Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters’ Tissue Content in Rats’ Hippocampus and Amygdala
Abstract
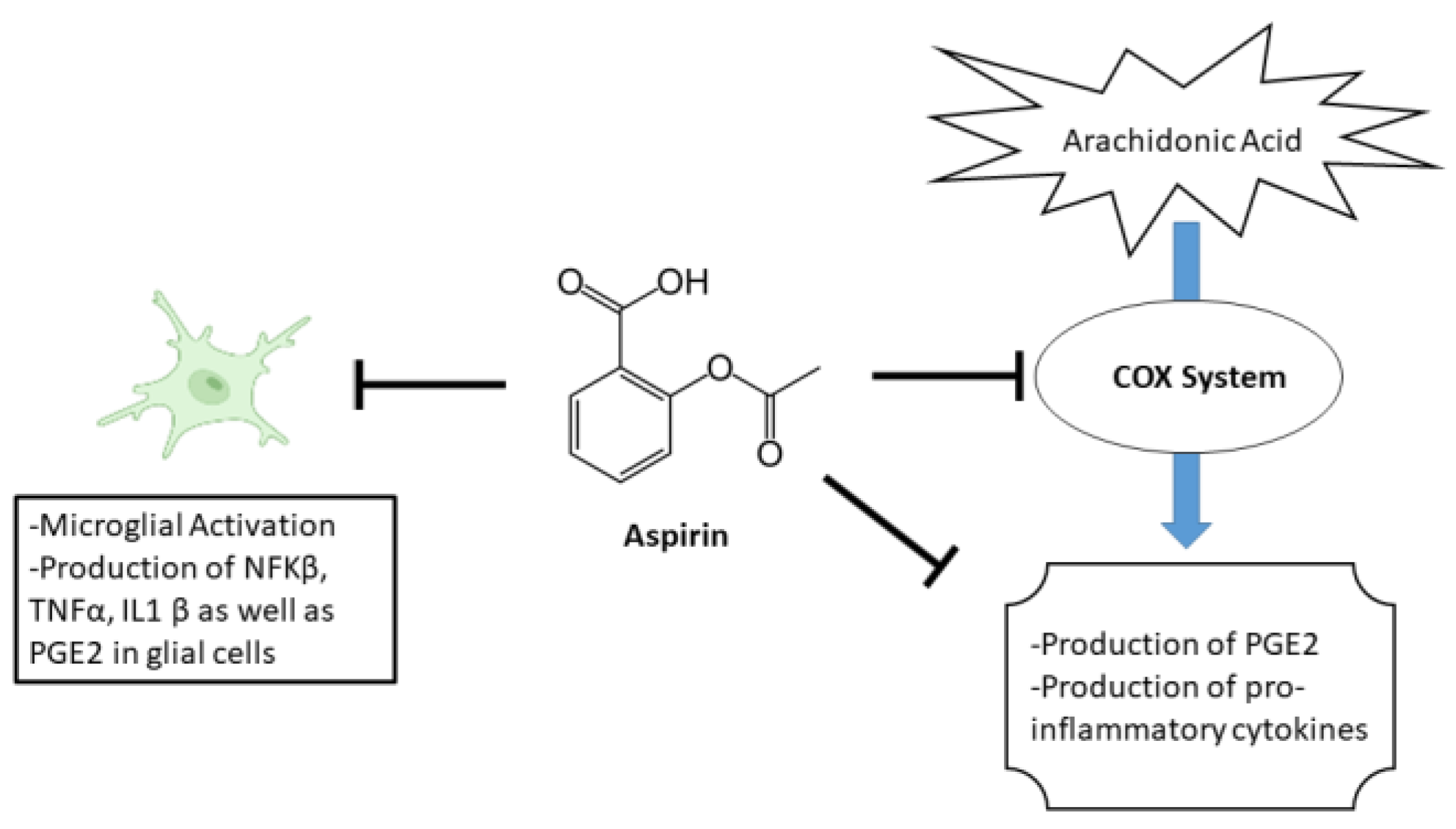
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals and Materials
2.3. Cigarette Smoke Exposure
