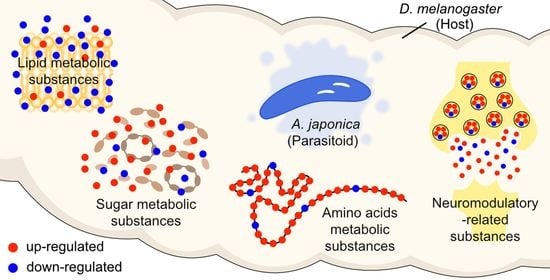
Metabolomics Provides New Insights into Host Manipulation Strategies by Asobara japonica (Hymenoptera: Braconidae), a Fruit Fly Parasitoid
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Experimental Procedures and Sample Collection
2.3. Sample Pretreatment
2.4. LC-MS/MS Analysis
2.5. Data Analysis
3. Results
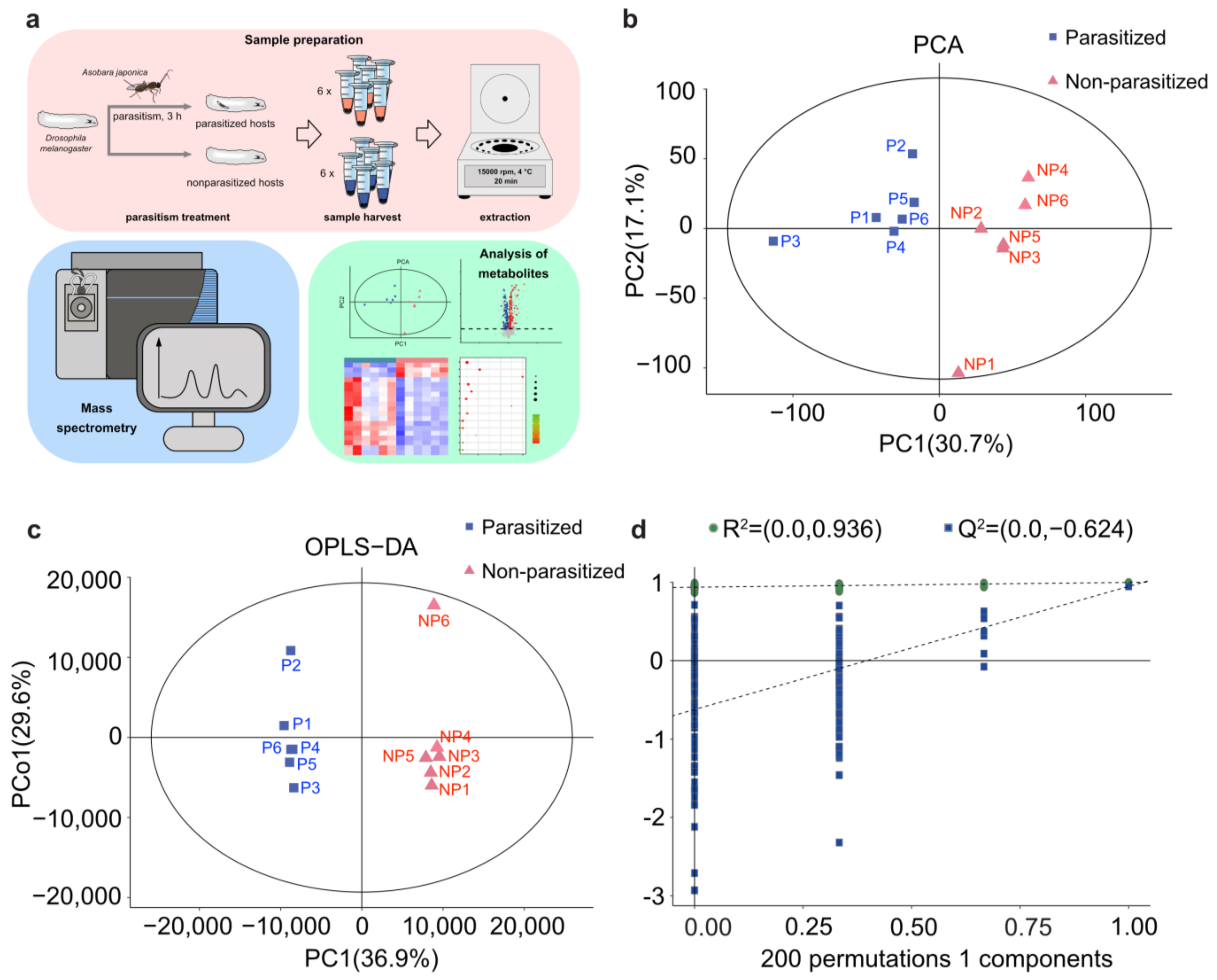
3.1. PCA and OPLS-DA Analyses of Metabolic Difference in Parasitized Host
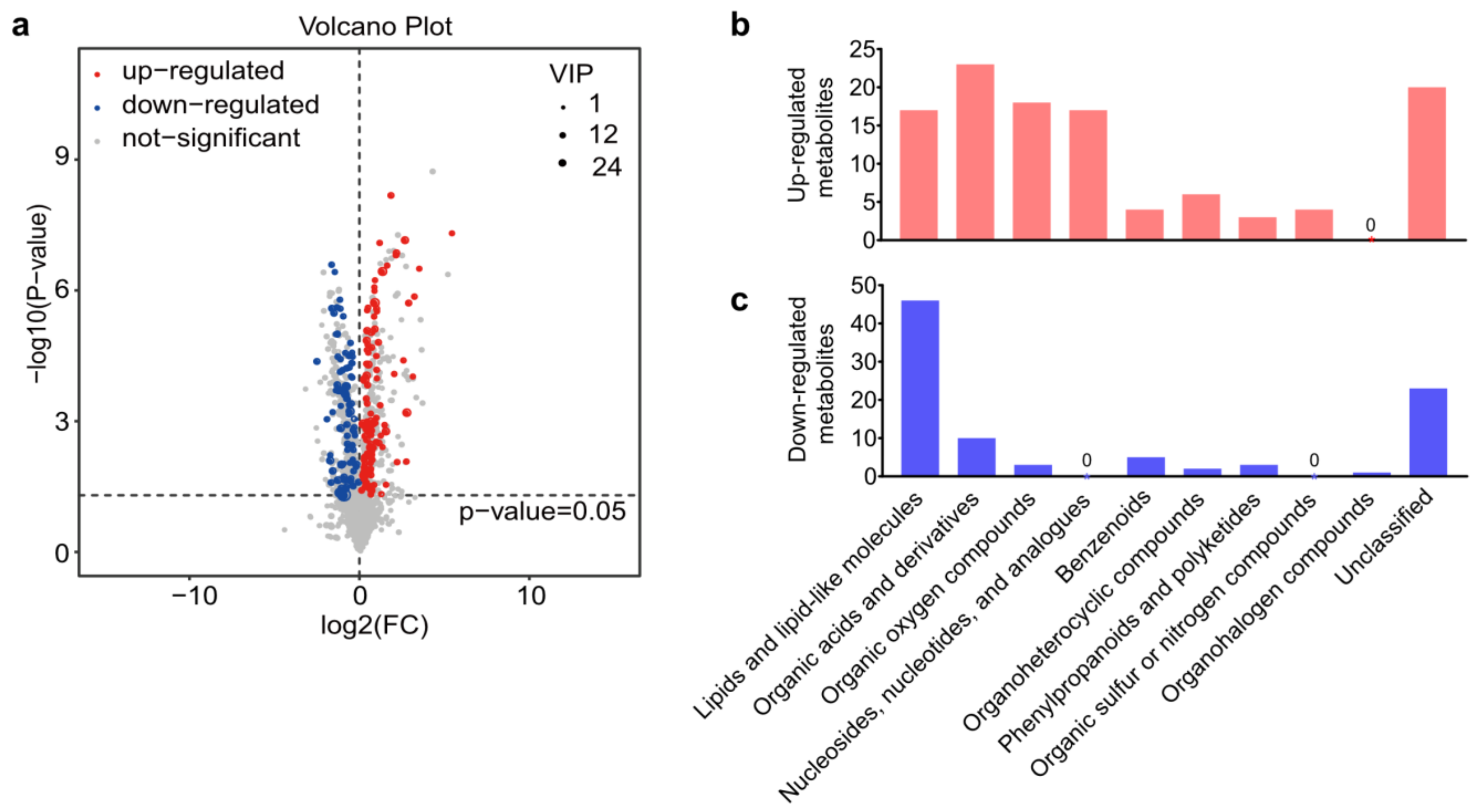
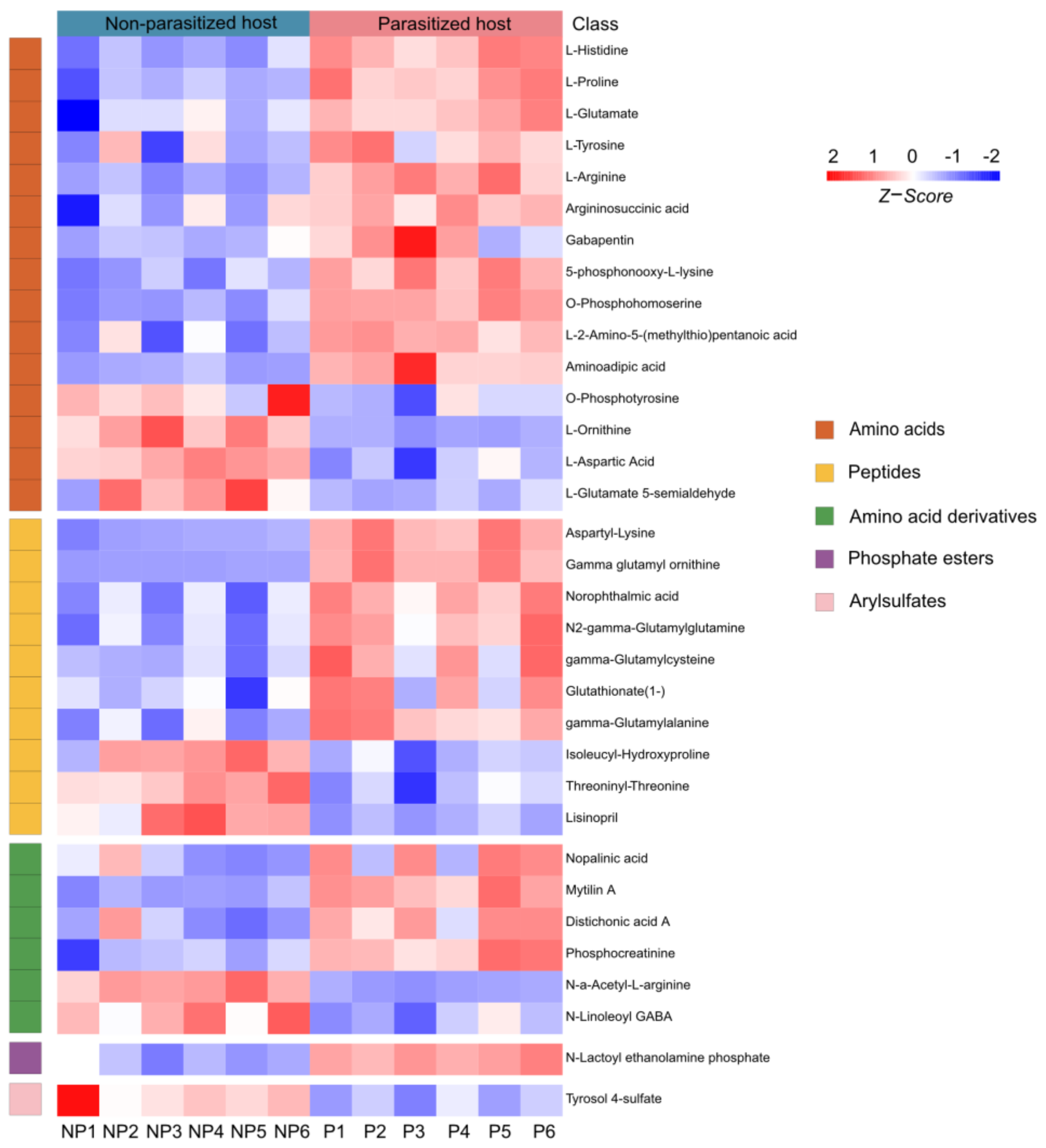
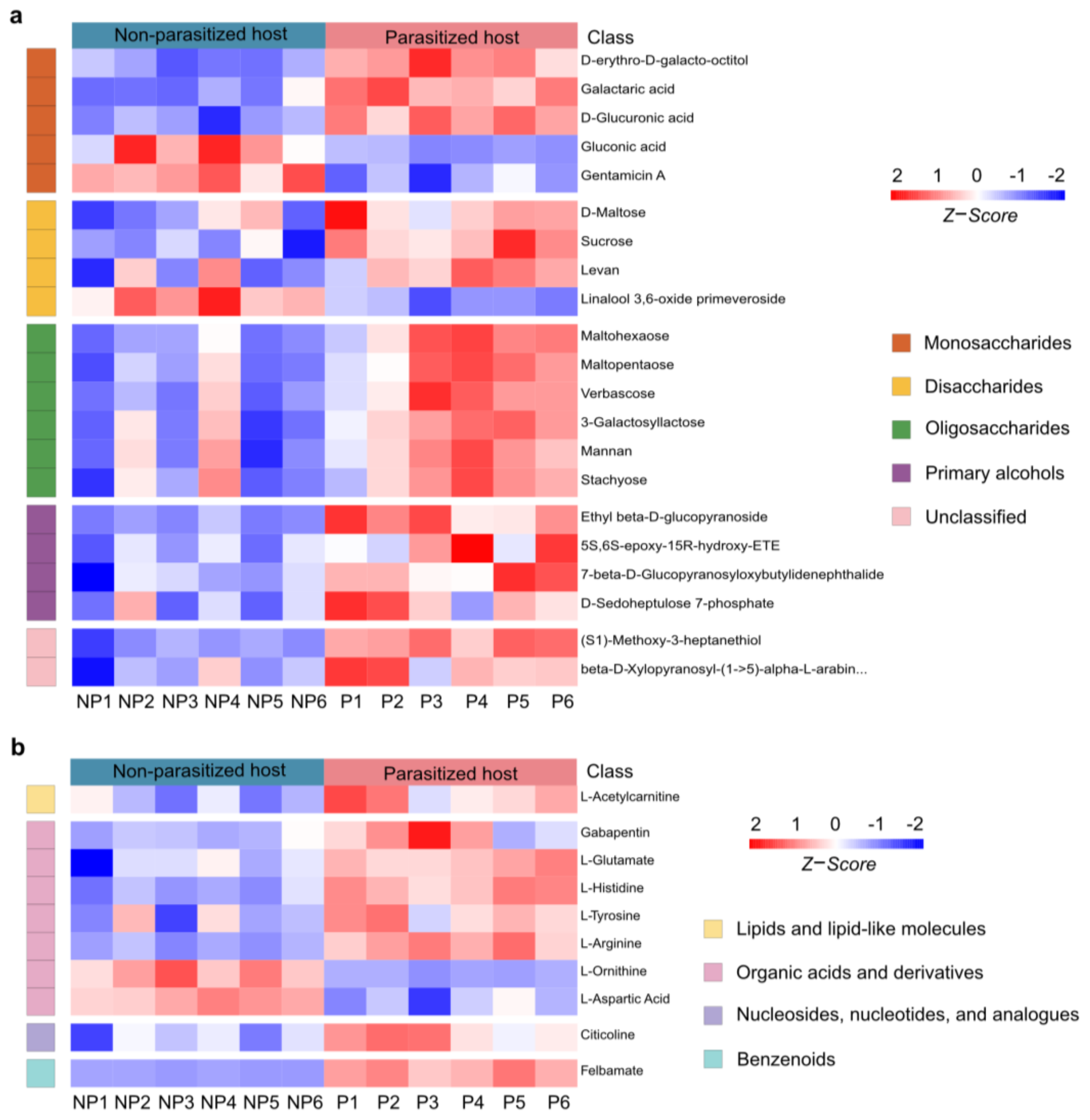
3.2. Changes in Metabolites Post A. japonica Parasitization
3.3. Differences in Lipids and Lipid-like Molecules Post A. japonica Parasitization
3.4. Differences in Organic Acids and Their Derivatives Post A. japonica Parasitization
3.5. Changes in Organic Oxygen Compounds Post A. japonica Parasitization
3.6. Changes in Neuromodulators Post A. japonica Parasitization
3.7. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pennacchio, F.; Strand, M.R. Evolution of developmental strategies in parasitic Hymenoptera. Annu. Rev. Èntomol. 2006, 51, 233–258. [Google Scholar] [CrossRef]
- Fei, M.; Gols, R.; Harvey, J.A. The Biology and Ecology of Parasitoid Wasps of Predatory Arthropods. Annu. Rev. Èntomol. 2022, 68, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Beckage, N.E.; Gelman, D.B. Wasp parasitoid disruption of host development: Implications for new biologically based strategies for insect control. Annu. Rev. Èntomol. 2004, 49, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Strand, M.R. The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology 2002, 83, 2439–2451. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Fang, G.; Pang, L.; Zhou, S.; Zhou, Y.; Pan, Z.; Zhang, Q.; Sheng, Y.; Lu, Y.; et al. Two novel venom proteins underlie divergent parasitic strategies between a generalist and a specialist parasite. Nat. Commun. 2021, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- A Schlenke, T.; Morales, J.; Govind, S.; Clark, A. Contrasting Infection Strategies in Generalist and Specialist Wasp Parasitoids of Drosophila melanogaster. PLoS Pathog. 2007, 3, 1486–1501. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Wang, Y.; Shi, M.; Chen, X.; Huang, J. Biocontrol characteristics of the fruit fly pupal parasitoid Trichopria drosophilae (Hymenoptera: Diapriidae) emerging from different hosts. Sci. Rep. 2018, 8, 13323. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom Proteins from Endoparasitoid Wasps and Their Role in Host-Parasite Interactions. Annu. Rev. Èntomol. 2011, 56, 313–335. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, Y.; Chen, J.; Pan, Z.; Pang, L.; Wang, Y.; Zhang, Q.; Strand, M.R.; Chen, X.-X.; Huang, J. Parasite reliance on its host gut microbiota for nutrition and survival. ISME J. 2022, 16, 2574–2586. [Google Scholar] [CrossRef]
