Abstract
Although there has been limited application in the field to date, human milk omics research continues to gain traction. Human milk lipidomics and metabolomics research is particularly important, given the significance of milk lipids and metabolites for infant health. For researchers conducting compositional milk analyses, it is important to consider the origins of these compounds. The current review aims to provide a summary of the existing evidence on the sources of human milk lipids and small metabolites. Here, we describe five major sources of milk lipids and metabolites: de novo synthesis from mammary cells, production by the milk microbiota, dietary consumption, release from non-mammary tissue, and production by the gut microbiota. We synthesize the literature to provide evidence and understanding of these pathways in the context of mammary gland biology. We recommend future research focus areas to elucidate milk lipid and small metabolite synthesis and transport pathways. Better understanding of the origins of human milk lipids and metabolites is important to improve translation of milk omics research, particularly regarding the modulation of these important milk components to improve infant health outcomes.
1. Introduction
As the first food for infants, human milk is a unique biofluid that provides the infant with all early-life requirements to thrive, including nutrient and bioactive factors. A wealth of evidence demonstrates that breastfeeding protects infants from acute and chronic illnesses and promotes the healthy development of various body systems [1,2,3,4,5]. Importantly, human milk forms the third arm of the mother-infant-milk triad [6], with human milk composition underpinning the complex relationship between mothers and infants. Various components of human milk contribute to healthy development and growth of the infant, including immune proteins, hormones, cytokines, leukocytes, microRNAs, macro- and micronutrients, microbiota, and stem cells [7,8,9,10]. Among the multitude of human milk components, lipids and small metabolites are of particular interest due to their likely importance in infant development, and the relative lack of research regarding their presence and function in milk [11,12].
Lipids are a major component of human milk, secreted as milk fat globules. These tri-layer structures deliver triacylglycerols, phospholipids, gangliosides, and many other lipid classes, to the infant [13]. The lipid portion of human milk is relatively complex, with wide intra- and inter-individual variation [14]. Although frequently thought to originate directly from maternal diet, the link between human milk lipids and diet is complex, with diet alone unable to account for the entire milk lipidome. Indeed, mammary tissue has significant lipogenic capacity. Human milk lipid composition is therefore a combination of incorporation from circulation and de novo synthesis. A number of human milk lipids have been linked to health and growth in early life. This includes many individual fatty acids, such as palmitic acid, which is present in higher abundance in human milk consumed by healthy infants compared to those who have cold-like illness [15,16,17]. The antimicrobial functions of human milk fatty acids are well known [17]. Additionally, a lower omega six to omega three (n−6:n−3) fatty acid ratio has been associated with increased lean body mass early in life and thus lowered risk of obesity [18]. Given the known importance of lipids in adult health and disease, human milk lipids have significant potential to modulate infant health and to program life-long health. A better understanding of the human milk lipidome will thereby aid in prediction or prevention of chronic health conditions [11].
Human milk also contains a multitude of small metabolites [19]. These include microbial metabolites such as 12,13-dihydroxyocatadecanoic acid (12,13-diHOME), short chain fatty acids (SCFAs), and trimethylamine (TMA), as well as microbial-host co-metabolites, such as taurine, polyamines, and hippuric acid. Many of these metabolites have only recently been identified in human milk, and their relationship to infant health is unknown. While there is an assumption that these metabolites derive from the maternal circulation (potentially originating from the maternal gut microbiome or maternal metabolism), local production of metabolites by the mammary cells and the milk microbiota is also likely [20,21]. The potential of some of these small metabolites to modulate infant health is already apparent. For example, human milk 12,13-diHOME has been associated with both lowered subcutaneous fat and slower body mass index (BMI) increase in early life, linking milk 12,13-diHOME to obesity [20]. Additionally, there is a correlation between infant fecal 12,13-diHOME and eczema and allergy, demonstrating the immune programming potential for this human milk metabolite [22]. Similarly, human milk SCFAs have also been linked to infant weight gain [23] and immunity [21]. Due to the causal role of the microbiome in many adult diseases, microbes and their small metabolite products are of high interest in early life, with human milk potential serving as a constant source of these important products [12].
To the best of our knowledge, the latest article reviewing similar content was published in 2013, focusing on the impact of maternal nutrition and genetics on human milk fatty acids [24]. Since then, many other lipids and small metabolites have been identified in human milk, and vast advancements have been made in human milk research techniques, including sample collection and storage [25], DNA and lipid extraction [26,27,28], mass spectrometry and nuclear magnetic resonance [29,30], DNA sequencing [31], and cell sequencing [32]. Thus, there is a need currently to review recent evidence regarding the origins of human milk lipids and small metabolites. With the uprise of omics research in the human milk field, it is critical that we consider the origins of milk lipids and small metabolites, as well as other components of milk. Importantly, such an understanding feeds into the framework of human milk as a complex biological system, recognizing its relationship with other maternal body sites and systems [33]. This will allow better interpretation of findings, and better application of research to translation. The purpose of this review is to present the existing evidence on the origins of human milk lipids and small metabolites to inform future research directions and application. A high degree of variability in lipid and small metabolite concentrations has been identified across milk samples from different individuals around the globe [14,34], suggesting that determination of milk composition is complex and dynamic. Human milk lipids and small metabolites may be synthesized locally within the mammary gland (by the mammary cells or the milk microbiota) or they may be incorporated from maternal circulation (originating from the maternal diet, maternal gut microbiota, or other non-mammary tissue) (Figure 1). The evidence for each of these pathways will be discussed.