2.4. Elevated plus Maze Test
2.5. Brian Regions Collection
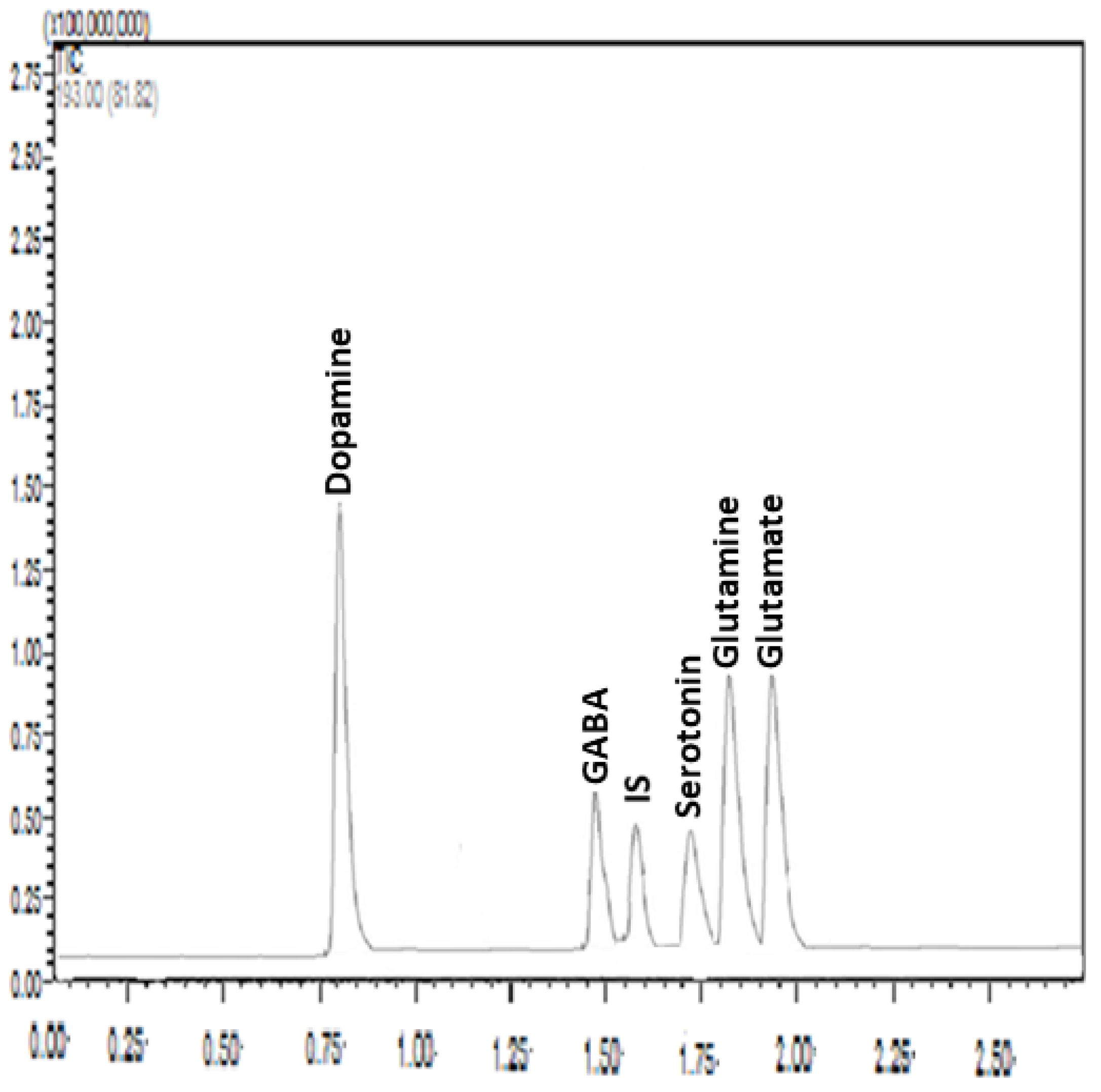
2.6. Sample Preparation and Chromatographic Analysis
2.7. Sample Size Calculation
2.8. Statistical Analysis
3. Results
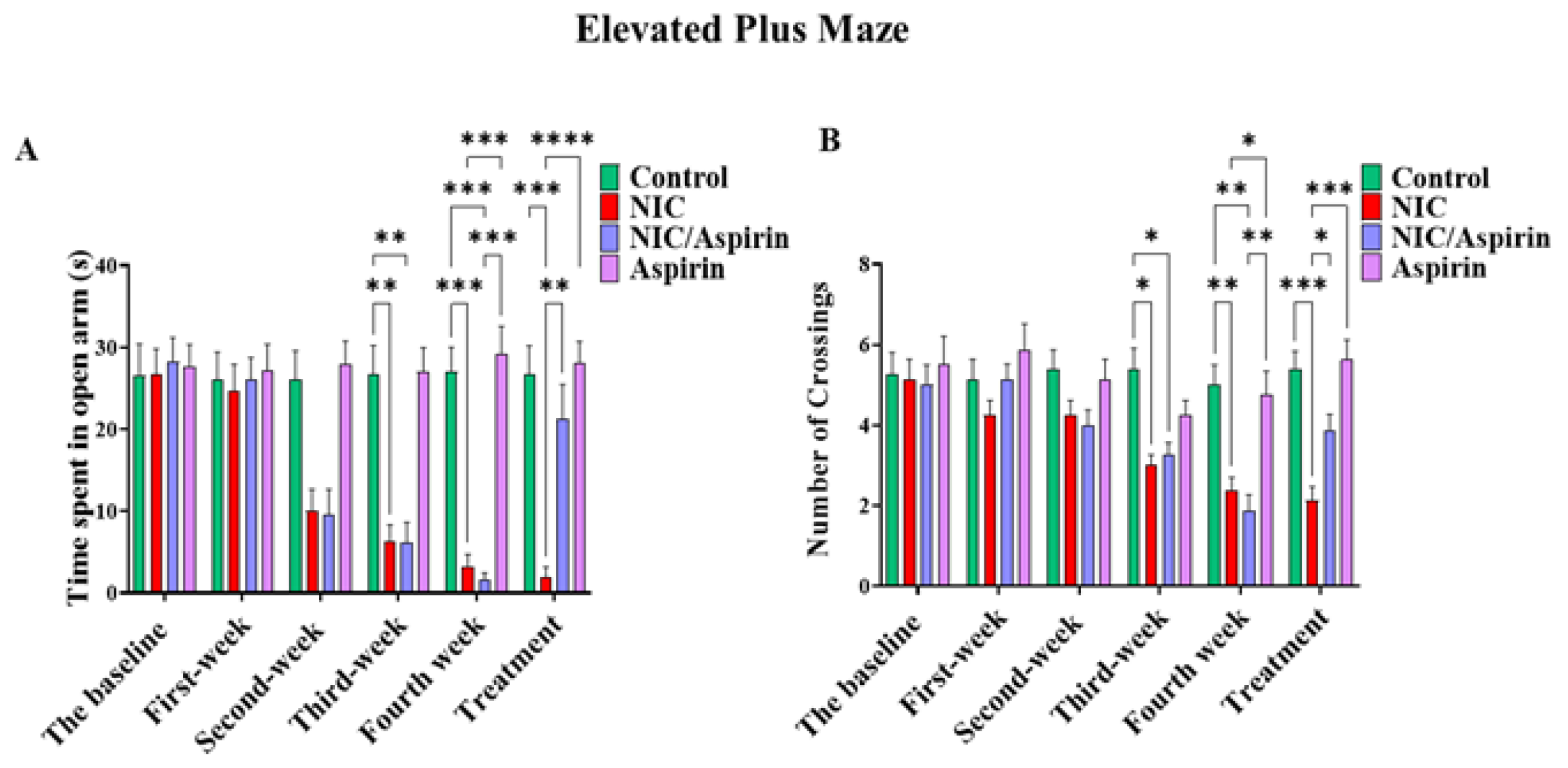