- Toledo, D.A.M.; D’Avila, H.; Melo, R.C.N. Host Lipid Bodies as Platforms for Intracellular Survival of Protozoan Parasites. Front. Immunol. 2016, 7, 174. [Google Scholar] [CrossRef]
- Pennacchio, F.; Caccia, S.; Digilio, M.C. Host regulation and nutritional exploitation by parasitic wasps. Curr. Opin. Insect Sci. 2014, 6, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Ellers, J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 2008, 54, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Le Lann, C.; Blanken, F.J.D.; Harvey, J.A.; van Alphen, J.J.M.; Ellers, J. Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 8677–8682. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.C.; Ganesalingam, V.K. Changes in the Composition of Host Haemolymph after Attack by an Insect Parasitoid. Nature 1970, 227, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Bischof, C.; Ortel, J. The effects of parasitism by Glyptapanteles liparidis (Braconidae: Hymenoptera) on the hemolymph and total body composition of gypsy moth larvae (Lymantria dispar, Lymantriidae: Lepidoptera). Parasitol. Res. 1996, 82, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Kaeslin, M.; Pfister-Wilhelm, R.; Lanzrein, B. Influence of the parasitoid Chelonus inanitus and its polydnavirus on host nutritional physiology and implications for parasitoid development. J. Insect Physiol. 2005, 51, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.C.; Herren, J.K.; Schüpfer, F.; Lemaitre, B. The Role of Lipid Competition for Endosymbiont-Mediated Protection against Parasitoid Wasps in Drosophila. Mbio 2016, 7, e01006-16. [Google Scholar] [CrossRef]
- Palm, W.; Sampaio, J.L.; Brankatschk, M.; Carvalho, M.; Mahmoud, A.; Shevchenko, A.; Eaton, S. Lipoproteins in Drosophila melanogaster—Assembly, function, and influence on tissue lipid composition. PLoS Genet. 2012, 8, e1002828. [Google Scholar] [CrossRef]
- Snart, C.J.; Hardy, I.C.; Barrett, D.A. Entometabolomics: Applications of modern analytical techniques to insect studies. Èntomol. Exp. Appl. 2015, 155, 1–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, D.Q.; Zhang, P.W.; Chen, X.T.; Liu, B.J.; Cheng, D.M.; Zhang, Z.X. Integrated LC–MS and GC–MS-based untargeted metabolomics studies of the effect of azadirachtin on Bactrocera dorsalis larvae. Sci. Rep. 2020, 10, 2306. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, H.; Meng, Q.; Wang, M.; Zhou, G.; Li, X.; Wang, H.; Miao, L.; Qin, Q.; Zhang, J. Metabolic insights into the cold survival strategy and overwintering of the common cutworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). J. Insect Physiol. 