Biological Characteristics and Energy Metabolism of Migrating Insects
Abstract
1. Introduction
2. Changes in Biological Characteristics during Insect Migration
2.1. Variations in Morphological Parameters
2.2. Changes in Flight Behavior
2.3. Feeding Behavior during Migratory Flight
2.4. Changes in Reproductive Status and Capacity
3. Regulatory Mechanism of Insect Migration and Reproduction Interaction
3.1. Relationship between Insect Migration and Reproduction
3.2. Regulation of Insulin in Relation to Insect Migration and Flight
3.3. Regulation of the Juvenile Hormone on Insect Migration and Reproduction
4. Energy and Substance Metabolism during Insect Migration Flight
4.1. Energy Substances during Insect Flight
4.2. Amino Acid Energy Metabolism during Flight
4.3. Carbohydrate Metabolism during Flight
4.4. Lipid Compound Metabolism during Flight
4.5. Hormonal Regulation of Energy Substances during Flight
5. Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alerstam, T.; Backman, J. Ecology of animal migration. Curr. Biol. 2018, 28, R968–R972. [Google Scholar] [CrossRef]
- May, R.M. How many species are there on earth? Science 1988, 241, 1441–1449. [Google Scholar] [CrossRef]
- Chapman, J.W.; Drake, V.A. Insect Migration. In Encyclopedia of Animal Behavior; Elsevier: Amsterdam, The Netherlands, 2010; pp. 161–166. ISBN 9780080453378. [Google Scholar]
- Wystrach, A.; Graham, P. What can we learn from studies of insect navigation? Anim. Behav. 2012, 84, 13–20. [Google Scholar] [CrossRef]
- Ronce, O. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 231–253. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Reynolds, D.R.; Gao, B.; Hu, G.; Chapman, J.W.; Wotton, K.R. Mechanisms and consequences of partial migration in insects. Front. Ecol. Evol. 2019, 7, 403. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Cheng, Y.X.; Zhang, L. Research advances and perspectives on migration-induced mechanisms promoting outbreaks of major Lepidopteran insect pests in China. Sci. Sin. Vitae 2016, 46, 565–572. [Google Scholar] [CrossRef]
- Liu, P.C.; Diao, Y.H.; Guo, J.W.; Gao, B.Y.; Hu, G. Insect migration behavior and its regulation. Chin. J. Appl. Entomol. 2021, 58, 520–529. [Google Scholar] [CrossRef]
- Rankin, M.A.; Burchsted, J.C.A. The cost of migration in insects. Annu. Rev. Entomol. 1992, 37, 533–559. [Google Scholar] [CrossRef]
- Auerswald, L.; Gade, G. Endocrine control of TAG lipase in the fat body of the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 2006, 36, 759–768. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.Q.; Feng, Q.L. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020, 11, 434. [Google Scholar] [CrossRef]
- Hou, L.; Guo, S.Y.; Ding, D.; Du, B.Z.; Wang, X.H. Neuroendocrinal and molecular basis of flight performance in locusts. Cell. Mol. Life Sci. 2022, 79, 325. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; Van Antwerpen, R. Lipid uptake by insect oocytes. Insect. Biochem. Mol. Biol. 2006, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Zhang, C.X.; Brisson, J.A.; Xu, H.J. Molecular mechanisms of wing polymorphism in insects. Annu. Rev. Entomol. 2019, 64, 297–314. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.F.; Luo, L.Z. Determination of sensitive stage for switching migrant oriental armyworms into resident. Environ. Entomol. 2008, 37, 1389–1395. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of migration in Mythimna separata (Walker) in China: A review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ. Entomol. 2011, 40, 516–533. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, L.L.; Chapman, J.W.; Sappington, T.W.; Liu, J.J.; Cheng, Y.X.; Jiang, X.F. Juvenile hormone regulates the shift from migrants to residents in adult oriental armyworm, Mythimna separata. Sci. Rep. 2020, 10, 11626. [Google Scholar] [CrossRef]
- Cohen, P. The twentieth century struggle to decipher insulin signalling. Nat. Rev. Mol. Cell. Biol. 2006, 7, 867–873. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef]
- Xing, Z.L. Wing Structure and Mechanical Properties of Some Species of Noctuid Moths. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Zhou, Y.; Wu, Q.L.; Zhao, S.Y.; Guo, J.L.; Wyckhuys, K.A.G.; Wu, K.M. Migratory Helicoverpa armigera (Lepidoptera: Noctuidae) exhibits marked seasonal variation in morphology and fitness. Environ. Entomol. 2019, 48, 755–763. [Google Scholar] [CrossRef]
- Altizer, S.; Davis, A.K. Populations of Monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 2010, 64, 1018–1028. [Google Scholar] [CrossRef]
- Korkmaz, R.; Rajabi, H.; Eshghi, S.; Gorb, S.N.; Buscher, T.H. The frequency of wing damage in a migrating butterfly. Insect Sci. 2022. Early View. [Google Scholar] [CrossRef]
- Yu, W.H.; Zhou, Y.; Guo, J.L.; Wyckhuys, K.A.G.; Shen, X.J.; Li, X.K.; Ge, S.S.; Liu, D.Z.; Wu, K.M. Interspecific and seasonal variation in wingbeat frequency among migratory Lepidoptera in northern China. J. Econ. Entomol. 2020, 113, 2134–2140. [Google Scholar] [CrossRef]