3.1. Effect of Whole-Body Tobacco Cigarette Smoke Exposure and Aspirin Treatment on Elevated plus Maze Behavioral Test
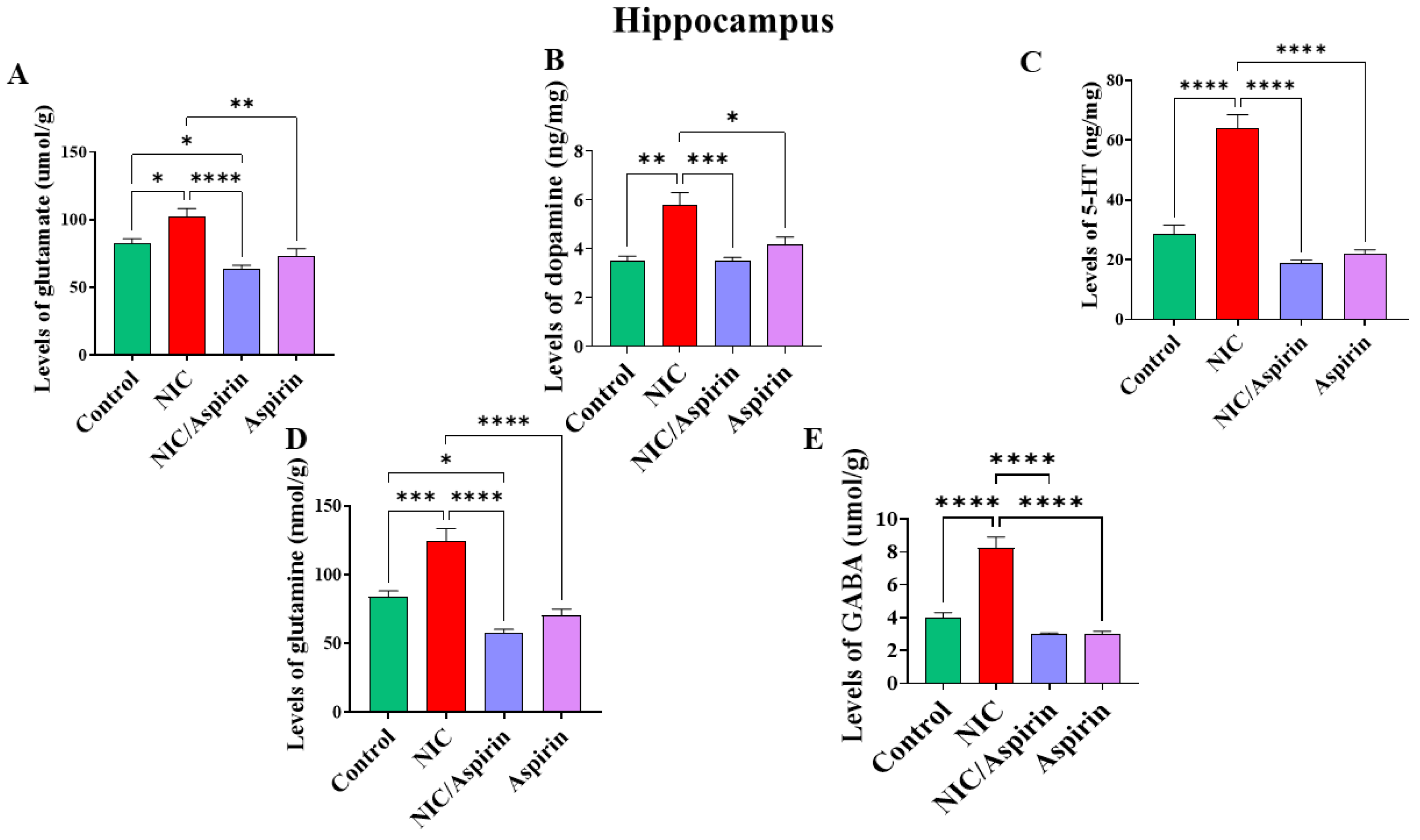
3.2. Effect of Whole-Body Cigarette Smoke Exposure and Aspirin Treatment on Tissue Content of Neurotransmitters in Hippocampus
