The Impact of Tannic Acid Consumption on Bone Mineralization
Abstract
:1. Introduction
2. Methods Used to Search for Relevant Scientific Information
3. Bone Remodeling
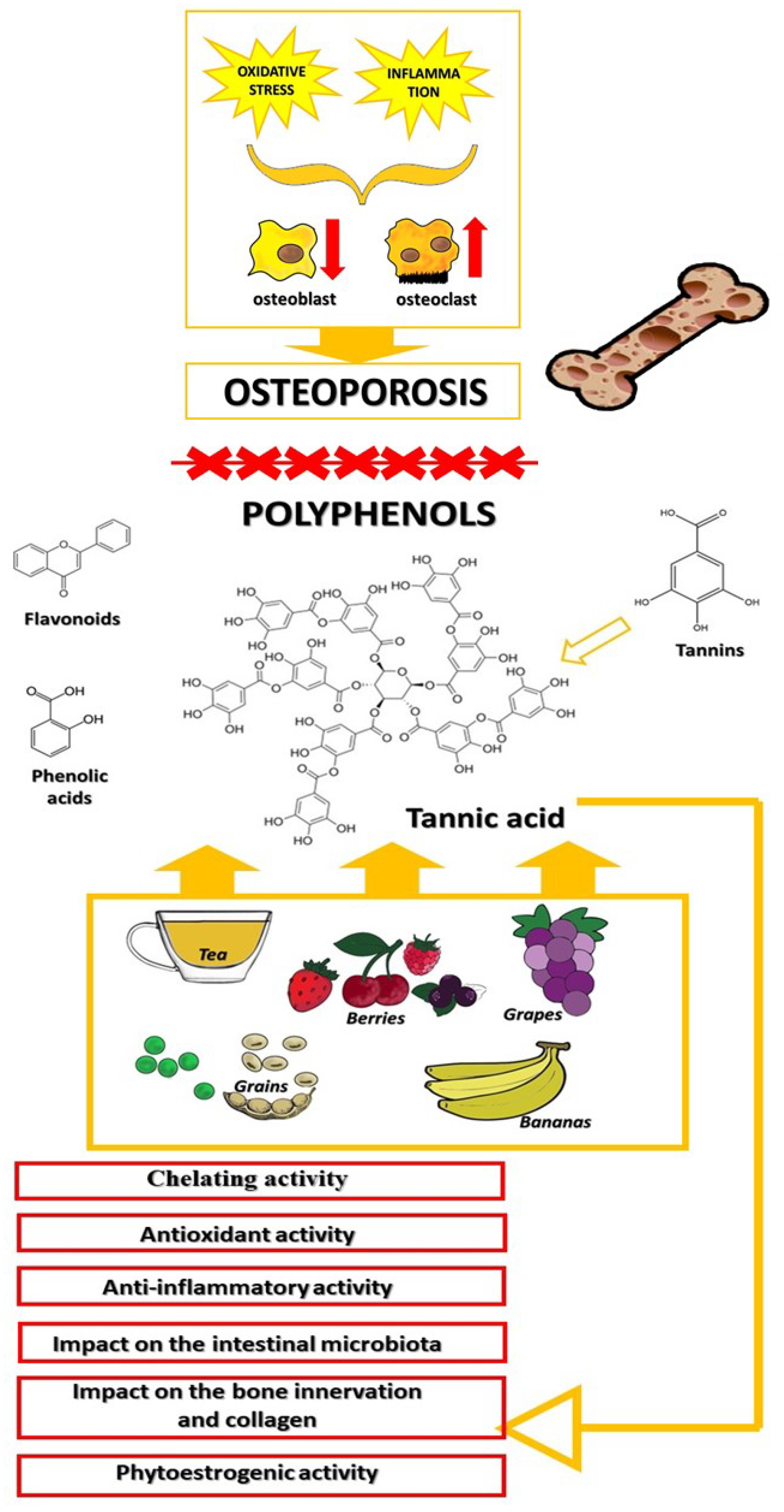
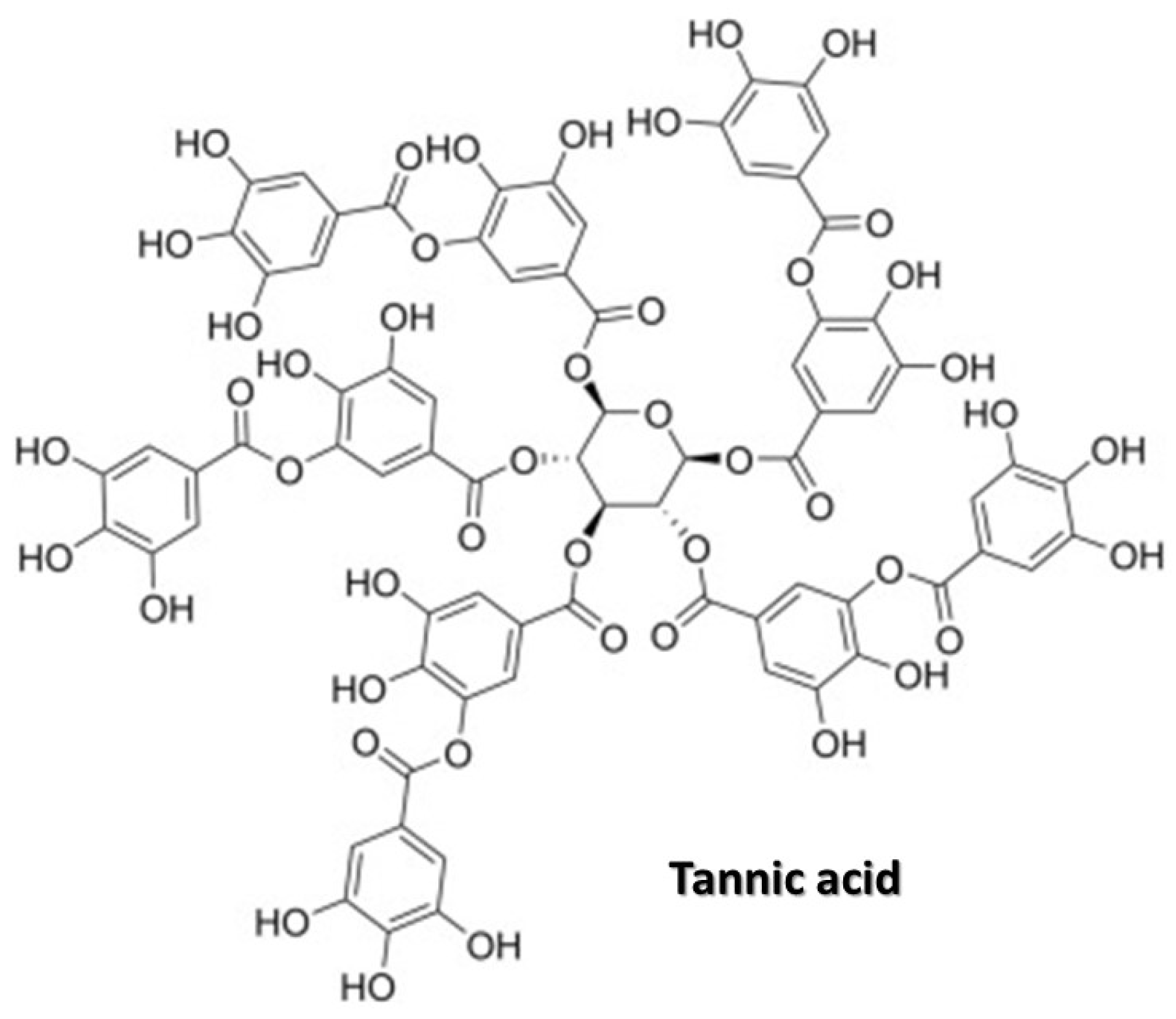
4. Tannic Acid
5. Influence of Tannic Acid on Bone Mineralization and Structure
5.1. Chelating Activity of Tannic Acid
5.2. Antioxidant Activity of Tannic Acid
5.3. Anti-Inflammatory Activity of Tannic Acid
5.4. The Relationship between Tannic Acid, the Gut Microbiota, and Bones
5.5. Tannic Acid as a Biomaterial in Bone Treatment
5.6. Tannic Acid and Bone Innervation
5.7. Tannic Acid and Collagen
5.8. Tannic Acid and Bone Loss in Menopause
6. Summary
7. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vielma, J.R.; Picon, D.; Gutiérrez, L.V.; Lara, N.D. Pathophysiology of Osteoporosis: Genes, Oxidative Stress and Immunopathogeny. A Qualitative Systematic Review. Av. Biomed. 2018, 7, 100–111. [Google Scholar]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. Biomed. Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; He, J.; Jiang, T. The correlation between estrogen receptor gene polymorphism and osteoporosis in Han Chinese women. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8084–8090. [Google Scholar]
- Prentice, A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004, 7, 227–243. [Google Scholar] [CrossRef]
- Li, Q.; Tian, C.; Liu, X.; Li, D.; Liu, H. Anti-inflammatory and antioxidant traditional Chinese Medicine in treatment and prevention of osteoporosis. Front. Pharmacol. 2023, 14, 203767. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—an update on their association and mechanistic links. Periodontology 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Bąkowski, M.; Krusiński, R.; Jachimowicz-Rogowska, K.; Demkowska-Kutrzepa, M.; Kiczorowska, B.; Krupa, W. Tannic Acid and Tea Prevents the Accumulation of Lead and Cadmium in the Lungs, Heart and Brain of Adolescent Male Wistar Rats—Possible Therapeutic Option. Animals 2022, 12, 2838. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Li, P.; Luo, Y.; Fu, J.; Gong, L.; Lv, Z.; Guo, Y. Effects of Tannic Acid Supplementation on the Intestinal Health, Immunity, and Antioxidant Function of Broilers Challenged with Necrotic Enteritis. Antioxidants 2023, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Lei, M.; Andargie, M.; Zeng, J.; Li, J. Antifungal activity and mechanism of action of tannic acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019, 107, 46–50. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, L.; Prasadam, I.; Crawford, R.; Zhou, Y.; Xiao, Y. Inflammatory macrophages interrupt osteocyte maturation and mineralization via regulating the Notch signaling pathway. Mol. Med. 2022, 28, 102. [Google Scholar] [CrossRef] [PubMed]
