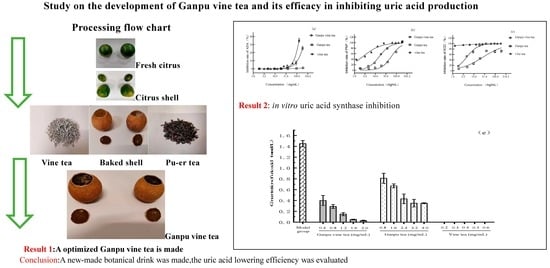
Research on the Efficacy of Ganpu Vine Tea in Inhibiting Uric Acid Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents
2.2. Preparation and Extraction of Ganpu Vine Tea
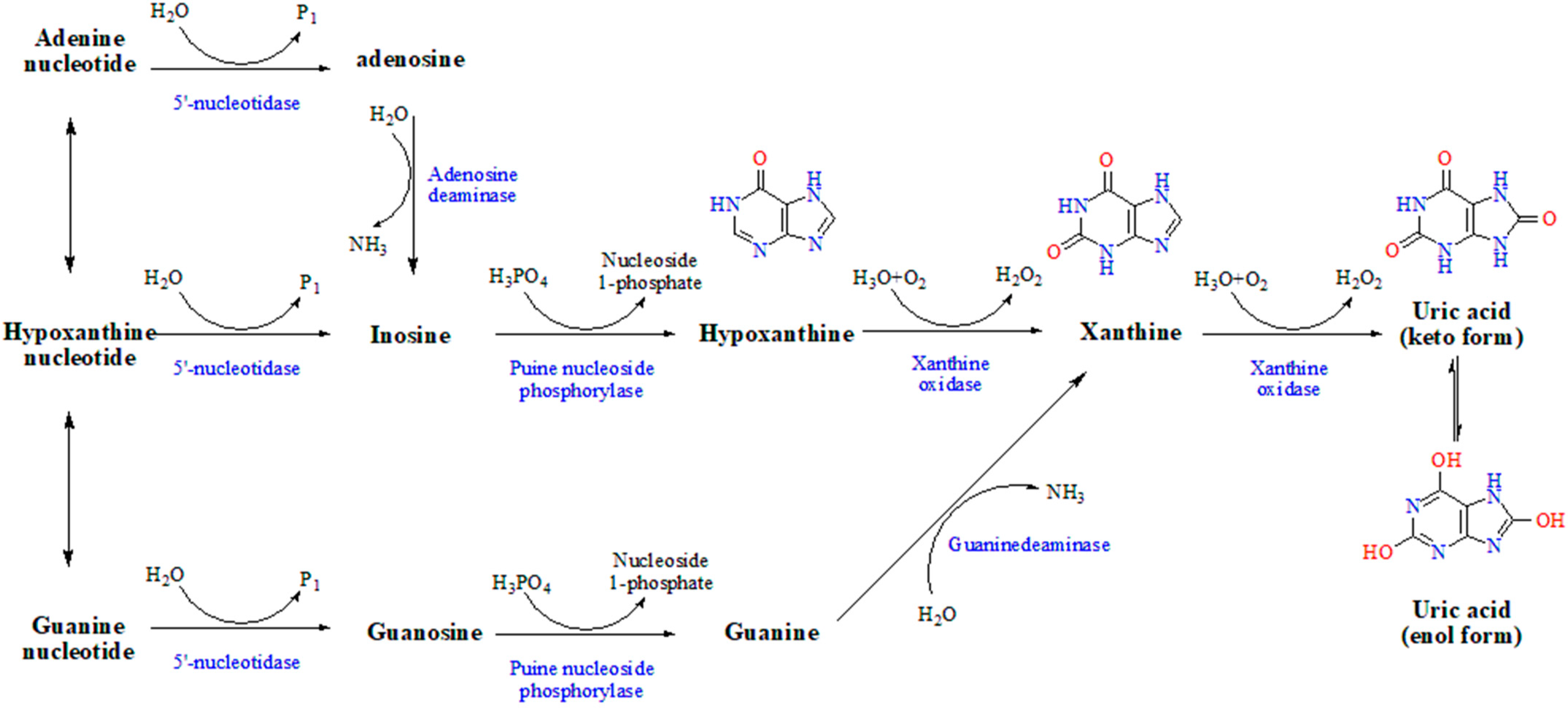
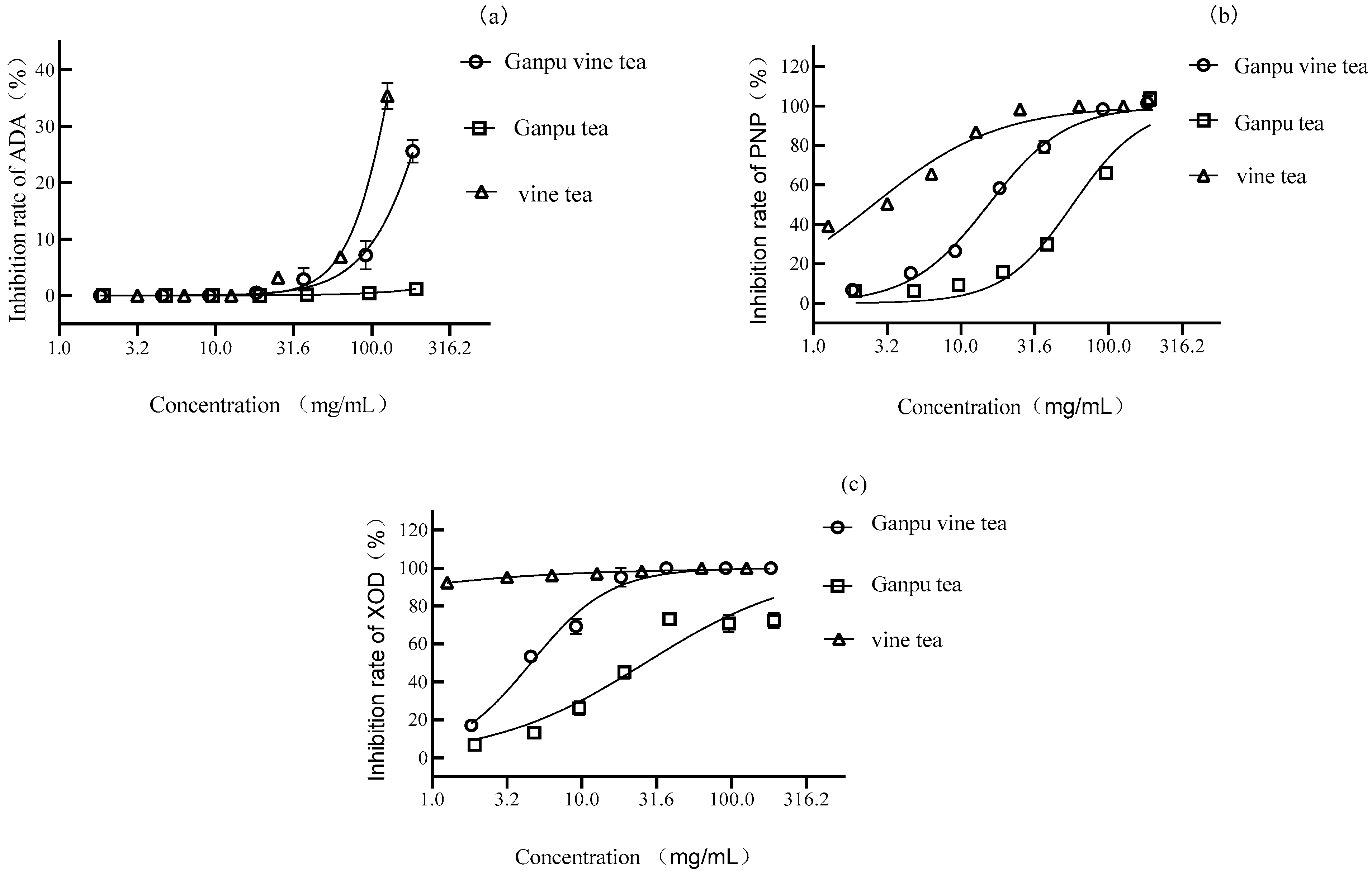
2.3. In Vitro Inhibition of ADA Reaction System
2.4. In Vitro Inhibition of PNP Reaction System
2.5. In Vitro Inhibition of XOD Reaction System
2.6. Uric Acid Inhibition through Ganpu Vine Tea in HepG2 Cells
2.7. HPLC Analysis
2.8. Data Analysis
3. Results
3.1. Results of Inhibition of Uric Acid Synthase Using Aqueous Extract
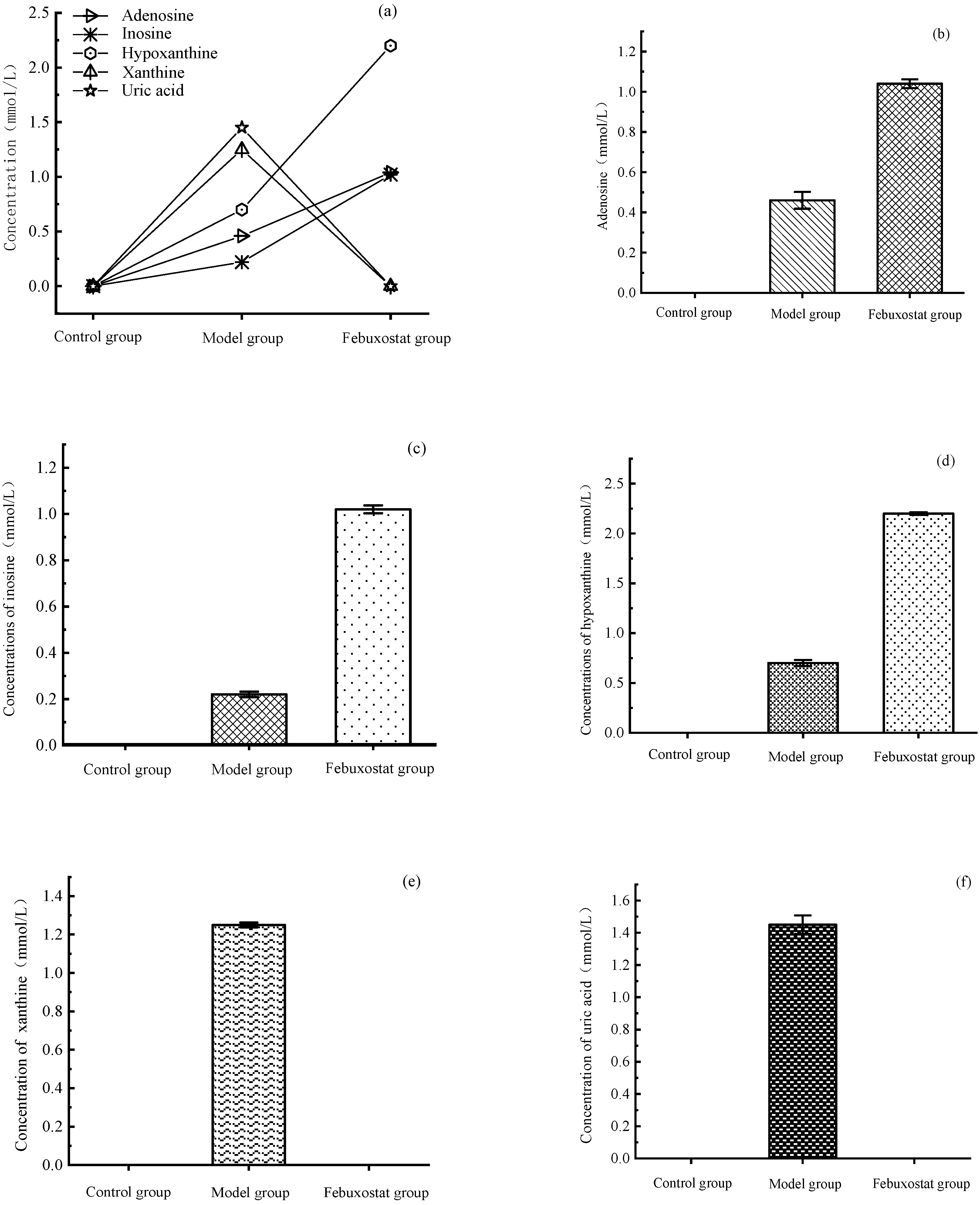
3.2. Results of Inhibition of Uric Acid Production Using Aqueous Extracts
3.2.1. High Uric Acid Cell Model Construction
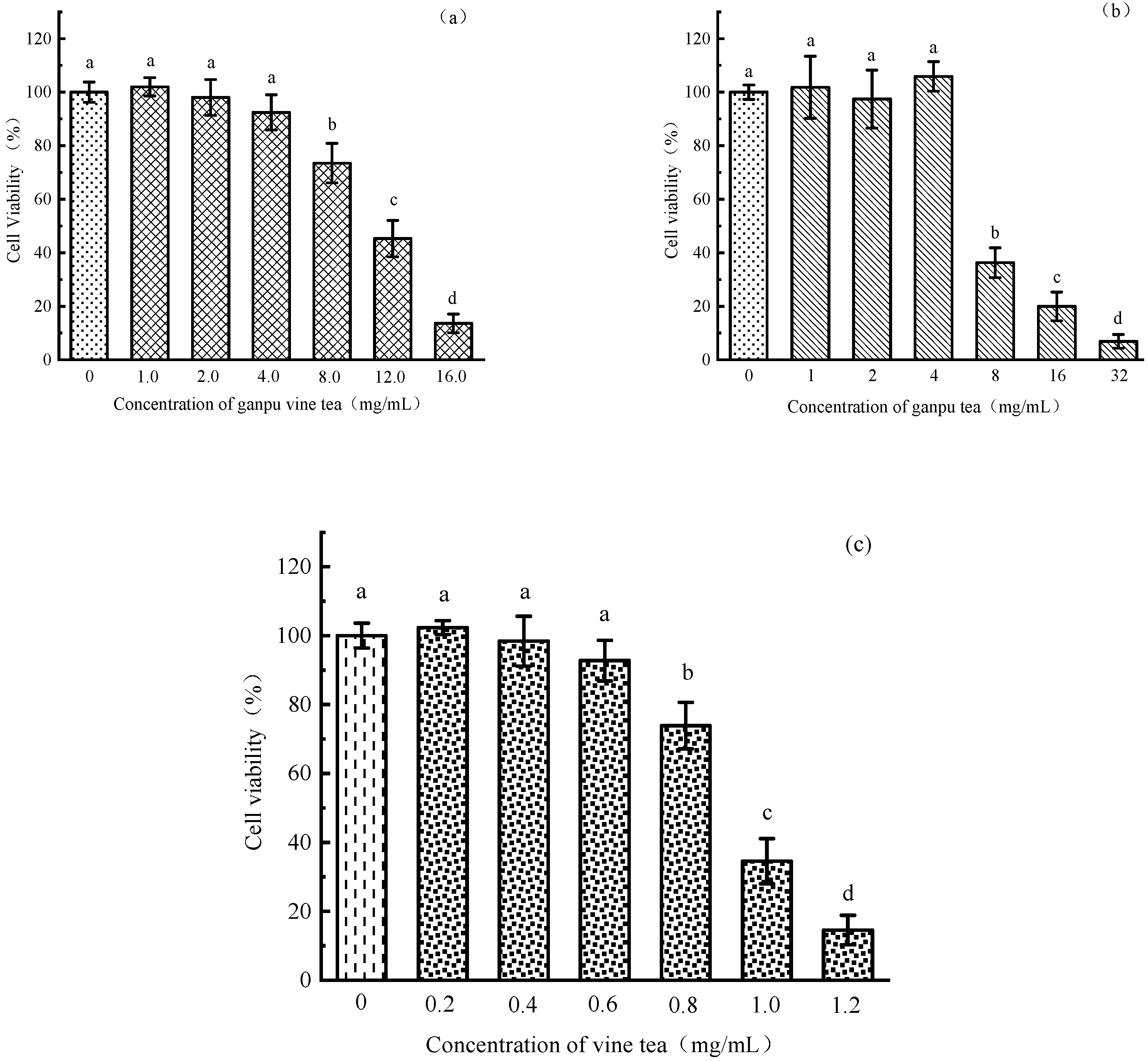
3.2.2. Results of Cytotoxicity Assay
3.2.3. Aqueous Extract Intervention Cell Model Experiment for High Uric Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Medical Association; Chinese Medical Journals Publishing House; Chinese Society of General Practice. Guideline for primary care of gout and hyperuricemia (2019). Chin. J. Gen. Pract. 2020, 19, 293–303. [Google Scholar]
- Huang, J.; Ma, Z.F.; Tian, Y.; Lee, Y.Y. Epidemiology and Prevalence of Gout in Mainland China: An Updated Systematic Review and Meta-Analysis. SN Compr. Clin. Med. 2020, 2, 1593–1606. [Google Scholar] [CrossRef]
