Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Anthropometric and Biochemical Analysis
2.3. Definitions
2.4. Metabolome Profile
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants
3.2. Serum Metabolic Profiles of MHO and MUO
3.3. Differential Metabolites
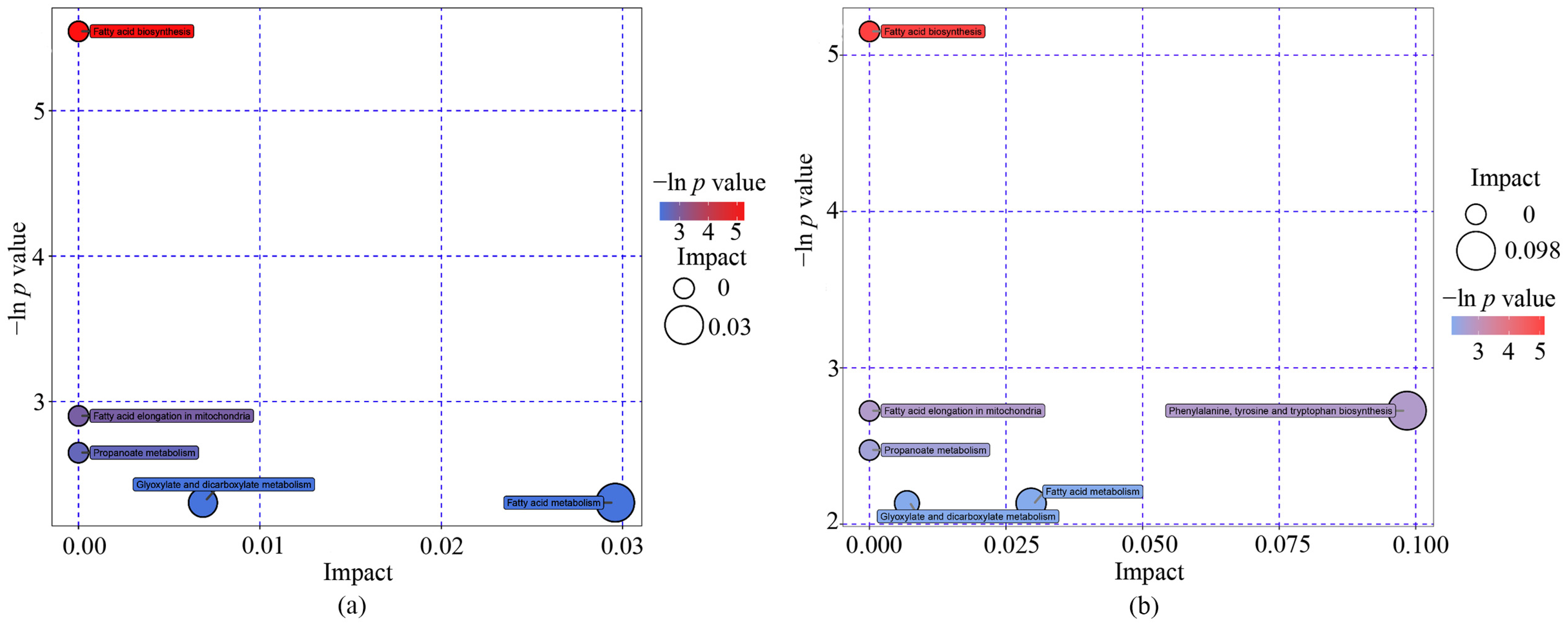
3.4. Metabolite Pathways of Differential Metabolites
3.5. Further Identification of Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danese, A.; Tan, M. Childhood maltreatment and obesity: Systematic review and meta-analysis. Mol. Psychiatry 2014, 19, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Chen, L.; Liu, J.Y.; Ma, T.; Zhang, Y.; Chen, M.M.; Zhong, P.L.; Shi, D.; Hu, P.J.; Li, J.; et al. Epidemiology and prediction of overweight and obesity among children and adolescents aged 7–18 years in China from 1985 to 2019. Chin. J. Prev. Med. 2023, 57, 11–19. [Google Scholar]
- Smith, G.I.; Mittendorfer, B.; Klein, S. Metabolically healthy obesity: Facts and fantasies. J. Clin. Investig. 2019, 129, 3978–3989. [Google Scholar] [CrossRef]
- Vinciguerra, F.; Tumminia, A.; Baratta, R.; Ferro, A.; Alaimo, S.; Hagnäs, M.; Graziano, M.; Vigneri, R.; Frittitta, L. Prevalence and Clinical Characteristics of Children and Adolescents with Metabolically Healthy Obesity: Role of Insulin Sensitivity. Life 2020, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Keihani, S.; Hosseinpanah, F.; Barzin, M.; Serahati, S.; Doustmohamadian, S.; Azizi, F. Abdominal obesity phenotypes and risk of cardiovascular disease in a decade of follow-up: The Tehran Lipid and Glucose Study. Atherosclerosis 2015, 238, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Q.; Liu, J.T.; Shang, Y.X.; Gao, B.; Zhao, X.Y.; Zhao, H.P.; Wu, W.J. DXA-measured visceral fat mass and lean body mass reflect abnormal metabolic phenotypes among some obese and nonobese Chinese children and adolescents. Nutr. Metab. Cardiovasc. 2018, 28, 618–628. [Google Scholar] [CrossRef]
- Chen, H.-H.; Tseng, Y.J.; Wang, S.-Y.; Tsai, Y.-S.; Chang, C.-S.; Kuo, T.-C.; Yao, W.-J.; Shieh, C.-C.; Wu, C.-H.; Kuo, P.-H. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int. J. Obes. 2015, 39, 1241–1248. [Google Scholar] [CrossRef]