- Yu, W.H.; Zhang, H.W.; Xu, R.B.; Sun, Y.S.; Wu, K.M. Characterization of wingbeat frequency of different taxa of migratory insects in northeast Asia. Insects 2022, 13, 520. [Google Scholar] [CrossRef]
- Shi, X.Y.; Feng, H.Q.; Li, J.D.; Liu, B. Comparison of wingbeat frequency between oriental armyworm Mythimna separate, cotton bollworm Helicoverpa armigera and black cutworm Agrotis ypsilon. Plant. Prot. 2013, 39, 31–35. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, K.M.; Cheng, D.F.; Guo, Y.Y. Spring migration and summer dispersal of Loxostege sticticalis (Lepidoptera: Pyralidae) and other insects observed with radar in northern China. Environ. Entomol. 2004, 33, 1253–1265. [Google Scholar] [CrossRef]
- Feng, H.Q.; Zhao, X.C.; Wu, X.F.; Wu, B.; Wu, K.M.; Cheng, D.F.; Guo, Y.Y. Autumn migration of Mythimna separata (Lepidoptera: Noctuidae) over the Bohai Sea in northern China. Environ. Entomol. 2008, 37, 774–781. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, K.M.; Cheng, D.F.; Guo, Y.Y. Radar observations of the autumn migration of the beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) and other moths in northern China. Bull. Entomol. Res. 2003, 93, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Q.; Wu, K.M.; Cheng, D.F.; Guo, Y.Y. Northward migration of Helicoverpa armigera (Lepidoptera: Noctuidae) and other moths in early summer observed with radar in northern China. J. Econ. Entomol. 2004, 97, 1874–1883. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, K.M.; Ni, Y.X.; Cheng, D.F.; Guo, Y.Y. Return migration of Helicoverpa armigera (Lepidoptera: Noctuidae) during autumn in northern China. Bull. Entomol. Res. 2005, 95, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.Q.; Wu, K.M.; Ni, Y.X.; Cheng, D.F.; Guo, Y.Y. High-altitude windborne transport of Helicoverpa armigera (Lepidoptera: Noctuidae) in mid-summer in northern China. J. Insect. Behav. 2005, 18, 335–349. [Google Scholar] [CrossRef]
- Feng, H.; Wu, X.; Wu, B.; Wu, K. Seasonal migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai Sea. J. Econ. Entomol. 2009, 102, 95–104. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, K.M.; Ni, Y.X.; Cheng, D.F.; Guo, Y.Y. Nocturnal migration of dragonflies over the Bohai Sea in northern China. Ecol. Entomol. 2006, 31, 511–520. [Google Scholar] [CrossRef]
- Wackers, F.L.; Romeis, J.; van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef]
- Jia, H.R.; Liu, Y.Q.; Li, X.K.; Li, H.; Pan, Y.F.; Hu, C.X.; Zhou, X.Y.; Wyckhuys, K.A.G.; Wu, K.M. Windborne migration amplifies insect-mediated pollination services. eLife 2022, 11, e76230. [Google Scholar] [CrossRef]
- He, L.M.; Liu, Y.Q.; Guo, J.L.; Chang, H.; Wu, K.M. Host plants and pollination regions for the long-distance migratory noctuid moth, Hadula trifolii Hufnagel in China. Ecol. Evol. 2022, 12, e8819. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.Y.; Wang, M.L.; Yu, W.H.; Wyckhuys, K.A.G.; Wu, K.M. Floral visitation can enhance fitness of Helicoverpa armigera (Lepidoptera: Noctuidae) long-distance migrants. J. Econ. Entomol. 2019, 112, 2655–2662. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Fu, X.W.; Mao, L.M.; Xing, Z.L.; Wu, K.M. Identification of host plant use of adults of a long-distance migratory insect, Mythimna separata. PLoS ONE 2017, 12, e0184116. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Guo, J.L.; Fu, X.W.; Liu, Y.Q.; Wyckhuys, K.A.G.; Hou, Y.M.; Wu, K.M. Molecular-assisted pollen grain analysis reveals spatiotemporal origin of long-distance migrants of a noctuid moth. Int. J. Mol. Sci. 2018, 19, 567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Fu, X.W.; Mao, L.M.; Xing, Z.L.; Wu, K.M. Host plants identification for adult Agrotis ipsilon, a long-distance migratory insect. Int. J. Mol. Sci. 2016, 17, 851. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Guo, Y.Y. Effects of food quality and larval density on flight capacity of cotton bollworm. Acta Entomol. Sin. 1997, 40, 51–57. [Google Scholar]
- He, L.M.; Zhao, S.Y.; He, W.; Wu, K.M. Pollen and nectar have different effects on the development and reproduction of noctuid moths. Front. Ecol. Evol. 2022, 10, 976987. [Google Scholar] [CrossRef]
- Fu, X.W.; Li, C.; Feng, H.Q.; Liu, Z.F.; Chapman, J.W.; Reynolds, D.R.; Wu, K.M. Seasonal migration of Cnaphalocrocis medinalis (Lepidoptera: Crambidae) over the Bohai Sea in northern China. Bull. Entomol. Res. 2014, 104, 601–609. [Google Scholar] [CrossRef]
- Fu, X.W.; Xing, Z.L.; Liu, Z.F.; Ali, A.; Wu, K.M. Migration of diamondback moth, Plutella xylostella, across the Bohai Sea in northern China. Crop Prot. 2014, 64, 143–149. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Feng, H.; Liu, Z.; Wu, K. Trans-regional migration of Agrotis ipsilon (Lepidoptera: Noctuidae) in north-east Asia. Ann. Entomol. Soc. Am. 2015, 108, 519–527. [Google Scholar] [CrossRef]