- Omnes, M.H.; Le Goasduff, J.; Le Delliou, H.; Le Bayon, N.; Quazuguel, P.; Robin, J.H. Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquacult. Rep. 2017, 6, 21–27. [Google Scholar] [CrossRef]
- Kraal, P.; Jansen, B.; Nierop, K.G.J.; Verstraten, J.M. Copper complexation by tannic acid in aqueous solution. Chemosphere 2006, 65, 2193–2198. [Google Scholar] [CrossRef]
- Jaramillo, Á.; Briones, L.; Andrews, M.; Arredondo, M.; Olivares, M.; Brito, A.; Pizarro, F. Effect of phytic acid, tannic acid and pectin on fasting iron bioavailability both in the presence and absence of calcium. J. Trace Elem. Med. Biol. 2015, 30, 112–117. [Google Scholar] [CrossRef]
- Baldwin, A.; Booth, B.W. Biomedical applications of tannic acid. J. Biomater. Appl. 2022, 36, 1503–1523. [Google Scholar] [CrossRef]
- Amalra, A.; Pius, A. Relative contribution of oxalic acid, phytate and tannic acid on the bioavailability of calcium from various calcium salts—an in vitro study. Int. Food Res. J. 2017, 24, 1278–1285. [Google Scholar]
- Terkawi, M.A.; Matsumae, G.; Shimizu, T.; Takahashi, D.; Kadoya, K.; Iwasaki, N. Interplay between Inflammation and Pathological Bone Resorption: Insights into Recent Mechanisms and Pathways in Related Diseases for Future Perspectives. Int. J. Mol. Sci. 2022, 23, 1786. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2021, 11, 511799. [Google Scholar] [CrossRef]
- Mazzaferro, S.; Bagordo, D.; De Martini, N.; Pasquali, M.; Rotondi, S.; Tartaglione, L.; Stenvinkel, P. Inflammation, Oxidative Stress, and Bone in Chronic Kidney Disease in the Osteoimmunology Era. Calcif. Tissue Int. 2021, 108, 452–460. [Google Scholar] [CrossRef]
- Roux, S.; Orcel, P. Bone loss. Factors that regulate osteoclast differentiation: An update. Arthritis Res. 2000, 2, 451–456. [Google Scholar] [CrossRef]
- Verborgt, O.; Gibson, G.J.; Schaffler, M.B. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J. Bone Miner. Res. 2000, 15, 60–67. [Google Scholar] [CrossRef]
- Janssens, K.; ten Dijke, P.; Janssens, S.; van Hul, W. Transforming growth factor-β1 to the bone. Endocr. Rev. 2005, 26, 743–774. [Google Scholar] [CrossRef]