- Tang, Q. Biochemistry and Molecular Biology; Fudan University Press: Shanhai, China, 2015; pp. 218–223. [Google Scholar]
- Wu, F.; Wang, L.; Li, H.; Qiao, P.; Zhang, A.; Zhou, H. Progress in hyperuricemia model establishment and uric acid-lowering drugs. Chin. J. Pathophysiol. 2021, 37, 1283–1294. [Google Scholar]
- Borghi, C.; Agabiti-Rosei, E.; Richard, J.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Redon, J.; Stack, A.G.; Tsioufis, K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jin, J.; Meng, X.; Liu, W. Research progress on relationship between hyperuricemia and its therapeutic drugs and kidney diseases. Drug Eval. Res. 2021, 44, 2013–2019. [Google Scholar]
- Gu, G.; Hu, H.; Guo, J. Research Progress of Drug Therapy for Hyperuricemia. Med. Recapitul. 2020, 26, 2829–2833. [Google Scholar]
- Otani, N.; Ouchi, M.; Kudo, H.; Tsuruoka, S.; Hisatome, I.; Anzai, N. Recent approaches to gout drug discovery: An update. Expert Opin. Drug Discov. 2020, 15, 943–954. [Google Scholar] [CrossRef]
- Rees, F.; Hui, M.; Doherty, M. Optimizing current treatment of gout. Nat. Rev. 2014, 10, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Ariyasinghe, J.T.N.; Thirumoorthy, T. Allopurinol hypersensitivity syndrome a preventable severe cutaneous adverse reaction. Singapore Med. J. 2008, 49, 384–387. [Google Scholar]