- Guo, J.L.; Fu, X.W.; Wu, X.; Zhao, X.C.; Wu, K.M. Annual migration of Agrotis segetum (Lepidoptera: Noctuidae): Observed on a small isolated island in northern China. PLoS ONE 2015, 10, e0131639. [Google Scholar] [CrossRef]
- He, L.M.; Fu, X.W.; Huang, Y.X.; Shen, X.J.; Sun, X.T.; Wu, K.M. Seasonal patterns of Scotogramma trifolii Rottemberg (Lepidoptera: Noctuidae) migration across the Bohai Strait in northern China. Crop Prot. 2018, 106, 34–41. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Fu, X.W.; Guo, J.L.; Zhou, Y.; Wyckhuys, K.A.G.; Wu, K.M. Seasonal patterns of Protoschinia scutosa (Lepidoptera: Noctuidae) migration across China’s Bohai Strait. Environ. Entomol. 2018, 47, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Wu, K. Frequency of migration of agricultural pests across the Bohai Sea in northern China and a control strategy for these species. Chin. J. Appl. Entomol. 2020, 57, 233–243. [Google Scholar] [CrossRef]
- Johnson, C.G. A basis for a general syatem of insect migration and dispersal by flight. Nature 1960, 186, 348–350. [Google Scholar] [CrossRef]
- Johnson, C.G. Physiological factors in insect migration by flight. Nature 1963, 198, 423–427. [Google Scholar] [CrossRef]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen & Co. Ltd.: London, UK, 1969. [Google Scholar]
- Luo, L.Z.; Li, G.B.; Hu, Y. Relationship between flight capacity and oviposition of oriental armyworm moths, Mythimna separata (Walker). Acta Entomol. Sin. 1995, 38, 284–289. [Google Scholar] [CrossRef]
- Guo, J.L.; Fu, X.W.; Zhao, X.C.; Wu, K.M. Preliminary study on the flight capacity of Agrotis segetum ( Lepidoptera: Noctuidae). J. Environ. Entomol. 2016, 38, 888–895. [Google Scholar] [CrossRef]
- Wu, X.; Fu, X.W.; Zhao, X.C.; Wu, K.M. Preliminary study of the flight capacity of the cabbage moth, Mamestra brassicae Linnaeus. Chin. J. Appl. Entomol. 2016, 53, 595–603. [Google Scholar] [CrossRef]
- Tigreros, N.; Davidowitz, G. Flight-fecundity tradeoffs in wing-monomorphic insects. Adv. Insect Physiol. 2019, 56, 1–41. [Google Scholar] [CrossRef]
- Gibbs, M.; Van Dyck, H. Butterfly flight activity affects reproductive performance and longevity relative to landscape structure. Oecologia 2010, 163, 341–350. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Sappington, T.W. Relationship of flight and reproduction in beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae), a migrant lacking the oogenesis-flight syndrome. J. Insect. Physiol. 2010, 56, 1631–1637. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Luo, L.Z.; Jiang, X.F.; Sappington, T.W. Synchronized oviposition triggered by migratory flight intensifies larval outbreaks of beet webworm. PLoS ONE 2012, 7, e31562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, K.M.; Wyckhuys, K.A.; Heimpel, G.E. Trade-offs between flight and fecundity in the soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2009, 102, 133–138. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, X.Q.; Xu, H.J.; Fan, H.W.; Huang, H.J.; Ma, X.F.; Wang, C.Y.; Chen, J.G.; Cheng, J.A.; Zhang, C.X. Molecular characterization of the flightin gene in the wing-dimorphic planthopper, Nilaparvata lugens, and its evolution in Pancrustacea. Insect Biochem. Mol. Biol. 2013, 43, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Denno, R.F.; Olmstead, K.L.; McCloud, E.S. Reproductive cost of flight capability: A comparison of life history traits in wing dimorphic planthoppers. Ecol. Entomol. 1989, 14, 31–44. [Google Scholar] [CrossRef]
- Denno, R.F.; Roderick, G.K. Population biology of planthoppers. Annu. Rev. Entomol. 1990, 35, 489–520. [Google Scholar] [CrossRef]
- Ge, S.S.; He, W.; He, L.M.; Yan, R.; Zhang, H.W.; Wu, K.M. Flight activity promotes reproductive processes in the fall armyworm, Spodoptera frugiperda. J. Integr. Agric. 2021, 20, 727–735. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, P.; Sappington, T.W.; Lu, W.X.; Luo, L.Z.; Jiang, X.F. Accelerated and synchronized oviposition induced by flight of young females may intensify larval outbreaks of the rice leaf roller. PLoS ONE 2015, 10, e0121821. [Google Scholar] [CrossRef]
- Russell, R.W.; May, M.L.; Soltesz, K.L. Massive swarm migrations of dragonflies (Odonata) in eastern north America. Am. Midl. Nat. 1998, 140, 325–342. [Google Scholar] [CrossRef]
- Han, L.Z.; Gu, H.N.; Zhai, B.P.; Zhang, X.X. Reproduction-flight relationship in the beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Entomol. 2008, 37, 374–381. [Google Scholar] [CrossRef]
- Ge, S.S.; Sun, X.X.; He, W.; Wyckhuys, K.A.G.; He, L.M.; Zhao, S.Y.; Zhang, H.W.; Wu, K.M. Potential trade-offs between reproduction and migratory flight in Spodoptera frugiperda. J. Insect Physiol. 2021, 132, 104248. [Google Scholar] [CrossRef]
- Nassel, D.R.; Vanden Broeck, J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell. Mol. Life Sci. 2016, 73, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.C.; Okech, S.H.; Liquido, N.J. Wing morphism in the brown planthopper, Nilaparvata lugens. Int. J. Trop. Insect Sc. 1981, 1, 343–348. [Google Scholar] [CrossRef]