- Chisari, E.; Shivappa, N.; Vyas, S. Polyphenol-Rich Foods and Osteoporosis. Curr. Pharm. Des. 2019, 25, 2459–2466. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Phiwchai, I.; Yuensook, W.; Sawaengsiriphon, N.; Krungchanuchat, S.; Pilapong, C. Tannic acid (TA): A molecular tool for chelating and imaging labile iron. Eur. J. Pharm. Sci. 2018, 114, 64–73. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Muszyński, S.; Tomczyk, A. The effect of tannic acid on bone mechanical and geometric properties, bone density, and trabecular histomorphometry as well as the morphology of articular and growth cartilages in rats co-exposed to cadmium and lead is dose dependent. Toxicol. Ind. Health 2017, 33, 855–866. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Tomczyk, A.; Muszyński, S. The effect of tannic acid on the bone tissue of adult male Wistar rats exposed to cadmium and lead. Exp. Toxicol. Pathol. 2017, 69, 131–141. [Google Scholar] [CrossRef]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, anti-inflammatory, anti-obesogenic, and antidiabetic properties of tea polyphenols—the positive impact of regular tea consumption as an element of prophylaxis and pharmacotherapy support in endometrial cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef]
- Kimbal, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. Am. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- León-Reyes, G.; Argoty-Pantoja, A.D.; Becerra-Cervera, A.; López-Montoya, P.; Rivera-Paredez, B.; Velázquez-Cruz, R. Oxidative-Stress-Related Genes in Osteoporosis: A Systematic Review. Antioxidants 2023, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. Protective effect of tannic acid on the brain of adult rats exposed to cadmium and lead. Environ. Toxicol. Phar. 2013, 36, 9–18. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A. The potential protective effect of green, black, red and white tea infusions against adverse effect of cadmium and lead during chronic exposure—A rat model study. Regul. Toxicol. Pharm. 2015, 73, 521–529. [Google Scholar] [CrossRef]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef]
- Zhang, J.; Lazarenko, O.P.; Kang, J.; Blackburn, M.L.; Ronis, M.J.; Badger, T.M.; Chen, J.R. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS ONE 2013, 8, e70438. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, Y.; Zhao, J. The Application of Tannic Acid in Orthopedics. Front. Mater. 2022, 8, 567. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, Y.; Lin, J.; Nguyen, T.D.; Huang, R.; Gu, Y.; Friis, T.; Crawford, R.; Xiao, Y. Notch expressed by osteocytes plays a critical role in mineralisation. J. Mol. Med. 2018, 96, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of MAPKs activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Ortiz, A.d.C.; Fideles, S.O.M.; Reis, C.H.B.; Bellini, M.Z.; Pereira, E.d.S.B.M.; Pilon, J.P.G.; de Marchi, M.Â.; Detregiachi, C.R.P.; Flato, U.A.P.; Trazzi, B.F.d.M.; et al. Therapeutic Effects of Citrus Flavonoids Neohesperidin, Hesperidin and Its Aglycone, Hesperetin on Bone Health. Biomolecules 2022, 12, 626. [Google Scholar] [CrossRef]
