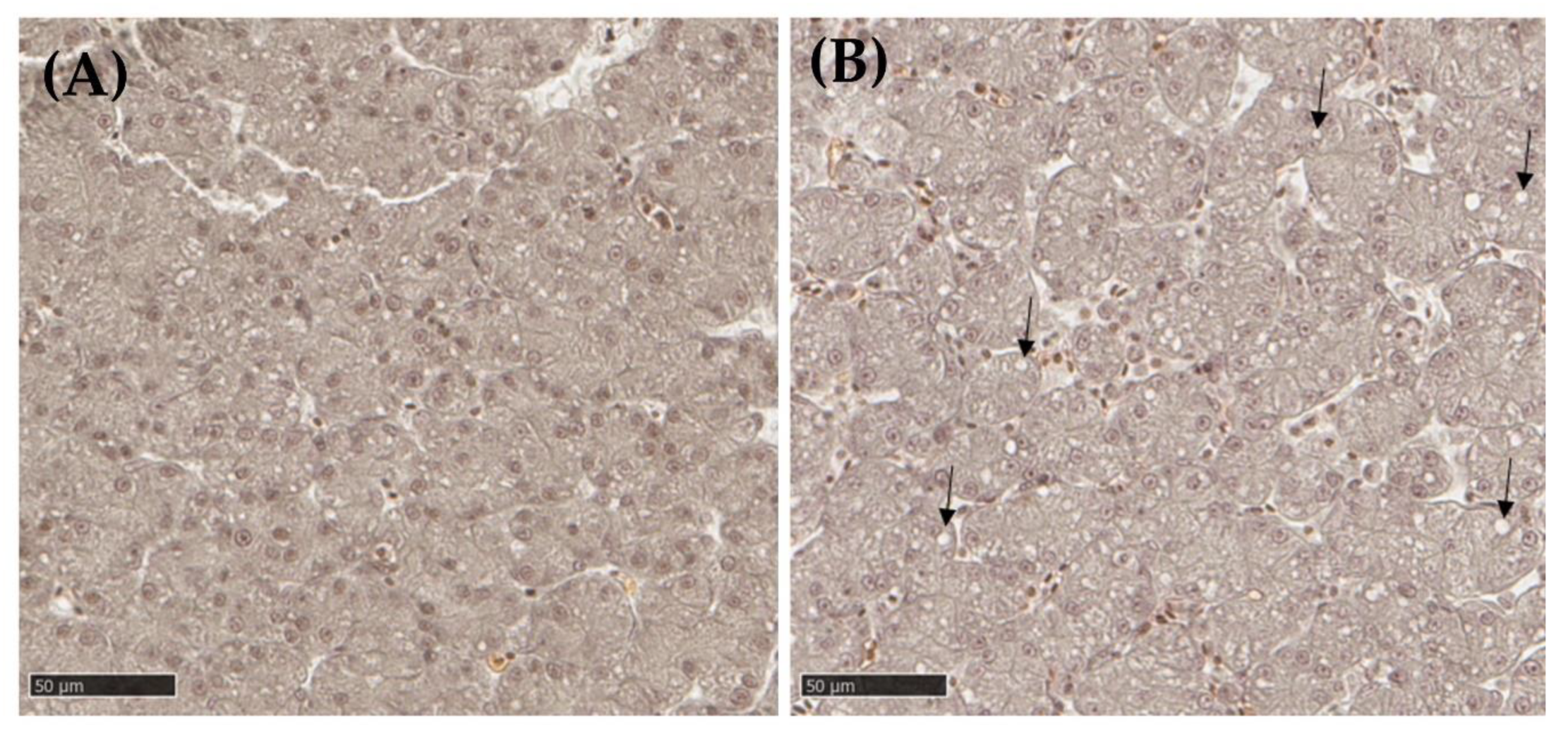
3.2.3. Liver Histopathology
4. Discussion
4.1. Evolution of the Allantoic Metabolome in Ex Ovo Conditions
4.2. Metabolomic Evaluation of VPA-Induced Hepatotoxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: http://bienetreanimal.wallonie.be/home/animaux/animaux-dexperience.html (accessed on 10 April 2023).