- Zhao, X.; Gang, X.; Liu, Y.; Sun, C.; Han, Q.; Wang, G. Using Metabolomic Profiles as Biomarkers for Insulin Resistance in Childhood Obesity: A Systematic Review. J. Diabetes Res. 2016, 2016, 8160545. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Michaliszyn, S.F.; De Las Heras, J.; Bacha, F.; Lee, S.; Chace, D.H.; DeJesus, J.V.; Arslanian, S.A. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: Evidence for enhanced mitochondrial oxidation. Diabetes Care 2012, 35, 605–611. [Google Scholar] [CrossRef]
- Ji, C. Report on childhood obesity in China (1)—Body mass index reference for screening overweight and obesity in Chinese school-age children. Biomed. Environ. Sci. 2005, 18, 390–400. [Google Scholar]
- Wan, N.-J.; Mi, J.; Wang, T.-Y.; Duan, J.-L.; Li, M.; Gong, C.-X.; Du, J.-B.; Zhao, X.-Y.; Cheng, H.; Hou, D.-Q.; et al. Metabolic syndrome in overweight and obese schoolchildren in Beijing. Chin. J. Pediatr. 2007, 45, 417–421. [Google Scholar]
- Ma, G.-S.; Ji, C.-Y.; Ma, J.; Mi, J.; Sung, R.Y.; Xiong, F.; Yan, W.-L.; Hu, X.-Q.; Li, Y.-P.; DU, S.-M.; et al. Waist Circumference Reference Values for Screening Cardiovascular Risk Factors in Chinese Children and Adolescents. Biomed. Environ. Sci. 2010, 23, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yan, Y.K.; Mi, J. Updating blood pressure references for Chinese children aged 3–17 years. Chin. J. Hypertens. 2017, 25, 428–435. [Google Scholar]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Van Rooijen, M.A.; Plat, J.; Zock, P.L.; Blom, W.A.; Mensink, R.P. Effects of two consecutive mixed meals high in palmitic acid or stearic acid on 8-h postprandial lipemia and glycemia in healthy-weight and overweight men and postmenopausal women: A randomized controlled trial. Eur. J. Nutr. 2021, 60, 3659–3667. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Guan, C.; Li, K.; Wang, C.; Feng, R.; Sun, C. Free fatty acid metabolic profile and biomarkers of isolated post-challenge diabetes and type 2 diabetes mellitus based on GC–MS and multivariate statistical analysis. J. Chromatogr. B 2010, 878, 2817–2825. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Deng, X.; Okekunle, A.P.; Wang, P.; Zhang, Q.; Ding, L.; Guo, X.; Lv, M.; Sun, C.; et al. Postprandial Saturated Fatty Acids Increase the Risk of Type 2 Diabetes: A Cohort Study in a Chinese Population. J. Clin. Endocrinol. Metab. 2018, 103, 1438–1446. [Google Scholar] [CrossRef]
