Abstract
We conducted a lipidomic analysis of the whole body of female Aedes aegypti mosquitoes at different time points over the course of feeding and reproduction. There were temporal biphasic increases of more than 80% of lipids identified at the time of feeding and from 16 h to 30 h post blood meal (PBM). During these two increases, the abundance of many lipids dropped while body weight remained stable, probably reflecting blood lipid digestion and the synthesis of vitellogenin in this period. A concerted temporal pattern was particularly strong at the second peak for membrane and signalling lipids such as phosphatidylethanolamine (PE), phosphatidylinositol (PI), cardiolipin (CL), hexosylceramide (HexCer) and lyso-phosphatidic acid (LPA). Lyso-glycerophospholipids showed three distinct change patterns that are functionally related: Lyso-PE and Lyso-phosphatidylcholine (LPC), which are membrane lipids, showed little change; LPA, a signalling lipid, showed a significant increase from 16 to 30 h PBM; Lyso-PI, a bioactive lipid, and both lyso-phosphatidylglycerol (LPG) and lyso-phosphatidylserine (LPS), which are bacterial membrane lipids, showed one significant increase from the time of feeding to 16 h post blood meal. The result of our study on the anautogenous insect Ae. aegypti point to specific lipids likely to be important in the reproductive process with a role in the formation and growth of ovarian follicles.
1. Introduction
The yellow fever mosquito, Aedes aegypti, is one of the major vectors that transmits arbovirus diseases in tropical and subtropical areas, including dengue and Zika [1,2,3]. Ae. aegypti females are anautogenous; they must ingest human/vertebrate blood to obtain unique nutrients to produce their eggs. Diseases are transmitted through the blood ingestion process [4,5].
The development of ovaries can be separated into the pre-vitellogenic (before blood meal, BBM) and vitellogenic (post blood meal, PBM) stages, depending on the timing of the blood meal. In the pre-vitellogenic stages, lipids are reserved in the fat body [6]; this is essential to support Ae. aegypti reproduction [7], particularly when a sugar source is compromised. Starving female mosquitoes before or after their blood feeding will not reduce their fecundity but will significantly reduce the amount of lipids in mothers after laying eggs [8], suggesting a trade-off between reproduction and female survival.
During the vitellogenic stage, consumption of blood triggers insulin-like peptide 3 (ILP3) from the brain to stimulate oogenesis [9]; this represents the insulin signaling pathway [10]. The secretion of 20-hydroxyecdysone reaches its peak at 16 h post blood meal [11], increasing the expression of vitellogenin genes in the fat body to support yolk protein accumulation and ovarian follicle development [12]. This is stimulated by gonadotropin [13] and lipids are transferred from the fat body to follicles [6]. Follicle lengths start to increase immediately after blood meal and this continues for 30 h [14]. Meanwhile, for the development of yolk, it takes up to 6 h to show a measurable increase of protein, lipid and glycogen [14]. Eggs start to mature and be oviposited at around three days post blood meal, and the whole gonotrophic cycle lasts for around five days [15,16].
Aside from activating the reproductive process through the modulation of hormones, human blood also provides essential nutrients, such as amino acids and cholesterol, to support mosquito reproduction [17,18,19]. The source and the quality of blood that females are fed can directly impact their fertility [20,21,22,23], though their nutritional components, such as amino acids and cholesterol, are considered similar [24,25]. Aside from these changes of amino acids, cholesterol and yolk protein, the vitellogenic cycle is also expected to involve lipid metabolism; lipids not only act as major energy resources in supporting egg development [6] and contribute 35% of ovarian dry weight [26,27], but also exhibit a variety of functions in the biological system [28,29].
Here we aim to understand lipid metabolism in Ae. aegypti during the reproductive process and how lipids with different functions act to support the development of eggs. In this study, we used liquid chromatography coupled with the mass spectrometry (LCMS)-based lipidomic method and a comprehensive lipid standard mixture covering nearly all major lipid groups to identify and analyze lipid components of whole female individuals before blood meal and post blood meal.
2. Material and Methods
2.1. Sample Preparation
For this experiment, Aedes aegypti mosquitoes were collected from Wolbachia-uninfected populations from Cairns, QLD, Australia, and maintained in laboratory conditions. Samples were prepared by hatching Ae. aegypti eggs stored in an incubator (PG50 Plant Growth Chambers, Labec Laboratory Equipment, Marrickville, NSW, Australia) at 22–30 ± 1 °C, with a 12 h:12 h photoperiod. Larvae were reared in reverse osmosis (RO) water with yeast and fed with Hikari Sinking Wafers fish food tablets until adult. All female mosquitoes were blood fed by the same volunteer’s forearm. The first blood meal was provided to adult females at 4 ± 1 days post-emergence. Females were isolated individually and observed for seven days to discard those that laid small numbers of eggs (<50). At the seventh day, a second blood meal was provided. Non- engorged individuals were removed from the experiment. Samples were then stored as different groups during their second vitellogenic cycle before blood meal (BBM) and at different times post blood meal (PBM): 0 h PBM (30 min after blood meal), 4 h PBM, 16 h PBM, 30 h PBM, 72 h PBM, and 120 h PBM. Sugar and oviposition cups were provided throughout the entire period of the experiment.
2.2. Female Mosquito Measurement
In addition to analysis of the lipidome, we measured the weight of different female individuals (four to seven) at each time point from the additional collected samples using a Sartorius Analytical balance BP 210 D (Sartorius, Gottigen, Germany, Readability: 0.01 mg). After weighing, eight mosquitoes were randomly selected from all time points and their wing lengths were measured to show female body size [30]. To further understand the development of female ovaries, we hatched batches of mosquito eggs and took photos of ovaries using an NIS Elements BR imaging microscope (Nikon Instruments, Tokyo, Japan) every day PBM.
2.3. Lipid Extraction
For each group, we extracted the whole body of six mosquito females, keeping individuals separate. We added 10 µL of Mouse SPLASH® LIPIDOMIX® Mass Spec Standard (cat. 330707, Avanti Polar Lipids, Alabaster, AL, USA) to each sample after 1 in 4 dilution prior the extraction and then homogenised samples in 200 μL ice-cold 60% methanol (MeOH) containing 0.01% (w/v) butylated hydroxytoluene (BHT) using TissueLyserII (Qiagen, Hilden, Germany) at 25 Hertz for one minute and incubating for 20 min in an ultrasonic bath (Soniclean Pty Ltd., Thebarton, Australia). Following the method previously reported by Lydic et al. [31], 120 μL of MilliQ water, 420 μL of MeOH with 0.01% (w/v) BHT and 260 μL of chloroform (CHCl3) were added to each tube and vortexed thoroughly before incubating in a Bioshake iQ (Quantifoil Instrument GmbH, Jena, Germany) setting at 1400 rpm for 30 min and centrifuged at 14,000 rpm for 15 min. The supernatant containing lipids was transferred to a new 2 mL tube. The remaining sediments were re-extracted with 100 μL of MilliQ water and 400 μL of CHCl3:MeOH (1:2, v:v) containing 0.01% (w/v) BHT as described above. Supernatants from the first and second extraction were pooled in the same 2 mL tube and then dried by evaporation under vacuum using a GeneVac miVac sample concentrator (SP Scientific, Warminster, PA, USA). Before HPLC, dried lipid extracts were resuspended in 50 μL of CHCl3:MeOH (1:9, v:v, containing 0.01% BHT).
2.4. Mass Spectrometry Analyses
Samples were analyzed using ultrahigh performance liquid chromatography (UHPLC) coupled to tandem mass spectrometry (MS/MS) employing a Vanquish UHPLC linked to an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA), with separate runs in positive and negative ion polarity. Solvent A was 6/4 (v/v) acetonitrile/water with 10 mM ammonium acetate and 5 µM medronic acid and solvent B was 9/1 (v/v) isopropanol/acetonitrile with 10 mM ammonium acetate. Each sample was injected into an RRHD Eclipse Plus C18 column (2.1 × 100 mm, 1.8 µm, Agilent Technologies, Santa Clara, CA, USA) at 50 °C at a flow rate of 350 μL/min for 3 min using 3% solvent B. During separation, the percentage of solvent B was increased from 3% to 70% in 5 min, from 70% to 99% in 16 min, from 99% to 99% in 3 min, and then decreased from 99% to 3% in 0.1 min and maintained at 3% for 3.9 min.
