Abstract
Cannabis belongs to the family Cannabaceae, and phytocannabinoids are produced by the Cannabis sativa L. plant. A long-standing debate regarding the plant is whether it contains one or more species. Phytocannabinoids are bioactive natural products found in flowers, seeds, and fruits. They can be beneficial for treating human diseases (such as multiple sclerosis, neurodegenerative diseases, epilepsy, and pain), the cellular metabolic process, and regulating biological function systems. In addition, several phytocannabinoids are used in various therapeutic and pharmaceutical applications. This study provides an overview of the different sources of phytocannabinoids; further, the biosynthesis of bioactive compounds involving various pathways is elucidated. The structural classification of phytocannabinoids is based on their decorated resorcinol core and the bioactivities of naturally occurring cannabinoids. Furthermore, phytocannabinoids have been studied in terms of their role in animal models and antimicrobial activity against bacteria and fungi; further, they show potential for therapeutic applications and are used in treating various human diseases. Overall, this review can help deepen the current understanding of the role of biotechnological approaches and the importance of phytocannabinoids in different industrial applications.
1. Introduction
Cannabis, scientifically known as Cannabis sativa L., is a genus within the family Cannabaceae; it is a wind-pollinated, dioecious herb (i.e., the male and female reproductive structures are on separate plants), although monoecious plants can occur in some populations [1]. Additionally, the species C. sativa L. is a potential source of fiber, food, oil, and protein [2]. The discovery of the curative virtues of plants is attributed to Chinese research dating back to 10,000 BC [3], and the first phytocannabinoids were isolated from C. sativa L. [4]. Phytocannabinoids are structurally diverse, naturally obtained compounds produced in several secondary metabolite plants, and they have a long, controversial history of use and abuse [4,5]. Cannabis has several varieties suitable for various purposes; hence, it has been widely used in industrial, ornamental, nutritional, recreational, and pharmaceutical applications and herbal medicine [6]. Cannabinoids are predominantly insoluble in water but soluble in alcohol and other nonpolar solvents [7]. According to the recent literature, over 200 phytocannabinoids have been identified in the cannabis plant [8].
Cannabis sativa L. subspecies are plants that contain a large variety of secondary metabolites, including phytocannabinoids, terpenoids, and flavonoids, which have profound anti-microbial activities, in addition to possessing anti-inflammatory, anti-oxidative, and neuromodulatory properties [8]. They are classified into different subclasses according to their chemical structure [9]. Cannabidiol (CBD), ∆9-tetrahydrocannabinol (∆9-THC), cannabigerol (CBG), and cannabinol (CBN) are the most studied [9]. During this research, numerous countries stopped growing plants [10]. According to the World Health Organization (WHO), an estimated 80% of people in developed countries rely on herbal medicine to meet their basic health needs and treat various diseases [11].
Nonetheless, these plants are perceived as criminal and unacceptable to communities, as most consumers cannot differentiate between psychoactive and non-psychoactive cannabis plants. By contrast, the use of cannabis plants with low levels of THC in medicine and foods is based on various potentially beneficial cannabinoid compounds [11], including CBD [12]. This is synthesized by postsynaptic neurons and acts as a retrograde messenger molecule for neurotransmitter release from CB1 expression, which is found in areas of the human brain with high densities of cannabinoid receptors (CBrs) [13]. The endocannabinoid system also plays a key role in neural and molecular control mechanisms. In fact, the endocannabinoid system plays a key role in the normal physiological functions of the gastrointestinal tract, including motility, gut–brain-mediated fat intake, hunger signaling, inflammation, and intestinal permeability [14,15]. Furthermore, cannabis fiber has been used in harmless, inexpensive, eco-friendly, biodegradable raw materials (all derived from plant-based products) to produce cellulose, biofuels, and bioethanol, such as paper towels, paper plates, ready-made clothes, and even natural fiber shoes [16]. It demonstrates its capability for sustainable agricultural production, aiming to protect the soil and environment through lack of water consumption in the production of hemp fibers, and the ability to grow without the need for fertilizer and pesticides [16]. Despite the ornamental feasibility of cannabis, its suitability for the ornamental industry remains largely unrecognized, and studies tend to focus primarily on botany, cultivation, propagation, and other agricultural aspects of cannabis [14]. Numerous developed countries, such as the UK, USA, Canada, and the Netherlands, have supported the production of cannabis [17]. Edible usage is classified into medicinal and recreational purposes [18]. However, the permitted amount of CBD per serving should not exceed 20 mg; and each package sold should not exceed 600 mg of CBD [15] and should contain less than 0.001% THC [18]. Whereas, in the USA, edibles should contain less than 0.3% THC and have CBD at levels of cannabis edibles for medicinal purposes [17]. Based on 2017 research, the total hemp wholesale market was valued at more than 700 million dollars in the USA. Despite the decline in the agriculture of cannabis owing to existing legal restrictions, standard procedures have been established to encourage the natural production of fiber from these plant species [18].
Moreover, these low levels of psychoactive components are what make pharmaceutical industries bet largely on hemp to obtain the non-psychoactive cannabinoid cannabidiol (CBD), which has shown a high therapeutic value in numerous diseases. Therefore, among cannabis products for skin care, CBD oil with high therapeutic potential and without undesirable psychotropic effects has been extracted from leaves [18]. Numerous chemicals are produced in hemp through secondary metabolism. They include cannabinoids, terpenes, and phenolic compounds [19]; these will be further described in the next sections. Although the pharmacological properties of cannabinoids have been extensively studied and are the most recognized hemp bioactives, other components have also been associated with potent health-promoting properties [19]. At the same time, these products are free of chemicals and toxins that are used in most synthetic cosmetics. CBD has a wide spectrum of biological activity, such as antioxidant and anti-inflammatory activity, and therefore can be used for the treatment of diseases associated with redox imbalance and inflammation. CBD can be used for the treatment of diabetes-related cardiomyopathy, (including stroke, arrhythmia, and hypertension), cancer, anxiety, psychosis, epilepsy, neurodegenerative disease, musculoskeletal pain, and skin disease [20]. A mixture of cannabinoids may produce tattoo ink with a low risk of infection and inflammation [21]. Certain natural plants possess at least some beneficial properties in mitigating biotic and abiotic stress [22]. However, the genetic mechanism of trichome development and, subsequently, the cannabinoid content have not been updated, and this remains a significant factor in cannabinoid production [23,24]. In the past several decades, a lack of knowledge of hemp and marijuana has reduced the growth of plants. Advanced research has focused primarily on the benefits of plants and other phytocannabinoids with weak or no psychoactivity with promise as therapeutic agents in human health. Thus, the terminology and important tools must be clarified, and clear descriptions must be elucidated for differentiating between therapeutic and other supplements derived from cannabis as technology advances [25]. This review presents the various sources of cannabis, the biosynthesis of phytocannabinoid pathways, the structures of the enzymes in the pathways, and the properties of phytocannabinoids that are important to animals and humans. Phytocannabinoids exhibit antimicrobial activities against bacteria and fungi [26]. Furthermore, the uses of cannabis are economically significant, owing to the various food industry applications and the development of therapeutic and pharmacological applications of phytocannabinoids. Therefore, the challenges associated with phytocannabinoids in advanced biotechnology and their most significant commercial interest is elucidated in this review.
2. Sources of Phytocannabinoids in C. sativa L.
Cannabis has many trichomes, which are collected from the protuberances, close to the plant’s leaves, flowers, seeds, and other important sections. These trichomes are found in C. sativa L., and they are divided into various types of glandular and non-glandular structures [27]. The secretory trichome’s numerous biologically active compounds are synthesized, which take the form of protuberances and cover the plant’s leaves and stems. Over 200 compounds with diverse biological activities, including flavonoids, terpenoids, and cannabinoids, have been confirmed in hemp. Many of the current common hemp varieties are individually similar and closely related [28]. The phytocannabinoid compound ∆9-THC is found in farming, some construction biomaterials, and certain textile industries and includes a negligible amount of the psychoactive cannabinoid compound [29]. Its secondary metabolites include phytocannabinoids, flavonoids, terpenoids, lignans, and alkaloids [29]. Phytocannabinoids are bioactive terpenoids that are thought to be exclusive to C. sativa L. [30]. They play a role in a variety of physiological and pathophysiological processes. Many of these effects are mediated by two G-protein-coupled receptors (GPCRs) in the CNS and are found in particularly high levels in the neocortex, basal ganglia, cerebellum, and brainstem [31]. Interestingly, CB1 receptors are highly enriched at presynaptic and axonal compartments and restrict their function to sites of synaptic activity [32]. The CB2 receptors exhibit a more defined pattern of expression in the brain than CB1 receptors and are found predominantly in cells and tissue of the immune system [33].