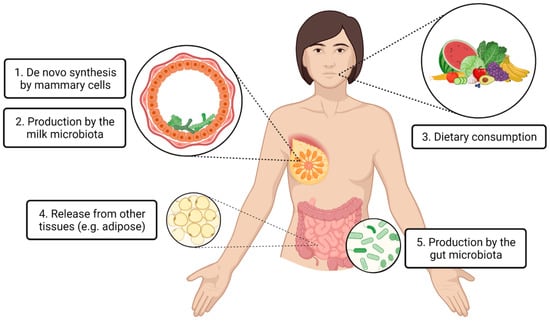
Figure 1.
Proposed origins of lipids and small metabolites found in human milk. Local production within the mammary gland (1,2) and transport from distal body locations via the circulatory system (3,4,5) are hypothesized.
2. Local Production within the Mammary Gland
2.1. De Novo Synthesis by Mammary Cells
During lactation, the human mammary gland is one of the most metabolically active organs, with many synthetic processes unique to mammary cells, or upregulated to a degree much higher than other lipogenic organs (such as the liver). Of particular interest, the capacity to esterify fatty acids to glycerol, forming triacylglycerols, and to position over 70% of the C16:0 in the sn−2 position, are quite unique to the mammary gland [35,36]. High gene expression has been measured from cells in human milk during lactation, including genes for lipolysis, fatty acid uptake, de novo fatty acid synthesis, elongation and saturation of fatty acids, and triacylglycerol and cholesterol synthesis [35].
De novo fatty acid synthesis occurs in the mammary cell cytosol, utilizing glucose and NADPH substrates, and fatty acid synthase. Thioesterase II is highly expressed only in mammary cells, and specifically controls the chain length of these fatty acids, uniquely favoring medium chain C14:0 [37]. Further to this, de novo mammary synthesis of C16:0 to C18:1, and its control, remain unclear. Much of the understanding of de novo synthesis is based on animal models, and mouse studies have indicated that between 15 and 40% of milk fatty acids are a result of de novo synthesis [38]. Evidence from fatty acid synthase knock-out mice demonstrates the importance of de novo mammary synthesis, with knock-out dams producing milk low in fatty acids and experiencing early involution, resulting in pups who cannot thrive [36]. Human evidence on de novo mammary lipid synthesis is scarcer due to methodological difficulties. In place of gene knock-out experiments, human studies rely on the estimation of fatty acid synthesis based on measurements of dietary intake and stored fatty acids. Regardless, a small number of human studies have suggested approximately similar portions in mice and humans. Data from one human tracer study, with only three participants, indicate that de novo fatty acid synthesis provides 10–12% of milk fatty acids [39].
Following de novo synthesis of fatty acids, they are esterified to produce triacylglycerols, phospholipids, and other lipids, through glycerol−3-phosphate and monoglyceride pathways [40]. Transcriptomics on mammary cells isolated from human milk has shown expression of many lipid metabolism genes, including desaturases (FADS 1–6) and elongases (ELOVL 1–6) suggesting that the mammary gland has the capacity to synthesize all types of fatty acids, including long chain polyunsaturated fatty acids, which have commonly been thought to originate from diet. Other lipid types may also be synthesized by mammary cells, including gangliosides and phosphatidylcholine, based on expression of ST3GAL5, which encodes GM3 synthase, and CHKA and CHKB, which encode choline kinase alpha and beta [32]. Increasing accessibility to rapidly advancing techniques in single cell omics, allowing identification of novel gene expression and synthesis pathways, will facilitate further understanding on this front; however, results from milk-derived cells should be interpreted with some caution, as they may not be entirely representative of in vivo mammary cells [32,35,41].
2.2. Production by the Milk Microbiota
Human milk harbors a low biomass, low diversity, viable bacterial microbiome, predominantly comprising Staphylococcus and Streptococcus species, along with lower abundances of typical oral, skin, and gut taxa, such as Cutibacerium, Gemella, and Bifidobacteria [42]. The milk microbiome varies both between individuals (in relation to factors such as delivery mode, parity, and maternal BMI [43]), and within individuals over time [44,45]. This community may actively produce lipids and metabolites within the mammary gland; however, to date, limited evidence supports this notion. Whether the bacteria detected in human milk represent a permanent mammary gland microbiome, or whether they are transiently present is still unconfirmed [42]. This is worth noting, as the nature of the milk microbiome has implications for our understanding of the metabolic activity of the milk microbiota within the lactating mammary gland. Bacterial metabolites are present in human milk [12,21,34,46,47], although it is currently unclear whether they are produced locally in the mammary gland by bacterial metabolism, or whether they are derived from the maternal gut microbiota (see Section 3.3, Production by gut microbiota). For instance, short chain fatty acids (SCFAs) are present in human milk [21,46] at concentrations and ratios that differ vastly from those seen in serum (Table 1) [48,49], potentially suggesting local production or degradation within the mammary gland. Indeed, propionate, which is present in serum at concentrations similar to butyrate [49] and has been detected in the blood of pregnant individuals [50], has only recently been detected in human milk and appears to be present in negligible concentrations compared to butyrate [51]. Bacteria that possess the capacity to produce SCFAs from metabolism of human milk oligosaccharides (HMOs), such as Bifidobacterium, are present in human milk [42]. However, SCFAs are also present in maternal serum [48], suggesting that gut-derived SCFAs may be distributed into milk via the circulation. To date, no direct evidence has demonstrated that HMOs are metabolized in situ in the lactating breast. The origins of human milk SCFAs therefore remain unknown.