All experiments were performed using a Heated Electrospray Ionization source. The spray voltages were 3.5 kV in positive ionization mode and 3.0 kV in negative ionization mode. In both polarities, the flow rates of sheath, auxiliary and sweep gases were 20 and 6 and 1 ‘arbitrary’ unit(s), respectively. The ion transfer tube and vaporizer temperatures were maintained at 350 °C and 400 °C, respectively, and the ion funnel radio frequency (RF) level was set at 50%. In both positive and negative ionization modes from 3 to 24 min, a top speed data-dependent scan with a cycle time of 1 s was used. Within each cycle, a full-scan MS spectrum was first acquired in the Orbitrap at a mass resolving power of 120,000 (at m/z 200) across a m/z range of 300–2000 using quadrupole isolation, an automatic gain control (AGC) target of 4 × 105 and a maximum injection time of 50 milliseconds, followed by higher-energy collisional dissociation (HCD)-MS/MS at a mass resolving power of 15,000 (at m/z 200), a normalized collision energy (NCE) of 27% at positive mode and 30% at negative mode, an m/z isolation window of 1, a maximum injection time of 22 milliseconds and an AGC target of 5 × 104. For the improved structural characterization of glycerophosphocholine (PC) in positive mode, a data-dependent product ion (m/z 184.0733)-triggered collision-induced dissociation (CID)-MS/MS scan was performed in the cycle using a q-value of 0.25 and a NCE of 30%, with other settings being the same as that for HCD-MS/MS. For the improved structural characterization of triacylglycerol (TG) lipid in positive mode, the fatty acid + NH3 neutral loss product ions observed by HCD-MS/MS were used to trigger the acquisition of the top-3 data-dependent CID-MS3 scans in the cycle using a q-value of 0.25 and a NCE of 30%, with other settings being the same as that for HCD-MS/MS.
2.5. Lipid Identification and Quantification
LC-MS/MS data was searched through MS Dial 4.80 [32,33]. The mass accuracy settings are 0.005 Da and 0.025 Da for MS1 and MS2, respectively. The minimum peak height is 50,000 and mass slice width is 0.05 Da. The identification score cut off is 80%. Post identification was performed with a text file containing the name and m/z of each standard in Mouse SPLASH® LIPIDOMIX® Mass Spec Standard. In positive mode, [M + H]+ and [M + NH4]+ were selected as ion forms. In negative mode, [M − H]− were selected as ion forms. All lipid classes available were selected for the search. PC, LPC, DG, TG, CE and SM were identified and quantified at positive mode while PE, LPE, PS, LPS, PG, LPG, PI, LPI, PA, LPA, Cer and CL were identified and quantified at negative mode. The retention time tolerance for alignment is 0.1 min. Lipids with maximum intensity less than 5-fold of average intensity in blank were removed. All other settings were default. All lipid LC-MS features were manually inspected and re-integrated when needed. We also removed four other types of lipids: (1) lipids with only sum composition except PC and SM; (2) lipids identified due to peak tailing; (3) retention time outliers within each lipid class; and (4) LPA and PA artifacts generated by in-source fragmentation of LPS and PS. The shorthand notation used for lipid classification and structural representation follows the nomenclature proposed previously [34].
Quantification of lipid species was achieved by comparison of the LC peak areas of identified lipids against peak areas and quantities of the corresponding internal lipid standards in the same or similar lipid class (Table 1); the concentrations of all lipids were reported at nmol/individual. Given that the commercially available stable isotope-labelled lipid standards are limited, some of the identified lipids were normalized against a standard from a different class or sub-class. No attempts were made to quantitatively correct for different ESI responses of individual lipids due to concentration, acyl chain length, degree of unsaturation or matrix effects caused by differences in chromatographic retention times compared with the relevant standards.

Table 1.
List of lipid standards, adduct type and number of lipids that were identified in each group.
2.6. Data Analysis
All data were analyzed through R v. 3.6.0 (R Core Team, Vienna, Austria). Female weight was analyzed by ANOVA across time followed by post-hoc Tukey HSD tests. The concentrations of different lipid groups (nmol/individual) were used as variables for principal component analysis (PCA). For specific comparisons between treatments for particular lipids, concentration was natural log-transformed before undertaking paired t-tests, and p values were corrected for lipid number considered in a comparison using the Benjamin–Hochberg method.
3. Results
3.1. Female Body Weight and Wing Length Measurement
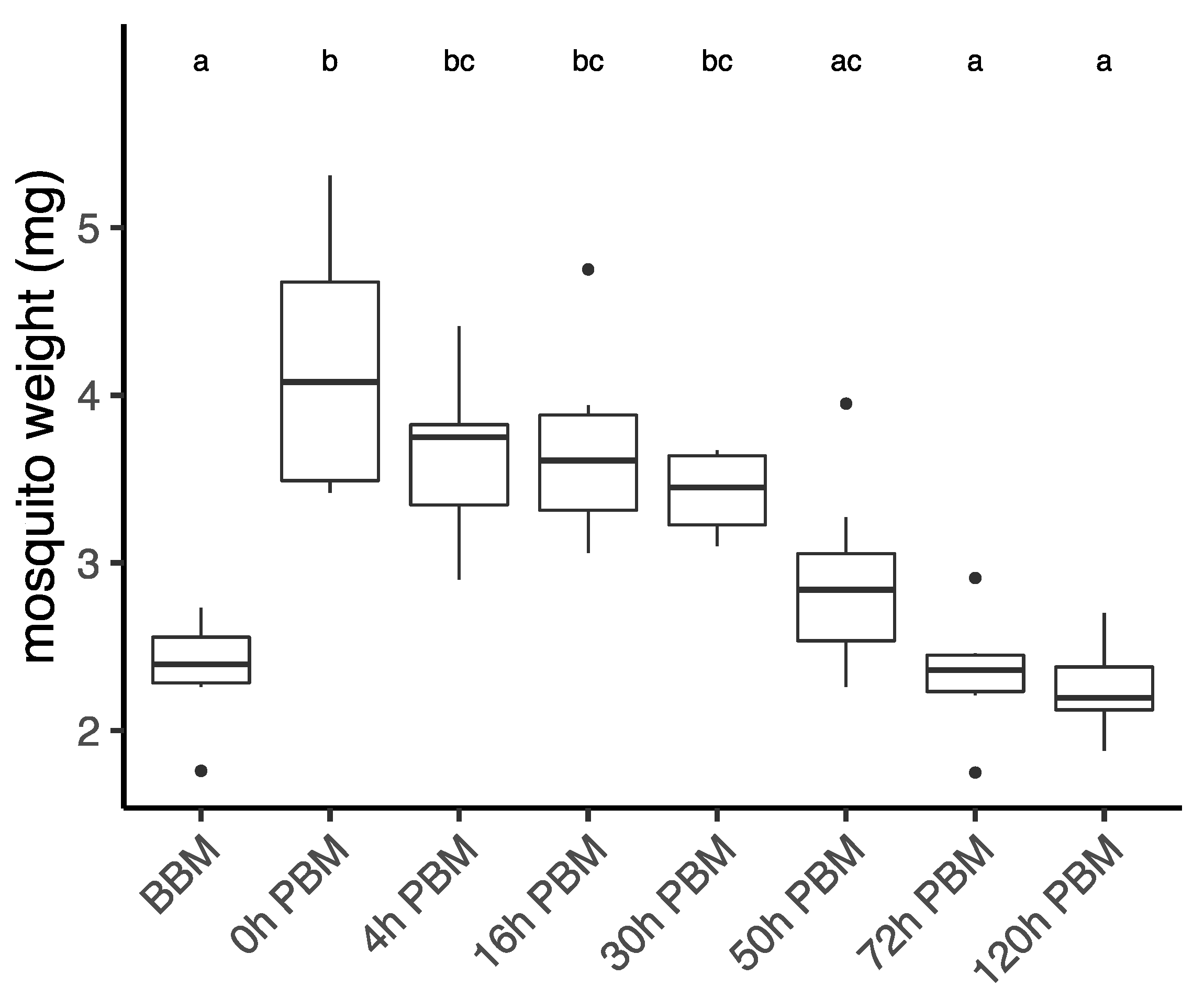
Aedes aegypti female mosquitoes were stored for lipidomic study in their second vitellogenic cycle before their blood meal (BBM) and at different times post blood meal (PBM): 0 h (30 min after blood meal), 4 h, 16 h, 30 h, 72 h, and 120 h. We first weighed female mosquitoes from samples collected at each time point (Figure 1), with the difference between BBM and 0 h PBM representing the amount of blood a female engorged. Sampling time had a significant effect on female weight (ANOVA, F7,41 = 13.06, p < 0.001). The weights of engorged female mosquitoes were approximately double their weights BBM. The females then started to digest blood and their weight dropped significantly from 0 h PBM to 4 h PBM, thereafter stabilizing until 30 h PBM, and then dropping again further to the BBM level at 72 h PBM. This change in weight reflects the process of blood digestion, which takes around 32 h after females are engorged [35,36]. After the oviposition period (120 h PBM), the weights of mosquitoes show little change when compared to the weights BBM. The appearance of female ovaries was captured every day under a microscope (Figure S1). We also calculated the coefficient of variation (CV; CV = (Standard Deviation/Mean) × 100) for female wing length for the eight samples we measured (mean ± se = 2.07 ± 0.06 mm, CV = 8.71) to indicate the spread of mosquito body sizes in this study.