2017, 100, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hao, F.; Hu, J.; Zhang, W.; Wan, L.; Zhu, L.; Tang, H.; He, G. Revealing Different Systems Responses to Brown Planthopper Infestation for Pest Susceptible and Resistant Rice Plants with the Combined Metabonomic and Gene-Expression Analysis. J. Proteome Res. 2010, 9, 6774–6785. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Wilinski, D.; Winzeler, J.; Duren, W.; Persons, J.L.; Holme, K.J.; Mosquera, J.; Khabiri, M.; Kinchen, J.M.; Freddolino, P.L.; Karnovsky, A.; et al. Rapid metabolic shifts occur during the transition between hunger and satiety in Drosophila melanogaster. Nat. Commun. 2019, 10, 4052. [Google Scholar] [CrossRef]
- Guan, X.L.; Cestra, G.; Shui, G.; Kuhrs, A.; Schittenhelm, R.B.; Hafen, E.; van der Goot, F.G.; Robinett, C.C.; Gatti, M.; Gonzalez-Gaitan, M.; et al. Biochemical Membrane Lipidomics during Drosophila Development. Dev. Cell 2013, 24, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Hunt, E.J.; Pritchard, J.; Bennett, M.J.; Zhu, X.; Barrett, D.A.; Allen, T.; Bale, J.S.; Newbury, H.J. The Arabidopsis thaliana/Myzus persicae model system demonstrates that a single gene can influence the interaction between a plant and a sap-feeding insect. Mol. Ecol. 2006, 15, 4203–4213. [Google Scholar] [CrossRef]
- Maag, D.; Erb, M.; Glauser, G. Metabolomics in plant-herbivore interactions: Challenges and applications. Èntomol. Exp. Appl. 2015, 157, 18–29. [Google Scholar] [CrossRef]
- Kang, K.; Yue, L.; Xia, X.; Liu, K.; Zhang, W. Comparative metabolomics analysis of different resistant rice varieties in response to the brown planthopper Nilaparvata lugens Hemiptera: Delphacidae. Metabolomics 2019, 15, 62. [Google Scholar] [CrossRef]
- Bertrand, C.; Gonzalez-Coloma, A.; Prigent-Combaret, C. Plant Metabolomics to the Benefit of Crop Protection and Growth Stimulation. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2021; Volume 98, pp. 107–132. [Google Scholar]
- Ideo, S.; Watada, M.; Mitsui, H.; Kimura, M.T. Host range of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of drosophilid flies. Èntomol. Sci. 2008, 11, 1–6. [Google Scholar] [CrossRef]
- Poyet, M.; Havard, S.; Prevost, G.; Chabrerie, O.; Doury, G.; Gibert, P.; Eslin, P. Resistance of Drosophila suzukii to the larval parasitoids Leptopilina heterotoma and Asobara japonica is related to haemocyte load. Physiol. Entomol. 2013, 38, 45–53. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Pan, Z.; Zhu, J.; Wang, Z.; Shi, M.; Chen, X.; Huang, J. The complete mitochondrial genome of Asobara japonica (Hymenoptera: Braconidae). Mitochondrial DNA Part B 2020, 5, 1279–1281. [Google Scholar] [CrossRef]
- Chen, J.; Fang, G.; Pang, L.; Sheng, Y.; Zhang, Q.; Zhou, Y.; Zhou, S.; Lu, Y.; Liu, Z.; Zhang, Y.; et al. Neofunctionalization of an ancient domain allows parasites to avoid intraspecific competition by manipulating host behaviour. Nat. Commun. 2021, 12, 5489. [Google Scholar] [CrossRef]