3. Structures of Phytocannabinoids
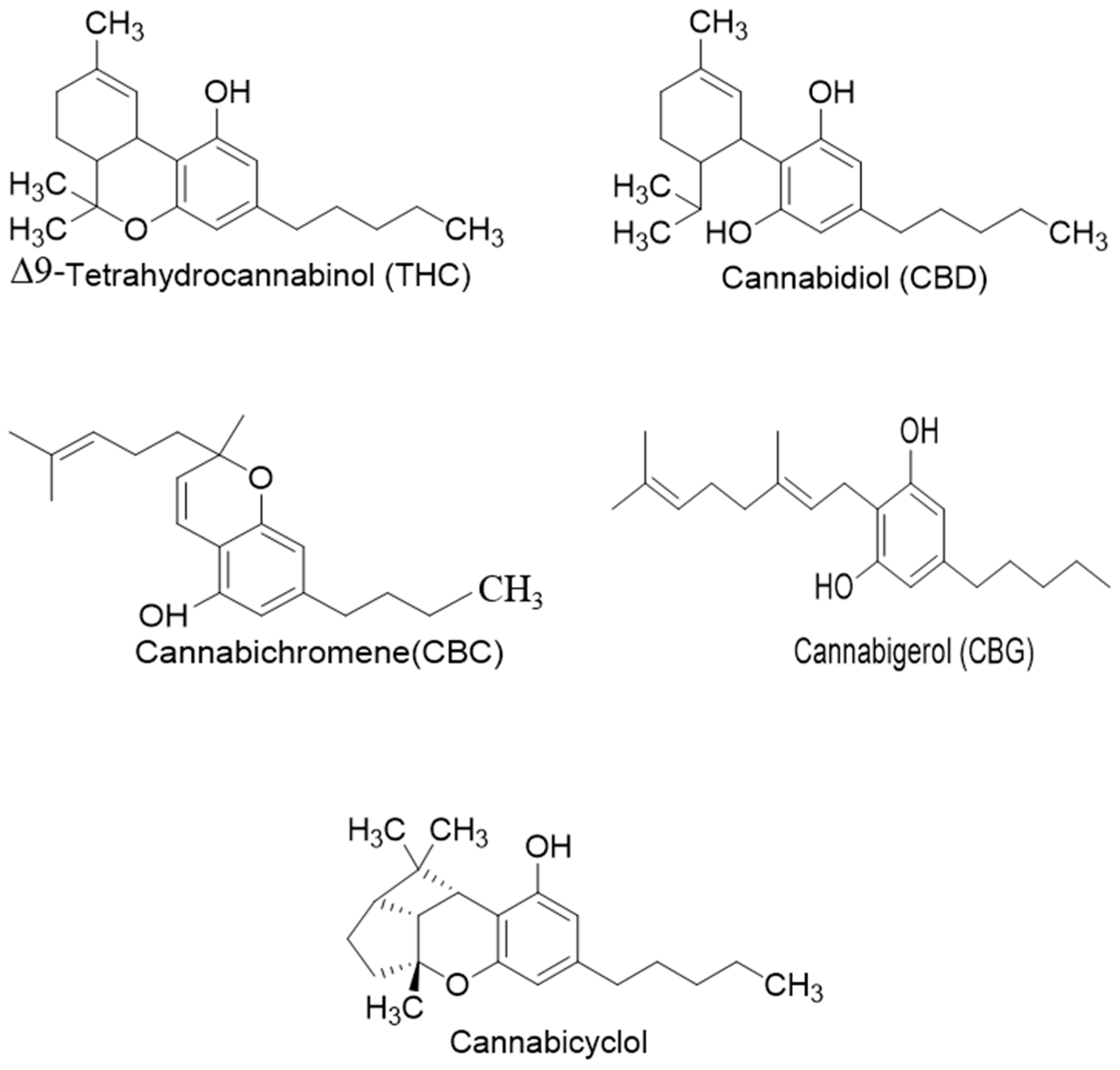
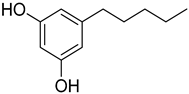
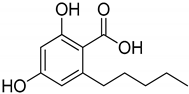
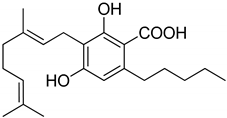
Phytocannabinoids have been found in different plant species of cannabis, including Echinacea purpurea, E. angustifolia, E. pallida, Acmella oleracea, Helichrysum umbraculigerum, and Radula marginata [12,33]. A group of C21 and C22 carboxylated forms of terpenophenolic compounds exhibit binding affinity at cannabinoid receptors. However, phytocannabinoid synthesis involves several structures of chemical compounds, such as THC, CBD, CBG, CBC, cannabicyclol (CBL), and cannabidiol type (CBND). Phytocannabinoids are represented by several motifs composed of various moieties: the isoprenyl residue, resorcinol core, and alkyl group [34] (Figure 1). Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the most abundant compounds in cannabis plants and are central to their therapeutic application [35,36]. There is limited access to low-THC/high-CBD products (i.e., products with low levels of THC and high levels of CBD) [36]. Of the conditions that allow for some access to cannabis compounds, cancer, HIV, multiple sclerosis, glaucoma, seizures/epilepsy, and pain are among the most recognized qualifying ailments [37]. Medical cannabis is also used for the treatment of any illness for which the drug provides relief for the individual. In the last few years, numerous studies have investigated natural and synthetic cannabinoids for targeting and killing tumors, to the point of collecting overwhelming evidence to suggest that cannabinoids can be used as adjuvant agents for the treatment of cancer [12].
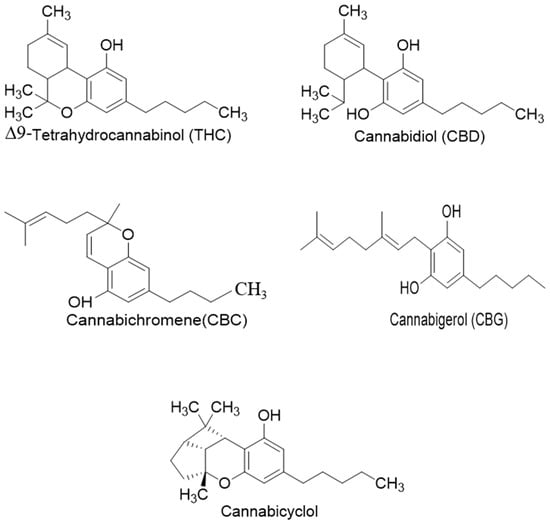
Figure 1.
Structure of phytocannabinoids.
4. Biosynthesis and Pathway of Phytocannabinoids
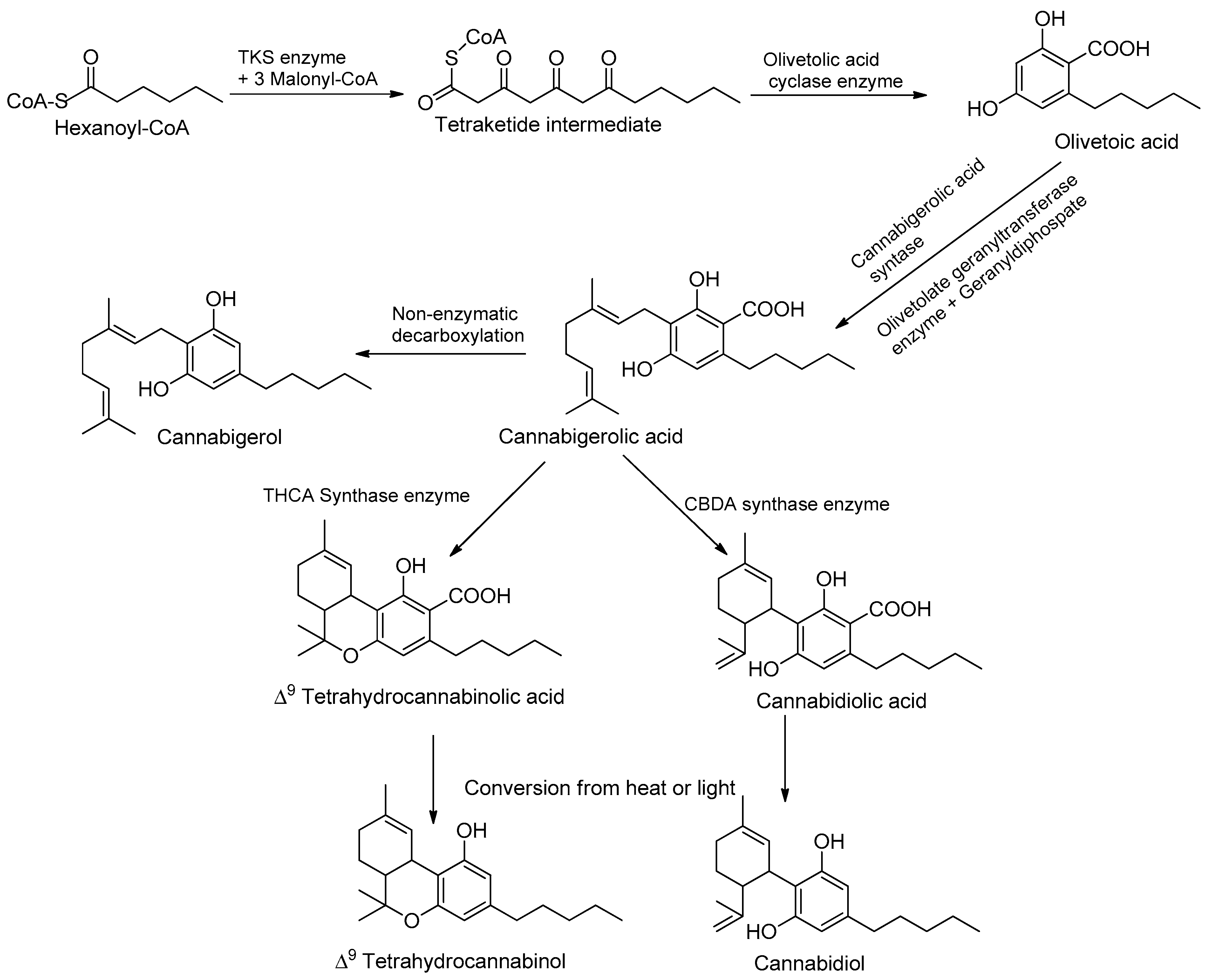
Phytocannabinoids are stored in glandular trichomes, located all over the aerial part of the plant; the root surface and root tissue do not produce phytocannabinoids [34]. Moreover, plants grown outdoors are exposed to a fair amount of ultraviolet UV-A and UV-B, and exposure to UV rays before harvest causes the plant to produce more THC compounds [35]. Adaptive responses to changing UV-B conditions also play an important role in plant–herbivore interactions. UVB-mediated changes in plant architecture, physiology, and/or chemistry can alter herbivorous arthropods’ performance and preference. In most cases, these UV-B-mediated induced physiological changes lead to the reinforcement of plant defenses. For example, increased production of UV-B-protective secondary metabolites and/or the reinforcement of plant cell walls induced by UV-B was proposed to affect plant colonization by herbivorous arthropods [35]. The pathways are split between different cell types and organelles, the cytoplasm of the gland cells, plastids, and storage of the extracellular cavity [12]. Many cannabinoids are formed in correlation with increased moisture, heat, temperature, and abiotic stress conditions, as well as low soil moisture content. The study demonstrated the potential of mineral nutrition to regulate cannabinoid metabolism and optimize the pharmacological quality of potassium, an essential plant nutrient that is required by plants in relatively high amounts and takes part in many key physiological processes [30]. Processes affected by K supply include stomatal regulation, protein synthesis, photosynthesis, enzyme activation, osmoregulation, and the uptake and accumulation of other essential cations such as Ca and Mg [36]. In addition to its involvement in the regulation of plant primary metabolism, K is known to have a considerable impact on the secondary metabolism of plants and is therefore considered a quality element [35]. As K is known to affect a wide range of secondary metabolites in plants, including phenolic compounds, flavonoids, carotenoids, and organic acids, we hypothesized that it has the potential to regulate the production of medical compounds in C. sativa [36]. UV rays at 320 nm provide an evolutionary benefit by acting as sunlight to flowering plants, thus resulting in the production of a greater number of phytocannabinoids conveying different biological benefits and activities; this represents a crucial light signal to which plants can respond and develop specific photomorphogenic responses [37,38]. Among these responses, changes in the morphology, physiology, and production of secondary metabolites are commonly described. The main psychoactive constituent in cannabis is THC, and the main non-psychoactive compound is CBD, which accumulates in glandular trichomes and is obtained from plant tissue, flowers, and a high density of plant constituents [38]. The cannabinoid pathways involve the hexanoyl-CoA produced by an acyl-activating enzyme (CsAAE1) derived from hexanoic acid through the fatty acid biosynthesis pathway [39]. Hereafter, type III polyketide synthase olivetol synthase elongates toward hexanoyl-CoA using three units of malonyl-CoA and has characteristics of another type III polyketide synthase (PKSs) in flowering plants; this method of production requires significant resources and is an unsustainable avenue for harvesting cannabinoids, given its recent rapid expansion in demand worldwide [40]. Instead, another enzyme, olivetolic acid cyclase (OAC), catalyzes the necessary C2 to C7 aldol condensation reaction to produce olivetolic acid (OLA) (Table 1).