- Soyocak, A.; Kurt, H.; Cosan, D.T.; Saydam, F.; Calis, I.U.; Kolac, Z.O.; Koroglu, U.K.; Degirmenci, I.; Mutlu, F.S.; Gunes, H.V. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum. Exp. Toxicol. 2019, 38, 1296–1301. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-κB activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Duan, P.; Bonewald, L.F. The role of the wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell. Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Jo, E.S.; Rugamba, A.; Kim, W.S.; Park, Y.M.; Hwang, D.Y.; Yoo, J.S.; Liu, Q.; Jang, K.J.; et al. Tannic Acid Promotes TRAIL-Induced Extrinsic Apoptosis by Regulating Mitochondrial ROS in Human Embryonic Carcinoma Cells. Cells 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, X.; Liu, Y.; Yu, X. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The role of gut microbiota in bone homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Tu, Y.; Yang, R.; Xu, X.; Zhou, X. The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 2021, 110, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Wołkowicz, T.; Januszkiewicz, A.; Szych, J. Gut microbiome and its dysbiosis as an important factor influencing the human health condition. Med. Doś. 2014, 66, 223–235. [Google Scholar]
- Gałęcka, M.; Basińska, A.M.; Bartnicka, A. The importance of intestinal microbiota in shaping human health—implications in the practice of the family physicia. Forum Med. Rod. 2018, 12, 50–59. [Google Scholar]
- Wang, J.; Wang, Y.; Gao, W.; Wang, B.; Zhao, H.; Zeng, Y.; Ji, Y.; Hao, D. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. PeerJ 2017, 5, e3450. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Gargiulo Isacco, C.; Inchingolo, A.D.; Nguyen, K.C.D.; Cantore, S.; Santacroce, L.; Scacco, S.; Cirulli, N.; Corriero, A.; Puntillo, F.; et al. The human microbiota key role in the bone metabolism activity. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2659–2670. [Google Scholar] [PubMed]
- Ni, J.J.; Yang, X.L.; Zhang, H.; Xu, Q.; Wei, X.-T.; Feng, G.-J.; Zhao, M.; Pei, Y.-F.; Zhang, L. Assessing causal relationship from gut microbiota to heel bone mineral density. Bone 2021, 143, 115652. [Google Scholar] [CrossRef] [PubMed]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef] [PubMed]
- Duffuler, P.; Bhullar, K.S.; Wu, J. Targeting gut microbiota in osteoporosis: Impact of the microbial based functional food ingredients. Food Sci. Hum. Wellness 2024, 13, 1–15. [Google Scholar] [CrossRef]