- Shi, C. Study on Regulation Mechanism of Natural Substances on Urate Metabolism Enzymes. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2018. [Google Scholar]
- Zhao, R. Study on the Effects of Dark Tea to Alleviate the Symptoms of Gout and Its Mechanism. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2017. [Google Scholar]
- Zhang, Y.; Fu, M.; Fan, H. Research Progress on Food with Uric Acid Lowering Effects. Food Nutr. China 2020, 26, 50–53. [Google Scholar]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef]
- Geng, Y.; Guo, A.; Jin, S.; Cheng, G.; Huang, D.; Zhang, L.; Zhu, Y. Research Progress in Effect and Use of Vine Tea. Bever. Ind. 2019, 22, 71–74. [Google Scholar]
- Chen, Y.; Luo, Z.; Xiang, D. Progress on Extraction and Isolation and Quantitative Methods of Flavonoids from Ampelopsis Grossedentata. J. Hubei Natl. (Nat. Sci. Edit.) 2008, 26, 277–281. [Google Scholar]
- Feng, H.; Li, N.; Wang, H. Research advances on the efficacy of flavanoids in Vine tea. J. Pub. Health Prevent Med. 2018, 29, 82–86. [Google Scholar]
- Wu, S.; Yu, H.; Yu, T.; Zhen, Z.; Ke, D. Study on Anti-hyperuricemia effect of Ampelopsis grossedentata extracts. Sci. Technol. Food Ind. 2021, 42, 350–355. [Google Scholar]