- Lu, H.; Hao, L.; Li, S.; Lin, S.; Lv, L.; Chen, Y.; Cui, H.; Zi, T.; Chu, X.; Na, L.; et al. Elevated circulating stearic acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway. Diabetologia 2016, 59, 1247–1257. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, R.; Zhang, Y.; Shi, H.; Sun, H.; Chu, X.; Wu, X.; Lu, H.; Sun, C. miRNA-mRNA profile and regulatory network in stearic acid-treated β-cell dysfunction. J. Endocrinol. 2020, 246, 13–27. [Google Scholar] [CrossRef]
- Borradaile, N.M.; Han, X.; Harp, J.D.; Gale, S.E.; Ory, D.S.; Schaffer, J.E. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006, 47, 2726–2737. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Zhang, W.Y.; Karnik, S.K.; Borwege, S.; Anand, V.R.; Laine, P.S.; Su, Y.; Reaven, P.D. Nutrient modification of the innate immune response: A novel mechanism by which saturated fatty acids greatly amplify monocyte inflammation. Arter. Thromb. Vasc. Biol. 2010, 30, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Minkler, P.E.; Lesnefsky, E.J.; Hoppel, C.L. Fatty acid chain elongation in palmitate-perfused working rat heart: Mitochondrial acetyl-CoA is the source of two-carbon units for chain elongation. J. Biol. Chem. 2014, 289, 10223–10234. [Google Scholar] [CrossRef] [PubMed]
- Welsh, N.; Cnop, M.; Kharroubi, I.; Bugliani, M.; Lupi, R.; Marchetti, P.; Eizirik, D.L. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes 2005, 54, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Prinsen, H.; Giannini, C.; De Graaf, R.; Juchem, C.; Li, F.; Caprio, S.; Santoro, N.; Herzog, R.I. Elevated α-Hydroxybutyrate and Branched-Chain Amino Acid Levels Predict Deterioration of Glycemic Control in Adolescents. J. Clin. Endocrinol. Metab. 2017, 102, 2473–2481. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Cunha, D.M.; Franco, C.; Teixeira, C.; Gojon, F.; Baylina, P.; Fernandes, R. Which Role Plays 2-Hydroxybutyric Acid on Insulin Resistance? Metabolites 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Dweikat, I.M.; Naser, E.N.; Abu Libdeh, A.I.; Naser, O.J.; Abu Gharbieh, N.N.; Maraqa, N.F.; Abu Libdeh, B.Y. Propionic acidemia mimicking diabetic ketoacidosis. Brain Dev. 2011, 33, 428–431. [Google Scholar] [CrossRef]