- Wallace, T.C.; Marzorati, M.; Spence, L.; Weaver, C.M.; Williamson, P.S. New Frontiers in Fibers: Innovative and Emerging Research on the Gut Microbiome and Bone Health. J. Am. Coll. Nutr. 2017, 36, 218–222. [Google Scholar] [CrossRef]
- Mineo, H.; Hara, H.; Tomita, F. Short-chain fatty acids enhance diffusional ca transport in the epithelium of the rat cecum and colon. Life Sci. 2001, 69, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Muszyński, S.; Tomaszewska, E. Bioactive compounds, antibiotics and heavy metals: Effects on the intestinal structure and microbiome of monogastric animals—a non-systematic review. Ann. Anim. Sci. 2023, 23, 289–313. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Navajas-Porras, B.; López-Maldonado, A.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Green tea and its relation to human gut microbiome. Molecules 2021, 26, 3907. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.A. Plant extracts as natural modulators of gut microbiota community structure and functionality. Heliyon 2020, 6, e05474. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Hu, Y.; Huang, J.; Yang, H.; Wang, L.; Chen, S.; Chen, C.; He, S. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J. Anim. Sci. 2020, 98, skaa112. [Google Scholar] [CrossRef]
- Choi, J.; Yadav, S.; Wang, J.; Lorentz, B.J.; Lourenco, J.M.; Callaway, T.R.; Kim, W.K. Effects of supplemental tannic acid on growth performance, gut health, microbiota, and fat accumulation and optimal dosages of tannic acid in broilers. Front. Physiol. 2022, 13, 912797. [Google Scholar] [CrossRef]
- Abbel-Monein, M.A. Effect of using green beans processing by-products with and without enzyme supplementation on broilers performance and blood parameters. J. Agrobiol. 2013, 30, 43–54. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Klebaniuk, R.; Kwiecień, M.; Tomczyk-Warunek, A.; Szymańczyk, S.; Kowalik, S.; Milczarek, A.; Blicharski, T.; Muszyński, S. Gut-bone axis response to dietary replacement of soybean meal with raw low-tannin faba bean seeds in broiler chickens. PLoS ONE 2018, 13, e0194969. [Google Scholar] [CrossRef]
- Hu, X.-F.; Wang, L.; Lu, Y.-Z.; Xiang, G.; Wu, Z.-X.; Yan, Y.-B.; Zhang, Y.; Zhao, X.; Zang, Y.; Shi, L.; et al. Adiponectin Improves the Osteointegration of Titanium Implant under Diabetic Conditions by Reversing Mitochondrial Dysfunction via the AMPK Pathway In Vivo and In Vitro. Acta Biomater. 2017, 61, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.; Jang, G.N.; Hong, M.H.; Yeo, J.; Shin, H.; Kim, W.J.; Shin, H. Biomimetic anti-inflammatory and osteogenic nanoparticles self-assembled with mineral ions and tannic acid for tissue engineering. Nano Converg. 2022, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Du, Q.; Qu, Y.; Shao, C.; Chen, C.; Sun, J.; Mao, C.; Tang, R.; Gu, X. Tannic acid induces dentin biomineralization by crosslinking and surface modification. RSC Adv. 2022, 12, 3454–3464. [Google Scholar] [CrossRef]