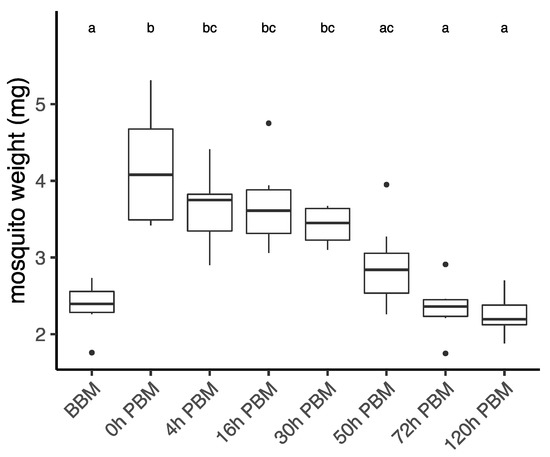
Figure 1.
Weights of Aedes aegypti females across different time points over the course of feeding and reproduction. Weights were measured at eight time points before blood meal (BBM) and post blood meal (PBM). The same letter at the top of the boxplots represents groups that are not significantly different following Tukey HSD post-hoc tests. Dots represent outliers.
3.2. Lipids Identification and Their Changes during Female Reproductive Process
We measured lipidomic changes averaged to individual female BBM and at different time points PBM covering the entire vitellogenic cycle. In total, we identified 456 lipids (Table 1) and divided them into six major groups: glycerolipids, diacyl-glycerophospholipids, lyso-glycerophospholipids, Cardiolipin (CL), Carnitine (CAR) and sphingolipids. The most abundant lipid group in female mosquitoes was the neutral glycerolipids, with 55 molecules comprising approximately 80% of the total lipid amount. Glycerophospholipids accounted for 55% of the molecules identified in this class, with 193 diacyl-glycerophospholipids and 48 lyso-glycerophospholipids. We also identified 66 CL, 15 CAR and 69 sphingolipids.
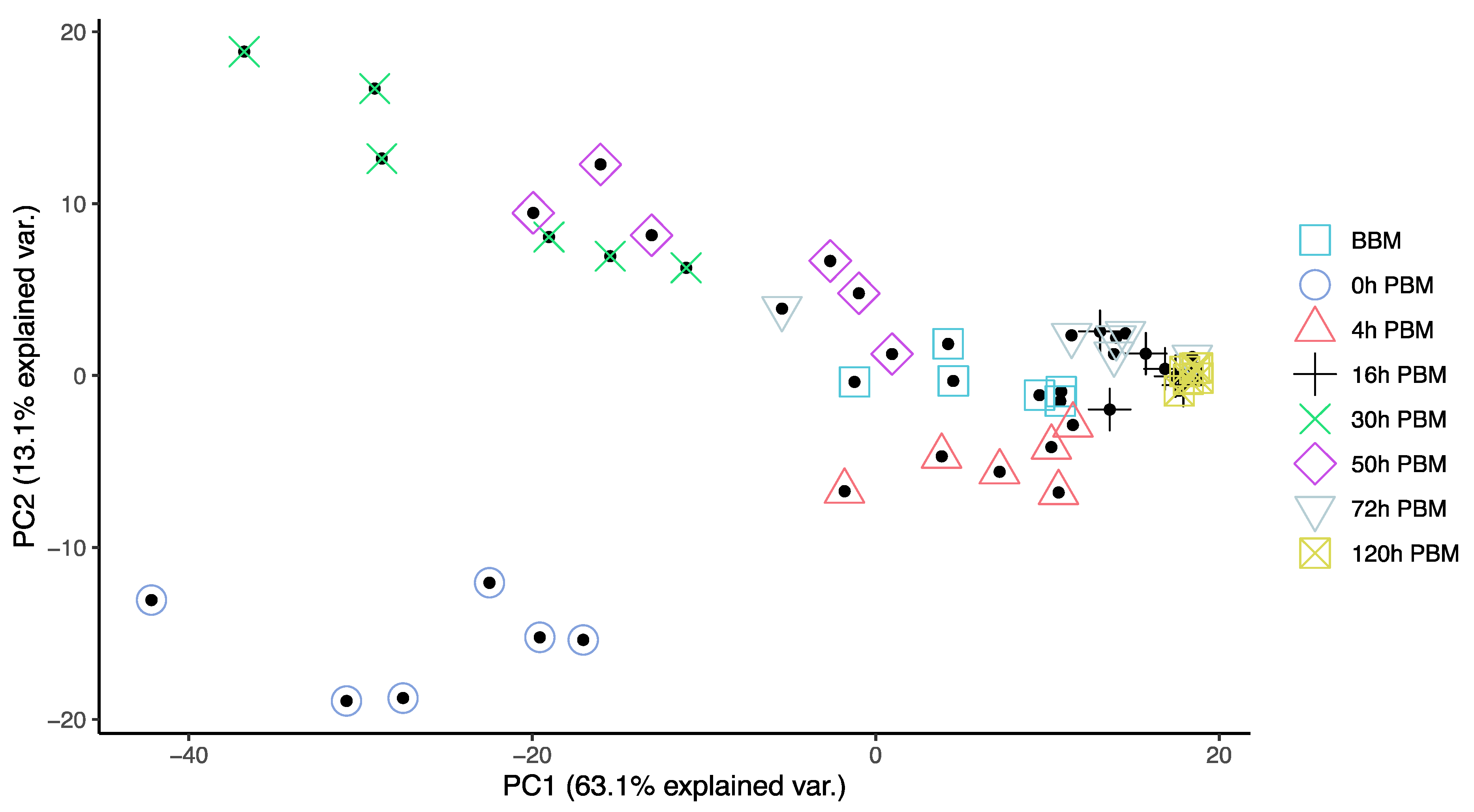