- Zhang, Z.Q. A study on the development of wing dimorphism in the rice brown planthopper, Nilaparvata lugens Stål. Acta Entomol. Sin. 1983, 26, 260–266. [Google Scholar] [CrossRef]
- Liu, J.N.; Gui, F.R.; Li, Z.Y. Factors of influencing the development of wing dimorphism in the rice white-backed planthopper, Sogatella furcifera Horvth. Acta Phytophy Sin. 2010, 37, 511–516. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Jiang, J.; Lavine, M.; Lavine, L.C. Host quality induces phenotypic plasticity in a wing polyphenic insect. Proc. Natl. Acad. Sci. USA 2018, 115, 7563–7568. [Google Scholar] [CrossRef]
- Gao, X.; Fu, Y.; Ajayi, O.E.; Guo, D.; Zhang, L.; Wu, Q. Identification of genes underlying phenotypic plasticity of wing size via insulin signaling pathway by network-based analysis in Sogatella furcifera. BMC Genom. 2019, 20, 396. [Google Scholar] [CrossRef]
- Zhuo, J.C.; Lei, C.; Shi, J.K.; Xu, N.; Xue, W.H.; Zhang, M.Q.; Ren, Z.W.; Zhang, H.H.; Zhang, C.X. Tra-2 mediates cross-talk between sex determination and wing polyphenism in female Nilaparvata lugens. Genetics 2017, 207, 1067–1078. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Ye, W.; Yuan, E.; Di, J.; Chen, X.; Xing, Y.; Sun, Y.; Ge, F. Functional evaluation of the insulin/insulin-like growth factor signaling pathway in determination of wing polyphenism in pea aphid. Insect Sci. 2022. Early View. [Google Scholar] [CrossRef]
- Ding, D.; Liu, G.; Hou, L.; Gui, W.; Chen, B.; Kang, L. Genetic variation in PTPN1 contributes to metabolic adaptation to high-altitude hypoxia in Tibetan migratory locusts. Nat. Commun. 2018, 9, 4991. [Google Scholar] [CrossRef]
- Tong, D.; Zhang, L.; Wu, N.; Xie, D.; Fang, G.; Coates, B.S.; Sappington, T.W.; Liu, Y.; Cheng, Y.; Xia, J.; et al. The oriental armyworm genome yields insights into the long-distance migration of noctuid moths. Cell. Rep. 2022, 41, 111843. [Google Scholar] [CrossRef]
- Jin, M.; Liu, B.; Zheng, W.; Liu, C.; Liu, Z.; He, Y.; Li, X.; Wu, C.; Wang, P.; Liu, K.; et al. Chromosome-level genome of black cutworm provides novel insights into polyphagy and seasonal migration in insects. BMC Biol. 2023, 21, 2. [Google Scholar] [CrossRef]
- Lv, W.; Zeng, L.; Zhang, Z.; He, H.; Wang, F.; Xie, X. Effects of juvenile hormone analog and days after emergence on the reproduction of oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae) populations. Insects 2022, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Z.; Li, K.B.; JIang, X.F.; Hu, Y. Regulation of flight capacity and contents of energy substances by methoprene in the moths of oriental armyworm, Mythimna separata. Insect Sci. 2001, 8, 63–72. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, L.; Jiang, X. Starvation influences allatotropin gene expression and juvenile hormone titer in the female adult oriental armyworm, Mythimna separata. Arch. Insect Biochem. Physiol. 2008, 68, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Jones, N.; Borst, D.W.; Rankin, M.A. Increased juvenile hormone levels after long-duration flight in the grasshopper, Melanoplus sanguinipes. J. Insect Physiol. 2004, 50, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A.; Fairbairn, D.J. The evolution and genetics of migration in insects. BioScience 2007, 57, 155–164. [Google Scholar] [CrossRef]
- Xiao, H.J.; Fu, X.W.; Liu, Y.Q.; Wu, K.M. Synchronous vitellogenin expression and sexual maturation during migration are negatively correlated with juvenile hormone levels in Mythimna separata. Sci. Rep. 2016, 6, 33309. [Google Scholar] [CrossRef]
- Santos, C.G.; Humann, F.C.; Hartfelder, K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019, 31, 43–48. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Davey, K.G. Cellular and molecular actions of juvenile hormone. II. roles of juvenile hormone in adult insects. Adv. Insect Physiol. 1996, 26, 1–155. [Google Scholar] [CrossRef]
- Cai, W.Z.; Pang, X.F.; Hua, B.Z.; Liang, G.W.; Song, D.L. General. Entomology; China Agriculture Press: Beijing, China, 2001. [Google Scholar]
- Tufail, M.; Takeda, M. Insect vitellogenin/lipophorin receptors: Molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol. 2009, 55, 87–103. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, N.; Yun, J.; Xu, H.; Yang, J.; Zhou, S. Juvenile hormone promotes paracellular transport of yolk proteins via remodeling zonula adherens at tricellular junctions in the follicular epithelium. PLoS Genet. 2022, 18, e1010292. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.P.; Wen, X.; Li, L.; Zhang, S.; Zhang, C.; Zhou, S. The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. USA 2021, 118, e2106908118. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Guo, W.; Jiang, F.; Kang, L.; Zhou, S. Argonaute 1 is indispensable for juvenile hormone mediated oogenesis in the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 2013, 43, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Z.; Wang, Z.; Deng, S.; Zhou, S. Kruppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect. Biochem. Mol. Biol. 2014, 52, 94–101. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, L.; Li, H.; Zhou, S. Kruppel-homolog 1 exerts anti-metamorphic and vitellogenic functions in insects via phosphorylation-mediated recruitment of specific cofactors. BMC Biol. 2021, 19, 222. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Z.; Song, J.; Jiang, F.; Wang, Z.; Deng, S.; Walker, V.K.; Zhou, S. Juvenile hormone-receptor complex acts on mcm4 and mcm7 to promote polyploidy and vitellogenesis in the migratory locust. PLoS Genet. 2014, 10, e1004702. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, W.; Xie, Y.; Zhou, S. Juvenile hormone activates the transcription of cell-division-cycle 6 (Cdc6) for polyploidy-dependent insect vitellogenesis and oogenesis. J. Biol. Chem. 2016, 291, 5418–5427. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, W.; Yang, L.; He, Q.; Zhou, S. Juvenile hormone promotes locust fat body cell polyploidization and vitellogenesis by activating the transcription of Cdk6 and E2f1. Insect. Biochem. Mol. Biol. 2018, 102, 1–10. [Google Scholar] [CrossRef]
- Wu, Z.; He, Q.; Zeng, B.; Zhou, H.; Zhou, S. Juvenile hormone acts through FoxO to promote Cdc2 and Orc5 transcription for polyploidy-dependent vitellogenesis. Development 2020, 147, dev188813. [Google Scholar] [CrossRef]
- Luo, M.; Li, D.; Wang, Z.; Guo, W.; Kang, L.; Zhou, S. Juvenile hormone differentially regulates two Grp78 genes encoding protein chaperones required for insect fat body cell homeostasis and vitellogenesis. J. Biol. Chem. 2017, 292, 8823–8834. [Google Scholar] [CrossRef]
- Skowronek, P.; Wojcik, L.; Strachecka, A. Fat body-Multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C. Insect Physiology; China Agriculture Press: Beijing, China, 2004. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, H.J.; Duch, C. Dynamic neural control of insect muscle metabolism related to motor behavior. Physiology (Bethesda) 2011, 26, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.W., Jr.; Teng, Y.M.; Deshpande, D.; Rankin, M.A. Mobilization of lipid and carbohydrate reserves in the migratory grasshopper Melanoplus sanguinipes. Physiol. Entomol. 1997, 22, 231–238. [Google Scholar] [CrossRef]
- Sappington, T.W.; Fescemyer, H.W.; Showers, W.B. Lipid and carbohydrate utilization during flight of the migratory moth, Agrotis ipsilon (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 1995, 29, 397–414. [Google Scholar] [CrossRef]
- Brower, L.P.; Fink, L.S.; Walford, P. Fueling the fall migration of the monarch butterfly. Integr. Comp. Biol. 2006, 46, 1123–1142. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, L.; Li, G.; Hu, Y. The relationship between utilization of energy materials and sustained flight in the moths of oriental armyworm, Mythimna separata (Walker). Acta Entomol. Sin. 1995, 38, 290–295. [Google Scholar]
- Li, K.; Gao, X.; Cao, Y.; Luo, L.; Jiang, X. Dynamics of energy reserves and utilization after tethered-flight in the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Acta Phytophy Sin. 2005, 32, 13–17. [Google Scholar] [CrossRef]
- Murata, M.; Tojo, S. Flight capability and fatty acid level in triacylglycerol of long-distance migratory adults of the common cutworm, Spodoptera litura. Zool. Sci. 2004, 21, 181–188. [Google Scholar] [CrossRef]
- Gunn, A.; Gatehouse, A.G. The development of enzymes involved in flight muscle metabolism in Spodoptera exempta and Mythimna separata. Comp. Biochem. Physiol. B. Comp. Biochem. 1988, 91, 315–324. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T.; Van der Horst, D.J.; Van Marrewijk, W.J.A. Insect flight muscle metabolism. Insect Biochem. 1984, 14, 243–260. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T. Carbohydrate and fat as a fuel for insect flight: A comparative study. J. Insect. Physiol. 1969, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.K. Energy metabolism during insect flight- biochemical design and physiological performance. Physiol. Biochem. Zool. 2000, 73, 765–771. [Google Scholar] [CrossRef]
- Brouwers, E.V.M.; de Kort, C.A.D. Amino acid metabolism during flight in the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 1979, 25, 411–414. [Google Scholar] [CrossRef]
- Weeda, E.; Kort, C.A.D.d.; Beenakkers, A.M.T. Fuels for energy metabolism in the Colorado potato beetle, Leptinotarsa decemlineata Say. J. Insect Physiol. 1979, 25, 951–955. [Google Scholar] [CrossRef]
- Suarez, R.K.; Darveau, C.A.; Welch, K.C., Jr.; O’Brien, D.M.; Roubik, D.W.; Hochachka, P.W. Energy metabolism in orchid bee flight muscles: Carbohydrate fuels all. J. Exp. Biol. 2005, 208, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Teulier, L.; Weber, J.M.; Crevier, J.; Darveau, C.A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. Biol. Sci. 2016, 283, 20160333. [Google Scholar] [CrossRef]
- Voss, R.H.; Ferro, D.N. Ecology of migrating Colorado potato beetles (Coleoptera: Chrysomelidae) in western Massachusetts. Environ. Entomol. 1990, 19, 123–129. [Google Scholar] [CrossRef]