- Qin, Q.; Lee, S.; Patel, N.; Walden, K.; Gomez-Salazar, M.; Levi, B.; James, A.W. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 2022, 54, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Leitão, L.; Neto, E.; Conceição, F.; Monteiro, A.; Couto, M.; Alves, C.J.; Sousa, D.M.; Lamghari, M. Osteoblasts are inherently programmed to repel sensory innervation. Bone Res. 2020, 8, 20. [Google Scholar] [CrossRef]
- Tabarowski, Z.; Gibson-Berry, K.; Felten, S.Y. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 1996, 98, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, P.; Priemel, M.; Rueger, J.M.; Amling, M. Bone remodeling: New aspects of a key process that controls skeletal maintenance and repair. Osteoporos. Int. 2005, 16, S18–S24. [Google Scholar] [CrossRef]
- Takeda, S. Central control of bone remodeling. Biochem. Biophys. Res. Commun. 2005, 18, 328, 697–699. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Xiong, Y.; Yang, L.; Wang, C.; Zhang, R.; Zhu, X. Postmenopausal osteoporosis is associated with the regulation of SP, CGRP, VIP, and NPY. Biomed. Pharmacother. 2018, 104, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Brazill, J.M.; Beeve, A.T.; Craft, C.S.; Ivanusic, J.J.; Scheller, E.L. Nerves in Bone: Evolving Concepts in Pain and Anabolism. J. Bone Miner. Res. 2019, 34, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.Q.; Qin, W.P.; Ma, Y.X.; Shen, M.J.; Li, J.; Zhang, Z.B.; Chen, J.H.; Tay, F.R.; Niu, L.N.; Jiao, K. Crosstalk between Bone and Nerves within Bone. Adv. Sci. 2021, 8, 2003390. [Google Scholar] [CrossRef] [PubMed]
- Juhász, T.; Helgadottir, S.L.; Tamás, A.; Reglődi, D.; Zákány, R. PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 2015, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Feng, L.; Zhu, M.L.; Yang, Z.M.; Wu, T.Y.; Xu, J.; Liu, Y.; Lin, W.P.; Lo, J.H.T.; Zhang, J.F.; et al. Vasoactive Intestinal Peptide Stimulates Bone Marrow-Mesenchymal Stem Cells Osteogenesis Differentiation by Activating Wnt/β-Catenin Signaling Pathway and Promotes Rat Skull Defect Repair. Stem. Cells Dev. 2020, 29, 655–666. [Google Scholar] [CrossRef]
- Imai, S.; Yoshitaka, M. Neuronal regulation of bone metabolism and anabolism: Calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc. Res. Techniq. 2002, 58, 61–69. [Google Scholar] [CrossRef]