- Schwab, M.A.; Sauer, S.W.; Okun, J.G.; Nijtmans, L.G.J.; Rodenburg, R.; Heuvel, L.P.V.D.; Dröse, S.; Brandt, U.; Hoffmann, G.F.; Ter Laak, H.; et al. Secondary mitochondrial dysfunction in propionic aciduria: A pathogenic role for endogenous mitochondrial toxins. Biochem. J. 2006, 398, 107–112. [Google Scholar] [CrossRef]
- Cao, L.-C.; Honeyman, T.W.; Cooney, R.; Kennington, L.; Scheid, C.R.; Jonassen, J.A. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int. 2004, 66, 1890–1900. [Google Scholar] [CrossRef]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Chen, M.H.; Keyes, M.; Rhee, E.P.; Clish, C.B.; Vasan, R.S.; Gerszten, R.E.; et al. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS ONE 2016, 11, e0148361. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, M.; Zhu, Y.; Wang, X.; Zheng, L.; Yin, Y. LC-MS-Based Metabolomics and Lipidomics Study of High-Density-Lipoprotein-Modulated Glucose Metabolism with an apoA-I Knockout Mouse Model. J. Proteome Res. 2019, 18, 48–56. [Google Scholar] [CrossRef]
- Yang, L.J.; Ding, X.X.; Ren, F.D.; Cai, F.; Fu, G.H.; Ren, D.B.; Zhao, Y.L.; Zhang, H. Liquid chromatography-mass spectrometry combined with chemometrics to analyze the metabolic characteristics of patients with coronary heart disease and coronary heart disease with hypertension. Chin. J. Anal. Chem. 2021, 49, 1649–1656. [Google Scholar]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.C.; Mehrabian, M.; Lusis, A.J. Sex differences in metabolism and cardiometabolic disorders. Curr. Opin. Infect. Dis. 2018, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.M.R.; Moliz, J.N.; Quesada, A.; Montoro-Molina, S.; Vargas-Tendero, P.; Osuna, A.; Wangensteen, R.; Vargas, F. l-Arginine metabolism in cardiovascular and renal tissue from hyper- and hypothyroid rats. Exp. Biol. Med. 2015, 241, 550–556. [Google Scholar] [CrossRef]

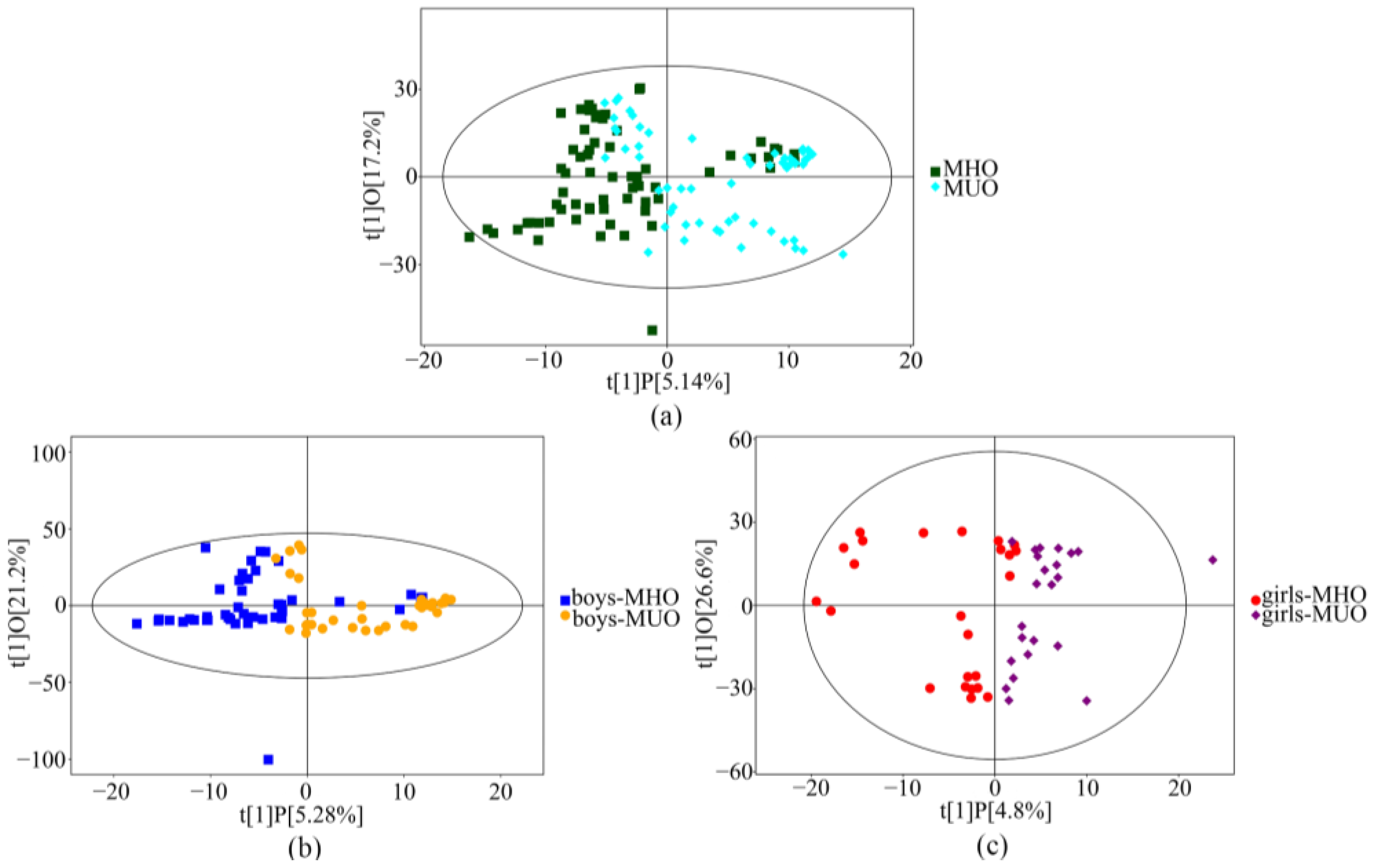
| Variables | MHO (n = 67) | MUO (n = 60) | p Value |
|---|---|---|---|