Table 1.
The Enzymatic Reaction in the biosynthesis of phytocannabinoids.
A native prenyltransferase from C. sativa L. that completes the step was identified as CsPT1 (or CsPT4), or geranylpyrophosphate: olivetolate geranyltransferase (GOT), in 1998. Geranylpyrophosphate (GPP) is assumed to be supplied through the 2-C-methyl-D-erythritol-4-phosphate (MEPs) pathways owing to cannabinoid synthases [47]. The pathway can be separated into three distinct functional activities, and the polyketide pathways can produce OLAs and isoprenoids and can produce cannabigerolic acid (CBGAs), as the first cannabinoids in collaboration with the prenyltransferase [48] (Figure 2). Recent work has focused on capitate stalked glandular (CSG) trichomes, which consist of two parts—a nearly spherical resin head (gland head) atop a multicellular stalk. The resin head incorporates a rosette of secretory disk cells at its base, covered by a thin, distensible sheath or cuticle. Cannabinoids and terpenoids accumulate in a secretory cavity between the disk cells and the cuticle [49]. Disk cells also secrete biosynthetic enzymes, such as THCA synthase, into the secretory cavity [46]. The pathway, with key chemical structures, is illustrated in Figure 2. See the chapter by Supaart Sirikantaramas et al. [49] for an elaboration. Cannabinoid content differs in terms of quantity and quality. Quantity and quality have different modes of inheritance [15]. Cannabinoid quantity (dry weight percentage) is polygenic and influenced by environmental factors. This is achieved through differential cyclization of the C10 moiety from GPP via the actions of independent synthases [49]. The discussion on synthetic biology approaches will be organized according to these individual enzymatic steps in the pathways, along with structural and mechanistic insights, to facilitate the subsequent framing of strategies for producing novel cannabinoids [50].
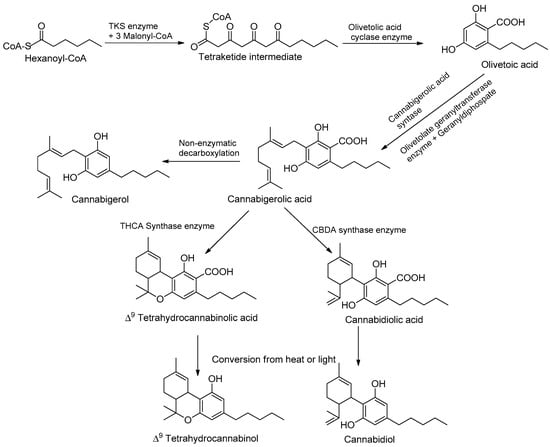
Figure 2.