- Jing, W.; Xiaolan, C.; Yu, C.; Feng, Q.; Haifeng, Y. Pharmacological effects and mechanisms of tannic acid. Biomed. Pharmacother. 2022, 154, 113561. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S. Natural polyphenols: An overview. Int. J. Food Prop. 2020, 20, 1689–1699. [Google Scholar] [CrossRef]
- Đudarić, L.; Fužinac-Smojver, A.; Muhvić, D.; Giacometti, J. The role of polyphenols on bone metabolism in osteoporosis. Food Res. Int. 2015, 77, 290–298. [Google Scholar] [CrossRef]
- Skiba, G.; Raj, S.; Sobol, M.; Kowalczyk, P.; Grela, E.R. Role of Polyphenols in the Metabolism of the Skeletal System in Humans and Animals—A Review. Ann. Anim. Sci. 2021, 21, 1275–1300. [Google Scholar] [CrossRef]
- Myllyharju, J.; Kivirikko, K. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of Collagen. Methods Mol. Biol. 2021, 2347, 17–25. [Google Scholar]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, B.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Ku, C.; Sathishkumar, M.; Mun, S. Binding affinity of proanthocyanidin from waste Pinus radiata bark onto proline-rich bovine achilles tendon collagen type I. Chemosphere 2007, 67, 1618–1627. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Mazur, O. Collagen-Based Materials Modified by Phenolic Acids-A Review. Materials 2020, 13, 3641. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Albu, M.G.; Popa, L.; Leca, M.; Brazdaru, L.; Cotrut, C.; Trandafir, V. Drug delivery systems based on collagen-tannic acid matrices. Rev. Roum. Chim. 2009, 54, 1103–1110. [Google Scholar]
- Albu, M.G.; Deselnicu, V.; Ioannidis, I.; Deselnicu, D.; Chelaru, C. Chemical functionalization and stabilization of type I collagen with organic tanning agents. Korean J. Chem. Eng. 2014, 32, 354–361. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The comparison of physic-chemical properties of chitosan/collagen/hyaluronic acid composites with nano-hydroxyapatite cross-linked by dialdehyde starch and tannic acid. Polym. Test. 2017, 62, 171–176. [Google Scholar] [CrossRef]
- Booth, B.W.; Inskeep, B.D.; Shah, H.; Park, J.P.; Hay, E.J.; Burg, K.J. Tannic Acid preferentially targets estrogen receptor-positive breast cancer. Int. J. Breast. Cancer 2013, 2013, 369609. [Google Scholar] [CrossRef]
- Nie, F.; Liang, Y.; Jiang, B.; Li, X.; Xun, H.; He, W.; Lau, H.T.; Ma, X. Apoptotic effect of tannic acid on fatty acid synthase over-expressed human breast cancer cells. Tumour Biol. 2016, 37, 2137–2143. [Google Scholar] [CrossRef]