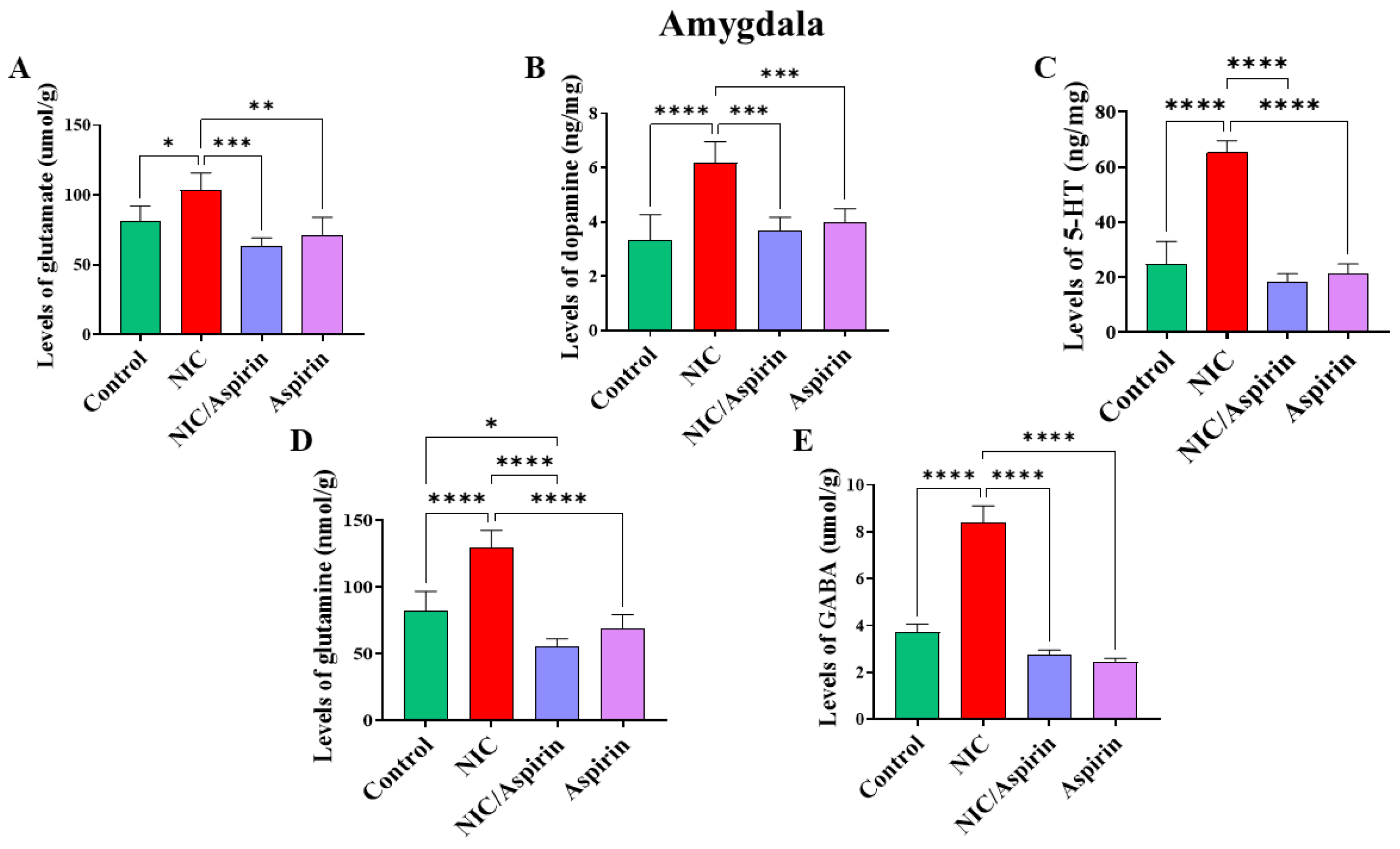
3.3. Effect of Whole-Body Cigarette Smoke Exposure and Aspirin Treatment on Tissue Content of Neurotransmitters in the Amygdala Brain Region
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 11 February 2023).