Cannabinoid biosynthetic pathway, leading to the two major phytocannabinoids, THC and CBD.
5. Structural Analysis of the Enzymes THCA and CBDA Synthase
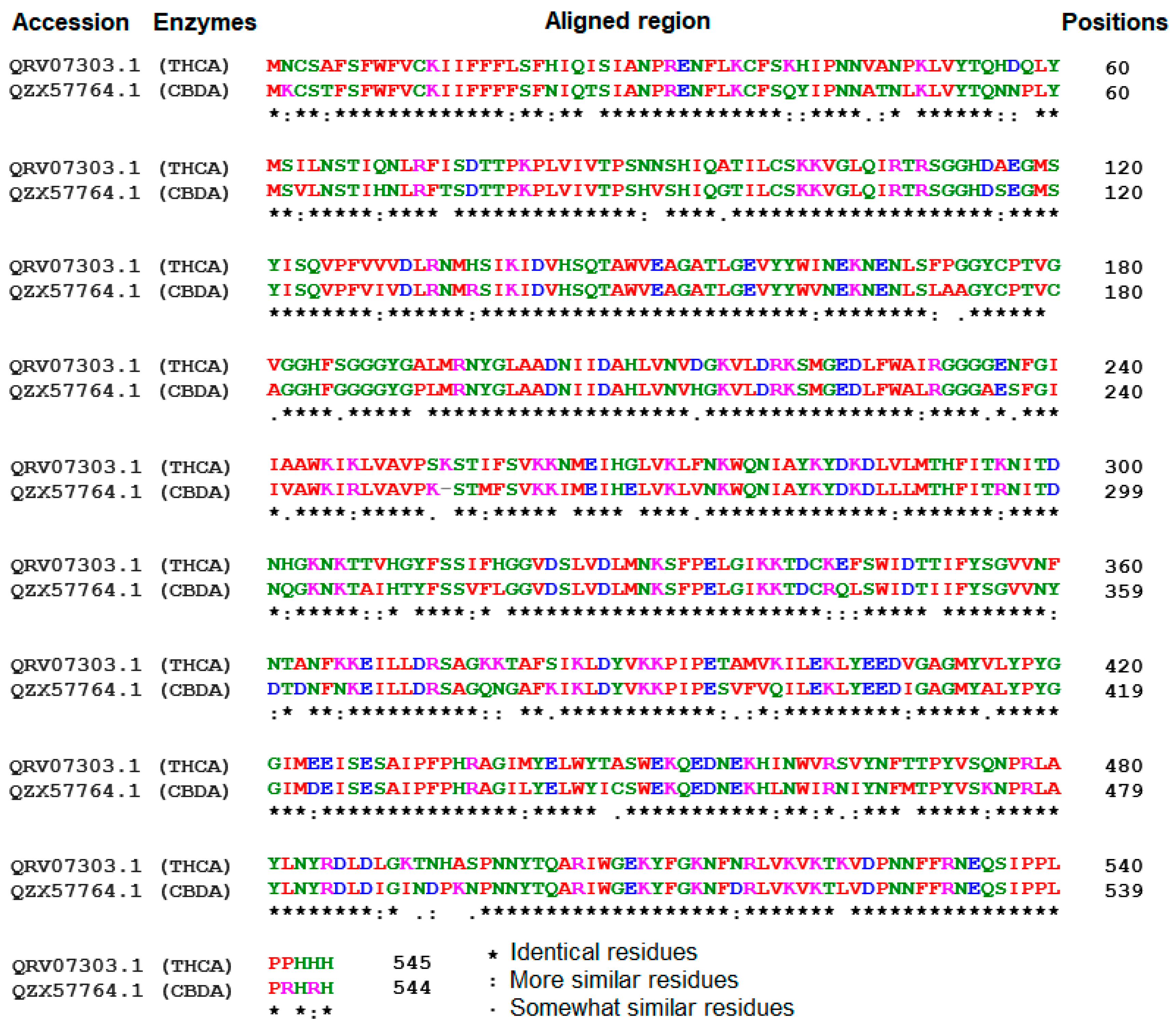
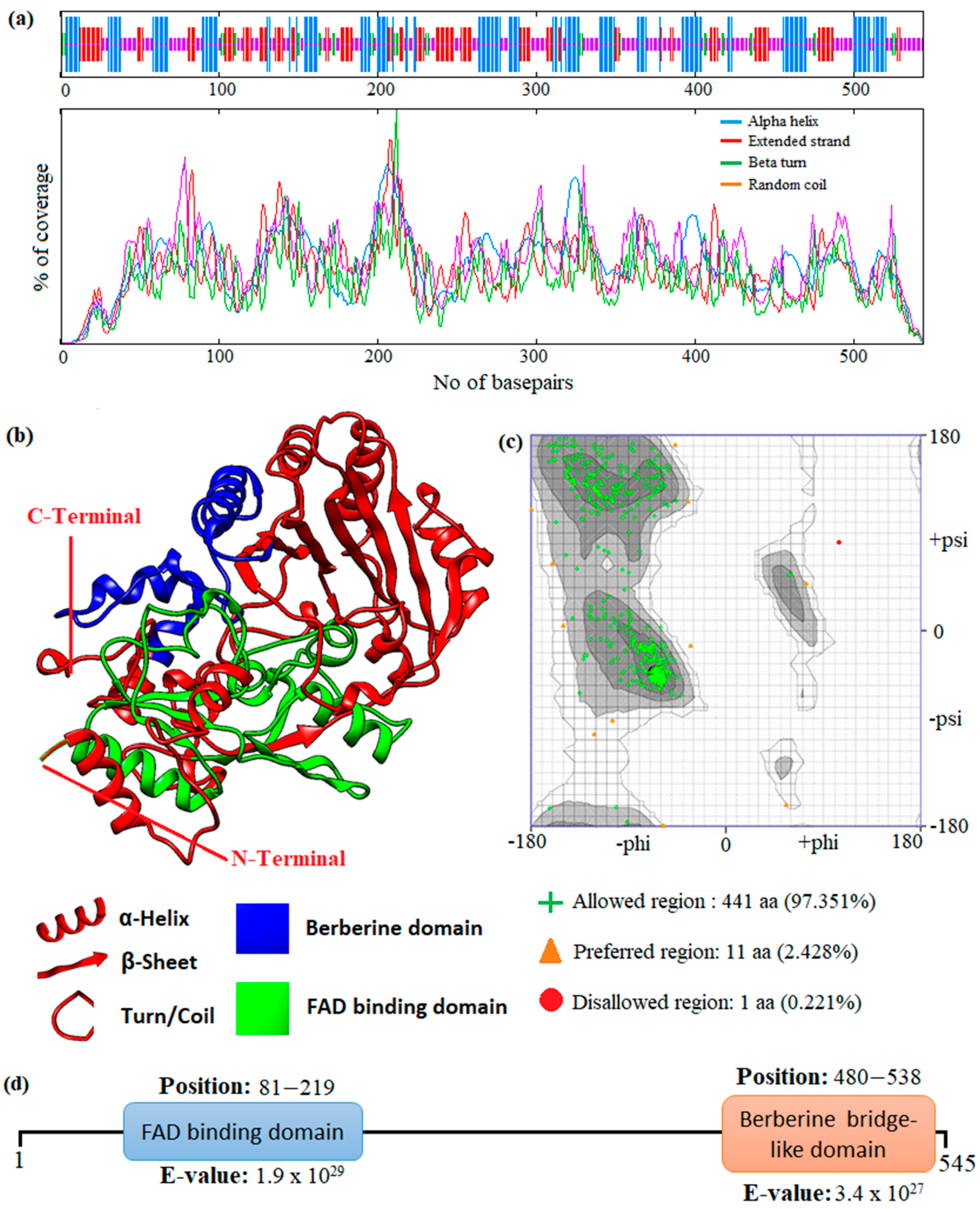
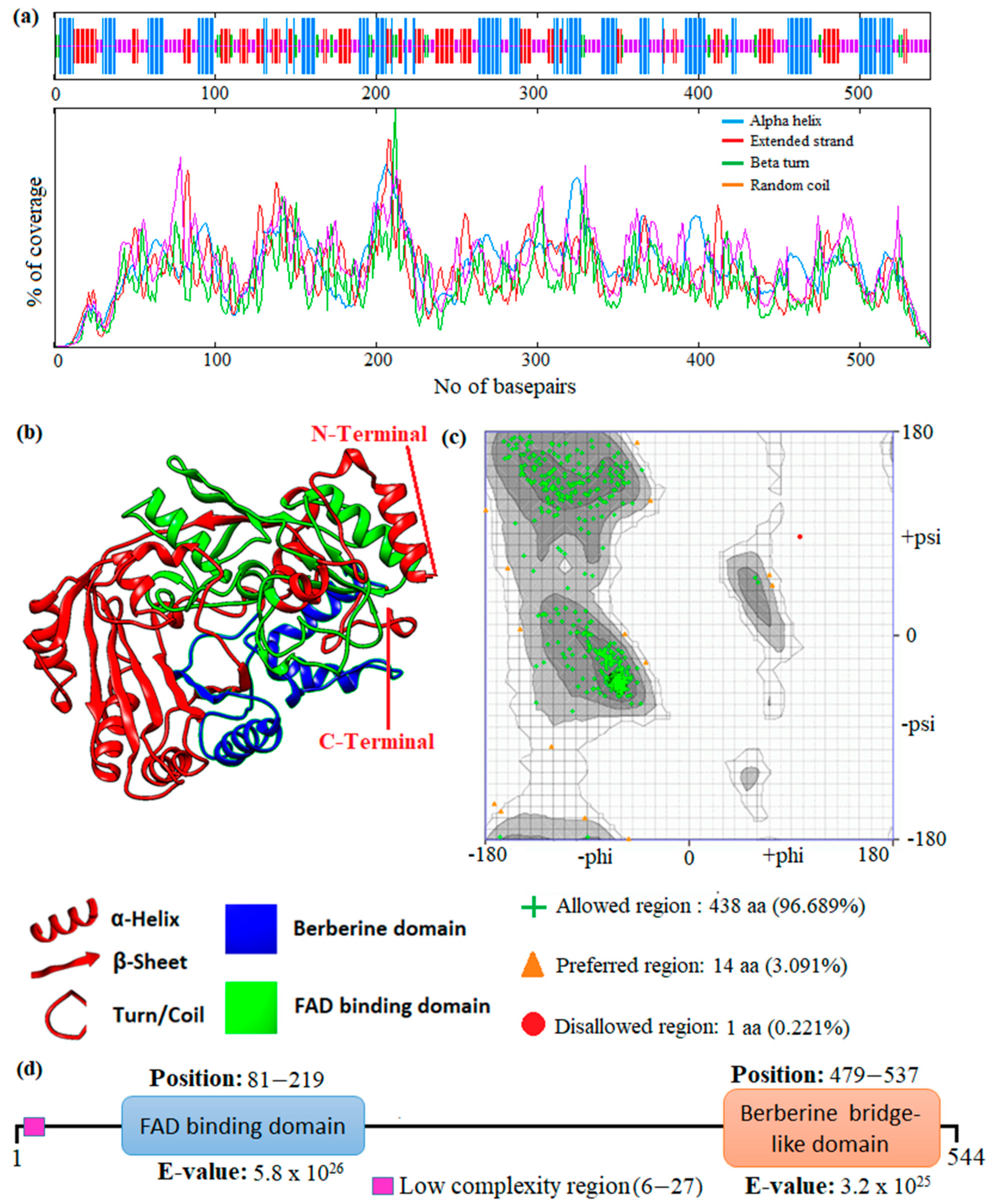
In this study, an in silico approach was used to perform structural analysis (1D, 2D, and 3D) of enzymes such as THC acid and CBD acid synthase. The primary compositions of the nucleotide and protein sequences were predicted using BioEdit V 7.2.6.1 [51] software, as represented in Supplementary Table S1. The pairwise sequence alignment of THC acid and CBD acid synthase was performed using the Clustal Omega server (https://www.ebi.ac.uk/Tools/msa/clustalo/ accessed on 15 February 2023). The alignment shows 90.6% of sequences are similar to one another, as depicted in Figure 3. Whereas the secondary structural elements of target enzymes were predicted using the (SOPMA) server (https://npsa-prabi.ibcp.fr/NPSA/npsa_sopma.html/accessed on 15 February 2023). The results revealed that a maximum number of residues formed a random coil, which determines the stability of enzymes. Further, 170 amino acid residues were found to be involved in alpha-helix and 132 for extended strand formation, as shown in Figure 4a and Figure 5a.
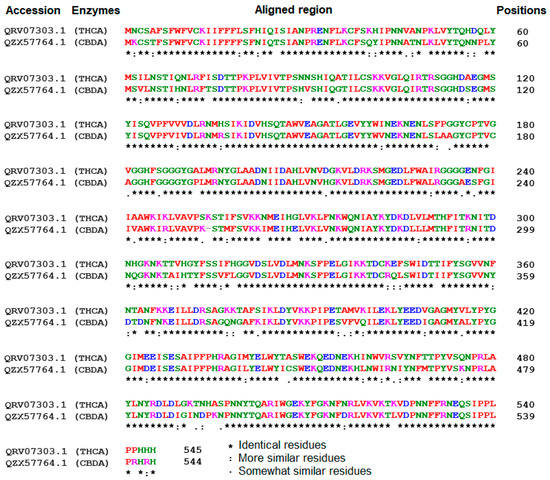
Figure 3.
Sequence alignment among tetrahydrocannabinolic acid (THCA) and Cannabidiolic acid (CBDA) synthases using the Clustal Omega alignment server.
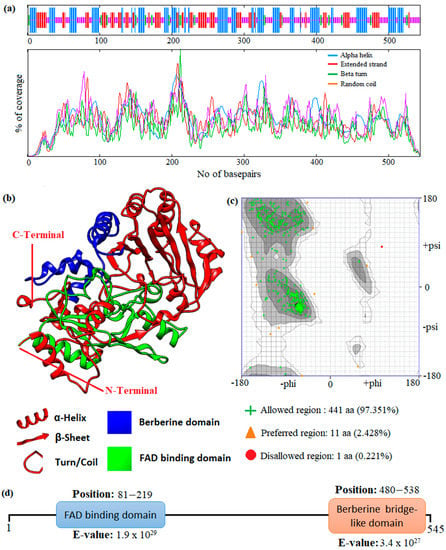
Figure 4.
Prediction of the 1D, 2D, and 3D structural profile of Tetrahydrocannabinolic acid synthase. (a) Secondary structural elements, (b) Predicted 3D model, (c) Validated model, and (d) Predicted motif and domain regions.
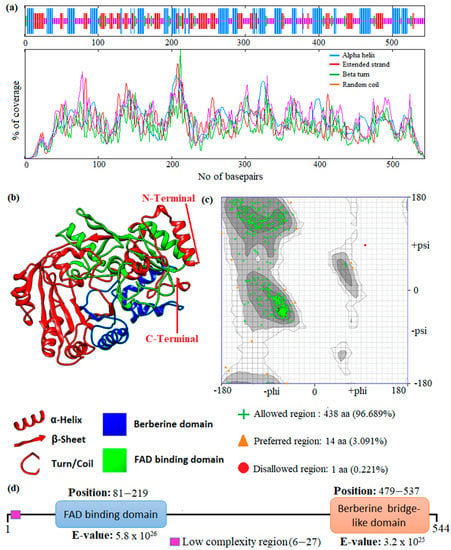
Figure 5.
Prediction of the 1D, 2D, and 3D structural profile of Cannabidiolic acid synthase. (a) Secondary structural elements, (b) Predicted 3D model, (c) Validated model, and (d) Predicted motif and domain regions.