- Feng, X.; Gao, G.; Yu, C.; Zhu, A.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Chen, P. Transcriptome and metabolome analysis reveals anthocyanin biosynthesis pathway associated with ramie (Boehmeria nivea (L.) Gaud.) leaf color formation. BMC Genom. 2021, 22, 684. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Pan, H.; Shao, H.; Qian, C.; Han, J.; Li, Y.; Lou, Y. UPLC/MS-based untargeted metabolomics reveals the changes in muscle metabolism of electron beam irradiated Solenocera melantho during refrigerated storage. Food Chem. 2022, 367, 130713. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, K.; Ji, W.; Xu, H.; Liu, Y.; Wang, S.; Wang, Z.; Gao, F.; Lin, Z.; Ji, T. Metabolomic analysis of honey bees (Apis mellifera) response to carbendazim based on UPLC-MS. Pestic. Biochem. Physiol. 2021, 179, 104975. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, Y.; Qin, D.; Chen, J.; Zhang, Z. Metabolic Changes in Larvae of Predator Chrysopa sinica Fed on Azadirachtin-Treated Plutella xylostella Larvae. Metabolites 2022, 12, 158. [Google Scholar] [CrossRef]
- Tao, S.; Wang, J.; Liu, M.; Sun, F.; Li, B.; Ye, C. Haemolymph metabolomic differences in silkworms (Bombyx mori L.) under mulberry leaf and two artificial diet rearing methods. Arch. Insect Biochem. 2022, 109, e21851. [Google Scholar] [CrossRef]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Jia, Z.; Moulson, C.L.; Pei, Z.; Miner, J.H.; Watkins, P.A. Fatty acid transport protein 4 is the principal very long chain fatty acyl-CoA synthetase in skin fibroblasts. J. Biol. Chem. 2007, 282, 20573–20583. [Google Scholar] [CrossRef]
- Consoli, F.L.; Vinson, S. Host regulation and the embryonic development of the endoparasitoid Toxoneuron nigriceps (Hymenoptera: Braconidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 137, 463–473. [Google Scholar] [CrossRef]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.D.; Shibata, N.; Podzorski, R.P.; Herron, M.J. Candida mannan: Chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin. Microbiol. Rev. 1991, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, D.; Gao, A.; Yang, X. Stachyose increases absorption and hepatoprotective effect of tea polyphenols in high fructose-fed mice. Mol. Nutr. Food Res. 2016, 60, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A. Parasites: Evolution’s neurobiologists. J. Exp. Biol. 2013, 216, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Haspel, G.; Gefen, E.; Ar, A.; Glusman, J.G.; Libersat, F. Parasitoid wasp affects metabolism of cockroach host to favor food preservation for its offspring. J. Comp. Physiol. A 2005, 191, 529–534. [Google Scholar] [CrossRef]
