Comparison of Local Metabolic Changes in Diabetic Rodent Kidneys Using Mass Spectrometry Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Models
2.3. Biochemical and Histopathological Analysis
2.4. AFADESI−MSI Analysis
2.5. Data Processing and Analysis
2.6. LC-MS/MS Analysis of Kidney Homogenates
2.7. Venn Diagram and Pathway Enrichment Analysis of Discriminating Metabolites
3. Results
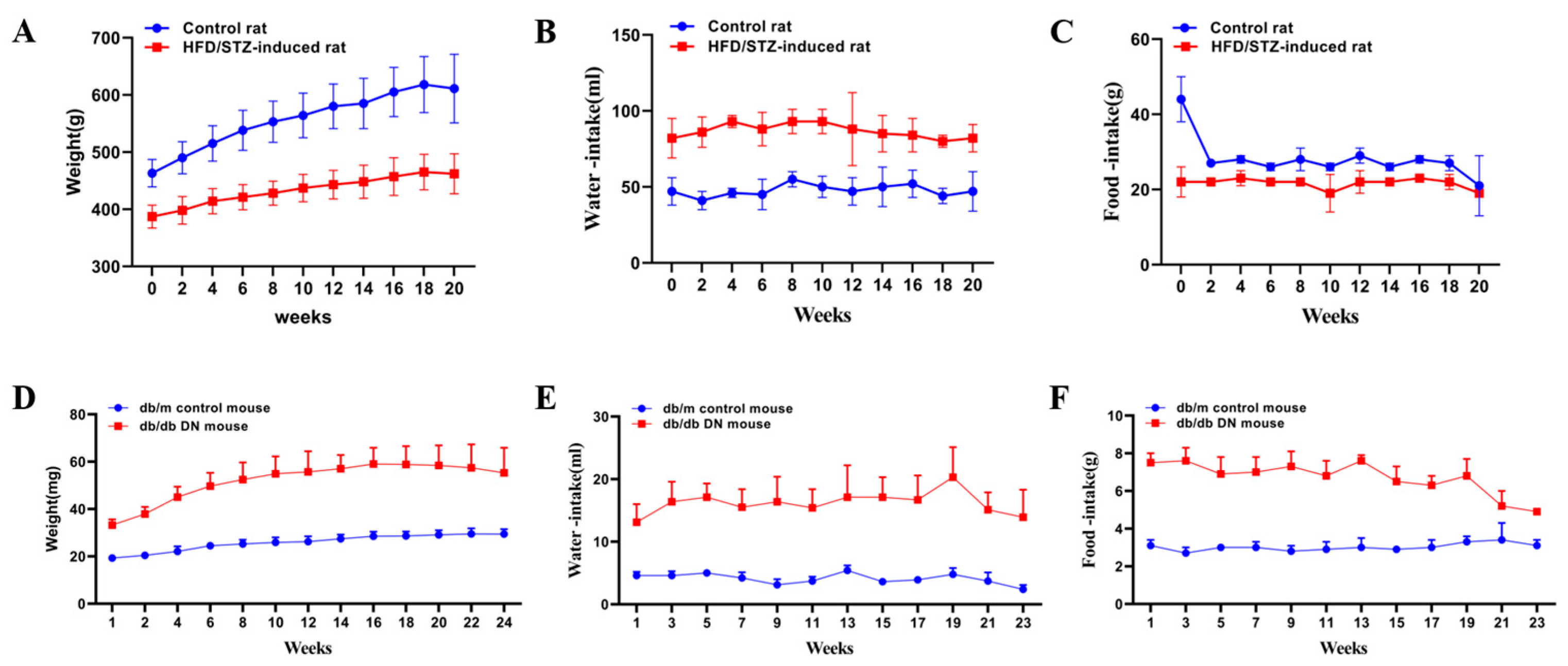
3.1. Assessment of Renal Injury in HFD/STZ-Induced Diabetic Rats and db/db Mice
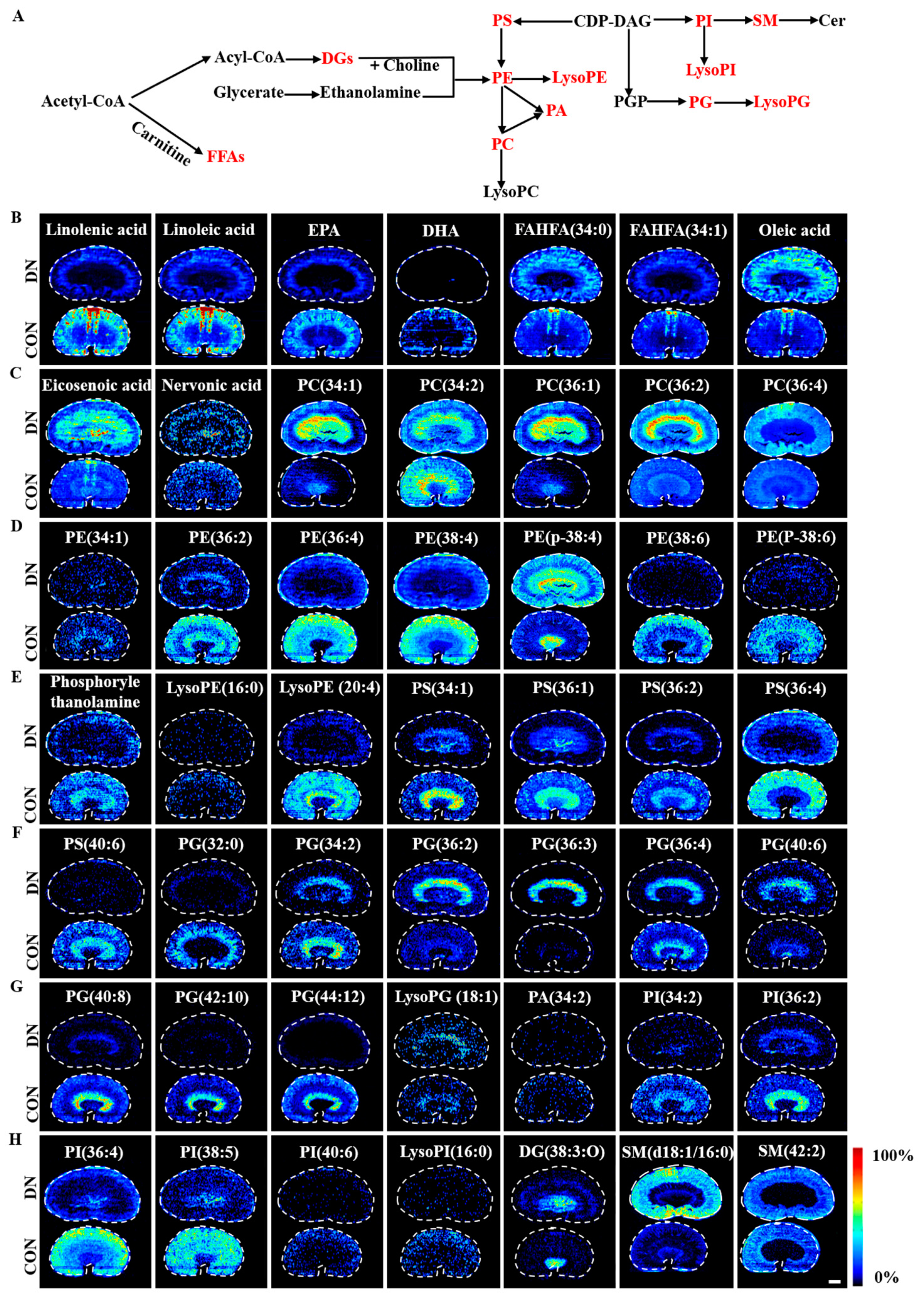
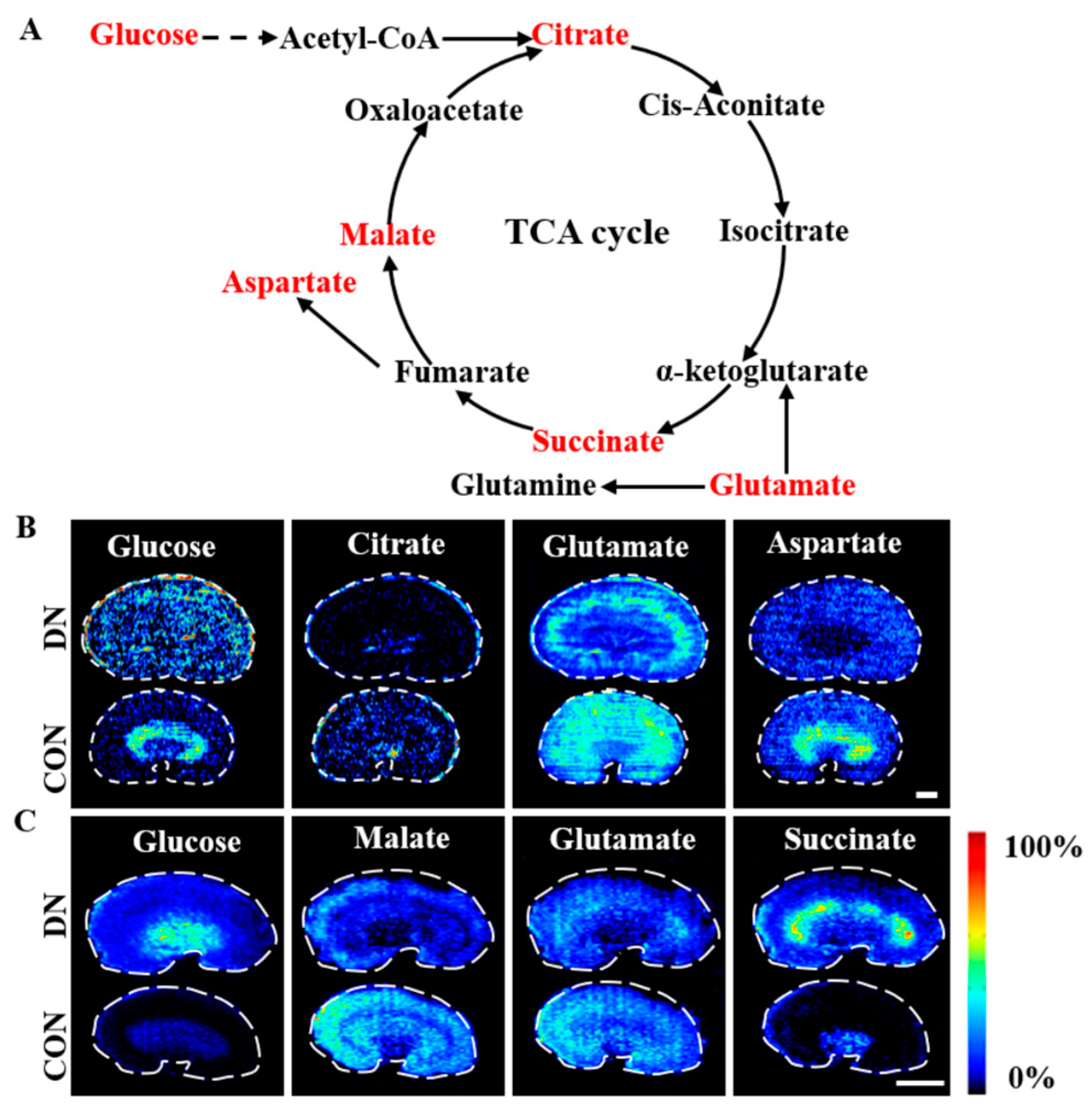
3.2. AFADESI-MSI Analysis of Kidneys of HFD/STZ-Induced DN Rats and db/db DN Mice
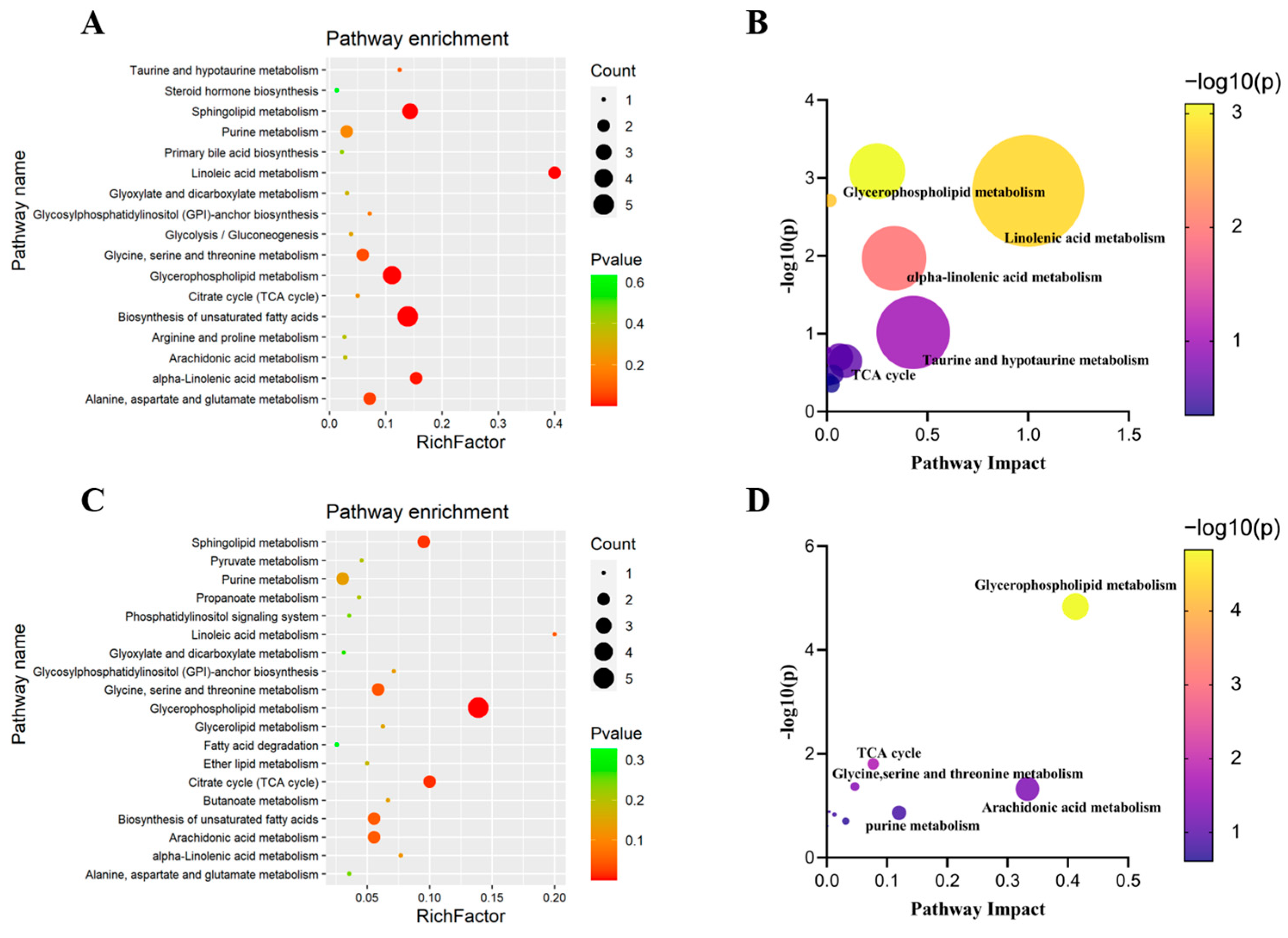
3.3. Pathway Enrichment Analysis
4. Discussion
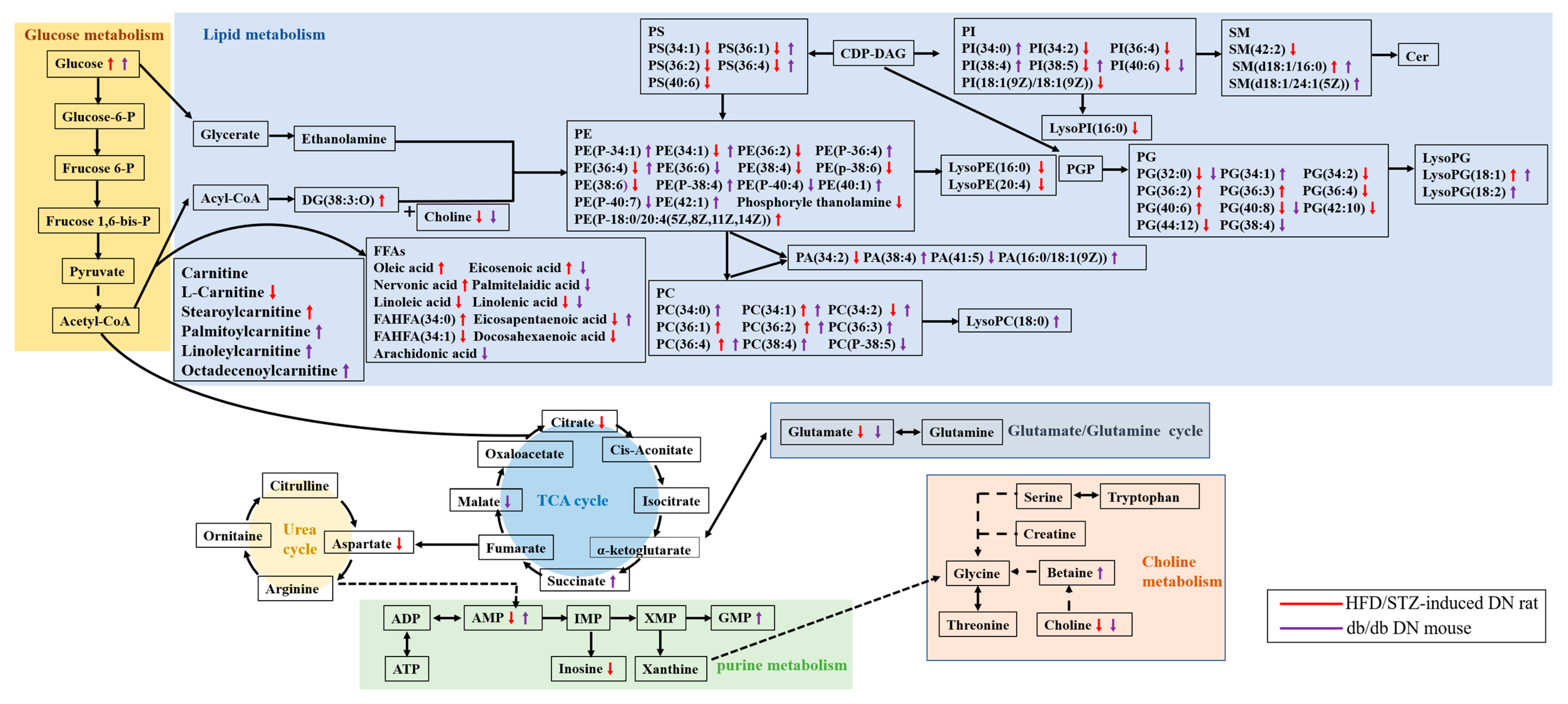
4.1. Alteration of Lipid Metabolism in HFD/STZ-Induced Diabetic Rats and db/db Mice
4.1.1. Fatty Acid Metabolism
4.1.2. Phospholipid Metabolism
4.2. Alteration of Glycolysis and the TCA Cycle in HFD/STZ-Induced Diabetic Rats and db/db Mice
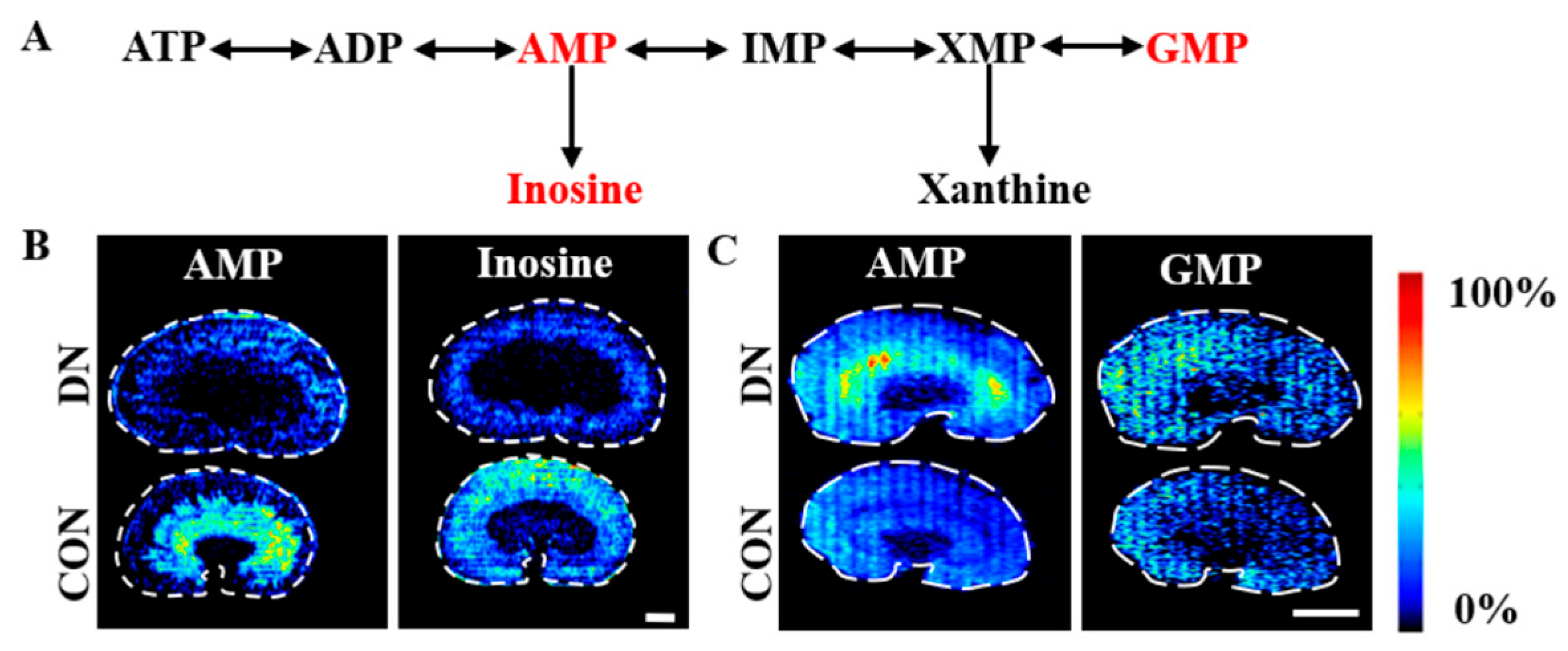
4.3. Alteration of Purine Metabolism in HFD/STZ-Induced Diabetic Rats and db/db Mice
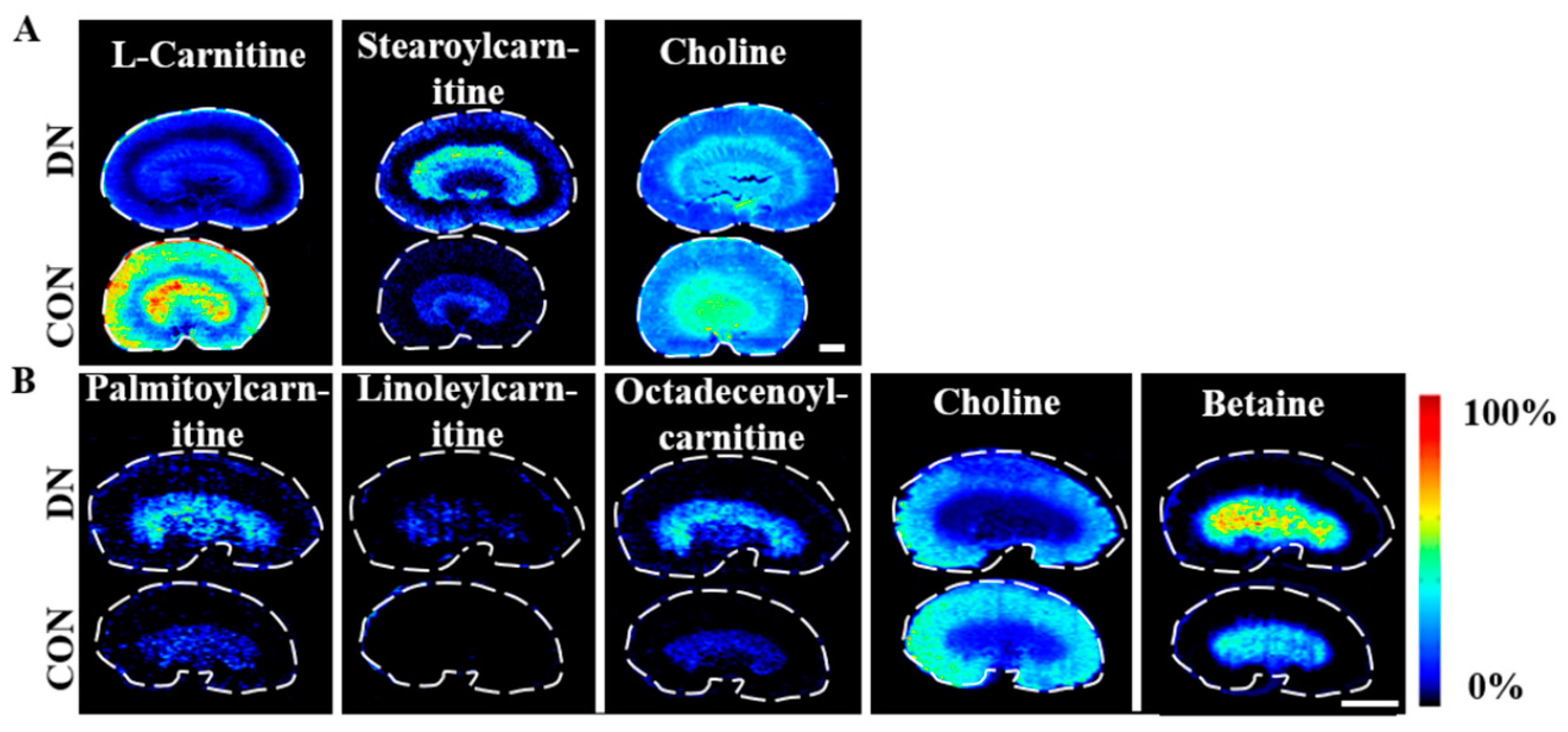
4.4. Alteration of Carnitine Metabolism in HFD/STZ-Induced Diabetic Rats and db/db Mice