Protein 3D structures were modeled with the Swiss Model [52], and the predicted structure was confirmed with the Ramachandran Plot server (https://zlab.umassmed.edu/bu/rama/accessed on 15 February 2023). The predicted model exhibited 100% similarity with the THCA enzymes and 83.72% for CBDAs, using the protein data bank (PDB) template 3VTE, as depicted in Figure 4b and Figure 5b, respectively. According to the structural validation results (Ramachandran plot), 441 and 438 amino acid residues were found in the allowed region, which occupies 97.351% and 96.689% for THCA and CBDA synthase, as represented in Figure 4c and Figure 5c, respectively. Our target enzyme’s motif and domain region were predicted using the smart server and Conserved Domain Database NCBI-CDD [53] search. The prediction revealed that two major domains, the FAD binding domain at positions 81–219 and Berberine bridge-like domain at positions 480–538, were found in both THCA and CBDA enzymes (Figure 4d and Figure 5d, respectively). Interestingly, a low-complexity region (repeats of single amino acids) was found in position 6–27 bases only in CBD [54]. In the 1960s and 1970s, numerous plausible hypotheses were advanced regarding the biosynthesis of THCA; however, they all were lacking experimental support. THCA was thought to arise from CBDA through cyclization. However, the reaction conditions of the transformation do differ from those present during natural biosynthesis in the plants; moreover, isomerase activity, which would be necessarily responsible for the conversion of CBDA into THCA, has never been detected in any enzyme assays using crude C. sativa enzyme extracts. Current thinking suggests it comes from CBGA instead, by either tetrahydrocannabinolic acid synthase (THCAs) or cannabidiolic acid synthase (CBDAs), both members of the p-cresol methyl-hydroxylase super family.
6. Bioactivities of Phytocannabinoids in Animals and Humans
Phytocannabinoids convey various biologically beneficial effects and properties; thus, they have been used as therapeutics since antiquity [54]. Further, they are linked to the modulation of the endocannabinoid mechanism and the class of receptors CB1 and CB2 distributed in various cells [55]. Additionally, some studies have reported that several phytocannabinoids exhibit potent antimicrobial activity against bacterial and fungal systems, and thus they can be used as antibiotics [55]. Moreover, phytocannabinoids might have therapeutic potential have antifungal properties, such as Phompsis ganjae [4], Stachybotrys bisbyi, Fusarium oxysporum [36], and Saccharomyces cerevisiae [37]. Their use in various industries is increasing worldwide and will likely increase in the next 10 years [56,57]. The endocannabinoid system in animals exposed to these contributes to the regulation of functions (such as learning and memory, emotional processing, sleep, temperature, diverse brain functions, and addiction) and cellular metabolic processes (such as glycolysis, lipolysis, and energy balance (Table 2).

Table 2.
Bioactivity and effect of different phytocannabinoids in animals.
Plant-based products found in various natural agro-industrial processes demonstrate their potential in enhancing animal and meat production and extension. This plant industry can produce seeds, leaves, seed oil, and cakes, and it is a valuable source of animal feed that is rich in protein for ruminant diets [66]. However, research on the bioavailability and leading bioactive compounds of cannabis species in ruminants, as well as the psychoactive effects of THC in humans, is limited [67]. The biotechnological use of phytocannabinoids can be enhanced through agro-industrial approaches to understand their role in medication. Moreover, apart from C. sativa, Nicotiana benthamiana also emerged as the favorable heterologous host to produce phytocannabinoids; in recent research, it was found to be a major biotechnological tool for phytocannabinoid production and for inducing genetic modification [68]. To achieve this, a biotechnological approach should be utilized to gain more knowledge on the pathways involved in phytocannabinoid production [69].
However, there is a lack of information on the bio-efficacy and bioactivity of cannabidiol in the meat milieu [70]; pilot clinical studies on animal models support the application of cannabidiol in inflammatory-based skin diseases. Additionally, non-psychotropic phytocannabinoids are used in the treatment of seborrheic dermatitis, and a recent national survey showed that among current adult users, 10.5 to 46.6% reported using cannabis solely for medical uses [71]. Of the states that allow for some access to cannabis compounds, cancer, HIV/AIDS, multiple sclerosis, glaucoma, seizures/epilepsy, and pain are among the most recognized qualifying ailments. CB1 (cannabinoid receptor type 1, first cloned in 1990) mRNA is highly expressed in the human brain [70]. It is also found in the heart, lungs, ovary, adrenal gland, thymus, bone marrow, and uterus. CB1 is also widely distributed inside non-neuronal tissues and inside various cells and tissues and co-exists with other CBRs [72]. CB1 is found inside the brain and displays high protein density and expression inside brain parts such as the cerebellar molecular layer, substantia nigra pars reticulate, globus pallidus external and internal, olfactory bulb, olfactory nucleus (anterior), hippocampus, and layers II–III, Va and VI of the cerebral cortexes [73]. Additionally, in humans, the highest CB1 level is found inside the cingulated gyrus, motor cortices, and frontal secondary somatosensory. CB1 is found in moderate levels inside the hypothalamus and ventral striatum and low levels in the brainstem; other respiratory control centers lack CB1. Moreover, this is the main reason phytocannabinoids have a low effect on respiratory and cardiovascular activities [74]. G-protein-coupled receptors (GPR) such as GPR18 and GPR55 serve as potent phytocannabinoid receptors. GPR18 is a “deorphanized” receptor present in cells and tissues of the thymus, spleen, small intestine, lymph nodes, leukocytes, and gametes [73]. Phytocannabinoids exerts strong therapeutic potential in humans assessed by the meta-analysis of several clinical trials; they possess a strong effect on health conditions such as nausea, vomiting, insomnia (sleeping disorder), depression, anxiety, paraplegia (paralyzes in a lower limb due to spinal cord injury), and psychosis (emotional metal disorder) and provide appetite stimulation in response to several syndromes [70]. Hemp predominantly produces bioactive compounds; it has resin glands with low THC and CBD activity. The antibacterial activity of these byproducts can be attributed to the terpenes and polyphenol compounds; phytocannabinoids are the main active compounds responsible for the antibacterial biocidal activity [72]. Another bibenzyl cis-THC, (−)-cis-perrottetinene (cis-PET), was isolated from the liverwort Radula perrottetii [72,73]. The cis configuration in the cyclohexene ring in cis-PET is comparable with ∆9-trans-THC. PET resembles ∆9-THC in its 3D shape and can bind to many of the same cannabinoid receptors (CBRs) as ∆9-THC. Moreover, research data on additives and the synergic and antagonistic activities of bioactive compounds are important to promote their use in feed as well as their industrial importance [73]. In addition, the cannabis industry is expected to grow much faster than expected, reaching USD 57 billion by 2027, with North America having the largest group of cannabis buyers; the global market is expected to cover at least 67% of the expenditure, and medicinal marijuana the remaining 33%. Other developed countries are expected to contribute to the medicinal cannabis market, thus making it the largest in the world. Several common diseases in several mammal species can be treated with phytocannabinoids, which can represent a translational improvement in bioactive phytocannabinoids; however, legislation must be passed to enable this [74,75].
7. Antimicrobial Activity of Phytocannabinoids
Antimicrobial resistance threatens the viability of modern medicine, which is largely dependent on the successful prevention and treatment of bacterial infections. Unfortunately, there are few new therapeutics [74]. A total of 150 phytocannabinoid compounds have been identified [75]. In addition, phytocannabinoids synthesized from cannabis and various Rhododendron sp. produce cannabinoids of the CBC type, whereas flowering plants, including Helichrysum umbraculigerum Less, Glycyrrhiza foetida, and Amorpha fruticosa, contain highly bioactive compounds with a cannabinoid backbone, carry an aralkyl side chain, and contain a resorcinol core [15]. Other cannabinoids, including bibenzyl analogs of ∆9-THC, have been isolated from liverworts, including Radula perrottetii [76]. Cannabinoids have been found in fungal species, including cannabiorchromenic acid from Cylindrocarpon oidium [77], which produces a considerable amount of biomass in a short duration. Compounds and hemp fibers have high amounts of lignin, cellulose, and other metabolites, which makes them suitable for animal feed [78]. Cannabidiol (CBD) is the major non-psychoactive component isolated from C. sativa and has been associated with multiple and potential biological activities, especially anxiolytic, antipsychotic, anti-inflammatory, analgesic, antioxidant, and neuroprotective properties [79]. The potential uses of cannabidiol in antibacterial therapies has recently emerged [80]. Since the 1950s, C. sativa-based preparations have also been investigated for their antibacterial activity [80]. Nonetheless, few studies have described the antibacterial activity of ultrapure CBD; CBD has a bacteriostatic as well as bactericidal effect against various Gram-positive bacterial species, such as Staphylococcus aeruginosa, Methicillin-resistant S. aureus (MRSA), and Streptococcus faecalis, and several fungal species, including Aspergillus niger and Candida albicans [81]. Recent research in the dental discipline suggests that CBD has a pronounced time-dependent inhibitory effect on biofilm formation as well as disrupts the mature biofilm of Candida albicans [82]. Antibacterial activity was tolerated owing to the nature of the prenyl moiety and its relative position compared with the pently moiety and carboxyl group [83]. Studies have reported that CBCA might exert antibacterial effects degrading the bacterial lipid membrane and altering the bacterial nucleoid [82]. Jin and Lee [81] investigated the antimicrobial and anti-lipogenic effects of hemp seed extract in HaCaT keratinocytes, as well as anti-inflammatory effects in stimulated HaCaT cells by reducing the expression of genes encoding inflammatory effects [84]. However, any potential relationship between cannabis use and microbial-induced diseases must be investigated and understood in detail [85]. Phytocannabinoids have a diverse roles in humans and exhibit antimicrobial and antibiotic activity. Apart from this, they also have some other biologically beneficial properties in mitigating biotic and abiotic stress in plants [12]. Cannabis trichomes possess phytocannabinoid in large quantities. High temperatures or herbivory cause trichome rupture and the release of the phytocannabinoid content, which protects the plant from desiccation and high-temperature stress [67]. It was also reported that phytocannabinoid production was enhanced in cannabis flowers after UV-B-induced stress. Significantly, phytocannabinoids inside oil bodies provided tolerance against several abiotic stresses, such as cold temperature, heat, excessive light, and UV radiation [71]. There is a lack of knowledge about the role of phytocannabinoids in biotic and abiotic stress mitigation. Thus, more in-depth studies are still required to understand the mechanism involved in stress tolerance via phytocannabinoids [85].