- Zvolensky, M.J.; Paulus, D.J.; Langdon, K.J.; Robles, Z.; Garey, L.; Norton, P.J.; Businelle, M.S. Anxiety sensitivity explains associations between anxious arousal symptoms and smoking abstinence expectancies, perceived barriers to cessation, and problems experienced during past quit attempts among low-income smokers. J. Anxiety Disord. 2017, 48, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Brunzell, D.H.; Caldarone, B.J. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport 2002, 13, 1097–1106. [Google Scholar] [CrossRef]
- Yehia, D.B.M.; Jacoub, S.M.; Eser, S.M. Predictors of Coping Strategies among Nursing College Students at AL-Zaytoonah University of Jordan. J. Educ. Pract. 2016, 7, 149–154. [Google Scholar]
- Garey, L.; Olofsson, H.; Garza, T.; Shepherd, J.M.; Smit, T.; Zvolensky, M.J. The role of anxiety in smoking onset, severity, and cessation-related outcomes: A review of recent literature. Curr. Psychiatry Rep. 2020, 22, 38. [Google Scholar] [CrossRef]
- Moylan, S. Cigarette Smoking and Increased Anxiety Symptoms and Disorders; Deakin University: Geelong, Australia, 2015. [Google Scholar]
- Morrell, H.E.; Cohen, L.M. Cigarette smoking, anxiety, and depression. J. Psychopathol. Behav. Assess. 2006, 28, 281–295. [Google Scholar] [CrossRef]
- McDermott, M.S.; Marteau, T.M.; Hollands, G.J.; Hankins, M.; Aveyard, P. Change in anxiety following successful and unsuccessful attempts at smoking cessation: Cohort study. Br. J. Psychiatry 2013, 202, 62–67. [Google Scholar] [CrossRef]
- Moylan, S.; Jacka, F.N.; Pasco, J.A.; Berk, M. Cigarette smoking, nicotine dependence and anxiety disorders: A systematic review of population-based, epidemiological studies. BMC Med. 2012, 10, 123. [Google Scholar] [CrossRef]
- Benowitz, N.L. Neurobiology of nicotine addiction: Implications for smoking cessation treatment. Am. J. Med. 2008, 121, S3–S10. [Google Scholar] [CrossRef]
- Moylan, S.; Jacka, F.N.; Pasco, J.A.; Berk, M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: A critical review of biological pathways. Brain Behav. 2013, 3, 302–326. [Google Scholar] [CrossRef]
- Morissette, S.B.; Tull, M.T.; Gulliver, S.B.; Kamholz, B.W.; Zimering, R.T. Anxiety, anxiety disorders, tobacco use, and nicotine: A critical review of interrelationships. Psychol. Bull. 2007, 133, 245. [Google Scholar] [CrossRef]
- Yasin, S.M. Workplace Smoking Cessation: Smoking Relapse, Sustained Cessation and Behavioural Attributes Following a Quit Attempt. Ph.D. Thesis, Department of Social and Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, 2013. [Google Scholar]
- Bystritsky, A.; Khalsa, S.S.; Cameron, M.E.; Schiffman, J. Current diagnosis and treatment of anxiety disorders. Pharm. Ther. 2013, 38, 30. [Google Scholar]
- Picciotto, M.R.; Lewis, A.S.; van Schalkwyk, G.I.; Mineur, Y.S. Mood and anxiety regulation by nicotinic acetylcholine receptors: A potential pathway to modulate aggression and related behavioral states. Neuropharmacology 2015, 96, 235–243. [Google Scholar] [CrossRef]
- Singer, S.; Rossi, S.; Verzosa, S.; Hashim, A.; Lonow, R.; Cooper, T.; Sershen, H.; Lajtha, A. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem. Res. 2004, 29, 1779–1792. [Google Scholar] [CrossRef]
- Dongelmans, M.; Durand-de Cuttoli, R.; Nguyen, C.; Come, M.; Duranté, E.K.; Lemoine, D.; Brito, R.; Ahmed Yahia, T.; Mondoloni, S.; Didienne, S. Chronic nicotine increases midbrain dopamine neuron activity and biases individual strategies towards reduced exploration in mice. Nat. Commun. 2021, 12, 6945. [Google Scholar] [CrossRef]
- Ryu, I.S.; Kim, J.; Seo, S.Y.; Yang, J.H.; Oh, J.H.; Lee, D.K.; Cho, H.-W.; Yoon, S.S.; Seo, J.-W.; Chang, S. Behavioral changes after nicotine challenge are associated with α7 nicotinic acetylcholine receptor-stimulated glutamate release in the rat dorsal striatum. Sci. Rep. 2017, 7, 15009. [Google Scholar] [CrossRef]
- Benowitz, N.L. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 2008, 83, 531–541. [Google Scholar] [CrossRef]
- Balfour, D.J. The neurobiology of tobacco dependence: A preclinical perspective on the role of the dopamine projections to the nucleus. Nicotine Tob. Res. 2004, 6, 899–912. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef]