- Emwas, A.H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-based metabolomics in human disease diagnosis: Applications, limitations, and recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Holmes, E.; Antti, H.; Bollard, M.E.; Keun, H.; Beckonert, O.; Ebbels, T.M.; Reily, M.D.; Robertson, D.; et al. Contemporary issues in toxicology: The role of metabonomics in toxicology and its evaluation by the COMET project. Toxicol. Appl. Pharm. 2003, 187, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chwalibog, A.; Sawosz, E.; Jaworski, S.; Kutwin, M.; Hotowy, A.; Wierzbicki, M.; Grodzik, M.; Kurantowicz, N.; Strojny, B.; Lipinska, L. Toxicity of pristine graphene in experiments in a chicken embryo model. Int. J. Nanomed. 2014, 9, 3913–3922. [Google Scholar] [CrossRef]
- Cavill, R.; Sidhu, J.K.; Kilarski, W.; Javerzat, S.; Hagedorn, M.; Timothy, M.D.E.; Bikfalvi, A.; Keun, H.C. A combined metabonomic and transcriptomic approach to investigate metabolism during development in the chick chorioallantoic membrane. J. Proteome Res. 2010, 9, 3126–3134. [Google Scholar] [CrossRef]
- Wang, M.; Rücklin, M.; Poelmann, R.E.; de Mooij, C.L.; Fokkema, M.; Lamers, G.E.; de Bakker, M.A.; Chin, E.; Bakos, L.J.; Marone, F.; et al. Nanoplastics causes extensive congenital malformations during embryonic development by passively targeting neural crest cells. Environ. Int. 2023, 173, 107865. [Google Scholar] [CrossRef]
- Sukumaran, V.; Mutlu, O.; Murtaza, M.; Alhalbouni, R.; Dubansky, B.; Yalcin, H.C. Experimental Assessment of Cardiovascular Physiology in the Chick Embryo. Dev. Dyn. 2023. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, M.I.; Marcacci, M.; Bravo, S.; Kurz, T.; Tremblay, J.; Rusing, J.C. Ex ovo model for directly visualizing chick embryo development. Am. Biol. Teach. 2012, 74, 628–634. [Google Scholar] [CrossRef]
- Alcântara, D.; Rodrigues, M.N.; Franciolli, A.L.R.; Da Fonseca, E.T.; Silva, F.M.O.; Carvalho, R.C.; Fratini, P.; Sarmento, C.A.P.; Ferreira, A.J.P.; Miglino, M.A. Embryonic development of endoderm in chicken (Gallus gallus domesticus). Microsc. Res. Tech. 2013, 76, 803. [Google Scholar] [CrossRef]
- Sheid, B.; Hirschberg, E.; Sheid, E.H. Glutamic Dehydrogenase and Aspartic and Alanine Aminotransferase Activities in Chick Embryo Liver. 1967. Available online: www.physiology.org/journal/ajplegacy (accessed on 10 April 2023).
- Ribatti’, D.; Vacca’, A.; Roncali’, L.; Dammacco’, F. The chick embryo chorioallantoic membrane as a model for in vivo research on angiogenesis. Int. J. Dev. Biol. 1996, 40, 1189–1197. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM) assay. Reprod. Toxicol. 2017, 70, 97–101. [Google Scholar] [CrossRef]
- Da Silva, M.; Labas, V.; Nys, Y.; Rehault-Godbert, S. Investigating proteins and proteases composing amniotic and allantoic fluids during chicken embryonic development. Poult. Sci. 2017, 96, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Cloney, K.; Franz-Odendaal, T.A. Optimized ex-ovo culturing of chick embryos to advanced stages of development. J. Vis. Exp. 2015, e52129. [Google Scholar] [CrossRef]
- Farzaneh, M.; Attari, F.; Khoshnam, S.E.; Mozdziak, P.E. The method of chicken whole embryo culture using the eggshell windowing, surrogate eggshell and ex ovo culture system. Br. Poult. Sci. 2018, 59, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.L.; Li, S.N.; He, Q.Q.; Zhao, J.L.; Li, L.L.; Ma, H.T. Based serum metabolomics analysis reveals simultaneous interconnecting changes during chicken embryonic development. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, X.; Ye, C.; Liu, M. Analysis of metabolites in allantoic fluid of chicken embryo by 900 MHz NMR spectroscopy. Appl. Magn. Reason. 2007, 32, 257–268. [Google Scholar] [CrossRef]