4.5. Alteration of Choline Metabolism in HFD/STZ-Induced Diabetic Rats and db/db Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 42. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Danne, T.; Garg, S.; Peters, A.L.; Buse, J.B.; Mathieu, C.; Pettus, J.H.; Alexander, C.M.; Battelino, T.; Ampudia-Blasco, F.J.; Bode, B.W.; et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium–Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care 2019, 42, 1147–1154. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Banu, R.; Chee, M.L.; Lee, R.; Wang, Y.X.; Tan, G.; Jonas, J.B.; Lamoureux, E.L.; Cheng, C.-Y.; Klein, B.E.K.; et al. Incidence and Progression of Diabetic Retinopathy: A Systematic Review. Lancet Diabetes Endocrinol. 2019, 7, 140–149. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of Diabetic Cardiomyopathy and Potential Therapeutic Strategies: Preclinical and Clinical Evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515. [Google Scholar] [CrossRef]
- Umanath, K.; Lewis, J.B. Update on Diabetic Nephropathy: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef]
- Srinivasan, K.; Ramarao, P. Animal Models in Type 2 Diabetes Research: An Overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar]
- Brosius, F.C.; Alpers, C.E.; Bottinger, E.P.; Breyer, M.D.; Coffman, T.M.; Gurley, S.B.; Harris, R.C.; Kakoki, M.; Kretzler, M.; Leiter, E.H.; et al. Mouse Models of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 2503–2512. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Koya, D. Rodent Models of Diabetic Nephropathy: Their Utility and Limitations. Int. J. Nephrol. Renovasc. Dis. 2016, 9, 279–290. [Google Scholar] [CrossRef]