Table 1.
Key health-modulating gut bacterial metabolites that have been identified in human milk. Previously reported levels from blood and milk samples are provided. Values are mean ± SD, or mean (range), depending on the reporting style of the study. Concentrations are µM unless stated otherwise.
Data on the metatranscriptome of human milk suggest that the bacteria within the mammary gland actively transcribe genes for biosynthesis of various compounds, including secondary metabolites, such as amines and chorismic acid, and aromatic compounds, such as phenols and indoles [59]. Given that human milk bacteria are transcriptionally active, and that substrates for bacterial metabolism (such as HMOs and glucose) are present in human milk, it is plausible that local production of bacterial metabolites occurs in the lactating mammary gland. While bacterial lipid synthesis pathways are well-described [60,61], uniquely bacterial-derived lipids are yet to be reported within human milk. Evidence from transcriptomic work has not yet described active transcription of microbial lipid synthesis genes in human milk. However, this is likely due to the limited number and nature of such studies rather than lack of such synthesis. Targeted polymerase chain reaction (PCR) for bacterial lipid synthesis genes may overcome this gap in the literature.
3. Incorporation from Maternal Circulation
Many components reach the mammary gland and are incorporated into human milk from the maternal circulation. During the colostrum phase, lipids and small metabolites from circulation may cross into milk via the paracellular pathway (free transport via openings between epithelial cells); however, following closure of the tight junctions between lactocytes and transition to mature milk, transport must occur via transcellular pathways (through epithelial cells) [62]. From the bloodstream, lipids and small metabolites, including approximately 60% of the utilized fatty acids [39], are either packaged or further metabolized in the mammary gland, ending up in the milk fat globule for secretion [62]. Metabolite transport mechanisms across the mammary epithelium likely depend on the size and properties of the metabolite, as well as other conditions, and may include pinocytosis-exocytosis (which has been demonstrated for molecules up to ~320 kDa) or receptor-mediated transport [62,63]. The uptake of many lipids from circulation by mammary cells has been demonstrated in labeled animal studies, where lipoprotein lipase presence allows uptake of fatty acids, cholesterol, and phosphatidylcholine in rats [64]. While this has not been demonstrated in humans, lipase increases dramatically at lactation initiation, with increasing milk production [65]. Further, many fatty acid transporter genes are expressed in mammary epithelial cells, allowing fatty acid uptake via transmembrane proteins such as FAT and FATP, although this remains an understudied process [35]. Understanding of transport of lipids from the circulation to the lactating mammary gland is also important, given evidence that these mechanisms may be disrupted in cases of obesity and low milk supply [66].
3.1. Dietary Consumption
Maternal diet has been most strongly linked to milk fatty acid composition, with the assumption that dietary intake reaches circulation and is transported to the mammary gland. It is estimated that 29% of fatty acids originate from the diet and thought that the high degree of variability in milk fatty acid composition between women is a reflection of dietary variation [39,67]. This is of particular interest as it implies that dietary modulation may affect human milk lipid composition, with potential effects on the health of breastfed infants. For instance, maternal consumption of linoleic acid leads to a spike in linoleic acid concentration in maternal milk 8–24 h later [68]. Similarly, maternal intake of docosahexaenoic acid (DHA) is strongly correlated to milk DHA [69,70], and many milk fatty acids have been shown to be positively correlated with maternal circulating lipids, including arachidonic, oleic, and linoleic acids [71]. However, studies reveal that this is not entirely clear; it appears that both habitual diet and acute diet can impact human milk fatty acid composition to varying degrees [67,72,73]. Tracer studies are used to investigate the incorporation of dietary fatty acids into human milk, which involve consumption of labeled fatty acids and subsequent measurement of their presence in human milk and blood over many hours following consumption. These studies are often limited by small sample sizes, population spread, and restrictive maternal diets; however, the proportion of fatty acid incorporation from diet in humans seems to match that of other animals relatively closely; therefore, the mechanisms involved in this process may be comparable across mammalian species [39].
Small intestine absorption of metabolites consumed in the diet varies depending on the nature of the metabolite. Typically, water-soluble species (such as carbohydrates and proteins) are able to enter the blood stream, while fat soluble species (such as lipids and some small metabolites) predominantly enter the lymphatic system through lacteals and are able to then enter the bloodstream via the thoracic vessel to the heart [74]. This means that, when ingested, the most water-soluble species (for example, SCFAs) may enter the bloodstream directly from the small intestine and reach the mammary gland via the circulation, whereas uptake of lipid and lipid-soluble species may occur via lymphatic absorption from the gut. Notably, very little research has characterized metabolite transport from the gut to the lactating mammary gland, with the entero-mammary route remaining relatively speculative [75].