- Nation, J.L. Insect Physiology and Biochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Thompson, S.N. Trehalose—The Insect ‘Blood’ Sugar. Adv. Insect Physiol. 2003, 19, 117–122. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Nakahara, Y.; Iwata, K.-i.; Tanaka, D.; Watanabe, M.; Okuda, T. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11585–11590. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.D.; Pan, B.Y.; Qiu, L.Y.; Huang, Z.; Zhou, T.; Ye, L.; Tang, B.; Wang, S.G. The structure characteristics and biologicaI functions on regulating trehalose metabolism of two NITret1s in Nilaparvata lugens. Sci. Agric. Sin. 2020, 53, 4802–4812. [Google Scholar] [CrossRef]
- Luo, Y.J.; Qiu, L.Y.; Liu, Y.K.; Pang, X.Q.; Yao, Q.; Wang, S.G.; Tang, B.; Xu, C.D. Functional analysis of two sugar transporters of the brown planthopper (Nilaparvata lugens) and their effects on regulating trehalose metabolism. J. Environ. Entomol. 2022, 44, 935–945. [Google Scholar] [CrossRef]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wei, P.; Chen, J.; Wang, S.; Zhang, W.Q. Progress in gene features and functions of insect trehalases. Acta Entomol. Sin. 2012, 55, 1315–1321. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Huang, J.H.; Shi, M.; Chen, X.X. Advances in research on the trehalose metabolism in insects. Chin. J. Appl. Entomol. 2018, 55, 145–152. [Google Scholar] [CrossRef]
- Silva, M.C.; Ribeiro, A.F.; Terra, W.R.; Ferreira, C. Sequencing of Spodoptera frugiperda midgut trehalases and demonstration of secretion of soluble trehalase by midgut columnar cells. Insect Mol. Biol. 2009, 18, 769–784. [Google Scholar] [CrossRef]
- Van der Horst, D.J.; Rodenburg, K.W. Locust flight activity as a model for hormonal regulation of lipid mobilization and transport. J. Insect. Physiol. 2010, 56, 844–853. [Google Scholar] [CrossRef]
- Wu, R.; Wu, Z.; Wang, X.; Yang, P.; Yu, D.; Zhao, C.; Xu, G.; Kang, L. Metabolomic analysis reveals that carnitines are key regulatory metabolites in phase transition of the locusts. Proc. Natl. Acad. Sci. USA 2012, 109, 3259–3263. [Google Scholar] [CrossRef]
- Auerswald, L.; Siegert, K.J.; Gade, G. Activation of triacylglycerol lipase in the fat body of a beetle by adipokinetic hormone. Insect Biochem. Mol. Biol. 2005, 35, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, D.J.; Roosendaal, S.D.; Rodenburg, K.W. Circulatory lipid transport: Lipoprotein assembly and function from an evolutionary perspective. Mol. Cell. Biochem. 2009, 326, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kawooya, J.K.; Keim, P.S.; Ryan, R.O.; Shapiro, J.P.; Samaraweera, P.; Law, J.H. Insect apolipophorin III. Purification and properties. J. Biol. Chem. 1984, 259, 10733–10737. [Google Scholar] [CrossRef]
- Prasad, S.V.; Fernando-Warnakulasuriya, G.J.; Sumida, M.; Law, J.H.; Wells, M.A. Lipoprotein biosynthesis in the larvae of the tobacco hornworm, Manduca sexta. J. Biol. Chem. 1986, 261, 17174–17176. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T.; Horst, D.J.V.d.; Marrewijk, W.J.V. Insect lipids and lipoproteins, and their role in physiological processes. Prog. Lipid Res. 1985, 24, 19–67. [Google Scholar] [CrossRef]
- Wheeler, C.H.; Horst, D.J.V.d.; Beenakkers, A.M.T. Lipolytic activity in the flight muscles of Locusta migratoria measured with haemolymph lipoproteins as substrates. Insect. Biochem. 1984, 14, 261–266. [Google Scholar] [CrossRef]
- Heusden, M.C.V.; Horst, D.J.V.d.; Doorn, J.M.V.; Wes, J.; Beenakkers, A.M.T. Lipoprotein lipase activity in the flight muscle of Locusta migratoria and its specificity for haemolymph lipoproteins. Insect Biochem. 1986, 16, 517–523. [Google Scholar] [CrossRef]
- Haunerland, N.H.; Chisholm, J.M. Fatty acid binding protein in flight muscle of the locust, Schistocerca gregaria. Biochim. Biophys. Acta 1990, 1047, 233–238. [Google Scholar] [CrossRef]
- van der Horst, D.J.; van Doorn, J.M.; Passier, P.C.C.M.; Vork, M.M.; Glatz, J.E.C. Role of fatty acid-binding protein in lipid metabolism of insect flight muscle. Mol. Cell. Biochem. 1993, 123, 145–152. [Google Scholar] [CrossRef]
- Veerkamp, J.H.; Peeters, R.A.; Maatman, R.G.H.J. Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim. Biophys. Acta 1991, 1081, 1–24. [Google Scholar] [CrossRef]
- Chen, X.; Haunerland, N.H. Fatty acid binding protein expression in locust flight muscle. Induction by flight, adipokinetic hormone, and low density lipophorin. Insect Biochem. Mol. Biol. 1994, 24, 573–579. [Google Scholar] [CrossRef]
- Rajapakse, S.; Qu, D.; Sayed Ahmed, A.; Rickers-Haunerland, J.; Haunerland, N.H. Effects of FABP knockdown on flight performance of the desert locust, Schistocerca gregaria. J. Exp. Biol. 2019, 222, jeb203455. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Gozalbo, M.E.; Bakker, J.A.; Waterham, H.R.; Wanders, R.J. Carnitine-acylcarnitine translocase deficiency, clinical, biochemical and genetic aspects. Mol. Asp. Med. 2004, 25, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef]