| Age (years) | 14.15 ± 1.66 | 14.73 ± 1.67 | 0.051 |
| Female, n (%) | 24 (35.8) | 23 (38.3) | 0.700 |
| Height (cm) | 168.9 ± 8.7 | 170.7 ± 8.6 | 0.259 |
| Weight (kg) | 80.6 ± 11.9 | 86.5 ± 13.7 | 0.010 |
| BMI (kg/m2) | 28.1 ± 2.5 | 29.6 ± 3.21 | 0.005 |
| WC (cm) | 91.8 ± 8.1 | 93.6 ± 10.2 | 0.335 |
| SBP (mmHg) | 119 ± 12 | 124 ± 12 | 0.013 |
| DBP (mmHg) | 69 ± 7 | 74 ± 7 | <0.001 |
| TC (mmol/L) | 3.68 ± 0.73 | 4.29 ± 0.79 | <0.001 |
| TG (mmol/L) * | 1.10 (0.92,1.32) | 2.13 (1.67,2.62) | <0.001 |
| HDL-C (mmol/L) | 1.24 ± 0.19 | 1.05 ± 0.21 | <0.001 |
| LDL-C (mmol/L) | 2.02 ± 0.66 | 2.47 ± 0.84 | 0.001 |
| Fasting glucose (mmol/L) | 4.77 ± 0.36 | 4.66 ± 0.40 | 0.173 |
| Metabolite | Mean Relative Quantitative Value in MUO Group | Mean Relative Quantitative Value in MHO Group | VIP | p Value | Fold Change |
|---|---|---|---|---|---|
| Total | |||||
| glycolic acid | 0.022 | 0.013 | 1.243 | 0.010 | 1.671 |
| palmitic acid | 1.293 | 1.668 | 2.804 | 0.002 | 0.775 |
| glucose | 0.078 | 0.056 | 1.735 | 0.035 | 1.391 |
| stearic acid | 1.243 | 1.582 | 2.667 | 0.003 | 0.786 |
| 2-hydroxybutanoic acid | 0.054 | 0.030 | 1.383 | 0.006 | 1.081 |
| galactose | 0.394 | 0.317 | 1.087 | 0.038 | 1.242 |
| phosphate | 0.443 | 0.681 | 1.949 | 0.010 | 0.651 |
| asparagine | 0.035 | 0.017 | 1.399 | 0.003 | 1.503 |
| alanine | 0.139 | 0.075 | 1.878 | 0.011 | 1.858 |
| glutamate | 0.008 | 0.013 | 2.117 | 0.034 | 0.609 |
| cyanoalanine | 0.030 | 0.020 | 2.433 | 0.029 | 1.510 |
| 3-hydroxypropionic acid | 0.167 | 0.102 | 1.266 | 0.015 | 1.639 |
| 2-hydroxypentanoic acid | 0.025 | 0.012 | 1.411 | 0.004 | 1.997 |
| hippuric acid | 0.067 | 0.039 | 1.521 | 0.020 | 1.715 |
| acetophenone | 0.076 | 0.046 | 1.877 | 0.015 | 1.663 |
| beta-gentiobiose | 0.029 | 0.018 | 0.854 | 0.022 | 1.605 |
| maltotriose | 0.006 | 0.004 | 0.843 | 0.021 | 1.540 |
| isoleucine | 0.031 | 0.019 | 1.187 | 0.021 | 1.645 |
| myristyl myristate | 0.000 | 0.001 | 1.511 | 0.046 | 0.710 |
| salicylaldehyde | 0.000 | 0.001 | 1.371 | 0.009 | 0.770 |
| Girls | |||||
| benzylalcohol | 0.001 | 0.000 | 0.686 | 0.040 | 2.407 |
| 2-ketoadipic acid | 0.000 | 0.000 | 1.074 | 0.028 | 1.819 |
| glycyl-proline | 0.000 | 0.000 | 3.020 | 0.009 | 0.582 |