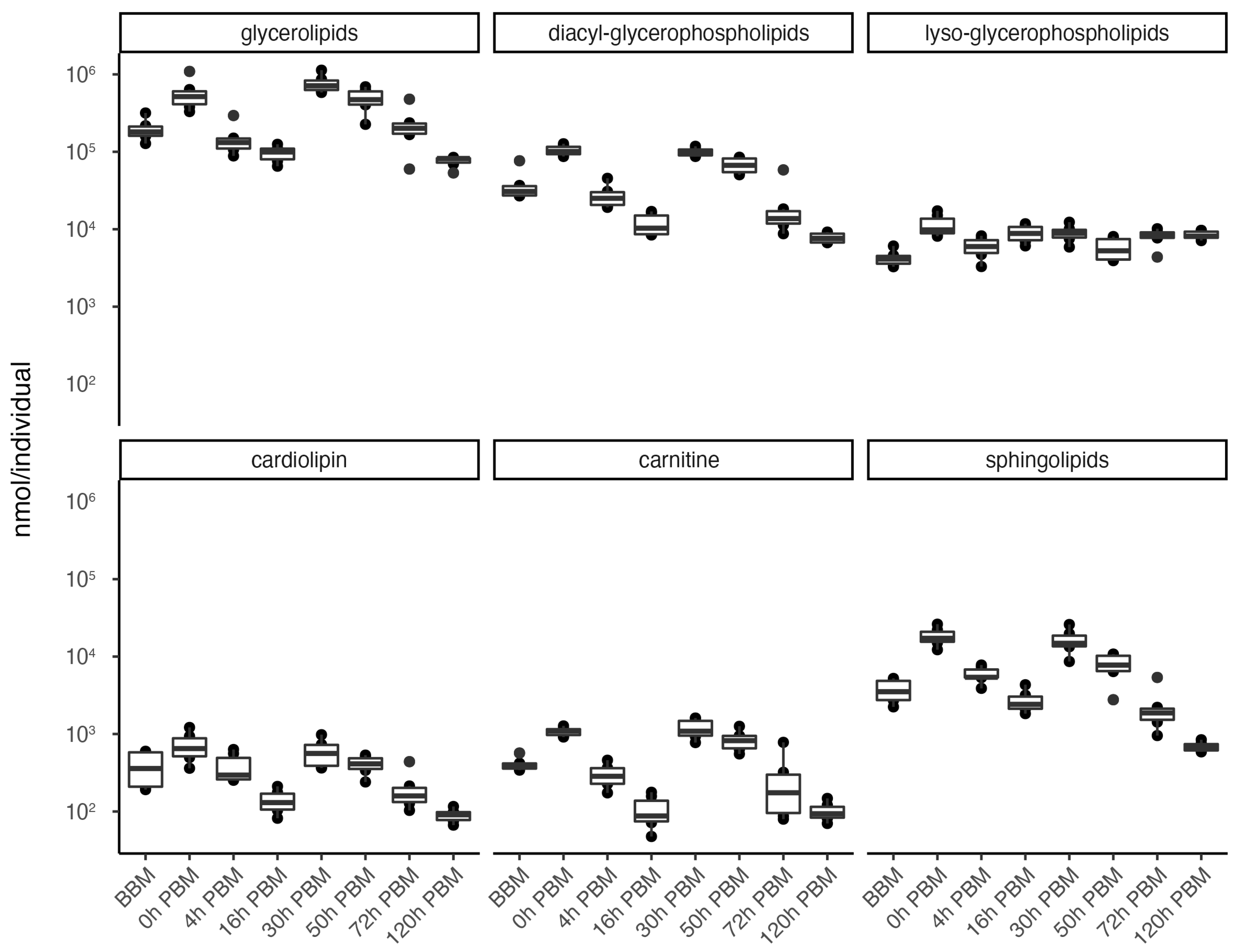
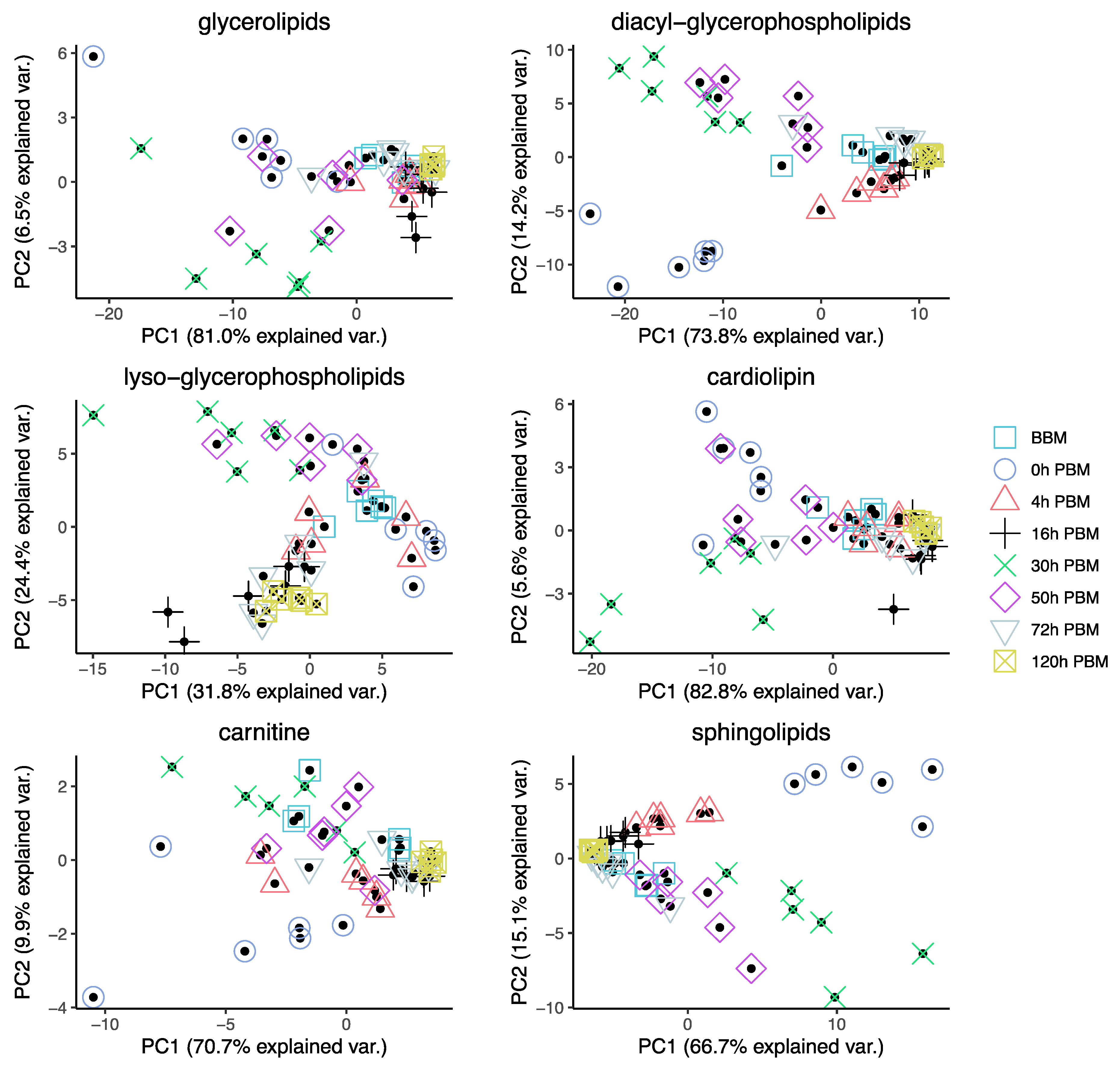
In order to obtain an overview of lipidomic changes during the reproductive process, we first undertook a principal component analysis (PCA) to describe overall patterns (Figure 2). This indicated that lipid components of female mosquitoes went through two primary changes: the first occurred immediately after a blood meal (0 h PBM), and the second occurred between 16 and 30 h PBM. Further analysis showed that the amounts of most lipids also started to increase at 0 h and 16 h PBM (Figure 3) except for lyso-glycerophospholipids, which remained relatively stable during the reproduction process. These results are also reflected in PCA analyses on specific classes of lipids (Figure 4); for most classes the first PC explained more than half of the variation, with the exception of the lyso-glycerophospholipids, where it only explained 31.8% of variation and there was no obvious change directly after blood feeding. However, there was one shift at a later time point (between 16 h PBM to 30 h PBM) for this group, indicating that most changes in lyso-glycerophospholipid reflect mosquito metabolism rather than direct blood ingestion.
