Metabolic Features of Increased Gut Permeability, Inflammation, and Altered Energy Metabolism Distinguish Agricultural Workers at Risk for Mesoamerican Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Methods
2.3. Data Analysis
2.4. Statistical Methods
3. Results
3.1. Non-Hypothesis-Based Explorations
3.2. Hypothesis-Based Explorations
3.3. Stability Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, R.J.; Wesseling, C.; Newman, L.S. Chronic Kidney Disease of Unknown Cause in Agricultural Communities. N. Engl. J. Med. 2019, 380, 1843–1852. [Google Scholar] [CrossRef]
- Wijkström, J.; González-Quiroz, M.; Hernandez, M.; Trujillo, Z.; Hultenby, K.; Ring, A.; Söderberg, M.; Aragón, A.; Elinder, C.-G.; Wernerson, A. Renal Morphology, Clinical Findings, and Progression Rate in Mesoamerican Nephropathy. Am. J. Kidney Dis. 2017, 69, 626–636. [Google Scholar] [CrossRef]
- Kupferman, J.; Amador, J.J.; Lynch, K.E.; Laws, R.L.; López-Pilarte, D.; Ramírez-Rubio, O.; Kaufman, J.S.; Lau, J.L.; Weiner, D.E.; Robles, N.V.; et al. Characterization of Mesoamerican Nephropathy in a Kidney Failure Hotspot in Nicaragua. Am. J. Kidney Dis. 2016, 68, 716–725. [Google Scholar] [CrossRef]
- Liu, X.; Locasale, J.W. Metabolomics: A Primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef]
- Abbiss, H.; Maker, G.L.; Trengove, R.D. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019, 9, 34. [Google Scholar] [CrossRef]
- Kalim, S.; Rhee, E.P. An Overview of Renal Metabolomics. Kidney Int. 2017, 91, 61–69. [Google Scholar] [CrossRef]
- Ralto, K.M.; Rhee, E.P.; Parikh, S.M. NAD(+) Homeostasis in Renal Health and Disease. Nat. Rev. Nephrol. 2020, 16, 99–111. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1α Drives NAD Biosynthesis Linking Oxidative Metabolism to Renal Protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef]
- Poyan Mehr, A.; Tran, M.T.; Ralto, K.M.; Leaf, D.E.; Washco, V.; Messmer, J.; Lerner, A.; Kher, A.; Kim, S.H.; Khoury, C.C.; et al. De Novo NAD + Biosynthetic Impairment in Acute Kidney Injury in Humans. Nat. Med. 2018, 24, 1351–1359. [Google Scholar] [CrossRef]
- Bignon, Y.; Rinaldi, A.; Nadour, Z.; Poindessous, V.; Nemazanyy, I.; Lenoir, O.; Fohlen, B.; Weill-Raynal, P.; Hertig, A.; Karras, A.; et al. Cell Stress Response Impairs de Novo NAD+ Biosynthesis in the Kidney. JCI Insight 2022, 7, e153019. [Google Scholar] [CrossRef]
- Katsyuba, E.; Mottis, A.; Zietak, M.; De Franco, F.; van der Velpen, V.; Gariani, K.; Ryu, D.; Cialabrini, L.; Matilainen, O.; Liscio, P.; et al. De Novo NAD+ Synthesis Enhances Mitochondrial Function and Improves Health. Nature 2018, 563, 354–359. [Google Scholar] [CrossRef]
- Fischer, R.S.B.; Vangala, C.; Truong, L.; Mandayam, S.; Chavarria, D.; Llanes, O.M.G.; Laguna, M.U.F.; Baez, A.G.; Garcia, F.; García-Trabanino, R.; et al. Early Detection of Acute Tubulointerstitial Nephritis in the Genesis of Mesoamerican Nephropathy. Kidney Int. 2018, 93, 681–690. [Google Scholar] [CrossRef]
- Sato, Y.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Jensen, T.; Tolan, D.R.; Sanchez-Lozada, L.G.; Newman, L.S.; Butler-Dawson, J.; Sorensen, C.; Glaser, J.; et al. Increase of Core Temperature Affected the Progression of Kidney Injury by Repeated Heat Stress Exposure. Am. J. Physiol. Ren. Physiol. 2019, 317, F1111–F1121. [Google Scholar] [CrossRef]