- Chiamulera, C. Cue reactivity in nicotine and tobacco dependence: A “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res. Rev. 2005, 48, 74–97. [Google Scholar] [CrossRef]
- Carobrez, A.; Bertoglio, L. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef]
- Davidson, R.J. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biol. Psychiatry 2002, 51, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.J.; Büchler, E.; Shabanpoor, F.; Hossain, M.A.; Wade, J.D.; Lawrence, A.J.; Gundlach, A.L. Central relaxin-3 receptor (RXFP3) activation decreases anxiety-and depressive-like behaviours in the rat. Behav. Brain Res. 2013, 244, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.; Rawlins, J.; McHugh, S.; Deacon, R.; Yee, B.; Bast, T.; Zhang, W.-N.; Pothuizen, H.; Feldon, J. Regional dissociations within the hippocampus—Memory and anxiety. Neurosci. Biobehav. Rev. 2004, 28, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Sjölander, A.; Lu, D.; Walker, A.K.; Sloan, E.K.; Fall, K.; Valdimarsdóttir, U.; Hall, P.; Smedby, K.E.; Fang, F. Aspirin and other non-steroidal anti-inflammatory drugs and depression, anxiety, and stress-related disorders following a cancer diagnosis: A nationwide register-based cohort study. BMC Med. 2020, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Crews, B.C.; Rowlinson, S.W.; Garner, C.; Seibert, K.; Marnett, L.J. Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science 1998, 280, 1268–1270. [Google Scholar] [CrossRef]
- Casolini, P.; Catalani, A.; Zuena, A.R.; Angelucci, L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J. Neurosci. Res. 2002, 68, 337–343. [Google Scholar] [CrossRef]
- Ng, Q.X.; Ramamoorthy, K.; Loke, W.; Lee, M.W.L.; Yeo, W.S.; Lim, D.Y.; Sivalingam, V. Clinical role of aspirin in mood disorders: A systematic review. Brain Sci. 2019, 9, 296. [Google Scholar] [CrossRef]
- Brune, K.; Patrignani, P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain Res. 2015, 8, 105–118. [Google Scholar] [CrossRef]
- Nilsson, S.E.; Johansson, B.; Takkinen, S.; Berg, S.; Zarit, S.; McClearn, G.; Melander, A. Does aspirin protect against Alzheimer’s dementia? A study in a Swedish population-based sample aged > or =80 years. Eur. J. Clin. Pharm. 2003, 59, 313–319. [Google Scholar] [CrossRef]
- Savitz, J.; Preskorn, S.; Teague, T.K.; Drevets, D.; Yates, W.; Drevets, W. Minocycline and aspirin in the treatment of bipolar depression: A protocol for a proof-of-concept, randomised, double-blind, placebo-controlled, 2x2 clinical trial. BMJ Open 2012, 2, e000643. [Google Scholar] [CrossRef]
- Laan, W.; Grobbee, D.E.; Selten, J.P.; Heijnen, C.J.; Kahn, R.S.; Burger, H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: Results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry 2010, 71, 520–527. [Google Scholar] [CrossRef]
- Berk, M.; Dean, O.; Drexhage, H.; McNeil, J.J.; Moylan, S.; O’Neil, A.; Davey, C.G.; Sanna, L.; Maes, M. Aspirin: A review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 2013, 11, 74. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.H.; Mittal, J.; Yan, D.; Eshraghi, A.A. Neurotransmitters: The critical modulators regulating gut–brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Moller, M.; Swanepoel, T.; Harvey, B. Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of schizophrenia: Implications for novel drug development. ACS Chem. Neurosci. 2015, 6, 987–1016. [Google Scholar] [CrossRef]
- Müller, N.; Myint, A.-M.; Schwarz, M.J. Inflammatory biomarkers and depression. Neurotox. Res. 2011, 19, 308–318. [Google Scholar] [CrossRef]
- Rizwan, S.; Idrees, A.; Ashraf, M.; Ahmed, T. Memory-enhancing effect of aspirin is mediated through opioid system modulation in an AlCl3-induced neurotoxicity mouse model. Exp. Ther. Med. 2016, 11, 1961–1970. [Google Scholar] [CrossRef]
- Israel, Y.; Quintanilla, M.E.; Ezquer, F.; Morales, P.; Santapau, D.; Berríos-Cárcamo, P.; Ezquer, M.; Olivares, B.; Herrera-Marschitz, M. Aspirin and N-acetylcysteine co-administration markedly inhibit chronic ethanol intake and block relapse binge drinking: Role of neuroinflammation-oxidative stress self-perpetuation. Addict. Biol. 2021, 26, e12853. [Google Scholar] [CrossRef]