3.2. Release from Other Tissues
Non-mammary tissues (such as liver and adipose tissue) also synthesize, store, and release lipids and small metabolites into circulation. Study of maternal intake of labeled fatty acids has indicated that body stores are the major source of many fatty acids in human milk [72,73]. In one such study, while approximately 88% of arachidonic acid (AA) did not originate from the diet, only a very small portion (~2.2% of the total milk AA) was synthesized by non-mammary tissue linoleic acid conversion, confirming that the remainder originated from non-mammary tissue stores [72]. An estimated 59% of milk fatty acids originate from non-mammary adipose tissue [39]. Another example is 12,13-diHOME, a linoleic acid metabolite that is released from brown adipose tissue following cold exposure or exercise [55,76]. Following moderate maternal exercise, 12,13-diHOME concentrations in human milk increase 1.4-fold in 90-min [20]. Incidentally, 12,13-diHOME is also of interest as a bacterial metabolite [12] and has been linked to infant adiposity and immune programming outcomes [20,77].
3.3. Production by Gut Microbiota
The maternal gut microbiota produces a slew of lipids and small metabolites, including SCFAs via fiber metabolism [60,61,78], which elicit both local and systemic effects [78,79,80]. One example of a gut microbiome metabolite that has recently garnered interest in the human milk field is 12,13-diHOME. This metabolite is an end product of linoleic acid metabolism. 12,13-diHOME production is encoded by epoxide hydrolase genes, which are present in human, bacterial, and fungal genomes [22,81,82,83]. While it is unclear the extent to which milk 12,13-diHOME is produced by human or microbial genes, bacterial epoxide hydrolase genes have been reported to be present in the infant gut microbiome [22]. Human milk and infant gut 12,13-diHOME have recently been linked to infant body composition [20] as well as infant allergy and asthma development [22,84]. Thus, bacterial metabolites in human milk are currently of great interest for infant health.
Gut microbial metabolites can be detected in maternal blood [48] and may therefore be transported to the lactating mammary gland (Table 1). For instance, SCFAs produced by gut bacteria are first absorbed into colonocytes, where they are used as a local energy source (primarily butyrate). Unused SCFAs then enter the liver via the portal vein where they are further utilized. Thus, only a small portion of overall SCFAs reach the maternal circulation and have the opportunity to be incorporated into the mammary gland [85,86]. However, transport of gut bacterial metabolites from the maternal circulation to milk is understudied, with little evidence regarding the extent to which they are able to cross the mammary epithelium. This is a critical gap in the understanding of the milk metabolome. Given the well-documented health-modulating effects of microbial metabolites [87,88,89,90], it is important to understand the extent to which maternal gut metabolites may integrate into milk and be ingested by the infant. Importantly, this may open opportunities to modulate the maternal gut microbiome to improve infant health. To better understand transport of gut microbial metabolites to human milk via the circulation, paired blood and milk samples should be analyzed to identify correlated metabolites. This work has been performed in dairy cows, finding that milk is a distinct metabolic compartment with a composition largely independent from that of plasma, with the exception of a small number of key metabolites that were found to be tightly correlated between plasma and milk. These correlations include the bacterial metabolite trimethylamine (TMA) and the host-bacterial co-metabolite dimethyl sulfone (DMSO2) [91] and suggests selective transport of certain metabolites from the maternal circulation into milk. As similar studies are lacking in humans, we have synthesized data from a variety of human studies to assess levels of key gut bacterial metabolites in the circulation compared to milk (Table 1). These data highlight that the human milk metabolome is distinct from that of blood, suggesting selective transport of bacterial metabolites across lactocytes. However, it should be noted that data on blood metabolite levels are sourced from the general adult population. Analysis of paired blood and milk samples from lactating mothers is necessary to verify these relationships.
4. Summary and Future Research Directions
Given the myriad of health effects of lipids and small metabolites for the breastfed infant [11,12], they are promising therapeutic targets to improve infant health. However, without an understanding of the origins of milk metabolites and lipids, we are limited in our ability to modulate these components. For instance, an understanding of the degree to which various maternal dietary lipids are able to reach the mammary gland may underpin future dietary recommendations for breastfeeding women. Here we have highlighted five sources for milk lipids and metabolites: de novo synthesis from mammary cells, production by the milk microbiota, dietary consumption, release from non-mammary tissue, and production by the gut microbiota. In order to further elucidate the origins of lipids and metabolites in human milk, we propose the following research strategies.
4.1. Transcriptome Analysis of Human Milk Cells
There is limited transcriptional data on human milk cells, due to the challenge involved in acquiring appropriate samples and the cost associated with such analyses. While uptake of such work is increasing in the field [32,35,92,93], further work is needed to define synthesis pathways for milk lipids and metabolites. Further analysis of transporter gene expression in mammary epithelial cells will also aid in defining transcellular transport mechanisms for circulating lipids and metabolites. It is important, however, to identify how closely cells isolated from milk represent those that produce milk in vivo, as prior data suggest important differences. Thus, other techniques are likely required, such as organoid modeling.
4.2. Paired Sample Analyses
It is necessary to analyze paired blood and milk samples from lactating individuals in order to better understand the degree to which circulating lipids and small metabolites are transported into the lactating mammary gland. While comparison of milk metabolite concentrations to circulating metabolite concentrations in non-lactating individuals suggests that the mammary gland is a distinct metabolic compartment, further paired analyses are needed to verify this finding. A recent example of a comprehensive study attempting to elucidate human milk component origins utilized milk, blood, and adipose tissue, and further solidified the evidence of milk fatty acids originating from adipose tissue [65]. Future studies could include other matched samples such as fecal samples, advanced dietary data collection and analysis, and labeling technologies, to add insight into component origin and final destination.