- Zheng, M.; Cai, J.; Liu, Z.; Shu, S.; Wang, Y.; Tang, C.; Dong, Z. Nicotinamide Reduces Renal Interstitial Fibrosis by Suppressing Tubular Injury and Inflammation. J. Cell Mol. Med. 2019, 23, 3995–4004. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Hu, T.; Wei, R.; Cai, C.; Wang, P.; Wang, L.; Qiao, W.; Feng, L. Endogenous Nampt Upregulation Is Associated with Diabetic Nephropathy Inflammatory-Fibrosis through the NF-ΚB P65 and Sirt1 Pathway; NMN Alleviates Diabetic Nephropathy Inflammatory-Fibrosis by Inhibiting Endogenous Nampt. Exp. Ther. Med. 2017, 14, 4181–4193. [Google Scholar] [CrossRef]
- Hallan, S.; Afkarian, M.; Zelnick, L.R.; Kestenbaum, B.; Sharma, S.; Saito, R.; Darshi, M.; Barding, G.; Raftery, D.; Ju, W.; et al. Metabolomics and Gene Expression Analysis Reveal Down-Regulation of the Citric Acid (TCA) Cycle in Non-Diabetic CKD Patients. EBioMedicine 2017, 26, 68–77. [Google Scholar] [CrossRef]
- Mándi, Y.; Vécsei, L. The Kynurenine System and Immunoregulation. J. Neural Transm. 2012, 119, 197–209. [Google Scholar] [CrossRef]
- Aregger, F.; Uehlinger, D.E.; Fusch, G.; Bahonjic, A.; Pschowski, R.; Walter, M.; Schefold, J.C. Increased Urinary Excretion of Kynurenic Acid Is Associated with Non-Recovery from Acute Kidney Injury in Critically Ill Patients. BMC Nephrol. 2018, 19, 44. [Google Scholar] [CrossRef]
- Raines, N.; González, M.; Wyatt, C.; Kurzrok, M.; Pool, C.; Lemma, T.; Weiss, I.; Marín, C.; Prado, V.; Marcas, E.; et al. Risk Factors for Reduced Glomerular Filtration Rate in a Nicaraguan Community Affected by Mesoamerican Nephropathy. MEDICC Rev. 2014, 16, 16–22. [Google Scholar]
- Hansson, E.; Glaser, J.; Weiss, I.; Ekström, U.; Apelqvist, J.; Hogstedt, C.; Peraza, S.; Lucas, R.; Jakobsson, K.; Wesseling, C.; et al. Workload and Cross-Harvest Kidney Injury in a Nicaraguan Sugarcane Worker Cohort. Occup. Environ. Med. 2019, 76, 818–826. [Google Scholar] [CrossRef]
- Laws, R.L.; Brooks, D.R.; Amador, J.J.; Weiner, D.E.; Kaufman, J.S.; Ramírez-Rubio, O.; Riefkohl, A.; Scammell, M.K.; López-Pilarte, D.; Sánchez, J.M.; et al. Changes in Kidney Function among Nicaraguan Sugarcane Workers. Int. J. Occup. Environ. Health 2015, 21, 241–250. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. Chronic Kidney Disease Epidemiology Collaboration. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Raines, N.H.; Inker, L.A.; Seegmiller, J.C.; Brooks, D.R.; Gonzalez-Quiroz, M.; Friedman, D.J. Estimated Versus Measured Glomerular Filtration Rate in Men at Risk for Mesoamerican Nephropathy. Am. J. Kidney Dis. 2022, 81, P370–P373. [Google Scholar] [CrossRef]
- O’Donnell, J.K.; Tobey, M.; Weiner, D.E.; Stevens, L.A.; Johnson, S.; Stringham, P.; Cohen, B.; Brooks, D.R. Prevalence of and Risk Factors for Chronic Kidney Disease in Rural Nicaragua. Nephrol. Dial. Transplant. 2011, 26, 2798–2805. [Google Scholar] [CrossRef]
- Gallo-Ruiz, L.; Sennett, C.M.; Sánchez-Delgado, M.; García-Urbina, A.; Gámez-Altamirano, T.; Basra, K.; Laws, R.L.; Amador, J.J.; Lopez-Pilarte, D.; Tripodis, Y.; et al. Prevalence and Risk Factors for CKD Among Brickmaking Workers in La Paz Centro, Nicaragua. Am. J. Kidney Dis. 2019, 74, 239–247. [Google Scholar] [CrossRef]
- Yih, W.K.; Kulldorff, M.; Friedman, D.J.; Leibler, J.H.; Amador, J.J.; López-Pilarte, D.; Galloway, R.L.; Ramírez-Rubio, O.; Riefkohl, A.; Brooks, D.R. Investigating Possible Infectious Causes of Chronic Kidney Disease of Unknown Etiology in a Nicaraguan Mining Community. Am. J. Trop. Med. Hyg. 2019, 101, 676–683. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Jacob, R.A.; Swendseid, M.E.; McKee, R.W.; Fu, C.S.; Clemens, R.A. Biochemical Markers for Assessment of Niacin Status in Young Men: Urinary and Blood Levels of Niacin Metabolites. J. Nutr. 1989, 119, 591–598. [Google Scholar] [CrossRef]