- Noshahr, Z.S.; Salmani, H.; Khajavi Rad, A.; Sahebkar, A. Animal Models of Diabetes-Associated Renal Injury. J. Diabetes Res. 2020, 2020, 9416419. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern Analytical Techniques in Metabolomics Analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef]
- Abbiss, H.; Maker, G.; Trengove, R. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019, 9, 34. [Google Scholar] [CrossRef]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A Metabolomic Comparison of Urinary Changes in Type 2 Diabetes in Mouse, Rat, and Human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef]
- Miyamoto, S.; Hsu, C.-C.; Hamm, G.; Darshi, M.; Diamond-Stanic, M.; Declèves, A.-E.; Slater, L.; Pennathur, S.; Stauber, J.; Dorrestein, P.C.; et al. Mass Spectrometry Imaging Reveals Elevated Glomerular ATP/AMP in Diabetes/Obesity and Identifies Sphingomyelin as a Possible Mediator. EBioMedicine 2016, 7, 121–134. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, W.; Huo, M.; He, B.; Liu, Y.; Tian, L.; Li, W.; Zhou, Z.; Wang, B.; Xia, J.; et al. Spatial-Resolved Metabolomics Reveals Tissue-Specific Metabolic Reprogramming in Diabetic Nephropathy by Using Mass Spectrometry Imaging. Acta Pharm. Sin. B 2021, 11, 3665–3677. [Google Scholar] [CrossRef]