- Hammad, A.M.; Al-Zaghari, L.; Alfaraj, M.; Al-Qerem, W.; Talib, W.H.; Alasmari, F.; Amawi, H.; Hall, F.S. Aspirin reduces cigarettes smoke withdrawal-induced anxiety in rats via modulating the expression of NFĸB, GLT-1, and xCT. Front. Pharmacol. 2023, 13, 5471. [Google Scholar] [CrossRef]
- Quintanilla González, M.E.; Morales Retamales, P.; Ezquer, F.; Ezquer, M.; Herrera-Marschitz Muller, M.; Israel, Y. Administration of N-acetylcysteine plus acetylsalicylic acid markedly inhibits nicotine reinstatement following chronic oral nicotine intake in female rats. Front. Behav. Neurosci. 2021, 14, 617418. [Google Scholar] [CrossRef]
- Rao, P.; Saternos, H.; Goodwani, S.; Sari, Y. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology 2015, 232, 2333–2342. [Google Scholar] [CrossRef]
- Berríos-Cárcamo, P.; Quezada, M.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y.; Ezquer, F. Oxidative stress and neuroinflammation as a pivot in drug abuse. A focus on the therapeutic potential of antioxidant and anti-inflammatory agents and biomolecules. Antioxidants 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, E.J.; Grady, C.C.; Crouch, R.A.; Lie, R.K.; Miller, F.G.; Wendler, D.D. The Oxford Textbook of Clinical Research Ethics; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Biedermann, S.V.; Biedermann, D.G.; Wenzlaff, F.; Kurjak, T.; Nouri, S.; Auer, M.K.; Wiedemann, K.; Briken, P.; Haaker, J.; Lonsdorf, T.B. An elevated plus-maze in mixed reality for studying human anxiety-related behavior. BMC Biol. 2017, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Alhusban, A.A.; Hammad, A.M.; Alzaghari, L.F.; Shallan, A.I.; Shnewer, K. Rapid and sensitive HPLC–MS/MS method for the quantification of dopamine, GABA, serotonin, glutamine and glutamate in rat brain regions after exposure to tobacco cigarettes. Biomed. Chromatogr. 2023, 37, e5513. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.M.; Aldhalaan, H.M.; Alshammari, T.K.; Alqasem, M.A.; Alshammari, M.A.; Albekairi, N.A.; AlSharari, S.D. The Role of Nicotinic Receptors in the Attenuation of Autism-Related Behaviors in a Murine BTBR T+ tf/J Autistic Model. Autism Res. 2020, 13, 1311–1334. [Google Scholar] [CrossRef]
- Honeycutt, S.C.; Garrett, P.I.; Barraza, A.G.; Maloy, A.N.; Hillhouse, T.M. Repeated nicotine vapor inhalation induces behavioral sensitization in male and female C57BL/6 mice. Behav. Pharmacol. 2020, 31, 583–590. [Google Scholar] [CrossRef]
- Alasmari, F.; Alotibi, F.M.; Alqahtani, F.; Alshammari, T.K.; Kadi, A.A.; Alghamdi, A.M.; Allahem, B.S.; Alasmari, A.F.; Alsharari, S.D.; Al-Rejaie, S.S. Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurobehaviors and Pre/Postsynaptic Neuron Markers. Toxics 2022, 10, 338. [Google Scholar] [CrossRef]
- Hammad, A.M.; Meknas, S.J.; Hall, F.S.; Hikmat, S.; Sari, Y.; Al-Qirim, T.; Alfaraj, M.; Amawi, H. Effects of waterpipe tobacco smoke and ceftriaxone treatment on the expression of endocannabinoid receptors in mesocorticolimbic brain regions. Brain Res. Bull. 2022, 185, 56–63. [Google Scholar] [CrossRef]
- Das, S.C.; Yamamoto, B.K.; Hristov, A.M.; Sari, Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology 2015, 97, 67–74. [Google Scholar] [CrossRef]
- Alajaji, M.; Bowers, M.; Knackstedt, L.; Damaj, M. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology 2013, 228, 419–426. [Google Scholar] [CrossRef]
- Li, X.; Semenova, S.; D’Souza, M.S.; Stoker, A.K.; Markou, A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology 2014, 76, 554–565. [Google Scholar] [CrossRef]
- Alasmari, F.; Bell, R.L.; Rao, P.; Hammad, A.M.; Sari, Y. Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol. Biochem. Behav. 2018, 170, 44–55. [Google Scholar] [CrossRef]
- Alasmari, F.; Alexander, L.E.C.; Hammad, A.M.; Horton, A.; Alhaddad, H.; Schiefer, I.T.; Shin, J.; Moshensky, A.; Sari, Y. E-cigarette aerosols containing nicotine modulate nicotinic acetylcholine receptors and astroglial glutamate transporters in mesocorticolimbic brain regions of chronically exposed mice. Chem. Biol. Interact. 2021, 333, 109308. [Google Scholar] [CrossRef]