- Ngobili, T.A.; Shah, H.; Park, J.P.; Kwist, K.W.; Inskeep, B.; Burg, K.J.; Booth, B.W. Remodeling of tannic acid crosslinked collagen type I induces apoptosis in ER+ breast cancer cells. Anticancer Res. 2015, 35, 1285–1290. [Google Scholar] [PubMed]
- Jordan, L.G.; Booth, B.W. HER2+ breast cancer cells undergo apoptosis upon exposure to tannic acid released from remodeled cross-linked collagen type I. J. Biomed. Mater. Res. A 2018, 106, 26–32. [Google Scholar] [CrossRef]
- AlMalki, F.A.; Hassan, A.M.; Klaab, Z.M.; Abdulla, S.; Pizzi, A. Tannin Nanoparticles (NP99) Enhances the Anticancer Effect of Tamoxifen on ER+ Breast Cancer Cells. J. Renew. Mater. 2021, 9, 2077–2092. [Google Scholar] [CrossRef]
- Xu, W.; Ning, Y.; Cao, S.; Wu, G.; Sun, H.; Chai, L.; Wu, S.; Li, J.; Luo, D. Insight into the interaction between tannin acid and bovine serum albumin from a spectroscopic and molecular docking perspective. RSC Adv. 2023, 13, 10592–10599. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of tannic acid when used as feed flavouring for all animal species. EFSA J. 2014, 12, 3828. [Google Scholar] [CrossRef]
- Onodera, H.; Kitaura, K.; Mitsumori, K.; Yoshida, J.; Yasuhara, K.; Shimo, T.; Takahashi, M.; Hayashi, Y. Study on the carcinogenicity of tannic acid in F344 rats. Food Chem. Toxicol. 1994, 32, 1101–1106. [Google Scholar] [CrossRef]
- Chung, M.Y.; Song, J.H.; Lee, J.; Shin, E.J.; Park, J.H.; Lee, S.H.; Hwang, J.T.; Choi, H.K. Tannic acid, a novel histone acetyltransferase inhibitor, prevents non-alcoholic fatty liver disease both in vivo and in vitro model. Mol. Metab. 2019, 19, 34–48. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Muszyński, S.; Tomaszewska, E.; Kwiecień, M.; Donaldson, J.; Tomczyk-Warunek, A.; Blicharski, T. The Impact of Tannic Acid Consumption on Bone Mineralization. Metabolites 2023, 13, 1072. https://doi.org/10.3390/metabo13101072
Winiarska-Mieczan A, Muszyński S, Tomaszewska E, Kwiecień M, Donaldson J, Tomczyk-Warunek A, Blicharski T. The Impact of Tannic Acid Consumption on Bone Mineralization. Metabolites. 2023; 13(10):1072. https://doi.org/10.3390/metabo13101072
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Siemowit Muszyński, Ewa Tomaszewska, Małgorzata Kwiecień, Janine Donaldson, Agnieszka Tomczyk-Warunek, and Tomasz Blicharski. 2023. "The Impact of Tannic Acid Consumption on Bone Mineralization" Metabolites 13, no. 10: 1072. https://doi.org/10.3390/metabo13101072
APA StyleWiniarska-Mieczan, A., Muszyński, S., Tomaszewska, E., Kwiecień, M., Donaldson, J., Tomczyk-Warunek, A., & Blicharski, T. (2023). The Impact of Tannic Acid Consumption on Bone Mineralization. Metabolites, 13(10), 1072. https://doi.org/10.3390/metabo13101072