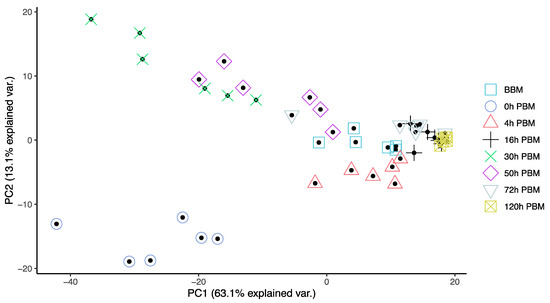
Figure 2.
Principal Component Analysis suggested lipidome differences of Aedes aegypti females at different time points over the course of feeding and reproduction. Color and shape symbols correspond to the eight time points before blood meal (BBM) and post blood meal (PBM). The first two principal axes explain 76% of the variance.
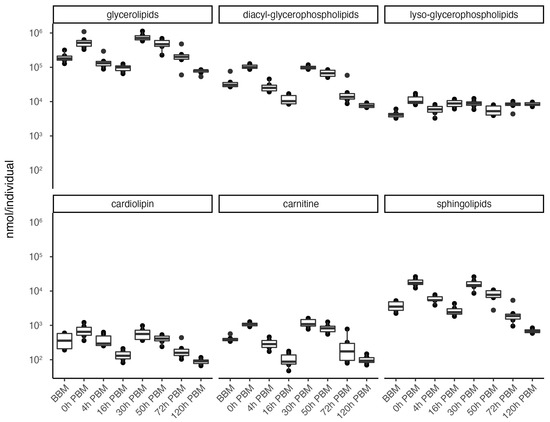
Figure 3.
Changes of lipid amounts in Aedes aegypti females over the course of feeding and reproduction. The total amount (nmol/individual) of glycerolipids, diacyl-glycerophospholipids, lyso-glycerophospholipids, cardiolipins, carnitines and sphingolipids were measured at eight time points before blood meal (BBM) and post blood meal (PBM). The significant level of differences between BBM and 0h PBM, and between 16 h PBM and 30 h PBM are marked.
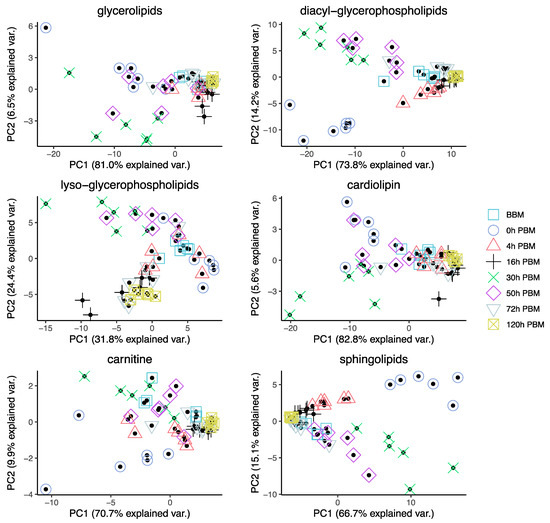
Figure 4.
Principal Component Analysis of different groups of lipids in Aedes aegypti females at different time points over the course of feeding and reproduction. Color and shape symbols correspond to the eight time points before blood meal (BBM) and post blood meal (PBM).
3.3. Identification of Change Patterns for Specific Lipids
The amount of blood a female mosquito takes in when fully engorged can be similar to the weight of the individual, or even nearly twice the individual’s weight [37,38]. By comparing the time points BBM and 0 h PBM for individual lipids, we were able to determine which lipids were taken in with human blood. We found 86% (392 molecules) of all the lipids identified significantly increased at the time of blood meal (Table S2). An exception occurred for glycerophospholipids and carnitines. We found 29 lyso-glycerophospholipids and 19 diacyl-glycerophospholipids that did not increase significantly after the blood meal; this might indicate that these lipids originated from mosquitoes instead of the human blood. These lipids include most of the LPA, LPG identified and all LPI identified. The carnitines generally showed a mild increase. The carnitines tetradecasphingenine (C14) and hexadecasphingenine (C16) CAR did not change significantly, while most of the other carnitines (7 out of 11) increased only slightly (adjusted 0.05 > p > 0.01); only one molecule (CAR 20:1) in this class increased significantly (adjusted p < 0.001).
From 0 h to 16 h PBM, the amount of lipids dropped while the weight of the mosquitoes did not decrease correspondingly. From 16 h to 30 h PBM, 87.7% of lipids (400 molecules) identified increased significantly (Table S3); 392 showed at least a two-fold increase and 139 showed an increase of at least ten-fold. There were 34 lyso-glycerophospholipids that did not increase significantly, and these lipids were distributed in all sub-classes of lyso-glycerophospholipids except for LPA. All LPA identified increased more than ten-fold, while none of the individual lipids identified from LPC and LPE significantly increased.
Unlike lyso-glycerophospholipids, almost all diacyl-glycerophospholipids had significantly increased, including all ether PI, oxidized PE, PE, PA, PG, PI and PS. In particular, 30 out of 43 PE and 18 out of 33 PI identified increased more than ten-fold. In addition, 42 out of 66 CL increased more than ten-fold and the four HexCer identified increased about ten-fold. In summary, lipids that increased by more than ten-fold include most of the PE, PI and CL, which act as membrane components, and all of HexCer and LPA, which act as signaling molecules and participate in a variety of physiological functions. These lipids are expected to be particularly important in mosquito egg production.
In general, most individual lipids have similar change patterns, regardless of the length of fatty acyl chain and number of double bonds. Outliers (those that did not increase significantly from BBM to 0 h PBM or from 16 to 30 h PBM) were found primarily in lyso-glycerophospholipids. Looking at the overall pattern, 21 lipid molecules identified did not increase from BBM to 0 h PBM or from 16 h to 30 h PBM; 19 of these are lyso-glycerophospholipids.