8. Phytocannabinoid Applications in Medicinal Therapies
The phytocannabinoids approved by the pharmaceutical industry and cannabinoids have been valuable starting compounds for the development of drugs, including Nabiximols (marketed as Sativex oral spray (2.7 mg/mL THC and 2.5 mg/mL CBD) by GW Pharmaceutical, Cambridge, UK). This drug has been approved in Canada, the USA, Europe, and several developing countries for treating muscle and neurological disorders, multiple sclerosis, and cancer [84]. Several synthetic drugs, such as cannabinoid-based drugs, have been approved for the relief of vomiting associated with cancer chemotherapy (Marinol, (Dronabinol), Solvay Pharmaceutical, and Cesamet) [85]. The numerous and versatile effects of cannabis contribute to the cannabinoid system (CBs) in different processes and help regulate several physiological and health diseases [86]. This plant treatment is a particularly valuable treatment for targeting the endocannabinoid system (eCBS); modulation by phytocannabinoids is currently being adopted to limit the biotherapeutic bioavailability of cannabis metabolites and treat a multitude of human diseases [87]. Phytocannabinoids have valuable efficacy in reducing the indicators of seizure, and several hypotheses dictate that endocannabinoid system (ECS) modulators affect neurogenesis. Where symptomatic benefit is observed, it is more commonly reflected in decreased agitation and aggression, increased appetite, sleep quality, objective mood, and pain control. Phytocannabinoids are promising candidates for treating symptoms of neurodegenerative and other diseases [88]. For example, administering CBD (30 mg/kg) intraperitoneally over 2 weeks increased hippocampal neurogenesis in rodents [89]. The effects of CBD were mediated by CB1 receptors, and with an increased level of the ECB ligand in the hippocampus with phytocannabinoid compounds, several effects on neurological factors were enhanced [90,91]. Owing to the evidence that cannabinoids are more effective at reducing nausea, in combination with the U.S. Food and Drug Administration (FDA)’s approval of dronabinol, the latter is now used therapeutically in the USA and other developed countries as a vomiting and nausea-related cancer treatment drug [92]. Additionally, phytocannabinoids have the ability to counteract anorexia, cachexia, and weight loss and act as an appetite stimulant in patients under chemotherapy [93]. Adverse effects are similar across diverse peoples, and it may be considered to mitigate these individual effects. The endocannabinoid system involved in the prominent subtype of the central nervous system (CNS) has garnered considerable attention as a potential therapeutic avenue for several pathological and neuropsychological disorders as well as neurodegenerative diseases. The possibility of patenting hemp cultivars with high yields in the pharmaceutical industry has led to the cultivation of hemp in aerobic environments, exclusively for the pharmaceutical industry.
9. Phytocannabinoids in Industrial Applications
Phytocannabinoids are naturally occurring cannabinoids found in the cannabis plant. Recently, numerous countries have increased their interest in the production and recreational use of marijuana as well as its tremendous medical and industrial applications. More than 100 naturally occurring compounds are produced by cannabinoids, the most abundant of which are Δ9-THC, CBD, terpenes, and flavonoids, which are used to treat various chronic diseases [83]. Cannabis’ medical and recreational uses, and its consequent legalization by the different government sectors of developed countries, enhance the development of cannabis worldwide. The plant produces a myriad of structurally and functionally diverse metabolites that play various roles in plant growth and development, and they are effective toxic compounds against herbivores. The production of cannabinoids has been modified for engineering in an in vitro cell-free system [94]. However, cannabis and cannabinoid products have evolved in response to the knowledge of profits from the phytocannabinoid system. They are highly complex owing to decades of use and abuse.
Phytocannabinoids are being investigated for their effect based on therapeutics in brain pathology. Several studies reported in previous databases on human tissues and animal models have highlighted the promising therapeutic potential of cannabinoids in different neurological disorders, including Parkinson’s and Alzheimer’s disease, and the mechanisms of action behind the reported improvement in the clinical outcome and disease progression are associated with their anti-inflammatory, immunosuppressive, antioxidant, and neuroprotective properties, due to the modulation of the endocannabinoid system [94]. Another important molecular feature found in neurodegenerative diseases is the failure of protein homeostasis mechanisms, resulting in undesirable aggregation of misfolded proteins [33]. In this context, CBD has exerted its protective effect over several signaling cascades involved with proteostasis, consequently reducing oxidative stress in cells [94]. The treatments are based on the pharmacophore of existing cannabinoids and treating the spasticity caused by multiple sclerosis in the USA, Canada, and Australia. Its contents, which include CBD and THC, have received regulatory approval in several European countries; however, CBD is a single therapeutic agent. Therefore, clinical studies are ongoing to confirm the efficacy of CBD [95]. Epidiolex (CBD) is in clinical trials for the treatment of resistance seizure disorders, such as Lennox-Gastaut and Dravet syndrome [96]. Based on clinical efficacy and regulation, it also appears to involve the receptor [96]. Phytocannabinoids and cannabinoids, which are effective and safe, are classed as drugs in clinical trials with drugs that fail to demonstrate their efficiency in vivo models. The anticipated legalization at the US federal level and the rapid development of legal cannabis production have led to a strong demand to incorporate cannabis and phytocannabinoids into medicine because of their benefits and low-level side effects on organic cognitive function [85]. With industry support and alternative medicinal research, cannabis is capable of producing an impressive number of cannabinoids exceeding 30% of the flower, bud, and dry weight [97]. Rivaling plant efficacy with microbial fermentation for producing phytocannabinoids such as THC and CBD is challenging, particularly as most significant resources have been put into cannabis agronomy research; in addition, the product sale prices for phytocannabinoids dropped steadily from 2019 to 2021 [5,98]. Because of the numerous potential benefits in the treatment of neurodegenerative disease and other known major diseases [99], synthetic cannabinoid analogs with the potential to exploit cannabinoid treatment without evoking undesirable psychotropic properties are highly desired and may advance research in this area [95].
10. Conclusions and Future Perspectives
Phytocannabinoids are bioactive natural products found in some flowering plants, liverworts, and fungi that can be beneficial for the treatment of humans and animals and present potent antibiotic effects; most uses are based on anti-inflammatory, neuroprotective, and anti-nociceptive activities. Numerous cannabinoids exhibit promising non-hallucinogenic bioactivities that are variable in nature owing to the side chain and prenyl group and are involved in the biosynthesis pathways. The most important phytocannabinoids possess therapeutic, antibacterial, and antimicrobial properties; thus, they are used in treating several human diseases, and these compounds can contribute to cold, heat, and UV radiation tolerance [100]. Phytocannabinoid biosynthesis can have a constructed nature, and it is used as the foundation of ornamental cannabis research by overcoming certain legal barriers. It has been introduced to the ornamental sector to further evaluate horticultural and cannabis-related research. The bioactive molecules show potential and are well suited to the novel treatment of various diseases in animals [101]. In the meantime, further studies are required for the acceptance of clinical uses for phytocannbinoids and other cannabis-related entities in the context of infectious diseases and antimicrobial activity. Various types of cannabinoids can be produced once additional biosynthetic engineering techniques have been elucidated and a large collection of enzyme molecules is established. However, mere determination of the influence of the abiotic factors on phytocannabinoids production is insufficient [98]. Hemp products, CBD, and THC, are the greatest wholesale potential markets and are highly attractive for their recreational and medicinal properties. However, the ideal extraction technology is expected to be cannabinoid-specific and selective toward the extraction of the CBD compounds [101].
Cannabis may improve plant performance in disease-resistant plants, which can increase the overall content of phytocannabinoids [102,103]. As individual plants are likely to be metabolically active in their cannabinoid formation, phytocannabinoids have versatile uses and beneficial effects. However, any type of cannabinoid can be produced once additional biosynthetic pathways have been elucidated [104,105]. The fate and durability of non-cannabinoids and cannabinoids will be determined by the therapeutic development of numerous rare and novel cannabinoids [106,107]. Synthetic and semi-synthetic cannabinoids are very important for the economy owing to demands from the wholesale market and various cannabis industries to enhance agri-business and prevent plant pathogens [108]. The hypothesis is that phytocannabinoids have versatile use and are beneficial for humans and plants if appropriately used [109,110]. Further investigations are needed on the genes and enzymes involved in the biosynthetic pathways in different plant species [111,112]. The main aim of the review focused on understanding phytocannabinoids through biosynthesis, structural elucidation, and the role of bioactivity in animals as well as whether they possess antimicrobial properties that are important in therapeutics [113,114]. In addition, further investigation is needed on genes and the enzymes involved in the biosynthetic pathways in various sources of plant species [115,116]. The activities of phytocannabinoids play a crucial role in their beneficial effects on animals and humans and provide knowledge that acts as a barrier to the acceptance and utility of cannabis-based antimicrobial therapeutics [117,118]. The physiological activity of cannabis has been largely restricted to THC and CBD compounds; however, some studies have reported that some of the effects arise from the other cannabinoids. Future research should focus on investigating these phytocannabinoids as a key to understanding their limitations. Moreover, information regarding their structural prediction is currently insufficient. The future use of these phytocannabinoids, including their safety in therapeutics, adverse effects, and economic demands, must be addressed. The production of this plant, which is beneficial to animal health, has no negative impact on the environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13030442/s1, Table S1: Predicted nucleotide composition among tetrahydrocanna-binolic acid (THCA) and Cannabidiolic acid (CBDA) synthase using BioEdit software.
Author Contributions
R.K.G.: conceptualization, resources, investigation, formal analysis, writing—original draft preparation. A.K.M.: resources, investigation, validation, writing—original draft and editing. K.-H.C.: resources, investigation, validation and draft preparation. K.-H.K. and K.M.Y.: resources, investigation, formal analysis, and draft preparation. K.-H.B.: supervision, resources, investigation, formal analysis, and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research fund from Yeungnam University in 2021.