4.3. Dietary Studies
Many of the metabolites discussed in this review are derived from bacterial metabolism of host dietary components, highlighting the need to couple metabolomic and microbiome analyses with dietary intake data. Advanced methods for dietary data analysis continue to emerge and will benefit this work greatly [94], particularly if labeled studies are incorporated [95], to improve our understanding of the impact of maternal diet on the breastfed infant.
4.4. Bacterial Gene Analysis
A current gap in the literature is the inability to identify whether microbial metabolites or lipids in human milk are produced by the gut or milk microbiota. Targeted PCR for known bacterial metabolite/lipid synthesis genes would demonstrate the capacity of milk bacteria to produce these components. Whole genome analysis would achieve a similar outcome but is made difficult by the low biomass of the milk microbiome and the relatively high portion of host DNA [31,96]. However, transcriptional analysis is necessary to demonstrate gene activity (and not just gene presence). Similar to whole genome sequencing, metatranscriptome analysis of the human milk microbiome is limited by biomass issues [31,96]. Targeted RNA-sequencing of genes of interest or use of reverse transcription PCR (RT-PCR) may therefore be necessary; however, such techniques require prior understanding of genes involved [97,98].
5. Conclusions
Human milk lipids and small metabolites are a critical component of human milk, with roles in infant growth, immunity, and development. Despite this, many gaps remain as to the origins of these, and thus translation of compositional research to improve infant health is hampered. Continued research into the maternal and microbial origins of human milk lipids and small metabolites is critical, and all compositional research should be conducted with the potential origins of components in mind.
Author Contributions
L.F.S. and A.D.G. contributed equally to writing—original draft preparation, Conceptualisation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work. L.F.S. is supported by an unrestricted research grant from Medela AG, administered by The University of Western Australia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Ladomenou, F.; Moschandreas, J.; Kafatos, A.; Tselentis, Y.; Galanakis, E. Protective effect of exclusive breastfeeding against infections during infancy: A prospective study. Arch. Dis. Child. 2010, 95, 1004–1008. [Google Scholar] [CrossRef]
- Vennemann, M.M.; Bajanowski, T.; Brinkmann, B.; Jorch, G.; Yucesan, K.; Sauerland, C.; Mitchell, E.A.; Ge, S.I.D.S.G. Does breastfeeding reduce the risk of sudden infant death syndrome? Pediatrics 2009, 123, e406–e410. [Google Scholar] [CrossRef]
- Lund-Blix, N.A.; Stene, L.C.; Rasmussen, T.; Torjesen, P.A.; Andersen, L.F.; Ronningen, K.S. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: The MIDIA Study. Diabetes Care 2015, 38, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Raman, A.S.; Murch, S.H.; Rollins, N.C.; Gordon, J.I. Understanding the mother-breastmilk-infant “triad”. Science 2020, 367, 1070–1072. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Bode, L.; McGuire, M.; Rodriguez, J.M.; Geddes, D.T.; Hassiotou, F.; Hartmann, P.E.; McGuire, M.K. It’s alive: Microbes and cells in human milk and their potential benefits to mother and infant. Adv. Nutr. 2014, 5, 571–573. [Google Scholar] [CrossRef]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.-J.; Hartmann, P.E. 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk—A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Burugupalli, S.; Paul, S.; Mansell, T.; Burgner, D.; Meikle, P.J. The Role of Human Milk Lipids and Lipid Metabolites in Protecting the Infant against Non-Communicable Disease. Int. J. Mol. Sci. 2022, 23, 7490. [Google Scholar] [CrossRef]
- Stinson, L.F.; Geddes, D.T. Microbial metabolites: The next frontier in human milk. Trends Microbiol. 2022, 30, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yang, X.; Cheng, Y.; Zhang, H.; Xu, X.; Zhou, J.; Chen, H.; Su, M.; Yang, Y.; et al. Human Milk Lipid Profiles around the World: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 2519–2536. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.S.; Rahman, I.A.; Lai, C.T.; Hepworth, A.; Trengove, N.; Hartmann, P.E.; Geddes, D.T. Changes in Fatty Acid Composition of Human Milk in Response to Cold-Like Symptoms in the Lactating Mother and Infant. Nutrients 2017, 9, 1034. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Wlodek, M.E.; Trengove, R.D.; Murray, K.; Geddes, D.T. Untargeted lipidomics using liquid chromatography-ion mobility-mass spectrometry reveals novel triacylglycerides in human milk. Sci. Rep. 2020, 10, 9255. [Google Scholar] [CrossRef]