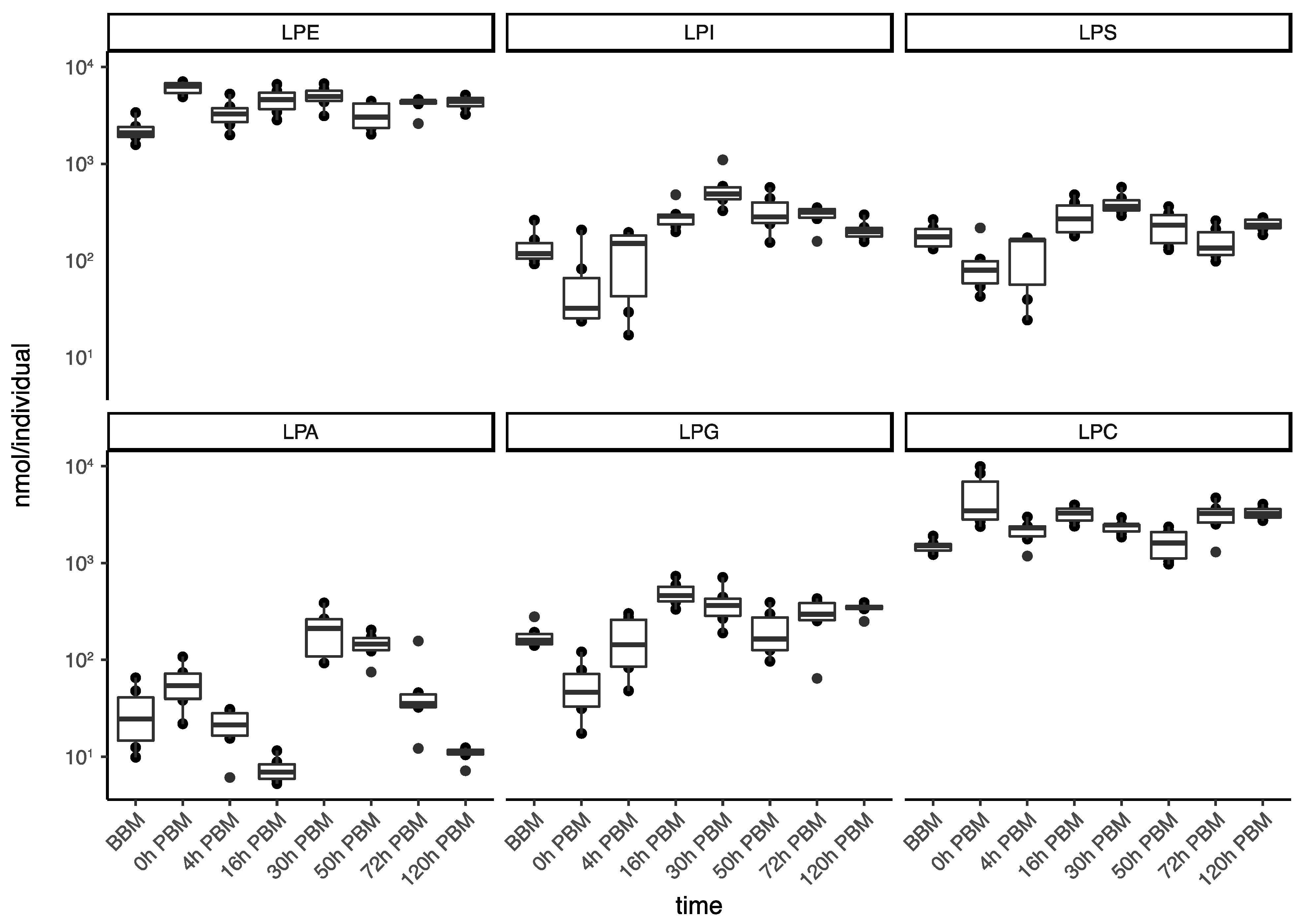
We then further analyzed the change patterns of lyso-glycerophospholipids; these represent a group composed of various sub-groups of lipids with different patterns of change (Figure 5) and diverse metabolic roles, including metabolic intermediates, extracellular signaling molecules and immune system activators. Specifically, the two most abundant lyso-glycerophospholipids, LPE and LPC, which play important roles in membrane composition, were relatively stable in terms of total amount during reproduction. LPA, an important group of lipids in regulating various signaling pathways, showed a significant increase from 16 to 30 h PBM; this is similar to the change patterns for other groups (other than lyso-glycerophospholipids) described previously. LPI, LPG and LPS, on the other hand, only had one significant increase during the time period from 0 to 16 h PBM, when most lipids in other groups decreased. LPI, a bioactive lipid, can initiate a variety of functions, including cell growth, differentiation and immunity [39,40]. LPS and LPG, membrane lipids in gram-positive bacteria and gram-negative bacteria, respectively, can act as microbial toxins to trigger mosquito immune response by activating the Toll and Imd pathway [41,42]. In this study, the changes of these two lipid groups most likely indicates the participation of gut bacteria in mosquito blood digestion.
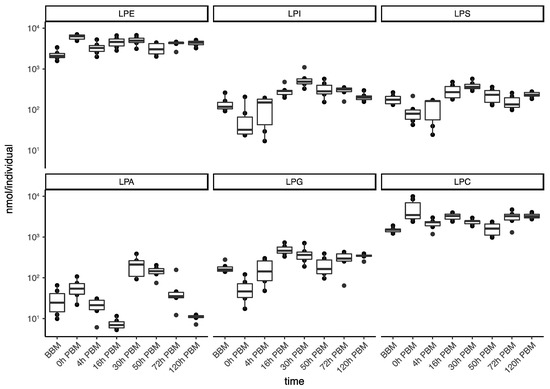
Figure 5.
Changes of lipid amounts in lyso-glycerophospholipids in Aedes aegypti females over the course of feeding and reproduction. The total amount (nmol/individual) of lyso-phosphatidylethanolamine (LPE), lyso- phosphatidylinositol (LPI), lyso-phosphatidylserine (LPS), lyso-phosphatidic acid (LPA), lyso-phosphatidylglycerol (LPG) and and lyso-phosphatidylcholine (LPC) were measured at eight time points before blood meal (BBM) and post blood meal (PBM).
By comparing the differences between the two periods (from BBM to 0 h PBM and from 16 h to 30 h PBM) when most increases occurred, we found that most lipids we identified were present in both human blood and mosquitoes; they were also affected by mosquito ovarian follicle development. Nevertheless, some lipids seemed to be present at a stable level in mosquitoes and were not significantly increased after blood feeding, including tetradecasphingenine (C14) and hexadecasphingenine (C16) CAR and most of the LPGs. Some lipids (such as oxidized PE and sphingomyelin) seemed to exist at a much higher abundance in human blood but were not used in mosquitoes to support egg development, which we define here as those that increased more than ten-fold after blood feeding but did not increase significantly (adjust p > 0.05) at the later period.
4. Discussion
In the current study, we focused on lipidomic changes of whole female mosquitoes during the reproductive process. Whole-body investigations provide an overall picture of lipid changes that might not be evident when focusing only on organs or cells. We found biphasic increases in more than 80% of lipids identified at the time of feeding and from 16 to 30 h PBM. The amount of lipids dropped between these periods of increase, as observed in the fat body [43]. The weight of the mosquitoes did not decrease at the first time point when lipids decreased, suggesting that lipids were transformed into different metabolites. Many metabolites can be produced within this period, such as glycogen and fatty acids [29,44], but it is more likely that the decrease of lipids from 0 to 16 h PBM reflects the synthesis of vitellogenin in the fat body, which is triggered by blood feeding. Previous studies found that the secretion of vitellogenin in the fat body reaches its highest peak at around 18 h PBM and decreases to the previtellogenic level at around 30 h PBM [45,46].
Pitch et al. [43] observed a relatively lower level of TG in the Ae. aegypti female fat body at around 30 h PBM; our study of whole body lipidome showed the highest level of TG at 30 h PBM. The differences may reflect the transportation of lipids from the fat body or midgut into oocytes during the insect reproductive process through lipophorin, a major lipid-carrying protein [47]. Ae. aegypti and some other Diptera predominantly transport TG via lipophorin, not DG as is the case of most other insects [48,49,50]. Moreover, a general observation for insects is that the midgut can convert DG to TG in the presence of high concentrations of potentially toxic fatty acids; this conversion could maintain low intracellular levels of DG and fatty acids to prevent lipotoxicity [29,51,52]. Additionally, there are other possibilities for lipid movement not based on DG transport, including evolutionarily conserved mechanisms for movement of fatty acids [53].