- Wang, Y. Study on the Mechanism of Anti-Hyperuricemia and Renal Function Protection of Vine Tea Extract. Ph.D. Thesis, Southwest Minzu University, Chengdu, China, 2020. [Google Scholar]
- Zeng, D.; Liu, M.; Zuo, Y. Analysis on Technological Innovation Ability and Competition Situation of Ampelopsis Grossedentata in China: Based on Patent Perspective. J. Lib. Inf. Sci. 2019, 4, 43–50. [Google Scholar]
- Chen, J.; Lin, Q.; Guo, Y. Exploration on the special flavors of different kinds of Teng Cha (Ampelopis grossedentata). Fujian Tea 2000, 3, 15–17+54. [Google Scholar]
- Zhou, C.; Zhang, M.; Mu, R.; Chen, W.P. Research progress of vine tea processing technology. Fujian Tea 2013, 35, 14–16. [Google Scholar]
- Long, H. Processing principle and method of Xinhui Green Citrus Tea. Guangdong Tea Ind. 2020, 4, 9–11. [Google Scholar]
- Chen, M.; Lian, X. Study on standardized production technology of Xinhui Mandarin tea. Chin. Food Safety Mag. 2018, 33, 140–141. [Google Scholar]
- Sun, Y.L.; Zhao, H.X.; Bai, H. In vitro screening of potential xanthine oxidase inhibitors by high performance liquid chromatography. Chin. J. Pharm. Anal. 2014, 34, 1391–1396. [Google Scholar]