- Adamo, S.; Linn, C.; Beckage, N. Correlation between changes in host behaviour and octopamine levels in the tobacco hornworm Manduca sexta parasitized by the gregarious braconid parasitoid wasp Cotesia congregata. J. Exp. Biol. 1997, 200, 117–127. [Google Scholar] [CrossRef]
- Libersat, F.; Delago, A.; Gal, R. Manipulation of Host Behavior by Parasitic Insects and Insect Parasites. Annu. Rev. Èntomol. 2009, 54, 189–207. [Google Scholar] [CrossRef]
- Engelstädter, J.; Hurst, G.D. The Ecology and Evolution of Microbes that Manipulate Host Reproduction. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 127–149. [Google Scholar] [CrossRef]
- Boots, M.; Sasaki, A. The evolutionary dynamics of local infection and global reproduction in host-parasite interactions. Ecol. Lett. 2000, 3, 181–185. [Google Scholar] [CrossRef]
- Pernas, L.; Bean, C.; Boothroyd, J.C.; Scorrano, L. Mitochondria Restrict Growth of the Intracellular Parasite Toxoplasma gondii by Limiting Its Uptake of Fatty Acids. Cell Metab. 2018, 27, 886–897.e4. [Google Scholar] [CrossRef]
- Charron, A.J.; Sibley, L.D. Host cells: Mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 2002, 115, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luo, J.-Y.; Lv, L.-M.; Wang, C.-Y.; Li, C.-H.; Zhu, X.-Z.; Cui, J.-J. Effects of Lysiphlebia japonica (Ashmead) on cotton-melon aphid Aphis gossypii Glover lipid synthesis. Insect Mol. Biol. 2015, 24, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, S.; Luo, J.; Lü, L.; Zhang, L.; Cui, J. Lipidomics and RNA-Seq study of lipid regulation in Aphis gossypii parasitized by Lysiphlebia japonica. Sci. Rep. 2017, 7, 1364. [Google Scholar]
- Zuo, W.; Li, C.; Luan, Y.; Zhang, H.; Tong, X.; Han, M.; Gao, R.; Hu, H.; Song, J.; Dai, F.; et al. Genome-wide identification and analysis of elongase of very long chain fatty acid genes in the silkworm, Bombyx mori. Genome 2018, 61, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Lee, K.C.; Cho, O.R.; Jung, I.Y.; Cho, S.Y.; Kim, S.Y. Sphingolipids from Bombycis Corpus 101A and Their Neurotrophic Effects. J. Nat. Prod. 2003, 66, 466–469. [Google Scholar] [CrossRef]
- Li, M.-J.; Jiang, G.-F.; Wang, W. Metabolite Changes in Orange Dead Leaf Butterfly Kallima inachus during Ontogeny and Diapause. Metabolites 2022, 12, 804. [Google Scholar] [CrossRef]
- Gage, S.L.; Kramer, C.; Calle, S.; Carroll, M.; Heien, M.; De Grandi-Hoffman, G. Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees (Apis mellifera). J. Exp. Biol. 2018, 221, jeb161489. [Google Scholar] [CrossRef]
- Schulz, D.; Morschel, J.; Schuster, S.; Eulenburg, V.; Gomeza, J. Inactivation of the Mouse L-Proline Transporter PROT Alters Glutamatergic Synapse Biochemistry and Perturbs Behaviors Required to Respond to Environmental Changes. Front. Mol. Neurosci. 2018, 11, 279. [Google Scholar] [CrossRef]