- Isaacs, C.E.; Kashyap, S.; Heird, W.C.; Thormar, H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch. Dis. Child. 1990, 65, 861–864. [Google Scholar] [CrossRef]
- Meyer, D.M.; Brei, C.; Stecher, L.; Much, D.; Brunner, S.; Hauner, H. Associations between long-chain PUFAs in maternal blood, cord blood, and breast milk and offspring body composition up to 5 years: Follow-up from the INFAT study. Eur. J. Clin. Nutr. 2019, 73, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Ojo-Okunola, A.; Cacciatore, S.; Nicol, M.P.; du Toit, E. The Determinants of the Human Milk Metabolome and Its Role in Infant Health. Metabolites 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Wolfs, D.; Lynes, M.D.; Tseng, Y.H.; Pierce, S.; Bussberg, V.; Darkwah, A.; Tolstikov, V.; Narain, N.R.; Rudolph, M.C.; Kiebish, M.A.; et al. Brown Fat-Activating Lipokine 12,13-diHOME in Human Milk Is Associated With Infant Adiposity. J. Clin. Endocrinol. Metab. 2021, 106, e943–e956. [Google Scholar] [CrossRef]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; du Toit, E.; Shimojo, N.; Munblit, D.; Campbell, D.E.; et al. Human Milk From Atopic Mothers Has Lower Levels of Short Chain Fatty Acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861. [Google Scholar] [CrossRef]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef]
- Innis, S.M. Maternal Nutrition, Genetics, and Human Milk Lipids. Curr. Nutr. Rep. 2013, 2, 151–158. [Google Scholar] [CrossRef]
- Stinson, L.F.; Trevenen, M.L.; Geddes, D.T. Effect of Cold Storage on the Viable and Total Bacterial Populations in Human Milk. Nutrients 2022, 14, 1875. [Google Scholar] [CrossRef]
- Douglas, C.A.; Ivey, K.L.; Papanicolas, L.E.; Best, K.P.; Muhlhausler, B.S.; Rogers, G.B. DNA extraction approaches substantially influence the assessment of the human breast milk microbiome. Sci. Rep. 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Garwolińska, D.; Hewelt-Belka, W.; Namieśnik, J.; Kot-Wasik, A. Rapid Characterization of the Human Breast Milk Lipidome Using a Solid-Phase Microextraction and Liquid Chromatography–Mass Spectrometry-Based Approach. J. Proteome Res. 2017, 16, 3200–3208. [Google Scholar] [CrossRef]
- Hewelt-Belka, W.; Garwolińska, D.; Belka, M.; Bączek, T.; Namieśnik, J.; Kot-Wasik, A. A new dilution-enrichment sample preparation strategy for expanded metabolome monitoring of human breast milk that overcomes the simultaneous presence of low- and high-abundance lipid species. Food Chem. 2019, 288, 154–161. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Ganeshalingam, M.; Enstad, S.; Sen, S.; Cheema, S.; Esposito, F.; Thomas, R. Role of lipidomics in assessing the functional lipid composition in breast milk. Front. Nutr. 2022, 9, 899401. [Google Scholar] [CrossRef]
- Stinson, L.F.; Ma, J.; Sindi, A.S.; Geddes, D.T. Methodological approaches for studying the human milk microbiome. Nutr. Rev. 2022, nuac082. [Google Scholar] [CrossRef] [PubMed]
- Twigger, A.J.; Engelbrecht, L.K.; Bach, K.; Schultz-Pernice, I.; Pensa, S.; Stenning, J.; Petricca, S.; Scheel, C.H.; Khaled, W.T. Transcriptional changes in the mammary gland during lactation revealed by single cell sequencing of cells from human milk. Nat. Commun. 2022, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Gay, M.C.L.; Koleva, P.T.; Slupsky, C.M.; Toit, E.D.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; Campbell, D.E.; Prescott, S.L.; et al. Worldwide Variation in Human Milk Metabolome: Indicators of Breast Physiology and Maternal Lifestyle? Nutrients 2018, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.A.; Haymond, M.W. Regulation of lipid synthesis genes and milk fat production in human mammary epithelial cells during secretory activation. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E700–E716. [Google Scholar] [CrossRef]
- Suburu, J.; Shi, L.; Wu, J.; Wang, S.; Samuel, M.; Thomas, M.J.; Kock, N.D.; Yang, G.; Kridel, S.; Chen, Y.Q. Fatty acid synthase is required for mammary gland development and milk production during lactation. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1132–E1143. [Google Scholar] [CrossRef]
- Smith, S.; Pasco, D.; Pawlak, J.; Thompson, B.J.; Stampfer, M.; Nandi, S. Thioesterase II, a new marker enzyme for human cells of breast epithelial origin. J. Natl. Cancer Inst. 1984, 73, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Gagné, H.T.; Pitelka, D.R.; Abraham, S. The effect of dietary fat on lipogenesis in mammary gland and liver from lactating and virgin mice. Biochem. J. 1969, 115, 807–815. [Google Scholar] [CrossRef]
- Hachey, D.L.; Thomas, M.R.; Emken, E.A.; Garza, C.; Brown-Booth, L.; Adlof, R.O.; Klein, P.D. Human lactation: Maternal transfer of dietary triglycerides labeled with stable isotopes. J. Lipid Res. 1987, 28, 1185–1192. [Google Scholar] [CrossRef]