- Haunerland, N.H. Transport and utilization of lipids in insect flight muscles. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1997, 117, 475–482. [Google Scholar] [CrossRef]
- Toprak, U.; Hegedus, D.; Dogan, C.; Guney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 2020, 104, e21682. [Google Scholar] [CrossRef]
- Hou, L.; Guo, S.; Wang, Y.; Nie, X.; Yang, P.; Ding, D.; Li, B.; Kang, L.; Wang, X. Neuropeptide ACP facilitates lipid oxidation and utilization during long-term flight in locusts. eLife 2021, 10, e65279. [Google Scholar] [CrossRef]
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006, 51, 1–24. [Google Scholar] [CrossRef]
- Lange, A.B. Tyramine: From octopamine precursor to neuroactive chemical in insects. Gen. Comp. Endocrinol. 2009, 162, 18–26. [Google Scholar] [CrossRef]
- Roeder, T. Tyramine and octopamine: Ruling behavior and metabolism. Annu. Rev. Entomol. 2005, 50, 447–477. [Google Scholar] [CrossRef]
- Roeder, T. Octopamine in invertebrates. Prog. Neurobiol. 1999, 55, 533–561. [Google Scholar] [CrossRef]
- Wolf, H.; Pearson, K.G. Comparison of motor patterns in the intact and deafferented flight system of the locust.III. Patterns of interneuronal activity. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1989, 165, 61–74. [Google Scholar] [CrossRef]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pfluger, H.J.; Blenau, W.; Broeck, J.V. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef] [PubMed]
- Wegener, G.; Michel, R.; Newsholme, E.A. Fructose 2,6-bisphosphate as a signal for changing from sugar to lipid oxidation during flight in locusts. FEBS Lett. 1986, 201, 129–132. [Google Scholar] [CrossRef]
- Wegener, G. Flying insects: Model systems in exercise physiology. Experientia 1996, 52, 404–412. [Google Scholar] [CrossRef]
- Blau, C.; Wegener, G. Metabolic integration in locust flight: The effect of octopamine on fructose 2,6-bisphosphate content of flight muscle in vivo. J. Comp. Physiol. B 1994, 164, 11–15. [Google Scholar] [CrossRef]
- Orchard, I. A multifunctional role for octopamine in locust flight. Annu. Rev. Entomol. 1993, 38, 227–249. [Google Scholar] [CrossRef]
- Duch, C.; Pflüger, H.-J. DUM neurons in locust flight: A model system for amine-mediated peripheral adjustments to the requirements of a central motor program. J. Comp. Physiol. A 1999, 184, 489–499. [Google Scholar] [CrossRef]
- Goosey, M.W.; Candy, D.J. The release and removal of octopamine by tissues of the locust Schistocerca americana Gregaria. Insect Biochem. 1982, 12, 681–685. [Google Scholar] [CrossRef]
- Mentel, T.; Duch, C.; Stypa, H.; Wegener, G.; Müller, U.; Pflüger, H.-J. Central modulatory neurons control fuel selection in flight muscle of migratory locust. J. Neurosci. 2003, 23, 1109–1113. [Google Scholar] [CrossRef]
- Orchard, I.; Loughton, B.G.; Webb, R.A. Octopamine and short-term hyperlipaemia in the locust. Gen. Comp. Endocrinol. 1981, 45, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Orchard, I.; Lange, A.B. Cyclic AMP in locust fat body: Correlation with octopamine and adipokinetic hormones during flight. J. Insect Physiol. 1984, 30, 901–904. [Google Scholar] [CrossRef]
- Staubli, F.; Jørgensen, T.J.D.; Cazzamali, G.; Williamson, M.; Lenz, C.; Søndergaard, L.; Roepstorff, P.; Grimmelikhuijzen, C.J.P. Molecular identification of the insect adipokinetichormone receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 3446–3451. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Gade, G. Hormonal regulation of energy metabolism in insects as a driving force for performance. Integr. Comp. Biol. 2009, 49, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Van Marrewijk, W.J.A.; Van den Broek, A.T.M.; Van der Horst, D.J. Adipokinetic hormone is dependent on extracellular Ca2+ for its stimulatory action on the glycogenolytic pathway in locust fat body in vitro. Insect Biochem. 1991, 21, 753–761. [Google Scholar] [CrossRef]
- Cheeseman, P.; Goldsworthy, G.J. The release of adipokinetic hormone during flight and starvation in Locusta. Gen. Comp. Endocrinol. 1979, 37, 35–43. [Google Scholar] [CrossRef]
- Lu, K.; Zhang, X.; Chen, X.; Li, Y.; Li, W.; Cheng, Y.; Zhou, J.; You, K.; Zhou, Q. Adipokinetic hormone receptor mediates lipid mobilization to regulate starvation resistance in the brown planthopper, Nilaparvata lugens. Front. Physiol. 2018, 9, 1730. [Google Scholar] [CrossRef]
- Mayer, R.J.; Candy, D.J. Control of haemolymph lipid concentration during locust flight: An adipokinetic hormone from the corpora cardiaca. J. Insect Physiol. 1969, 15, 611–620. [Google Scholar] [CrossRef]
- Stone, J.V.; Mordue, W.; Batley, K.E.; Morris, H.R. Structure of locust adipokinetic hormone, a neurohormone that regulates lipid utilisation during flight. Nature 1976, 263, 207–211. [Google Scholar] [CrossRef]
- Vroemen, S.F.; Horst, D.J.V.d.; Marrewijk, W.J.A.V. New insights into adipokinetic hormone signaling. Mol. Cell. Endocrinol. 1998, 141, 7–12. [Google Scholar] [CrossRef]