- He, J.; Tang, F.; Luo, Z.; Chen, Y.; Xu, J.; Zhang, R.; Wang, X.; Abliz, Z. Air Flow Assisted Ionization for Remote Sampling of Ambient Mass Spectrometry and Its Application: Air Flow Assisted Ionization for Remote Sampling. Rapid Commun. Mass Spectrom. 2011, 25, 843–850. [Google Scholar] [CrossRef]
- Lv, Y.; Li, T.; Guo, C.; Sun, C.; Tang, F.; Huang, L.; Luo, Z.; Li, X.; Zhang, R.; Zang, Q.; et al. A High-Performance Bio-Tissue Imaging Method Using Air Flow-Assisted Desorption Electrospray Ionization Coupled with a High-Resolution Mass Spectrometer. Chin. Chem. Lett. 2019, 30, 461–464. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, J.-Q.; Huang, W.-Q.; Li, W.; Huang, Y.; Zhang, Z.-J.; Xu, F.-G. Renal Medulla Is More Sensitive to Cisplatin than Cortex Revealed by Untargeted Mass Spectrometry-Based Metabolomics in Rats. Sci. Rep. 2017, 7, 44804. [Google Scholar] [CrossRef]
- Linnan, B.; Yanzhe, W.; Ling, Z.; Yuyuan, L.; Sijia, C.; Xinmiao, X.; Fengqin, L.; Xiaoxia, W. In Situ Metabolomics of Metabolic Reprogramming Involved in a Mouse Model of Type 2 Diabetic Kidney Disease. Front. Physiol. 2021, 12, 779683. [Google Scholar] [CrossRef]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enter. Nutr. 2015, 39 (Suppl. 1), 18S–32S. [Google Scholar] [CrossRef]
- Kim, D.-H.; Yoo, T.-H.; Lee, S.H.; Kang, H.Y.; Nam, B.Y.; Kwak, S.J.; Kim, J.-K.; Park, J.T.; Han, S.H.; Kang, S.-W. Gamma Linolenic Acid Exerts Anti-Inflammatory and Anti-Fibrotic Effects in Diabetic Nephropathy. Yonsei Med. J. 2012, 53, 1165. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Arany, I.; Clark, J.S.; Reed, D.K.; Juncos, L.A.; Dixit, M. Role of P66shc in Renal Toxicity of Oleic Acid. Am. J. Nephrol. 2013, 38, 226–232. [Google Scholar] [CrossRef]