- Wu, G.; Liu, J.; Li, M.; Xiao, Y.; Yi, Y. Prior infection of Galleria mellonella with sublethal dose of Bt elicits immune priming responses but incurs metabolic changes. J. Insect Physiol. 2022, 139, 104401. [Google Scholar] [CrossRef]
- Li, X.-J.; Dong, G.-P.; Fang, J.-M.; Liu, H.-J.; Guo, W.-L. Parasitic behaviour of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae) induced changes in free animo acid pools in hemolymph of host Monochamus alternatus Hope (Coleoptera: Cerambycidae). Orient. Insects 2018, 52, 329–341. [Google Scholar] [CrossRef]
- Rahbé, Y.; Digilio, M.; Febvay, G.; Guillaud, J.; Fanti, P.; Pennacchio, F. Metabolic and symbiotic interactions in amino acid pools of the pea aphid, Acyrthosiphon pisum, parasitized by the braconid Aphidius ervi. J. Insect Physiol. 2002, 48, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, T.L.; Starkey, S.R.; Beckage, N.E. Tyrosine and catecholamine levels in the hemolymph of tobacco hornworm larvae, Manduca sexta, parasitized by the braconid wasp, Cotesia congregata, and in the developing parasitoids. Arch. Insect Biochem. 1998, 38, 193–201. [Google Scholar] [CrossRef]
- Idris, A.B.; Grafius, E. Wildflowers as Nectar Sources for Diadegma insulare (Hymenoptera: Ichneumonidae), a Parasitoid of Diamondback Moth (Lepidoptera: Yponomeutidae). Environ. Èntomol. 1995, 24, 1726–1735. [Google Scholar] [CrossRef]
- Wäckers, F.L. A comparison of flowering herbs with respect to their nectar accessibility for the parasitoid Pimpla turionellae. Exp. Appl. Entomol. 1996, 7, 177–182. [Google Scholar]
- Baker, H.G. Floral Nectar Sugar Constituents in Relation to Pollinator Type. In Handbook of Experimental Pollination Biology; Springer: New York, NY, USA, 1983; pp. 117–141. [Google Scholar]
- Nation, J.L. Insect Physiology and Biochemistry; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Mrinalini; Siebert, A.L.; Wright, J.; Martinson, E.; Wheeler, D.; Werren, J.H. Parasitoid venom induces metabolic cascades in fly hosts. Metabolomics 2015, 11, 350–366. [Google Scholar] [CrossRef]
- Thompson, S. Effect of the insect parasite Hyposoter exiguae (Viereck) on the carbohydrate metabolism of its host, Trichoplusia ni (Hübner). J. Insect Physiol. 1986, 32, 287–293. [Google Scholar] [CrossRef]
- Dahlman, D. Trehalose and glucose levels in hemolymph of diet-reared, tobacco leaf-reared and parasitized tobacco hornworm larvae. Comp. Biochem. Physiol. Part A Physiol. 1975, 50, 165–167. [Google Scholar] [CrossRef]
- Mattson, W.J. Herbivory in Relation to Plant Nitrogen Content. Annu. Rev. Ecol. Syst. 1980, 11, 119–161. [Google Scholar] [CrossRef]