- Chatterjee, D.; Subba Reddy, K.V.; Ray, T.K. Lipid synthesis in lactating mammary gland. Trends Biochem. Sci. 1979, 4, 278–280. [Google Scholar] [CrossRef]
- Boutinaud, M.; Herve, L.; Lollivier, V. Mammary epithelial cells isolated from milk are a valuable, non-invasive source of mammary transcripts. Front. Genet. 2015, 6, 323. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Sindi, A.S.M.; Cheema, A.S.; Lai, C.T.; Muhlhausler, B.S.; Wlodek, M.E.; Payne, M.S.; Geddes, D.T. The human milk microbiome: Who, what, when, where, why, and how? Nutr. Rev. 2020, 79, 529–543. [Google Scholar] [CrossRef]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.S.; Trevenen, M.L.; Turlach, B.A.; Furst, A.J.; Roman, A.S.; Bode, L.; Gridneva, Z.; Lai, C.T.; Stinson, L.F.; Payne, M.S.; et al. Exclusively Breastfed Infant Microbiota Develops over Time and Is Associated with Human Milk Oligosaccharide Intakes. Int. J. Mol. Sci. 2022, 23, 2804. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lonnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.O.; Meng, F.; Lanfranchi, E.; Young, J.F.; Stanton, C.; Ryan, C.A.; Kelly, A.L.; Sundekilde, U.K. Dynamic Changes in the Human Milk Metabolome Over 25 Weeks of Lactation. Front. Nutr. 2022, 9, 917659. [Google Scholar] [CrossRef]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef]
- Lentner, C.; West Cadwell, N.J. (Eds.) Geigy Scientific Tables, 8th ed.; Medical Education Div., Ciba-Geigy Corp: Basel, Switzerland, 1992; pp. 165–177. [Google Scholar]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Li, H.; Jia, K.; Wu, M.; Chen, M. Unveiling the influence of ultrasonic-assisted lipolysis: A pilot study of short-chain fatty acid profiling in human milk based on mass spectrometry. Food Chem. Adv. 2022, 1, 100097. [Google Scholar] [CrossRef]
- Gomez-Gallego, C.; Kumar, H.; Garcia-Mantrana, I.; du Toit, E.; Suomela, J.P.; Linderborg, K.M.; Zhang, Y.; Isolauri, E.; Yang, B.; Salminen, S.; et al. Breast Milk Polyamines and Microbiota Interactions: Impact of Mode of Delivery and Geographical Location. Ann. Nutr. Metab. 2017, 70, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gouveia-Figueira, S.; Domellof, M.; Zivkovic, A.M.; Nording, M.L. Oxylipins, endocannabinoids, and related compounds in human milk: Levels and effects of storage conditions. Prostagland. Other Lipid Mediat. 2016, 122, 28–36. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef]
- Lichtenberger, L.M.; Gardner, J.W.; Barreto, J.C.; Morriss, F.H., Jr. Evidence for a role of volatile amines in the development of neonatal hypergastrinemia. J. Pediatr. Gastroenterol. Nutr. 1991, 13, 342–346. [Google Scholar] [CrossRef]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Trabado, S.; Al-Salameh, A.; Croixmarie, V.; Masson, P.; Corruble, E.; Feve, B.; Colle, R.; Ripoll, L.; Walther, B.; Boursier-Neyret, C.; et al. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS ONE 2017, 12, e0173615. [Google Scholar] [CrossRef]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial lipids: Metabolism and membrane homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E.; Thomas, J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 2009, 459, 395–433. [Google Scholar] [CrossRef]
- Neville, M.C. The physiological basis of milk secretion. Ann. N. Y. Acad. Sci. 1990, 586, 1–11. [Google Scholar] [CrossRef]
- Shennan, D.B.; Boyd, C.A. The functional and molecular entities underlying amino acid and peptide transport by the mammary gland under different physiological and pathological conditions. J. Mammary Gland Biol. Neoplasia 2014, 19, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Scow, R.O.; Chernick, S.S.; Fleck, T.R. Lipoprotein lipase and uptake of triacylglycerol, cholesterol and phosphatidylcholine from chylomicrons by mammary and adipose tissue of lactating rats in vivo. Biochim. Biophys. Acta 1977, 487, 297–306. [Google Scholar] [CrossRef]
- Freed, L.M.; Berkow, S.E.; Hamosh, P.; York, C.M.; Mehta, N.R.; Hamosh, M. Lipases in human milk: Effect of gestational age and length of lactation on enzyme activity. J. Am. Coll. Nutr. 1989, 8, 143–150. [Google Scholar] [CrossRef]
- Walker, R.E.; Harvatine, K.J.; Ross, A.C.; Wagner, E.A.; Riddle, S.W.; Gernand, A.D.; Nommsen-Rivers, L.A. Fatty Acid Transfer from Blood to Milk is Disrupted in Mothers with Low Milk Production, Obesity, and Inflammation. J. Nutr. 2022, 152, 2716–2726. [Google Scholar] [CrossRef] [PubMed]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Andersons, J.; Volkovs, V.; Ceļmalniece, K. Impact of Maternal Diet on Human Milk Composition Among Lactating Women in Latvia. Medicina 2019, 55, 173. [Google Scholar] [CrossRef]