- Diederich, M.; Chateauvieux, S.; Morceau, F.; Dicato, M. Molecular and therapeutic potential and toxicity of valproic acid. J. Biomed. Biotechnol. 2010, 2010, 479364. [Google Scholar] [CrossRef]
- Van Breda, S.G.J.; Claessen, S.M.; van Herwijnen, M.; Theunissen, D.H.; Jennen, D.G.; de Kok, T.M.; Kleinjans, J.C. Integrative omics data analyses of repeated dose toxicity of valproic acid in vitro reveal new mechanisms of steatosis induction. Toxicology 2018, 393, 160–170. [Google Scholar] [CrossRef]
- Zareba, G.; Cernichiari, E.; Hojo, R.; Mc Nitt, S.; Weiss, B.; Mumtaz, M.M.; Jones, D.E.; Clarkson, T.W. Thimerosal distribution and metabolism in neonatal mice: Comparison with methyl mercury. J. Appl. Toxicol. 2007, 27, 511–518. [Google Scholar] [CrossRef]
- Silva, M.F.B.; Aires, C.C.P.; Luis, P.B.M.; Ruiter, J.P.N.; Ijlst, L.; Duran, M.; Wanders, R.J.A.; de Almeida, I.T. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: A review. J. Inherit. Metab. Dis. 2008, 31, 205–216. [Google Scholar] [CrossRef]
- Cuykx, M.; Claes, L.; Rodrigues, R.M.; Vanhaecke, T.; Covaci, A. Metabolomics profiling of steatosis progression in HepaRG® cells using sodium valproate. Toxicol. Lett. 2018, 286, 22–30. [Google Scholar] [CrossRef]
- Sun, J.; Schnackenberg, L.K.; Hansen, D.K.; Beger, R.D. Study of valproic acid-induced endogenous and exogenous metabolite alterations using LC-MS-based metabolomics. Bioanalysis 2010, 2, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, D.; Wang, C.; An, G.; Zhu, L.; Cui, C. Comprehensive Analysis of Metabolic Changes in Male Mice Exposed to Sodium Valproate Based on GC-MS Analysis. Drug Des. Dev. 2022, 16, 1915–1930. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Jung, B.H.; Chung, B.C.; Cho, S.H.; Kim, K.Y.; Kwon, O.S.; Nugraha, B.; Lee, Y.-J. Metabolomics study with gas chromatography-mass spectrometry for predicting valproic acid-induced hepatotoxicity and discovery of novel biomarkers in rat urine. Int. J. Toxicol. 2009, 28, 392–404. [Google Scholar] [CrossRef]
- Huo, T.; Chen, X.; Lu, X.; Qu, L.; Liu, Y.; Cai, S. An effective assessment of valproate sodium-induced hepatotoxicity with UPLC-MS and 1HNMR-based metabonomics approach. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 969, 109–116. [Google Scholar] [CrossRef]
- Kohl, A.; Golan, N.; Cinnamon, Y.; Genin, O.; Chefetz, B.; Sela-Donenfeld, D. A proof of concept study demonstrating that environmental levels of carbamazepine impair early stages of chick embryonic development. Environ. Int. 2019, 129, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Afzal, E.; Khorsand, M.; Khoshnood, M.J.; Takhshid, M.A. Effects of Two-by-Two Combination Therapy with Valproic Acid, Lithium Chloride, and Celecoxib on the Angiogenesis of the Chicken Chorioallantoic Membrane. Iran. J. Med. Sci. 2018, 43, 506. [Google Scholar] [PubMed]
- Tagliatti, V.; Colet, J.-M. Drug-Induced Impairment of Mitochondrial Fatty Acid Be- ta-Oxidation: A Metabonomic Evaluation in Rats. J. Med. Genom. Biomark. 2016, 3, 005. [Google Scholar]
- Lowenstein, P.R.; Castro, M.G. Uncertainty in the Translation of Preclinical Experiments to Clinical Trials. Why do Most Phase III Clinical Trials Fail? Curr. Gene Ther. 2009, 9, 368–374. [Google Scholar] [CrossRef]
- Available online: https://policy.brown.edu/policy/avian-embryo-use (accessed on 10 April 2023).