- Miklankova, D.; Markova, I.; Hüttl, M.; Stankova, B.; Malinska, H. The Different Insulin-Sensitising and Anti-Inflammatory Effects of Palmitoleic Acid and Oleic Acid in a Prediabetes Model. J. Diabetes Res. 2022, 2022, 458790. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose Tissue Regulates Insulin Sensitivity: Role of Adipogenesis, de Novo Lipogenesis and Novel Lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Tan, D.; Ertunc, M.E.; Konduri, S.; Zhang, J.; Pinto, A.M.; Chu, Q.; Kahn, B.B.; Siegel, D.; Saghatelian, A. Discovery of FAHFA-Containing Triacylglycerols and Their Metabolic Regulation. J. Am. Chem. Soc. 2019, 141, 8798–8806. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-Inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Et Biophys. Acta BBA Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef]
- Allen, H.G.; Allen, J.C.; Boyd, L.C.; Alston-Mills, B.P.; Fenner, G.P. Determination of Membrane Lipid Differences in Insulin Resistant Diabetes Mellitus Type 2 in Whites and Blacks. Nutrition 2006, 22, 1096–1102. [Google Scholar] [CrossRef]
- Xu, T.; Xu, X.; Zhang, L.; Zhang, K.; Wei, Q.; Zhu, L.; Yu, Y.; Xiao, L.; Lin, L.; Qian, W.; et al. Lipidomics Reveals Serum Specific Lipid Alterations in Diabetic Nephropathy. Front. Endocrinol. 2021, 12, 781417. [Google Scholar] [CrossRef]