Lyso-glycerophospholipids were highlighted as a lipid group of interest by the present study due to their unusual temporal pattern in abundance. Lyso-glycerophospholipids have diverse functions and they are also found in cell membranes [54]; as precursors of phospholipid biosynthesis, they may act as signal molecules involved in a broad range of biological processes [55,56]. However, they might also reflect a highly regulated reservoir of lipids with accessible utility and concentration-dependent lipid toxicity liability [57] that can result from lipid overload generated by dietary excess [58] as might be the case when mosquitoes blood feed. LPE and LPC incorporate into membranes and change membrane permeability and stability. The change in permeability and stability can result in membrane toxicity unless concentrations of lyso-glycerophospholipids are kept below a cytotoxic limit [57]; this may explain the stability of these two lipid groups. Also, we found substantial increases in cell membrane lipids between 16 and 30 h PBM as in the cases of PE and PC, reflecting the growth of embryos during the reproductive process. Moreover, lyso-glycerophospholipids could act as a collective reserve that provides acyl chains for beta-oxidation when needed [53], or they could be a source of partially assembled molecules for further biosynthesis; this may explain the unusual increase of LPI between 0 and 16 h PBM followed by increase of nearly all major lipid groups from 16 to 30 h PBM. LPS and LPG also increase between 0 and 16 h PBM; this might represent the growth of gut bacteria during the blood digestion process [59]. Gut bacteria can also contribute to exploit lipids from blood meal [60,61]. LPA is the only group from lyso-glycerophospholipids showing a bimodal pattern with increases. These lipids are essential extracellular signaling molecules via cognate G protein-coupled receptors [62].
We also found that many lipids from groups PI and CL increased significantly from 16 to 30 h PBM. These lipids are closely connected with metabolic activity and signaling. For example, PIs are important in regulating phototransduction, cell growth and the developmental process of fruit flies [63,64,65]. CLs are integral to mitochondrial membranes, with important roles in ATP-synthase and phospholipid remodeling [66,67]. Moreover, SM play significant roles in mammal cells, as they are involved in diverse signaling pathways [68,69]. Meanwhile, for dipteran insects such as Drosophila, there is a lack of SM; instead, they synthesize the SM analogue phosphoethanolamine ceramide [70]. Though we did not look into phosphoethanolamine ceramide in the present study, we also note that most SMs were either relatively constant or changed only slightly between 16 h and 30 h PBM.
Finally, it is worth noting that approximately a quarter of the lipids, including almost all SHexCer we identified, contained odd chains. Pinch et al. [43] investigated the fat body of mosquitoes feeding on bovine blood and considered that odd-chain lipids may come from ruminal microflora, microbes in larval rearing water or branched chain amino acids from hemoglobin. Our study showed that the last hypothesis is most likely correct, as we observed these odd-chain lipids from human blood, and these also increased in mosquitoes between 16 h and 30 h PBM. These odd chain fatty acid-containing lipids did not show any distinct change patterns comparable to even chain fatty acid-containing lipids from the same lipid group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13030421/s1, Figure S1: Ovarian appearance of female mosquitoes dissected at (A) BBM, (B) 1 d PBM, (C) 2 d PBM, (D) 3 d PBM, (E) 4 d PBM and (F) 5 d PBM. There is some variation in the stages of development of the ovaries among individuals as captured by variation in weight (Figure 1); Table S2: List of individual lipids and their changes before and after female Aedes aegypti mosquitoes blood fed (between BBM and 0 h PBM); Table S3: List of individual lipids and their changes between 16 h and 30 h after female Aedes aegypti mosquitoes blood fed (between 16 h and 30 h PBM).
Author Contributions
Conceptualization, M.-J.L., Q.Y. and A.A.H.; Methodology, M.-J.L. and S.N.; Software, S.N. and N.A.W.; Validation, M.-J.L., S.N., C.M. and A.A.H.; Formal analysis, M.-J.L. and S.N.; Investigation, M.-J.L.; Resources, N.A.W. and A.A.H.; Data curation, M.-J.L. and S.N.; Writing—original draft, M.-J.L., S.N. and L.G.H.; Writing—review and editing, M.-J.L., S.N., Q.Y., L.G.H., C.M. and A.A.H.; Visualization, M.-J.L.; Supervision, S.N. and A.A.H.; Project administration, A.A.H.; Funding acquisition, A.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Health and Medical Research Council (1132412, 1118640, www.nhmrc.gov.au). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Female mosquitoes were blood fed on a volunteer in this experiment, with ethics approval from the University of Melbourne Human Ethics committee (approval 0723847).
Informed Consent Statement
The process of mosquito females feeding on human volunteers is approved by the University of Melbourne Human Ethics committee (approval 0723847). All adult subjects provided informed written consent (no children were involved).
Data Availability Statement
Data available in a publicly accessible repository: The mass spectrometry raw files that presented in this study are openly available in the MASS Spectrometry Interactive Virtual Environment (MassIVE) and are accessible via the identifier MSV000091430.
Acknowledgments
We thank Cameron Patrick from Statistical Consulting Centre and Melbourne Statistical Consulting Platform, the University of Melbourne, for statistical advice. We also thank Perran A. Ross and Moshe Jasper from Pest and Environmental Adaptation Research Group, University of Melbourne, for their assistance in mosquito blood feeding and advice in data visualization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gould, E.A.; Higgs, S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Evans, B.R.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 2014, 68, 514–525. [Google Scholar] [CrossRef]
- Gulia-Nuss, M.; Elliot, A.; Brown, M.R.; Strand, M.R. Multiple factors contribute to anautogenous reproduction by the mosquito Aedes aegypti. J. Insect Physiol. 2015, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Naksathit, A.; Day, J.F.; Kittayapong, P.; Edman, J.D. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am. J. Trop. Med. Hyg. 1997, 57, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; Ibrahim, M.M. Formation of lipid reserves in fat body and eggs of the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 2001, 47, 623–627. [Google Scholar] [CrossRef]
- Zhou, G.; Pennington, J.E.; Wells, M.A. Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochem Mol. Biol. 2004, 34, 919–925. [Google Scholar] [CrossRef]
- Zhou, G.; Flowers, M.; Friedrich, K.; Horton, J.; Pennington, J.; Wells, M.A. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J. Insect Physiol. 2004, 50, 337–349. [Google Scholar] [CrossRef]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef]
- Hagedorn, H.H.; O’Connor, J.D.; Fuchs, M.S.; Sage, B.; Schlaeger, D.A.; Bohm, M.K. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc. Natl. Acad. Sci. USA 1975, 72, 3255–3259. [Google Scholar] [CrossRef]
- Belles, X.; Piulachs, M.D. Ecdysone signalling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim. Biophys. Acta 2015, 1849, 181–186. [Google Scholar] [CrossRef]
- Klowden, M.J. Endocrine aspects of mosquito reproduction. Physiol. Insect Biochem. Physiol. 1997, 35, 491–512. [Google Scholar] [CrossRef]
- Briegel, H.; Gut, T.; Lea, A.O. Sequential deposition of yolk components during oogenesis in an insect, Aedes aegypti (Diptera: Culicidae). J. Insect Physiol. 2003, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Casas-Martínez, M.; Tamayo-Domínguez, R.; Bond-Compeán, J.G.; Rojas, J.C.; Weber, M.; Ulloa-García, A. Oogenic development and gonotrophic cycle of Aedes aegypti and Aedes albopictus in laboratory. Salud Publica Mex. 2020, 62, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.W.; Knapek, K.J.; Kendall, L.V. Rearing Aedes aegypti mosquitoes in a laboratory setting. Lab. Animal Sci. Prof. 2020, 55, 42–45. [Google Scholar]