Acknowledgments
The authors appreciate the research funding provided by Yeungnam University in 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kanabus, J.; Bryła, M.; Roszko, M.; Modrzewska, M.; Pierzgalski, A. Cannabinoids-Characteristics and potential for use in food production. Molecules 2021, 26, 6723. [Google Scholar] [CrossRef]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Bot. Mus. Lealf. Harv. Univ. 1974, 23, 337–367. [Google Scholar] [CrossRef]
- Chen, C.; Pan, Z. Cannabidiol and terpenes from hemp–ingredients for future foods and processing technologies. J. Futur. Foods 2021, 1, 113–127. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Bajguz, A.; Hayat, S. Phytocannabinoids biosynthesis in angiosperms, fungi, and liverworts and their versatile role. Plants 2021, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Blatt-Janmaat, K.; Qu, Y. The biochemistry of phytocannabinoids and metabolic engineering of their production in heterologous systems. Int. J. Mol. Sci. 2021, 22, 2454. [Google Scholar] [CrossRef]
- Balant, M.; Gonzalez, R.R.; Garcia, S.; Garnatje, T.; Pellicer, J.; Valles, J.; Vitales, D.; Hidalgo, O. Novel Insights into the Nature of Intraspecific Genome Size Diversity in Cannabis sativa L. Plants 2022, 11, 2736. [Google Scholar] [CrossRef]
- Karche, T. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Jones, A.M.P. Current status and future prospects in cannabinoid production through in vitro culture and synthetic biology. Biotechnol. Adv. 2022, 62, 108074. [Google Scholar] [CrossRef]
- Klahn, P. Cannabinoids-Promising Antimicrobial Drugs or Intoxicants with Benefits? Antibiotics 2020, 9, 297. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Rahman, M.H.; Roy, B.; Chowdhury, G.M.; Hasan, A.; Saimun, M.S.R. Medicinal plant sources and traditional healthcare practices of forest-dependent communities in and around Chunati Wildlife Sanctuary in southeastern Bangladesh. Environ. Sustain. 2022, 5, 207–241. [Google Scholar] [CrossRef]
- Vines, G. Herbal Harvests with a Future: Towards Sustainable Sources for Medicinal Plants; Plantlife International: Wiltshire, UK, 2004. [Google Scholar]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Barrales-Cureño, H.J.; López-Valdez, L.G.; Reyes, C.; Cetina-Alcala, V.M.; Vasquez-García, I.; Diaz-Lira, O.F.; Herrera-Cabrera, B.E. Chemical characteristics, therapeutic uses, and legal aspects of the cannabinoids of Cannabis sativa: A review. Brazilian Arch. Biol. Technol. 2020, 63, e20190222. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Jones, A.M.P. Recent advances in cannabis biotechnology. Ind. Crops Prod. 2020, 158, 113026. [Google Scholar] [CrossRef]
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis sativa research trends, challenges, and new-age perspectives. Iscience 2021, 24, 103391. [Google Scholar] [CrossRef]
- Hesami, M.; Baiton, A.; Alizadeh, M.; Pepe, M.; Torkamaneh, D.; Jones, A.M.P. Advances and perspectives in tissue culture and genetic engineering of cannabis. Int. J. Mol. Sci. 2021, 22, 5671. [Google Scholar] [CrossRef]
- Livingston, S.J.; Rensing, K.H.; Page, J.E.; Samuels, A.L. A polarized supercell produces specialized metabolites in cannabis trichomes. Curr. Biol. 2022, 32, 4040–4047. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Khanongnuch, C.; Mathivanan, K.; Shyu, D.J.; Sharma, K.P.; De Mandal, S. In-vitro biotransformation of tea using tannase produced by Enterobacter cloacae 41. J. Food Sci. Technol. 2021, 58, 3235–3242. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It is our turn to get cannabis high: Put cannabinoids in food and health baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef]
- Rubin, G. Tattoo Ink Containing Cannabis or Hemp Derived Cannabinoids or Mixture of Both. U.S. Patent 16/560,914, 4 September 2019. [Google Scholar]
- Gertsch, J.; Pertwee, R.G.; Di Marzo, V. Phytocannabinoids beyond the Cannabis plant–do they exist? Br. J. Pharmacol. 2010, 160, 523–529. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef]
- Thomas, F.; Schmidt, C.; Kayser, O. Bioengineering studies and pathway modeling of the heterologous biosynthesis of tetrahydrocannabinolic acid in yeast. Appl. Microbiol. Biotechnol. 2020, 104, 9551–9563. [Google Scholar] [CrossRef] [PubMed]
- Hurgobin, B.; Tamiru-Oli, M.; Welling, M.T.; Doblin, M.S.; Bacic, A.; Whelan, J.; Lewsey, M.G. Recent advances in Cannabis sativa genomics research. N. Phytol. 2021, 230, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. The case for the entourage effect and conventional breeding of clinical cannabis: No “strain,” no gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Huchelmann, A.; Boutry, M.; Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological interest. Plant Physiol. 2017, 175, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Bernstein, N.; Gorelick, J.; Koch, S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2019, 129, 185–194. [Google Scholar] [CrossRef]
- Vasincu, A.; Rusu, R.N.; Ababei, D.C.; Larion, M.; Bild, W.; Stanciu, G.D.; Solcan, C.; Bild, V. Endocannabinoid Modulation in Neurodegenerative Diseases: In Pursuit of Certainty. Biology 2022, 11, 440. [Google Scholar] [CrossRef]
- Svizenska, I.; Dubovy, P.; Sulcova, A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—A short review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. In Phytocannabinoids. Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 103–131. ISBN 978-3-319-45541-9. [Google Scholar]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis glandular trichomes alter morphology and metabolite content during flower maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- Bilodeau, S.E.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- Schauer, G.L.; King, B.A.; Bunnell, R.E.; Promoff, G.; McAfee, T.A. Toking, vaping, and eating for health or fun: Marijuana use patterns in adults, U.S., 2014. Am. J. Prev. Med. 2016, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Belendiuk, K.A.; Baldini, L.L.; Bonn-Miller, M.O. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict. Sci. Clin. Pract. 2015, 10, 10. [Google Scholar] [CrossRef]
- Rodziewicz, P.; Loroch, S.; Marczak, Ł.; Sickmann, A.; Kayser, O. Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes. Plant Sci. 2019, 284, 108–116. [Google Scholar] [CrossRef]
- Lim, K.J.H.; Lim, Y.P.; Hartono, Y.D.; Go, M.K.; Fan, H.; Yew, W.S. Biosynthesis of Nature-Inspired Unnatural Cannabinoids. Molecules 2021, 26, 2914. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Pacchetti, B.; Anand, P.; Sodergren, M.H. Cannabis-based medicines and pain: A review of potential synergistic and entourage effects. Pain Manag. 2021, 11, 395–403. [Google Scholar] [CrossRef]
- Taura, F.; Tanaka, S.; Taguchi, C.; Fukamizu, T.; Tanaka, H.; Shoyama, Y.; Morimoto, S. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 2009, 583, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef]
- Stout, J.M.; Boubakir, Z.; Ambrose, S.J.; Purves, R.W.; Page, J.E. The hexanoyl-CoA precursor for cannabinoid biosynthesis is formed by an acyl-activating enzyme in Cannabis sativa trichomes. Plant J. 2012, 71, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Komatsu, K.; Taura, F.; Shoyama, Y. Purification and characterization of cannabichromenic acid synthase from Cannabis sativa. Phytochemistry 1998, 49, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef]
- Degenhardt, F.; Stehle, F.; Kayser, O. The biosynthesis of cannabinoids. In Handbook of Cannabis and Related Pathologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 13–23. [Google Scholar]
- Hanus, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Compton, D.R.; Rice, K.C.; De Costa, B.R.; Razdan, R.K.; Melvin, L.S.; Johnson, M.R.; Martin, B.R. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J. Pharmacol. Exp. Ther. 1993, 265, 218–226. [Google Scholar] [PubMed]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- Grassi, G.; McPartland, J.M. Chemical and morphological phenotypes in breeding of Cannabis sativa L. Cannabis Sativa L. Bot. Biotechnol. 2017, 137–160. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’ Agli, M.; Piazza, S.; Sangiovanni, E. Cannabis sativa and skin health: Dissecting the role of phytocannabinoids. Planta Med. 2022, 88, 492–506. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatolog. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef]
- Fuentes, P.; Armarego-Marriott, T.; Bock, R. Plastid transformation and its application in metabolic engineering. Curr. Opin. Biotechnol. 2018, 49, 10–15. [Google Scholar] [CrossRef]
- Pellechia, T. Legal Cannabis Industry Poised for Big Growth, in North America and around the World. Available online: https://www.forbes.com/sites/thomaspellechia/2018/03/01/double-digit-billions-puts-north-america-in-the-worldwide-cannabis-market-lead/# (accessed on 8 December 2022).