- Blanco, C.A.; Portilla, M.; Abel, C.A.; Winters, H.; Ford, R.; Streett, D.; Cohen, A. Soybeanflour and wheat germ proportions in artificial diet and their effect on the growth rates of the tobacco budworm, Heliothis virescens. J. Insect Sci. 2009, 9, 1536–2442. [Google Scholar] [CrossRef]
- Mabiala-Moundoungou, A.; Doury, G.; Eslin, P.; Cherqui, A.; Prévost, G. Deadly venom of Asobara japonica parasitoid needs ovarian antidote to regulate host physiology. J. Insect Physiol. 2010, 56, 35–41. [Google Scholar] [CrossRef]
- Wang, X.; Biondi, A.; Nance, A.H.; Zappalà, L.; Hoelmer, K.A.; Daane, K.M. Assessment of Asobara japonica as a potential biological control agent for the spotted wing drosophila, Drosophila suzukii. Èntomol. Gen. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Pech, L.L. Immunological compatibility in parasitoid-host relationships. Annu. Rev. Entomol. 1995, 40, 31–56. [Google Scholar] [CrossRef] [PubMed]

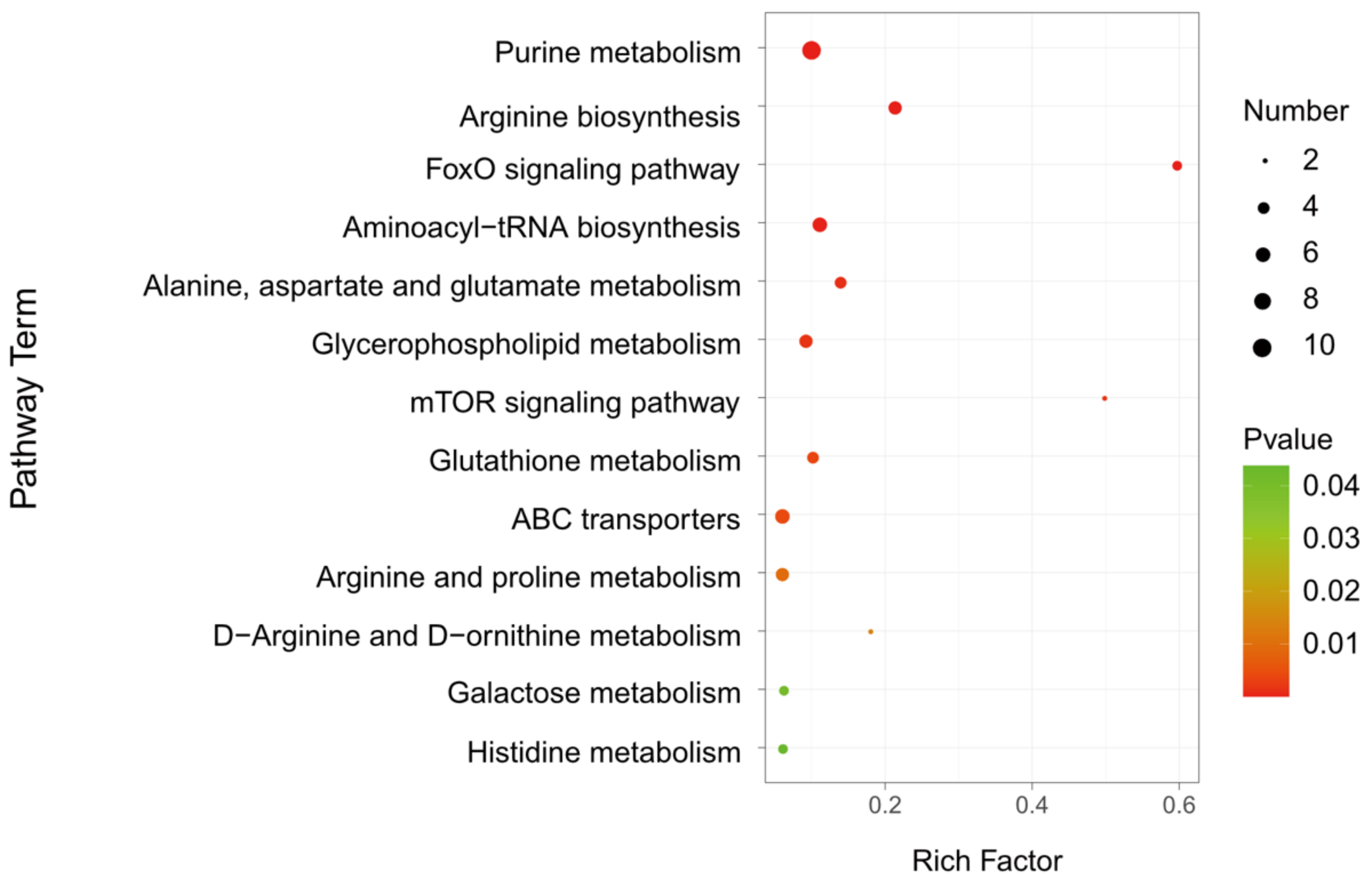
| Pathway | Metabolites | Regulation | p-Value | VIP |
|---|---|---|---|---|
| Purine metabolism (p = 2.51 × 10−6) | Adenosine 3’-monophosphate | up | 9.05 × 10−5 | 5.54 |
| Adenosine monophosphate | up | 1.21 × 10−3 | 3.33 | |
| Adenylsuccinic acid | up | 4.35 × 10−4 | 1.51 | |
| ADP | up | 6.31 × 10−3 | 1.02 | |
| Guanosine | up | 2.57 × 10−2 | 1.16 | |
| Guanosine monophosphate | up | 6.34 × 10−3 | 1.50 | |
| Hypoxanthine | up | 3.33 × 10−3 | 2.07 | |
| Inosine | up | 1.65 × 10−3 | 2.37 | |
| Inosinic acid | up | 7.76 × 10−6 | 3.61 | |
| Uric acid | up | 1.31 × 10−2 | 2.54 | |
| Arginine biosynthesis (p = 3 × 10−5) | Argininosuccinic acid | up | 9.89 × 10−3 | 1.12 |
| L-Arginine | up | 2.51 × 10−6 | 1.48 | |
| L-Aspartic Acid | down | 4.18 × 10−4 | 1.15 | |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| L-Ornithine | down | 1.01 × 10−5 | 1.50 | |
| FoxO signaling pathway (p = 4.44 × 10−5) | L-Glutamate | up | 7.75 × 10−3 | 2.50 |
| Adenosine monophosphate | up | 1.21 × 10−3 | 3.33 | |
| ADP | up | 6.31 × 10−3 | 1.02 | |
| Aminoacyl-tRNA biosynthesis (p = 0.00019) | L-Arginine | up | 2.51 × 10−6 | 1.48 |
| L-Aspartic Acid | down | 4.18 × 10−4 | 1.15 | |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| L-Histidine | up | 1.42 × 10−5 | 4.66 | |
| L-Proline | up | 5.07 × 10−5 | 5.37 | |
| L-Tyrosine | up | 3.09 × 10−2 | 1.36 | |
| Alanine, aspartate and glutamate metabolism (p = 0.00110) | Adenylsuccinic acid | up | 4.35 × 10−4 | 1.51 |
| Argininosuccinic acid | up | 9.89 × 10−3 | 1.12 | |
| L-Aspartic Acid | down | 4.18 × 10−4 | 1.15 | |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| Glycerophospholipid metabolism (p = 0.00160) | Citicoline | up | 5.49 × 10−3 | 1.04 |
| Glycerylphosphorylethanolamine | up | 2.05 × 10−5 | 2.22 | |
| LysoPC(15:0) | down | 9.43 × 10−3 | 1.04 | |
| PC(16:1(9Z)/0:0) | down | 9.97 × 10−3 | 6.62 | |
| PC(16:0/16:0) | up | 1.73 × 10−3 | 5.91 | |