- Moutsioulis, A.A.; Rule, D.C.; Murrieta, C.M.; Bauman, D.E.; Lock, A.L.; Barbano, D.M.; Carey, G.B. Human breast milk enrichment in conjugated linoleic acid after consumption of a conjugated linoleic acid-rich food product: A pilot study. Nutr. Res. 2008, 28, 437–442. [Google Scholar] [CrossRef]
- Juber, B.A.; Jackson, K.H.; Johnson, K.B.; Harris, W.S.; Baack, M.L. Breast milk DHA levels may increase after informing women: A community-based cohort study from South Dakota USA. Int. Breastfeed. J. 2016, 12, 7. [Google Scholar] [CrossRef]
- Fidler, N.; Sauerwald, T.; Pohl, A.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid transfer into human milk after dietary supplementation: A randomized clinical trial. J. Lipid Res. 2000, 41, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, F.; Fleith, M.; Goyer, A.; Samuel, T.M.; Elmelegy-Masserey, I.; Fontannaz, P.; Cruz-Hernandez, C.; Thakkar, S.K.; Monnard, C.; De Castro, C.A.; et al. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe. Eur. J. Nutr. 2022, 61, 2167–2182. [Google Scholar] [CrossRef]
- Del Prado, M.; Villalpando, S.; Elizondo, A.; Rodríguez, M.; Demmelmair, H.; Koletzko, B. Contribution of dietary and newly formed arachidonic acid to human milk lipids in women eating a low-fat diet. Am. J. Clin. Nutr. 2001, 74, 242–247. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Jasińska-Melon, E.; Mojska, H.; Olędzka, G.; Wesołowska, A.; Szostak-Węgierek, D. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients 2019, 11, 1585. [Google Scholar] [CrossRef]
- Dixon, J.B. Mechanisms of chylomicron uptake into lacteals. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. 1), E52–E57. [Google Scholar] [CrossRef]
- Sakwinska, O.; Bosco, N. Host Microbe Interactions in the Lactating Mammary Gland. Front. Microbiol. 2019, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef]
- Lin, D.I.N.; Rackaityte, E.; Magnaye, K.; Porsche, C.; Özçam, M.; Levan, S.; Lynch, S. Gut bacterial-derived 12,13-diHOME promotes inflammatory macrophage polarization and epigenetic modifications. J. Allergy Clin. Immunol. 2022, 149, AB231. [Google Scholar] [CrossRef]
- Descamps, H.C.; Herrmann, B.; Wiredu, D.; Thaiss, C.A. The path toward using microbial metabolites as therapies. EBioMedicine 2019, 44, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.K.; Morisseau, C.; Garen, G.; Cherney, M.M.; Garen, C.; Niu, C.; Hammock, B.D.; James, M.N. The molecular structure of epoxide hydrolase B from Mycobacterium tuberculosis and its complex with a urea-based inhibitor. J. Mol. Biol. 2008, 381, 897–912. [Google Scholar] [CrossRef]
- Decker, M.; Arand, M.; Cronin, A. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch. Toxicol. 2009, 83, 297–318. [Google Scholar] [CrossRef]
- Morisseau, C. Role of epoxide hydrolases in lipid metabolism. Biochimie 2013, 95, 91–95. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, R.W.P.; Martens, E.C. In sickness and health: Effects of gut microbial metabolites on human physiology. PLoS Pathog. 2020, 16, e1008370. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.D.; Hayes, B.; Cocks, B.; Marett, L.; Wales, W.J.; Rochfort, S.J. Latent biochemical relationships in the blood-milk metabolic axis of dairy cows revealed by statistical integration of 1H NMR spectroscopic data. J. Proteome Res. 2013, 12, 1428–1435. [Google Scholar] [CrossRef]
- Nyquist, S.K.; Gao, P.; Haining, T.K.J.; Retchin, M.R.; Golan, Y.; Drake, R.S.; Kolb, K.; Mead, B.E.; Ahituv, N.; Martinez, M.E.; et al. Cellular and transcriptional diversity over the course of human lactation. Proc. Natl. Acad. Sci. USA 2022, 119, e2121720119. [Google Scholar] [CrossRef]
- Lemay, D.G.; Ballard, O.A.; Hughes, M.A.; Morrow, A.L.; Horseman, N.D.; Nommsen-Rivers, L.A. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS ONE 2013, 8, e67531. [Google Scholar] [CrossRef]
- Tanweer, A.; Khan, S.; Mustafa, F.N.; Imran, S.; Humayun, A.; Hussain, Z.-U.-N. Improving dietary data collection tools for better nutritional assessment—A systematic review. Comput. Methods Programs Biomed. Update 2022, 2, 100067. [Google Scholar] [CrossRef]
- Demmelmair, H.; Kuhn, A.; Dokoupil, K.; Hegele, V.; Sauerwald, T.; Koletzko, B. Human lactation: Oxidation and maternal transfer of dietary 13C-labelled α-linolenic acid into human milk. Isot. Environ. Health Stud. 2016, 52, 270–280. [Google Scholar] [CrossRef]
- Ruiz, L.; Garcia-Carral, C.; Rodriguez, J.M. Unfolding the Human Milk Microbiome Landscape in the Omics Era. Front. Microbiol. 2019, 10, 1378. [Google Scholar] [CrossRef]
- Martinez-Gomez, K.; Flores, N.; Castaneda, H.M.; Martinez-Batallar, G.; Hernandez-Chavez, G.; Ramirez, O.T.; Gosset, G.; Encarnacion, S.; Bolivar, F. New insights into Escherichia coli metabolism: Carbon scavenging, acetate metabolism and carbon recycling responses during growth on glycerol. Microb. Cell Factories 2012, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).