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa–From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Nadal, X.; Del Río, C.; Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sánchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef]
- Pryce, G.; Riddall, D.R.; Selwood, D.L.; Giovannoni, G.; Baker, D. Neuroprotection in experimental autoimmune encephalomyelitis and progressive multiple sclerosis by cannabis-based cannabinoids. J. Neuroimmune Pharmacol. 2015, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. In Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 61–101. ISBN 978-3-319-45541-9. [Google Scholar]
- Devinsky, O.; Verducci, C.; Thiele, E.A.; Laux, L.C.; Patel, A.D.; Filloux, F.; Szaflarski, J.P.; Wilfong, A.; Clark, G.D.; Park, Y.D. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018, 86, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1934578X1801300922. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A. Chemical constituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2017, 81, 641–660. [Google Scholar] [CrossRef]
- Semwogerere, F.; Katiyatiya, C.L.F.; Chikwanha, O.C.; Marufu, M.C.; Mapiye, C. Bioavailability and bioefficacy of hemp by-products in ruminant meat production and preservation: A review. Front. Vet. Sci. 2020, 7, 572906. [Google Scholar] [CrossRef]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S. Cannabinoids for medical use: A systematic review and meta-analysis. Jama 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- FEEDAP. Scientific Opinion on the safety of hemp (Cannabis genus) for use as animal feed. EFSA J. 2011, 9, 2011. [Google Scholar]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. Var. Sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.; Zadik-Weiss, L.; Almogi-Hazan, O.; Or, R. Cannabis, One Health, and Veterinary Medicine: Cannabinoids’ Role in Public Health, Food Safety, and Translational Medicine. Rambam Maimonides Med. J. 2020, 11, e0006. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.; Neira Agonh, D.; Lehmann, C. Antibacterial Effects of Phytocannabinoids. Life 2022, 12, 1394. [Google Scholar] [CrossRef]
- Toyota, M.; Kinugawa, T.; Asakawa, Y. Bibenzyl cannabinoid and bisbibenzyl derivative from the liverwort Radula perrottetii. Phytochemistry 1994, 37, 859–862. [Google Scholar] [CrossRef]
- Quaghebeur, K.; Coosemans, J.; Toppet, S.; Compernolle, F. Cannabiorci-and 8-chlorocannabiorcichromenic acid as fungal antagonists from Cylindrocarpon olidum. Phytochemistry 1994, 37, 159–161. [Google Scholar] [CrossRef]
- Barak, T.; Sharon, E.; Steinberg, D.; Feldman, M.; Sionov, R.V.; Shalish, M. Anti-Bacterial Effect of Cannabidiol against the Cariogenic Streptococcus mutans Bacterium: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 15878. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Steinberg, D. Anti-microbial activity of phytocannabinoids and endocannabinoids in the light of their physiological and pathophysiological roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Biofilm Activity of Cannabigerol against Streptococcus mutans. Microorganisms 2021, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Gildea, L.; Ayariga, J.; Ajayi, O.; Xu, J.; Villafane, R.; Samuel-Foo, M. Cannabis sativa CBD Extract Shows Promising Antibacterial Activity against S. typhimurium and S. newington. Molecules 2022, 27, 2699. [Google Scholar] [CrossRef]
- Abichabki, N.; Zacharias, L.V.; Moreira, N.C.; Bellissimo-Rodrigues, F.; Moreira, F.L.; Benzi, J.R.; Ogasawara, T.M.; Ferreira, J.C.; Ribeiro, C.M.; Pavan, F.R.; et al. Potential cannabidiol (CBD) repurposing as antibacterial and promising therapy of CBD plus polymyxin B (PB) against PB-resistant gram-negative bacilli. Sci. Rep. 2022, 12, 6454. [Google Scholar] [CrossRef]
- Russo, C.; Lavorgna, M.; Nugnes, R.; Orlo, E.; Isidori, M. Comparative assessment of antimicrobial, antiradical and cytotoxic activities of cannabidiol and its propyl analogue cannabidivarin. Sci. Rep. 2021, 11, 22494. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules. 2020, 10, 900. [Google Scholar] [CrossRef]
- de Fonseca, F.R.; Del Arco, I.; Bermudez-Silva, F.J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol Alcohol. 2005, 40, 2–14. [Google Scholar] [CrossRef]
- Uziel, A.; Gelfand, A.; Amsalem, K.; Berman, P.; Lewitus, G.M.; Meiri, D.; Lewitus, D.Y. Full-spectrum cannabis extract microdepots support controlled release of multiple phytocannabinoids for extended therapeutic effect. ACS Appl. Mater. Interfaces 2020, 12, 23707–23716. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutiérrez, S.O.; Van der Stelt, M. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Marchalant, Y.; Rosi, S.; Wenk, G.L. Anti-inflammatory property of the cannabinoid agonist WIN-55212-2 in a rodent model of chronic brain inflammation. Neuroscience 2007, 144, 1516–1522. [Google Scholar] [CrossRef]
- Liano, H.C.; Zakowicz, P.; Mikołajczak, P. Cannabinoids as antiemetics: A short review. Acta Pol. Pharm. Res. 2018, 75, 1063–1068. [Google Scholar]
- Schleider, L.B.-L.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef]
- Meija, J.; McRae, G.; Miles, C.O.; Melanson, J.E. Thermal stability of cannabinoids in dried cannabis: A kinetic study. Anal. Bioanal. Chem. 2022, 414, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Prandi, C.; Blangetti, M.; Namdar, D.; Koltai, H. Structure-activity relationship of cannabis derived compounds for the treatment of neuronal activity-related diseases. Molecules 2018, 23, 1526. [Google Scholar] [CrossRef]
- Lago-Fernandez, A.; Redondo, V.; Hernandez-Folgado, L.; Figuerola-Asencio, L.; Jagerovic, N. New Methods for the Synthesis of Cannabidiol Derivatives. Methods Enzymol. 2017, 593, 237–257. [Google Scholar]
- Ferreira, J.F.S.; Benedito, V.A.; Sandhu, D.; Marchese, J.A.; Liu, S. Seasonal and differential sesquiterpene accumulation in Artemisia annua suggest selection based on both artemisinin and dihydroartemisinic acid may increase artemisinin in planta. Front. Plant Sci. 2018, 9, 1096. [Google Scholar] [CrossRef]
- Stern, E.; Lambert, D.M. Medicinal chemistry endeavors around the phytocannabinoids. Chem. Biodivers. 2007, 4, 1707–1728. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2009, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Rosa Franco, G.; Smid, S.; Viegas, C. Phytocannabinoids: General Aspects and Pharmacological Potential in Neurodegenerative Diseases. Curr. Neuropharmacol. 2021, 19, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Mathivanan, K.; Selva, R.; Chandirika, J.U.; Govindarajan, R.K.; Srinivasan, R.; Annadurai, G.; Duc, P.A. Biologically synthesized silver nanoparticles against pathogenic bacteria: Synthesis, calcination and characterization. Biocatal. Agric. Biotechnol. 2019, 22, 101373. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional quality and potential functionality for human health and nutrition. Nutrients. 2020, 12, 1935. [Google Scholar] [CrossRef]
- Iftikhar, A.; Zafar, U.; Ahmed, W.; Shabbir, M.A.; Sameen, A.; Sahar, A.; Bhat, Z.F.; Kowalczewski, P.Ł.; Jarzębski, M.; Aadil, R.M. Applications of Cannabis Sativa L. in Food and Its Therapeutic Potential: From a Prohibited Drug to a Nutritional Supplement. Molecules. 2021, 26, 7699. [Google Scholar] [CrossRef]
- Ranalli, P.; Di Candilo, M.; Mandolino, G.; Grassi, G.; Carboni, A. Hemp for sustainable agricultural systems. Agro Food Ind. Hi-Tech 1999, 10, 33–38. [Google Scholar]
- Tutek, K.; Masek, A. Hemp and Its Derivatives as a Universal Industrial Raw Material (with Particular Emphasis on the Polymer Industry)—A Review. Materials 2022, 15, 2565. [Google Scholar] [CrossRef]
- Pane, A.; Cosentino, S.L.; Copani, V.; Cacciola, S.O. First report of southern blight caused by Sclerotium rolfsii on hemp (Cannabis sativa) in Sicily and Southern Italy. Plant Dis. 2007, 91, 636. [Google Scholar] [CrossRef]
- Casiraghi, A.; Roda, G.; Casagni, E.; Cristina, C.; Musazzi, U.M.; Franze, S.; Rocco, P.; Giuliani, C.; Fico, G.; Minghetti, P. Extraction method and analysis of cannabinoids in cannabis olive oil preparations. Planta Med. 2018, 84, 242–249. [Google Scholar] [CrossRef]
- Salehi, A.; Puchalski, K.; Shokoohinia, Y.; Zolfaghari, B.; Asgary, S. Differentiating cannabis products: Drugs, food, and supplements. Front. Pharmacol. 2022, 13, 906038. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, R.K.; Mathivanan, K.; Khanongnuch, C.; Srinivasan, R.; Unban, K.; Deepak, A.C.; Al Farraj, D.A.; Alarjani, K.M.; Al Qahtany, F.S. Tannin acyl-hydrolase production by Bacillus subtilis KMS2-2: Purification, characterization, and cytotoxicity studies. J. King Saud Univ. Sci. 2021, 33, 101359. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. Biomed. Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef] [PubMed]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The antimicrobial activity of cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Kamil, S.; Shah, M.R.; Bhimanadham, N.N.; Imran, S. Pros and cons of marijuana in treatment of Parkinson’s disease. Cureus 2019, 11, e4813. [Google Scholar] [CrossRef]
- Paes-Colli, Y.; Aguiar, A.F.; Isaac, A.R.; Ferreira, B.K.; Campos, R.M.P.; Trindade, P.M.P.; de Melo Reis, R.A.; Sampaio, L.S. Phytocannabinoids and Cannabis-Based Products as Alternative Pharmacotherapy in Neurodegenerative Diseases: From Hypothesis to Clinical Practice. Front. Cell. Neurosci. 2022, 16, 273. [Google Scholar] [CrossRef]
- Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in neurological diseases: Could they restore a physiological GABAergic transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef]
- Escobar-Bravo, R.; Klinkhamer, P.G.; Leiss, K.A. Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front. Plant Sci. 2017, 8, 278. [Google Scholar] [CrossRef]
- Thomas, B.F.; ElSohly, M.A. Biosynthesis and pharmacology of phytocannabinoids and related chemical constituents. In The Analytical Chemistry of Cannabis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–41. [Google Scholar]
- Kiran, G.S.; Sajayan, A.; Priyadharshini, G.; Balakrishnan, A.; Prathiviraj, R.; Sabu, A.; Selvin, J. A novel anti-infective molecule nesfactin identified from sponge associated bacteria Nesterenkonia sp. MSA31 against multidrug resistant Pseudomonas aeruginosa. Microb. Pathog. 2021, 157, 104923. [Google Scholar] [CrossRef]
- Chellapandi, P.; Hussain, M.M.K.; Prathiviraj, R. CPSIR-CM: A database for structural properties of proteins identified in cyanobacterial C1 metabolism. Algal Res. 2017, 22, 135–139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).