| Phosphocholine | up | 1.50 × 10−3 | 6.53 | |
| mTOR signaling pathway (p = 0.00165) | L-Arginine | up | 2.51 × 10−6 | 1.48 |
| Adenosine monophosphate | up | 1.21 × 10−3 | 3.33 | |
| Glutathione metabolism (p = 0.00349) | gamma-Glutamylalanine | up | 6.73 × 10−4 | 1.13 |
| gamma-Glutamylcysteine | up | 3.95 × 10−3 | 1.12 | |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| L-Ornithine | down | 1.01 × 10−5 | 1.50 | |
| ABC transporters (p = 0.00426) | D-Maltose | up | 1.78 × 10−2 | 8.29 |
| L-Arginine | up | 2.51 × 10−6 | 1.48 | |
| L-Aspartic Acid | down | 4.18 × 10−4 | 1.15 | |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| L-Histidine | up | 1.42 × 10−5 | 4.66 | |
| Sucrose | up | 1.17 × 10−3 | 10.22 | |
| Arginine and proline metabolism (p = 0.00937) | L-Arginine | up | 2.51 × 10−6 | 1.48 |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| L-Glutamate 5-semialdehyde | down | 9.51 × 10−3 | 1.04 | |
| L-Ornithine | down | 1.01 × 10−5 | 1.50 | |
| L-Proline | up | 5.07 × 10−5 | 5.37 | |
| D-Arginine and D-ornithine metabolism (p = 0.01398) | L-Arginine | up | 2.51 × 10−6 | 1.48 |
| L-Ornithine | down | 1.01 × 10−5 | 1.50 | |
| Galactose metabolism (p = 0.04148) | Stachyose | up | 3.34 × 10−2 | 2.63 |
| Sucrose | up | 1.17 × 10−3 | 10.22 | |
| UDP-D-galactose | up | 2.70 × 10−2 | 1.39 | |
| Histidine metabolism (p = 0.04379) | L-Aspartic Acid | down | 4.18 × 10−4 | 1.15 |
| L-Glutamate | up | 7.75 × 10−3 | 2.50 | |
| L-Histidine | up | 1.42 × 10−5 | 4.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, J.; Sheng, Y.; Feng, T.; Shi, W.; Lu, Y.; Guan, X.; Chen, X.; Huang, J.; Chen, J. Metabolomics Provides New Insights into Host Manipulation Strategies by Asobara japonica (Hymenoptera: Braconidae), a Fruit Fly Parasitoid. Metabolites 2023, 13, 336. https://doi.org/10.3390/metabo13030336
Liu S, Zhang J, Sheng Y, Feng T, Shi W, Lu Y, Guan X, Chen X, Huang J, Chen J. Metabolomics Provides New Insights into Host Manipulation Strategies by Asobara japonica (Hymenoptera: Braconidae), a Fruit Fly Parasitoid. Metabolites. 2023; 13(3):336. https://doi.org/10.3390/metabo13030336
Chicago/Turabian StyleLiu, Shengmei, Junwei Zhang, Yifeng Sheng, Ting Feng, Wenqi Shi, Yueqi Lu, Xueying Guan, Xuexin Chen, Jianhua Huang, and Jiani Chen. 2023. "Metabolomics Provides New Insights into Host Manipulation Strategies by Asobara japonica (Hymenoptera: Braconidae), a Fruit Fly Parasitoid" Metabolites 13, no. 3: 336. https://doi.org/10.3390/metabo13030336
APA StyleLiu, S., Zhang, J., Sheng, Y., Feng, T., Shi, W., Lu, Y., Guan, X., Chen, X., Huang, J., & Chen, J. (2023). Metabolomics Provides New Insights into Host Manipulation Strategies by Asobara japonica (Hymenoptera: Braconidae), a Fruit Fly Parasitoid. Metabolites, 13(3), 336. https://doi.org/10.3390/metabo13030336