| glucosamine | 0.000 | 0.000 | 2.555 | 0.007 | 0.552 |
| pyrophosphate | 0.000 | 0.000 | 1.100 | 0.042 | 0.575 |
| 2-ketobutyric acid | 0.000 | 0.000 | 3.156 | 0.015 | 0.583 |
| Boys | |||||
| 2-hydroxy-2-methylbutanoic acid | 0.021 | 0.035 | 1.867 | 0.036 | 0.594 |
| 2-hydroxybutanoic acid | 0.064 | 0.034 | 1.053 | 0.016 | 1.869 |
| 2-hydroxypentanoic acid | 0.030 | 0.014 | 0.902 | 0.006 | 2.183 |
| 3-hydroxypropionic acid | 0.198 | 0.114 | 1.283 | 0.024 | 1.743 |
| 5-methyluridine | 0.035 | 0.024 | 1.171 | 0.006 | 1.452 |
| acetophenone | 0.090 | 0.052 | 1.638 | 0.021 | 1.746 |
| alanine | 0.167 | 0.088 | 1.482 | 0.023 | 1.898 |
| asparagine | 0.045 | 0.021 | 2.004 | 0.003 | 2.105 |
| beta-gentiobiose | 0.036 | 0.019 | 0.603 | 0.011 | 1.860 |
| cyanoalanine | 0.039 | 0.025 | 3.286 | 0.017 | 1.602 |
| furoylglycine | 0.000 | 0.000 | 0.981 | 0.036 | 0.822 |
| galactinol | 0.047 | 0.030 | 0.904 | 0.020 | 1.538 |
| galactose | 0.400 | 0.306 | 1.044 | 0.039 | 1.307 |
| glutamate | 0.008 | 0.015 | 1.649 | 0.024 | 0.549 |
| glycerol-alpha-phosphate | 0.017 | 0.026 | 0.895 | 0.010 | 0.681 |
| glycolic acid | 0.026 | 0.015 | 2.050 | 0.027 | 1.674 |
| isocitric acid | 0.090 | 0.066 | 1.218 | 0.025 | 1.351 |
| isogluconic acid | 0.113 | 0.065 | 1.230 | 0.031 | 1.745 |
| isoleucine | 0.041 | 0.024 | 1.079 | 0.025 | 1.701 |
| maltotriose | 0.007 | 0.004 | 1.479 | 0.029 | 1.631 |
| palmitic acid | 1.205 | 1.667 | 2.854 | 0.002 | 0.723 |
| phosphate | 0.433 | 0.690 | 1.675 | 0.032 | 0.628 |
| salicylaldehyde | 0.000 | 0.001 | 1.633 | 0.003 | 0.731 |
| shikimic acid | 0.050 | 0.028 | 0.807 | 0.013 | 1.816 |
| stearic acid | 1.155 | 1.555 | 2.705 | 0.003 | 0.743 |
| Pathway Name | Total Number of Compounds in the Pathways | Enrichment Analysis p Value | Topology Analysis Impact Factor | Name of Metabolites and KEGG ID |
|---|---|---|---|---|
| Total (N = 127) | ||||
| Fatty acid biosynthesis | 49 | 0.004 | 0.000 | Stearic acid cpd:C01530; Palmitic acid cpd:C00249 |
| Fatty acid elongation in mitochondria | 27 | 0.055 | 0.000 | Palmitic acid cpd:C00249 |
| Propanoate metabolism | 35 | 0.071 | 0.000 | 2-Hydroxybutyric acid cpd:C05984 |