- Hansen, I.A.; Attardo, G.M.; Park, J.H.; Peng, Q.; Raikhel, A.S. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 10626–10631. [Google Scholar] [CrossRef]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 2017, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Attardo, G.M.; Hansen, I.A.; Raikhel, A.S. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Mol. Biol. 2005, 35, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Phasomkusolsil, S.; Tawong, J.; Monkanna, N.; Pantuwatana, K.; Damdangdee, N.; Khongtak, W.; Kertmanee, Y.; Evans, B.P.; Schuster, A.L. Maintenance of mosquito vectors: Effects of blood source on feeding, survival, fecundity, and egg hatching rates. J. Vector Ecol. 2013, 38, 38–45. [Google Scholar] [CrossRef]
- Ross, P.A.; Lau, M.-J.; Hoffmann, A.A. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS ONE 2019, 14, e0224268. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; Rodrigues, S.L.; Mansur, S.B.; de Oliveira, S.P.; Caragata, E.P.; Moreira, L.A. Development and physiological effects of an artificial diet for Wolbachia-infected Aedes aegypti. Sci. Rep. 2017, 7, 15687. [Google Scholar] [CrossRef]
- Talyuli, O.A.; Bottino-Rojas, V.; Taracena, M.L.; Soares, A.L.; Oliveira, J.H.; Oliveira, P.L. The use of a chemically defined artificial diet as a tool to study Aedes aegypti physiology. J. Insect Physiol. 2015, 83, 1–7. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rancès, E.; O’Neill, S.L.; McGraw, E.A. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol. 2014, 67, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H.; Hefti, M.; DiMarco, E. Lipid metabolism during sequential gonotrophic cycles in large and small female Aedes aegypti. J. Insect Physiol. 2002, 48, 547–554. [Google Scholar] [CrossRef]
- Troy, S.; Anderson, W.A.; Spielman, A. Lipid content of maturing ovaries of Aedes aegypti mosquitoes. Comp. Biochem. Physiol. B 1975, 50, 457–461. [Google Scholar] [CrossRef]
- Vrablik, T.L.; Watts, J.L. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Mol. Reprod. Dev. 2013, 80, 244–259. [Google Scholar] [CrossRef]
- Toprak, U.; Hegedus, D.; Doğan, C.; Güney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 2020, 104, e21682. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990, 36, 165–172. [Google Scholar] [CrossRef]
- Lydic, T.A.; Townsend, S.; Adda, C.G.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–95. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid. Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Oshaghi, M.A.; Chavshin, A.R.; Vatandoost, H.; Yaaghoobi, F.; Mohtarami, F.; Noorjah, N. Effects of post-ingestion and physical conditions on PCR amplification of host blood meal DNA in mosquitoes. Exp. Parasitol. 2006, 112, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Mukabana, W.R.; Takken, W.; Seda, P.; Killeen, G.F.; Hawley, W.A.; Knols, B.G. Extent of digestion affects the success of amplifying human DNA from blood meals of Anopheles gambiae (Diptera: Culicidae). Bull. EntoMol. Res. 2002, 92, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.C.; Edman, J.D.; Scott, T.W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. EntoMol. 2001, 38, 411–422. [Google Scholar] [CrossRef]
- Roy, D. On the role of blood in ovulation in Aedes aegypti, Linn. Bull. EntoMol. Res. 1936, 27, 423–429. [Google Scholar] [CrossRef]
- Arifin, S.A.; Falasca, M. Lysophosphatidylinositol signalling and metabolic diseases. Metabolites 2016, 6, 6. [Google Scholar] [CrossRef]
- Piñeiro, R.; Falasca, M. Lysophosphatidylinositol signalling: New wine from an old bottle. Biochim. Biophys. Acta 2012, 1821, 694–705. [Google Scholar] [CrossRef]
- Kim, T.; Kim, Y.J. Overview of innate immunity in Drosophila. J. Biochem. Mol. Biol. 2005, 38, 121–127. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Galindo-Lagunas, K.A.; Guan, Z.; Vinuesa, P.; Robinson, S.; Thomas-Oates, J.; Raetz, C.R.; Geiger, O. The lipid lysyl-phosphatidylglycerol is present in membranes of Rhizobium tropici CIAT899 and confers increased resistance to polymyxin B under acidic growth conditions. Mol. Plant Microbe Interact. 2007, 20, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Pinch, M.; Mitra, S.; Rodriguez, S.D.; Li, Y.; Kandel, Y.; Dungan, B.; Holguin, F.O.; Attardo, G.M.; Hansen, I.A. Fat and happy: Profiling mosquito fat body lipid storage and composition post-blood meal. Front. Insect Sci. 2021, 1, 693168. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.L.; Saha, T.T.; Roy, S.; Zhao, B.; Raikhel, A.S.; Zou, Z. Temporal coordination of carbohydrate metabolism during mosquito reproduction. PLoS Genet. 2015, 11, e1005309. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, H.H.; Fallon, A.M.; Laufer, H. Vitellogenin synthesis by the fat body of the mosquito Aedes aegypti: Evidence of transcriptional control. Dev. Biol. 1973, 31, 285–294. [Google Scholar] [CrossRef]
- Sun, J.; Hiraoka, T.; Dittmer, N.T.; Cho, K.H.; Raikhel, A.S. Lipophorin as a yolk protein precursor in the mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2000, 30, 1161–1171. [Google Scholar] [CrossRef]
- Kawooya, J.K.; Law, J.H. Role of lipophorin in lipid transport to the insect egg. J. Biol. Chem. 1988, 263, 8748–8753. [Google Scholar] [CrossRef]
- Ford, P.S.; Van Heusden, M.C. Triglyceride-rich lipophorin in Aedes aegypti (Diptera: Culicidae). J. Med. EntoMol. 1994, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.E.; Nussenzveig, R.H.; Van Heusden, M.C. Lipid transfer from insect fat body to lipophorin: Comparison between a mosquito triacylglycerol-rich lipophorin and a sphinx moth diacylglycerol-rich lipophorin. J. Lipid. Res. 1996, 37, 1144–1152. [Google Scholar] [CrossRef]
- Arrese, E.L.; Canavoso, L.E.; Jouni, Z.E.; Pennington, J.E.; Tsuchida, K.; Wells, M.A. Lipid storage and mobilization in insects: Current status and future directions. Insect Biochem. Mol. Biol. 2001, 31, 7–17. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Wells, M.A. Metabolic pathways for diacylglycerol biosynthesis and release in the midgut of larval Manduca sexta. Insect Biochem. Mol. Biol. 2000, 30, 1173–1180. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; DiRusso, C.C. Transmembrane movement of exogenous long-chain fatty acids: Proteins, enzymes, and vectorial esterification. MicroBiol. Mol. Biol. Rev. 2003, 67, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid receptors: Signaling and biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid. Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid. Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Levental, K.R.; Malmberg, E.; Symons, J.L.; Fan, Y.Y.; Chapkin, R.S.; Ernst, R.; Levental, I. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nat. Commun. 2020, 11, 1339. [Google Scholar] [CrossRef]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 2013, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Njoroge, T.M.; Dunlap, C.; Cáceres, C.E. Blood meal source and mixed blood-feeding influence gut bacterial community composition in Aedes aegypti. Parasit. Vectors 2021, 14, 83. [Google Scholar] [CrossRef]
- Gaio Ade, O.; Gusmão, D.S.; Santos, A.V.; Berbert-Molina, M.A.; Pimenta, P.F.; Lemos, F.J. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: Culicidae) (L.). Parasit. Vectors 2011, 4, 105. [Google Scholar] [CrossRef]
- Luquain, C.; Sciorra, V.A.; Morris, A.J. Lysophosphatidic acid signaling: How a small lipid does big things. Trends. Biochem. Sci. 2003, 28, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Toscano, S.; Trivedi, D.; Jones, D.R.; Mathre, S.; Clarke, J.H.; Divecha, N.; Raghu, P. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc. Natl. Acad. Sci. USA 2013, 110, 5963–5968. [Google Scholar] [CrossRef]
- Milligan, S.C.; Alb, J.G., Jr.; Elagina, R.B.; Bankaitis, V.A.; Hyde, D.R. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol. 1997, 139, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Murillas, I.; Pettitt, T.; Macdonald, E.; Okkenhaug, H.; Georgiev, P.; Trivedi, D.; Hassan, B.; Wakelam, M.; Raghu, P. lazaro encodes a lipid phosphate phosphohydrolase that regulates phosphatidylinositol turnover during Drosophila phototransduction. Neuron 2006, 49, 533–546. [Google Scholar] [CrossRef]
- Schlame, M.; Ren, M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim. Biophys. Acta 2009, 1788, 2080–2083. [Google Scholar] [CrossRef]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.L.; Schlame, M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.; Acharya, J.K. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol. Life Sci. 2005, 62, 128–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).