- Bogerd, J.; Kooiman, F.P.; Pijnenburg, M.A.; Hekking, L.H.; Oudejans, R.C.; Van der Horst, D.J. Molecular cloning of three distinct cDNAs, each encoding a different adipokinetic hormone precursor, of the migratory locust, Locusta migratoria. Differential expression of the distinct adipokinetic hormone precursor genes during flight activity. J. Biol. Chem. 1995, 270, 23038–23043. [Google Scholar] [CrossRef] [PubMed]
- Candy, D.J. Adipokinetic hormones concentrations in the haemolymph of Schistocerca gregaria, measured by radioimmunoassay. Insect. Biochem. Mol. Biol. 2002, 32, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Flowers, M.T.; Gazard, J.L.; Wells, M.A. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lipid Res. 1999, 40, 556–564. [Google Scholar] [CrossRef]
- Gade, G.; Auerswald, L. Mode of action of neuropeptides from the adipokinetic hormone family. Gen. Comp. Endocrinol. 2003, 132, 10–20. [Google Scholar] [CrossRef]
- Auerswald, L.; Gäde, G. The role of Ins (1, 4, 5) P3 in signal transduction of the metabolic neuropeptide Mem-CC in the cetoniid beetle. Insect Biochem. Mol. Biol. 2002, 32, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Bednarova, A.; Kodrik, D.; Krishnan, N. Adipokinetic hormone exerts its anti-oxidative effects using a conserved signal-transduction mechanism involving both PKC and cAMP by mobilizing extra- and intracellular Ca2+ stores. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 142–149. [Google Scholar] [CrossRef]
- Worm, R.A.A.; Beenakkers, A.M.T. Regulation of substrate utilization in the flight muscle of the locust, Locusta migratoria, during flight. Insect Biochem. 1980, 10, 53–59. [Google Scholar] [CrossRef]
- Orchard, I.; Lange, A.B. Release of identified adipokinetic hormones during flight and following neural stimulation in Locusta migratoria. J. Insect Physiol. 1983, 29, 425–429. [Google Scholar] [CrossRef]
- Ziegler, R.; Eckart, K.; Law, J.H. Adipokinetic hormone controls lipid metabolism in adults and carbohydrate metabolism in larvae of Manduca sexta. Peptides 1990, 11, 1037–1040. [Google Scholar] [CrossRef]
- Kollisch, G.V.; Lorenz, M.W.; Kellner, R.; Verhaert, P.D.; Hoffmann, K.H. Structure elucidation and biological activity of an unusual adipokinetic hormone from corpora cardiaca of the butterfly, Vanessa cardui. Eur. J. Biochem. 2000, 267, 5502–5508. [Google Scholar] [CrossRef]
- Hansen, K.K.; Stafflinger, E.; Schneider, M.; Hauser, F.; Cazzamali, G.; Williamson, M.; Kollmann, M.; Schachtner, J.; Grimmelikhuijzen, C.J. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J. Biol. Chem. 2010, 285, 10736–10747. [Google Scholar] [CrossRef] [PubMed]
- Siegert, K.J. Locust corpora cardiaca contain an inactive adipokinetic hormone. FEBS Lett. 1999, 447, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Zhao, S.Y.; Yang, X.M.; Wang, R.; Cang, X.Z.; Zhang, H.W.; Hu, C.; Wyckhuys, K.A.G.; Wu, K.M. Radar monitoring unveils migration dynamics of the yellow-spined bamboo locust (Orthoptera: Arcypteridae). Comput. Electron. Agric. 2021, 187, 106306. [Google Scholar] [CrossRef]
- Wu, Q.L.; Zeng, J.; Wu, K.M. Research and application of crop pest monitoring and early warning technology in China. Front. Agric. Sci. Eng. 2022, 9, 19–36. [Google Scholar] [CrossRef]
- Jones, C.M.; Papanicolaou, A.; Mironidis, G.K.; Vontas, J.; Yang, Y.; Lim, K.S.; Oakeshott, J.G.; Bass, C.; Chapman, J.W. Genomewide transcriptional signatures of migratory flight activity in a globally invasive insect pest. Mol. Ecol. 2015, 24, 4901–4911. [Google Scholar] [CrossRef]
- Kvist, J.; Mattila, A.L.; Somervuo, P.; Ahola, V.; Koskinen, P.; Paulin, L.; Salmela, L.; Fountain, T.; Rastas, P.; Ruokolainen, A.; et al. Flight-induced changes in gene expression in the Glanville fritillary butterfly. Mol. Ecol. 2015, 24, 4886–4900. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Zhang, W.; Niitepold, K.; Hsu, J.; Haeger, J.F.; Zalucki, M.P.; Altizer, S.; de Roode, J.C.; Reppert, S.M.; Kronforst, M.R. The genetics of monarch butterfly migration and warning colouration. Nature 2014, 514, 317–321. [Google Scholar] [CrossRef]
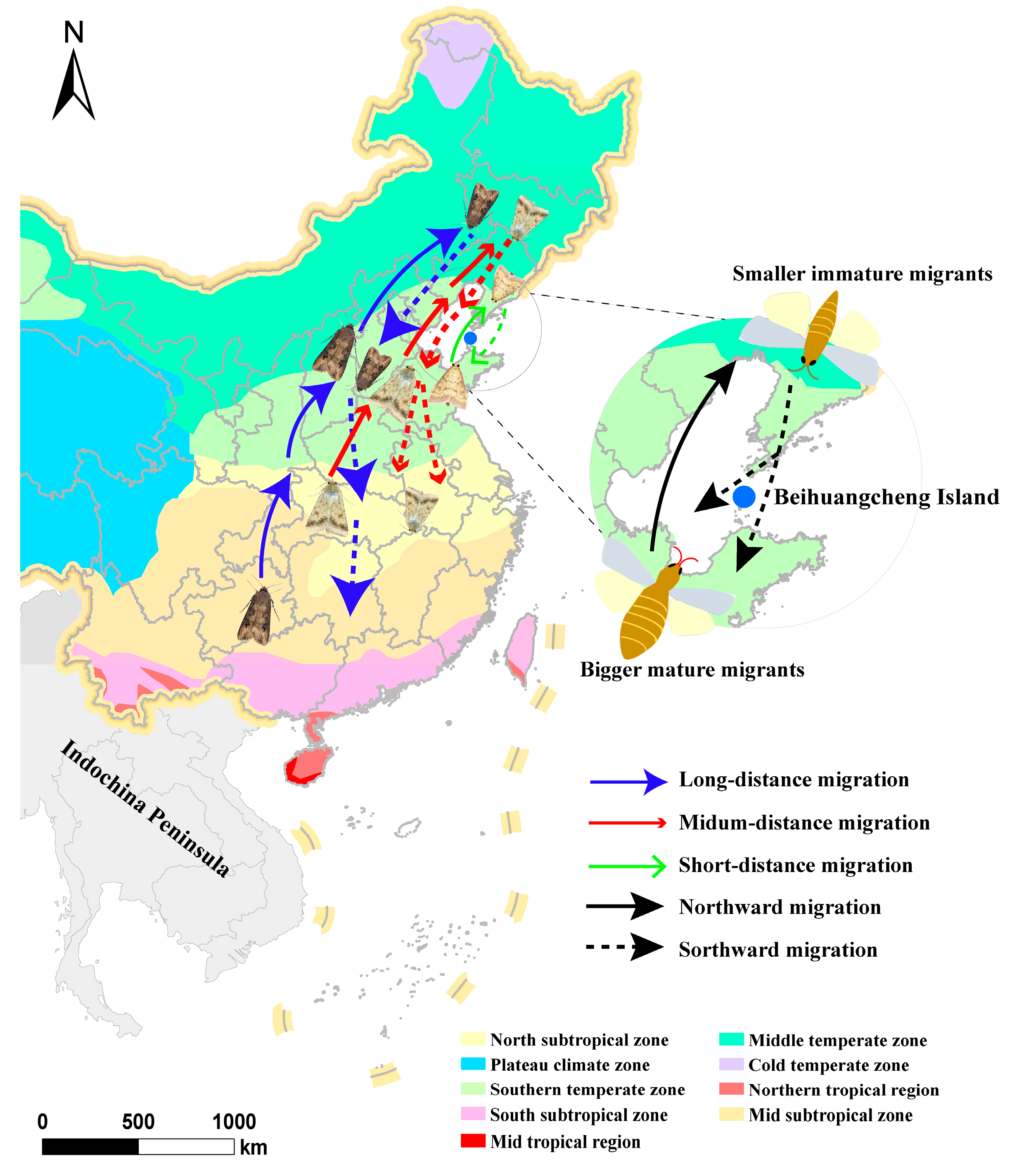
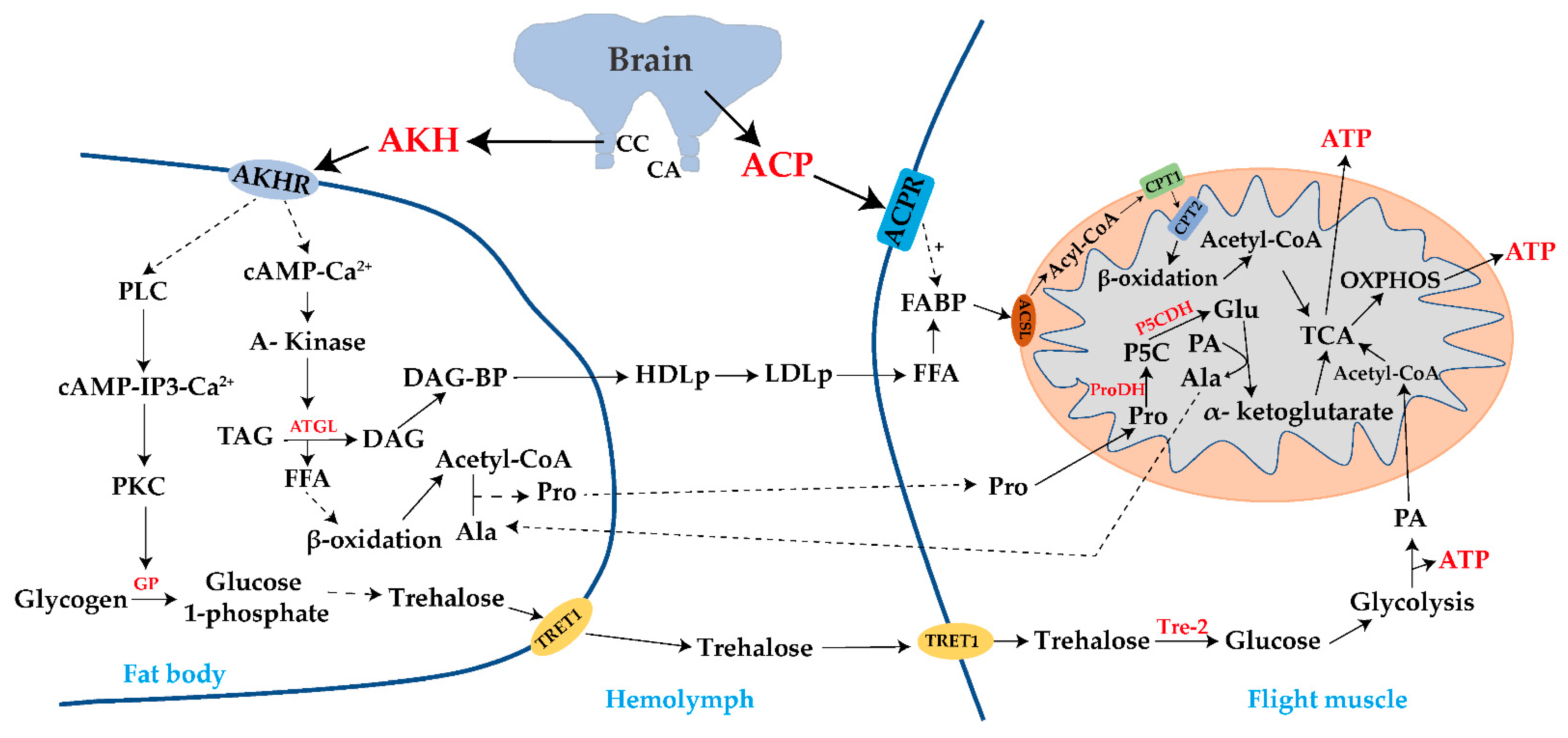
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, Y.; Wu, K. Biological Characteristics and Energy Metabolism of Migrating Insects. Metabolites 2023, 13, 439. https://doi.org/10.3390/metabo13030439
Li X, Zhou Y, Wu K. Biological Characteristics and Energy Metabolism of Migrating Insects. Metabolites. 2023; 13(3):439. https://doi.org/10.3390/metabo13030439
Chicago/Turabian StyleLi, Xiaokang, Yan Zhou, and Kongming Wu. 2023. "Biological Characteristics and Energy Metabolism of Migrating Insects" Metabolites 13, no. 3: 439. https://doi.org/10.3390/metabo13030439
APA StyleLi, X., Zhou, Y., & Wu, K. (2023). Biological Characteristics and Energy Metabolism of Migrating Insects. Metabolites, 13(3), 439. https://doi.org/10.3390/metabo13030439