| Glyoxylate and dicarboxylate metabolism | 50 | 0.099 | 0.007 | Glycolic acid cpd:C00160 |
| Fatty acid metabolism | 50 | 0.099 | 0.030 | Palmitic acid cpd:C00249 |
| Boys (n = 80) | ||||
| Fatty acid biosynthesis | 49 | 0.006 | 0.000 | Stearic acid cpd:C01530;Palmitic acid cpd:C00249 |
| Fatty acid elongation in mitochondria | 27 | 0.066 | 0.000 | Palmitic acid cpd:C00249 |
| Propanoate metabolism | 35 | 0.084 | 0.000 | 2-Hydroxybutyric acid cpd:C05984 |
| Glyoxylate and dicarboxylate metabolism | 50 | 0.118 | 0.007 | Glycolic acid cpd:C00160 |
| Fatty acid metabolism | 50 | 0.118 | 0.030 | Palmitic acid cpd:C00249 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 27 | 0.067 | 0.098 | Shikimic acid cpd:C00493 |
| Metabolite | β | S.E. | Wald | p Value |
|---|---|---|---|---|
| Total a | ||||
| glycolic acid | −27.820 | 12.448 | 4.995 | 0.025 |
| palmitic acid | 0.856 | 0.360 | 5.657 | 0.017 |
| glucose | −7.386 | 3.997 | 3.145 | 0.065 |
| stearic acid | 0.834 | 0.372 | 5.024 | 0.025 |
| 2-hydroxybutanoic acid | 9.024 | 7.527 | 1.438 | 0.231 |
| galactose | −1.380 | 1.123 | 1.509 | 0.219 |
| phosphate | 0.761 | 0.379 | 4.026 | 0.045 |
| asparagine | −15.913 | 6.284 | 6.412 | 0.011 |
| alanine | −3.000 | 1.418 | 4.475 | 0.034 |
| glutamate | 22.905 | 16.295 | 1.976 | 0.160 |
| cyanoalanine | −15.101 | 7.701 | 3.845 | 0.050 |
| 3-hydroxypropionic acid | −3.230 | 1.409 | 5.259 | 0.022 |
| 2-hydroxypentanoic acid | −9.604 | 4.186 | 5.263 | 0.022 |
| hippuric acid | −5.234 | 3.003 | 3.037 | 0.081 |
| acetophenone | −5.504 | 2.883 | 3.646 | 0.056 |
| beta-gentiobiose | −14.408 | 7.883 | 3.340 | 0.068 |
| maltotriose | −89.458 | 48.507 | 3.401 | 0.065 |
| isoleucine | −16.832 | 8.126 | 4.291 | 0.038 |
| myristyl myristate | 576.787 | 485.312 | 1.412 | 0.235 |
| salicylaldehyde | 1354.748 | 794.353 | 2.909 | 0.088 |
| Girls b | ||||
| benzylalcohol | −1090.490 | 657.516 | 2.751 | 0.097 |
| 2-ketoadipic acid | 9133.505 | 4866.245 | 3.523 | 0.061 |
| glycyl-proline | 4800.051 | 2407.659 | 3.975 | 0.046 |
| glucosamine | 5353.060 | 2541.747 | 4.435 | 0.035 |
| pyrophosphate | 4161.921 | 2452.639 | 2.944 | 0.086 |
| 2-ketobutyric acid | 9133.505 | 4866.245 | 3.523 | 0.061 |
| Boys b | ||||