- Alasmari, F.; Alexander, L.E.C.; Nelson, J.A.; Schiefer, I.T.; Breen, E.; Drummond, C.A.; Sari, Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 1–8. [Google Scholar] [CrossRef]
- Alasmari, F.; Crotty Alexander, L.E.; Hammad, A.M.; Bojanowski, C.M.; Moshensky, A.; Sari, Y. Effects of chronic inhalation of electronic cigarette vapor containing nicotine on neurotransmitters in the frontal cortex and striatum of C57BL/6 mice. Front. Pharmacol. 2019, 10, 885. [Google Scholar] [CrossRef]
- Wang, B.W.; Liao, W.N.; Chang, C.T.; Wang, S.J. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse 2006, 59, 491–501. [Google Scholar] [CrossRef]
- Konradsson-Geuken, Å.; Gash, C.R.; Alexander, K.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A.; Bruno, J.P. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse 2009, 63, 1069–1082. [Google Scholar] [CrossRef]
- Alasmari, F.; Goodwani, S.; McCullumsmith, R.E.; Sari, Y. Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence. Prog. Neurobiol. 2018, 171, 32–49. [Google Scholar] [CrossRef]
- Sonnewald, U.; Schousboe, A. Introduction to the glutamate–glutamine cycle. In The Glutamate/GABA-Glutamine Cycle; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–7. [Google Scholar]
- Shameem, M.; Patel, A.B. Glutamatergic and GABAergic metabolism in mouse brain under chronic nicotine exposure: Implications for addiction. PLoS ONE 2012, 7, e41824. [Google Scholar] [CrossRef]
- Changeux, J.-P. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nat. Rev. Neurosci. 2010, 11, 389–401. [Google Scholar] [CrossRef]
- Tizabi, Y.; Copeland Jr, R.L.; Louis, V.A.; Taylor, R.E. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol. Clin. Exp. Res. 2002, 26, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Perez, X.A.; Ly, J.; McIntosh, J.M.; Quik, M. Long-term nicotine exposure depresses dopamine release in nonhuman primate nucleus accumbens. J. Pharmacol. Exp. Ther. 2012, 342, 335–344. [Google Scholar] [CrossRef]
- Fuxe, K.; Jansson, A.; Jansson, A.; Andersson, K.; Eneroth, P.; Agnati, L. Chronic nicotine treatment increases dopamine levels and reduces dopamine utilization in substantia nigra and in surviving forebrain dopamine nerve terminal systems after a partial di-mesencephalic hemitransection. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1990, 341, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Semba, J.i.; Wakuta, M. Chronic effect of nicotine on serotonin transporter mRNA in the raphe nucleus of rats: Reversal by co-administration of bupropion. Psychiatry Clin. Neurosci. 2008, 62, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Takada, Y.; Nagai, N.; Urano, T.; Takada, A. Nicotine increases stress-induced serotonin release by stimulating nicotinic acetylcholine receptor in rat striatum. Synapse 1998, 28, 212–219. [Google Scholar] [CrossRef]
- Awtry, T.L.; Werling, L.L. Acute and chronic effects of nicotine on serotonin uptake in prefrontal cortex and hippocampus of rats. Synapse 2003, 50, 206–211. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, A.M.; Alhusban, A.A.; Alzaghari, L.F.; Alasmari, F.; Sari, Y. Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters’ Tissue Content in Rats’ Hippocampus and Amygdala. Metabolites 2023, 13, 515. https://doi.org/10.3390/metabo13040515
Hammad AM, Alhusban AA, Alzaghari LF, Alasmari F, Sari Y. Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters’ Tissue Content in Rats’ Hippocampus and Amygdala. Metabolites. 2023; 13(4):515. https://doi.org/10.3390/metabo13040515
Chicago/Turabian StyleHammad, Alaa M., Ala A. Alhusban, Lujain F. Alzaghari, Fawaz Alasmari, and Youssef Sari. 2023. "Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters’ Tissue Content in Rats’ Hippocampus and Amygdala" Metabolites 13, no. 4: 515. https://doi.org/10.3390/metabo13040515
APA StyleHammad, A. M., Alhusban, A. A., Alzaghari, L. F., Alasmari, F., & Sari, Y. (2023). Effect of Cigarette Smoke Exposure and Aspirin Treatment on Neurotransmitters’ Tissue Content in Rats’ Hippocampus and Amygdala. Metabolites, 13(4), 515. https://doi.org/10.3390/metabo13040515