- Yokouchi, Y. Establishment of a chick embryo model for analyzing liver development and a search for candidate genes. Dev. Growth Differ. 2005, 47, 357–366. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A Series of Normal Stages in the Development of the Chick Embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Sivanesan, S.; Taylor, A.; Zhang, J.; Bakovic, M. Betaine and Choline Improve Lipid Homeostasis in Obesity by Participation in Mitochondrial Oxidative Demethylation. Front. Nutr. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Chhibber-Goel, J.; Gaur, A.; Singhal, V.; Parakh, N.; Bhargava, B.; Sharma, A. The complex metabolism of trimethylamine in humans: Endogenous and exogenous sources. Expert Rev. Mol. Med. 2016, 18, e8. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, N.; López-Rosas, I.; Román-Cisneros, G.; Velázquez-Arellano, A. Biotin deficiency affects both synthesis and degradation of pyruvate carboxylase in rat primary hepatocyte cultures. Mol. Genet. Metab. 2007, 92, 222–228. [Google Scholar] [CrossRef] [PubMed]
- León-Del-Río, A. Biotin in metabolism, gene expression, and human disease. J. Inherit. Metab. Dis. 2019, 42, 647–654. [Google Scholar] [CrossRef]
- Lheureux, P.E.R.; Penaloza, A.; Zahir, S.; Gris, M. Science review: Carnitine in the treatment of valproic acid-induced toxicity—What is the evidence? Crit. Care 2005, 9, 431–440. [Google Scholar] [CrossRef]
- Nanau, R.M.; Neuman, M.G. Adverse drug reactions induced by valproic acid. Clin. Biochem. 2013, 46, 1323–1338. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Mani, U. Valproic acid induced liver injury: An insight into molecular toxicological mechanism. Environ. Toxicol. Pharmacol. 2022, 95, 103967. [Google Scholar] [CrossRef]
- Kesterson, J.W.; Granneman, G.R.; Machinist, J.M. The Hepatotoxicity of Valproic Acid and Its Metabolites in Rats. I. Toxicologic, Biochemical and Histopathologic Studies. Hepatology 1984, 4, 1143–1152. [Google Scholar] [CrossRef]
- Baéza, E.; Gondret, F.; Chartrin, P.; Le Bihan-Duval, E.; Berri, C.; Gabriel, I.; Narcy, A.; Lessire, M.; Métayer-Coustard, S.; Collin, A.; et al. The ability of genetically lean or fat slow-growing chickens to synthesize and store lipids is not altered by the dietary energy source. Animal 2015, 9, 1643–1652. [Google Scholar] [CrossRef]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken embryo development: Metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Pecere, S.; Gasbarrini, A.; Ojetti, V. Physiology and pathophysiology of liver lipid metabolism. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Szalowska, E.; Van Der Burg, B.; Man, H.Y.; Hendriksen, P.J.M.; Peijnenburg, A.A.C.M. Model steatogenic compounds (amiodarone, valproic acid, and tetracycline) alter lipid metabolism by different mechanisms in mouse liver slices. PLoS ONE 2014, 9, e86795. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Burgberger, M.; Wojtasik, W. 3-hydroxybutyrate as a metabolite and a signal molecule regulating processes of living organisms. Biomolecules 2021, 11, 402. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y.; Tang, Q.; Leng, Y.; Cai, W. The effects of choline on hepatic lipid metabolism, mitochondrial function and antioxidative status in human hepatic C3A cells exposed to excessive energy substrates. Nutrients 2014, 6, 2552–2571. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.I.; Ross, L.H.; Bozovic, G.; Grant, J.P. The Effect of Choline Supplementation on Hepatic Steatosis in the Parenterally Fed Rat. J. Parenter. Enter. Nutr. 1985, 9, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Tong, V.; Teng, X.W.; Chang, T.K.H.; Abbott, F.S. Valproic acid II: Effects on oxidative stress, mitochondrial membrane potential, and cytotoxicity in glutathione-depleted rat hepatocytes. Toxicol. Sci. 2005, 86, 436–443. [Google Scholar] [CrossRef]
- McCann, M.R.; De la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-carnitine and acylcarnitines: Mitochondrial biomarkers for precision medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Luís, P.B.M.; Ruiter, J.P.; Ijlst, L.; Diogo, L.; Garcia, P.; De Almeida, I.T.; Duran, M.; Wanders, R.J.; Silva, M.F.B. Inhibition of 3-methylcrotonyl-CoA carboxylase explains the increased excretion of 3-hydroxyisovaleric acid in valproate-treated patients. J. Inherit. Metab. Dis. 2012, 35, 443–449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagliatti, V.; Descamps, C.; Lefèvre, M.; Colet, J.-M. Predicting Valproate-Induced Liver Injury Using Metabolomic Analysis of Ex Ovo Chick Embryo Allantoic Fluid. Metabolites 2023, 13, 721. https://doi.org/10.3390/metabo13060721
Tagliatti V, Descamps C, Lefèvre M, Colet J-M. Predicting Valproate-Induced Liver Injury Using Metabolomic Analysis of Ex Ovo Chick Embryo Allantoic Fluid. Metabolites. 2023; 13(6):721. https://doi.org/10.3390/metabo13060721
Chicago/Turabian StyleTagliatti, Vanessa, Caroline Descamps, Margaux Lefèvre, and Jean-Marie Colet. 2023. "Predicting Valproate-Induced Liver Injury Using Metabolomic Analysis of Ex Ovo Chick Embryo Allantoic Fluid" Metabolites 13, no. 6: 721. https://doi.org/10.3390/metabo13060721
APA StyleTagliatti, V., Descamps, C., Lefèvre, M., & Colet, J.-M. (2023). Predicting Valproate-Induced Liver Injury Using Metabolomic Analysis of Ex Ovo Chick Embryo Allantoic Fluid. Metabolites, 13(6), 721. https://doi.org/10.3390/metabo13060721