| 2-hydroxy-2-methylbutanoic acid | 14.974 | 8.681 | 2.975 | 0.085 |
| 2-hydroxybutanoic acid | −10.303 | 4.774 | 4.657 | 0.031 |
| 2-hydroxypentanoic acid | −25.500 | 10.365 | 6.053 | 0.014 |
| 3-hydroxypropionic acid | −3.964 | 1.716 | 5.337 | 0.021 |
| 5-methyluridine | −47.939 | 18.506 | 6.711 | 0.010 |
| acetophenone | −7.110 | 3.528 | 4.061 | 0.044 |
| alanine | −3.398 | 1.698 | 4.004 | 0.045 |
| asparagine | −19.827 | 7.431 | 7.119 | 0.008 |
| beta-gentiobiose | −19.175 | 9.517 | 4.060 | 0.044 |
| cyanoalanine | −20.389 | 9.125 | 4.992 | 0.025 |
| furoylglycine | 2916.777 | 1479.492 | 3.887 | 0.049 |
| galactinol | −19.112 | 8.661 | 4.869 | 0.027 |
| galactose | −2.548 | 1.552 | 2.694 | 0.101 |
| glutamate minor | 38.914 | 20,759 | 3.514 | 0.061 |
| glycerol-alpha-phosphate | 38.874 | 17.372 | 5.007 | 0.025 |
| glycolic acid | −32.656 | 15.120 | 4.664 | 0.031 |
| isocitric acid minor | −12.577 | 6.127 | 4.152 | 0.042 |
| isogluconic acid | −4.772 | 2.591 | 3.391 | 0.066 |
| isoleucine | −18.384 | 9.066 | 4.112 | 0.043 |
| maltotriose | −111.404 | 59.697 | 3.483 | 0.062 |
| palmitic acid | 1.408 | 0.534 | 6.952 | 0.008 |
| phosphate | 0.868 | 0.466 | 3.476 | 0.062 |
| salicylaldehyde | 2711.686 | 1206.927 | 5.048 | 0.025 |
| shikimic acid | −14.479 | 7.244 | 3.995 | 0.046 |
| stearic acid | 1.404 | 0.566 | 6.163 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Tian, M.; Ma, X.; Bai, L.; Zhou, J.; Ding, W. Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years. Metabolites 2023, 13, 641. https://doi.org/10.3390/metabo13050641
Tong L, Tian M, Ma X, Bai L, Zhou J, Ding W. Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years. Metabolites. 2023; 13(5):641. https://doi.org/10.3390/metabo13050641
Chicago/Turabian StyleTong, Lingling, Mei Tian, Xiaoyan Ma, Ling Bai, Jinyu Zhou, and Wenqing Ding. 2023. "Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years" Metabolites 13, no. 5: 641. https://doi.org/10.3390/metabo13050641
APA StyleTong, L., Tian, M., Ma, X., Bai, L., Zhou, J., & Ding, W. (2023). Metabolome Profiling and Pathway Analysis in Metabolically Healthy and Unhealthy Obesity among Chinese Adolescents Aged 11–18 Years. Metabolites, 13(5), 641. https://doi.org/10.3390/metabo13050641