- Yuan, M.; Liu, Y.; Xiao, A.; Leng, J.; Liao, L.; Ma, L.; Liu, L. The interaction of dietary flavonoids with xanthine oxidase in vitro: Molecular property-binding affinity relationship aspects. RSC Adv. 2019, 9, 10781–10788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kwon, S.H.; Hwang, S.H.; Kang, Y.H.; Lee, J.Y.; Lim, S.S. Competitive binding experiments can reduce the false positive results of affinity-based ultrafiltration-HPLC: A case study for identification of potent xanthine oxidase inhibitors from Perilla frutescens extract. J. Chromatogr. B 2017, 1048, 30–37. [Google Scholar] [CrossRef]
- Ma, E.; Li, S.; Cheng, X.; Wang, W.; Wang, Y. Protective effect of total flavonoids from ampelopsis grossedentata on renal function and anti-hyperuricemia effect. Pharmacol. Clin. Chin. Mater. 2021, 37, 80–85. [Google Scholar]
- Wang, Y.; Hong, Z.; Yang, K.; Zeng, C. Research progress of dihydromyricetin in Ampelopsis grossedentata. J. Shenyang Pharm. Univ. 2020, 37, 569–576. [Google Scholar]
- Zeng, N. Inhibition Dffect and Mwchanism of Four Flavones on Xanthine Oxidase. Ph.D. Thesis, Nanchang University, Nanchang, China, 2020. [Google Scholar]
- Wang, X. Molecular Docking Study on Multi-Target Mechanism of Chicory in the Treatment of Hyperuricemia. Ph.D. Thesis, Beijing University of Chinese Medicine, Beijing, China, 2016. [Google Scholar]

| Tea Name | Constitute | Main Chemical Components |
|---|---|---|
| Pu er tea | Tea leaves (fermented) | Polysaccharides, catechin, tea polyphenol |