- Maciel, E.; da Silva, R.N.; Simões, C.; Melo, T.; Ferreira, R.; Domingues, P.; Domingues, M.R.M. Liquid Chromatography–Tandem Mass Spectrometry of Phosphatidylserine Advanced Glycated End Products. Chem. Phys. Lipids 2013, 174, 1–7. [Google Scholar] [CrossRef]
- Zhu, C.; Liang, Q.; Hu, P.; Wang, Y.; Luo, G. Phospholipidomic Identification of Potential Plasma Biomarkers Associated with Type 2 Diabetes Mellitus and Diabetic Nephropathy. Talanta 2011, 85, 1711–1720. [Google Scholar] [CrossRef]
- Le Barz, M.; Boulet, M.M.; Calzada, C.; Cheillan, D.; Michalski, M.-C. Alterations of Endogenous Sphingolipid Metabolism in Cardiometabolic Diseases: Towards Novel Therapeutic Approaches. Biochimie 2020, 169, 133–143. [Google Scholar] [CrossRef]
- Ussher, J.R.; Koves, T.R.; Cadete, V.J.J.; Zhang, L.; Jaswal, J.S.; Swyrd, S.J.; Lopaschuk, D.G.; Proctor, S.D.; Keung, W.; Muoio, D.M.; et al. Inhibition of De Novo Ceramide Synthesis Reverses Diet-Induced Insulin Resistance and Enhances Whole-Body Oxygen Consumption. Diabetes 2010, 59, 2453–2464. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Mallela, S.K.; Ducasa, G.M.; Yoo, T.H.; Rosenfeld-Gur, E.; Zelnik, I.D.; Molina, J.; Varona Santos, J.; Ge, M.; Sloan, A.; et al. SMPDL3b Modulates Insulin Receptor Signaling in Diabetic Kidney Disease. Nat. Commun. 2019, 10, 2692. [Google Scholar] [CrossRef]
- Zhang, G.; Darshi, M.; Sharma, K. The Warburg Effect in Diabetic Kidney Disease. Semin. Nephrol. 2018, 38, 111–120. [Google Scholar] [CrossRef]
- Vallon, V.; Rose, M.; Gerasimova, M.; Satriano, J.; Platt, K.A.; Koepsell, H.; Cunard, R.; Sharma, K.; Thomson, S.C.; Rieg, T. Knockout of Na-Glucose Transporter SGLT2 Attenuates Hyperglycemia and Glomerular Hyperfiltration but Not Kidney Growth or Injury in Diabetes Mellitus. Am. J. Physiol.-Ren. Physiol. 2013, 304, F156–F167. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial Dysfunction in Diabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef]
- Hallan, S.; Sharma, K. The Role of Mitochondria in Diabetic Kidney Disease. Curr. Diab. Rep. 2016, 16, 61. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Guo, Y.; Ran, Z.; Zhang, Y.; Song, Z.; Wang, L.; Yao, L.; Zhang, M.; Xin, J.; Mao, X. Marein Ameliorates Diabetic Nephropathy by Inhibiting Renal Sodium Glucose Transporter 2 and Activating the AMPK Signaling Pathway in Db/Db Mice and High Glucose–Treated HK-2 Cells. Biomed. Pharmacother. 2020, 131, 110684. [Google Scholar] [CrossRef]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Komlósi, K.; Havasi, V.; Bene, J.; Süle, N.; Pajor, L.; Nicolai, R.; Benatti, P.; Calvani, M.; Melegh, B. Histopathologic Abnormalities of the Lymphoreticular Tissues in Organic Cation Transporter 2 Deficiency: Evidence for Impaired B Cell Maturation. J. Pediatr. 2007, 150, 109–111.e2. [Google Scholar] [CrossRef]