| Ganpu tea | Citrus shell and Pu er tea | Polysaccharides, catechin, tea polyphenol, nobiletin, hesperidin |
| Vine tea | Leaves of vine (dried) | Dihydromyricetin, polyphenols |
| Ganpu vine tea | Citrus shell, Pu er tea and vine tea | Polysaccharides, catechin, polyphenol, nobiletin, hesperidin, dihydromyricetin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-X.; Mo, R.-M.; Liu, D.-B.; Liu, Y.-S.; Liu, C.-H.; Li, Y.-S.; Liu, Z.-H.; Qin, D. Research on the Efficacy of Ganpu Vine Tea in Inhibiting Uric Acid Production. Metabolites 2023, 13, 704. https://doi.org/10.3390/metabo13060704
Zhang Z-X, Mo R-M, Liu D-B, Liu Y-S, Liu C-H, Li Y-S, Liu Z-H, Qin D. Research on the Efficacy of Ganpu Vine Tea in Inhibiting Uric Acid Production. Metabolites. 2023; 13(6):704. https://doi.org/10.3390/metabo13060704
Chicago/Turabian StyleZhang, Zhi-Xu, Run-Ming Mo, Dong-Bo Liu, Yi-Song Liu, Cong-Hui Liu, Yong-Shen Li, Zhong-Hua Liu, and Dan Qin. 2023. "Research on the Efficacy of Ganpu Vine Tea in Inhibiting Uric Acid Production" Metabolites 13, no. 6: 704. https://doi.org/10.3390/metabo13060704
APA StyleZhang, Z.-X., Mo, R.-M., Liu, D.-B., Liu, Y.-S., Liu, C.-H., Li, Y.-S., Liu, Z.-H., & Qin, D. (2023). Research on the Efficacy of Ganpu Vine Tea in Inhibiting Uric Acid Production. Metabolites, 13(6), 704. https://doi.org/10.3390/metabo13060704