- Schreiber, B. Levocarnitine and Dialysis: A Review. Nutr. Clin. Pract. 2005, 20, 218–243. [Google Scholar] [CrossRef]
- Hoppel, C.L.; Genuth, S.M. Carnitine Metabolism in Normal-Weight and Obese Human Subjects during Fasting. Am. J. Physiol.-Endocrinol. Metab. 1980, 238, E409–E415. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Y.; Yang, S.; Gao, X.; Wang, S.; Wang, Z.; Zhang, C.; Zhou, Z.; Chen, Y.; Wang, Z.; et al. Comparison of Local Metabolic Changes in Diabetic Rodent Kidneys Using Mass Spectrometry Imaging. Metabolites 2023, 13, 324. https://doi.org/10.3390/metabo13030324
Zhang X, Liu Y, Yang S, Gao X, Wang S, Wang Z, Zhang C, Zhou Z, Chen Y, Wang Z, et al. Comparison of Local Metabolic Changes in Diabetic Rodent Kidneys Using Mass Spectrometry Imaging. Metabolites. 2023; 13(3):324. https://doi.org/10.3390/metabo13030324
Chicago/Turabian StyleZhang, Xin, Yanhua Liu, Shu Yang, Xin Gao, Shuo Wang, Zhaoying Wang, Chen Zhang, Zhi Zhou, Yanhua Chen, Zhonghua Wang, and et al. 2023. "Comparison of Local Metabolic Changes in Diabetic Rodent Kidneys Using Mass Spectrometry Imaging" Metabolites 13, no. 3: 324. https://doi.org/10.3390/metabo13030324
APA StyleZhang, X., Liu, Y., Yang, S., Gao, X., Wang, S., Wang, Z., Zhang, C., Zhou, Z., Chen, Y., Wang, Z., & Abliz, Z. (2023). Comparison of Local Metabolic Changes in Diabetic Rodent Kidneys Using Mass Spectrometry Imaging. Metabolites, 13(3), 324. https://doi.org/10.3390/metabo13030324