- Reinhold, D.; Pielke-Lombardo, H.; Jacobson, S.; Ghosh, D.; Kechris, K. Pre-Analytic Considerations for Mass Spectrometry-Based Untargeted Metabolomics Data. Methods Mol. Biol. 2019, 1978, 323–340. [Google Scholar] [CrossRef]
- Contreras-Jodar, A.; Nayan, N.H.; Hamzaoui, S.; Caja, G.; Salama, A.A.K. Heat Stress Modifies the Lactational Performances and the Urinary Metabolomic Profile Related to Gastrointestinal Microbiota of Dairy Goats. PLoS ONE 2019, 14, e0202457. [Google Scholar] [CrossRef]
- Hansson, E.; Glaser, J.; Jakobsson, K.; Weiss, I.; Wesseling, C.; Lucas, R.A.I.; Wei, J.L.K.; Ekström, U.; Wijkström, J.; Bodin, T.; et al. Pathophysiological Mechanisms by Which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers. Nutrients 2020, 12, E1639. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s Metabolites in Exercise, Inflammation, and Mental Health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Strasser, B.; Geiger, D.; Schauer, M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Effects of Exhaustive Aerobic Exercise on Tryptophan-Kynurenine Metabolism in Trained Athletes. PLoS ONE 2016, 11, e0153617. [Google Scholar] [CrossRef]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a Marker for Immune System Activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef]
- Kumar, R.; Adiga, A.; Novack, J.; Etinger, A.; Chinitz, L.; Slater, J.; de Loor, H.; Meijers, B.; Holzman, R.S.; Lowenstein, J. The Renal Transport of Hippurate and Protein-bound Solutes. Physiol. Rep. 2020, 8, e14349. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Vafai, S.B.; Delaney, N.F.; Clish, C.B.; Deik, A.A.; Pierce, K.A.; Ludwig, D.S.; Mootha, V.K. Effects of Sodium Benzoate, a Widely Used Food Preservative, on Glucose Homeostasis and Metabolic Profiles in Humans. Mol. Genet. Metab. 2015, 114, 73–79. [Google Scholar] [CrossRef]
- Gräber, T.; Kluge, H.; Hirche, F.; Broz, J.; Stangl, G.I. Effects of Dietary Benzoic Acid and Sodium-Benzoate on Performance, Nitrogen and Mineral Balance and Hippuric Acid Excretion of Piglets. Arch. Anim. Nutr. 2012, 66, 227–236. [Google Scholar] [CrossRef]
- Audrito, V.; Messana, V.G.; Brandimarte, L.; Deaglio, S. The Extracellular NADome Modulates Immune Responses. Front. Immunol. 2021, 12, 704779. [Google Scholar] [CrossRef]
- Clark, A.J.; Parikh, S.M. Mitochondrial Metabolism in Acute Kidney Injury. Semin. Nephrol. 2020, 40, 101–113. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef]
- Lee, H.W.; Handlogten, M.E.; Osis, G.; Clapp, W.L.; Wakefield, D.N.; Verlander, J.W.; Weiner, I.D. Expression of Sodium-Dependent Dicarboxylate Transporter 1 (NaDC1/SLC13A2) in Normal and Neoplastic Human Kidney. Am. J. Physiol. Ren. Physiol. 2017, 312, F427–F435. [Google Scholar] [CrossRef]
- Lever, M.; Sizeland, P.C.; Bason, L.M.; Hayman, C.M.; Chambers, S.T. Glycine Betaine and Proline Betaine in Human Blood and Urine. Biochim. Biophys. Acta 1994, 1200, 259–264. [Google Scholar] [CrossRef]
- Khamis, M.M.; Holt, T.; Awad, H.; El-Aneed, A.; Adamko, D.J. Comparative Analysis of Creatinine and Osmolality as Urine Normalization Strategies in Targeted Metabolomics for the Differential Diagnosis of Asthma and COPD. Metabolomics 2018, 14, 115. [Google Scholar] [CrossRef]
- Refsum, H.E.; Strömme, S.B. Urea and Creatinine Production and Excretion in Urine during and after Prolonged Heavy Exercise. Scand. J. Clin. Lab. Investig. 1974, 33, 247–254. [Google Scholar] [CrossRef]
- Laparre, J.; Kaabia, Z.; Mooney, M.; Buckley, T.; Sherry, M.; Le Bizec, B.; Dervilly-Pinel, G. Impact of Storage Conditions on the Urinary Metabolomics Fingerprint. Anal. Chim. Acta 2017, 951, 99–107. [Google Scholar] [CrossRef]
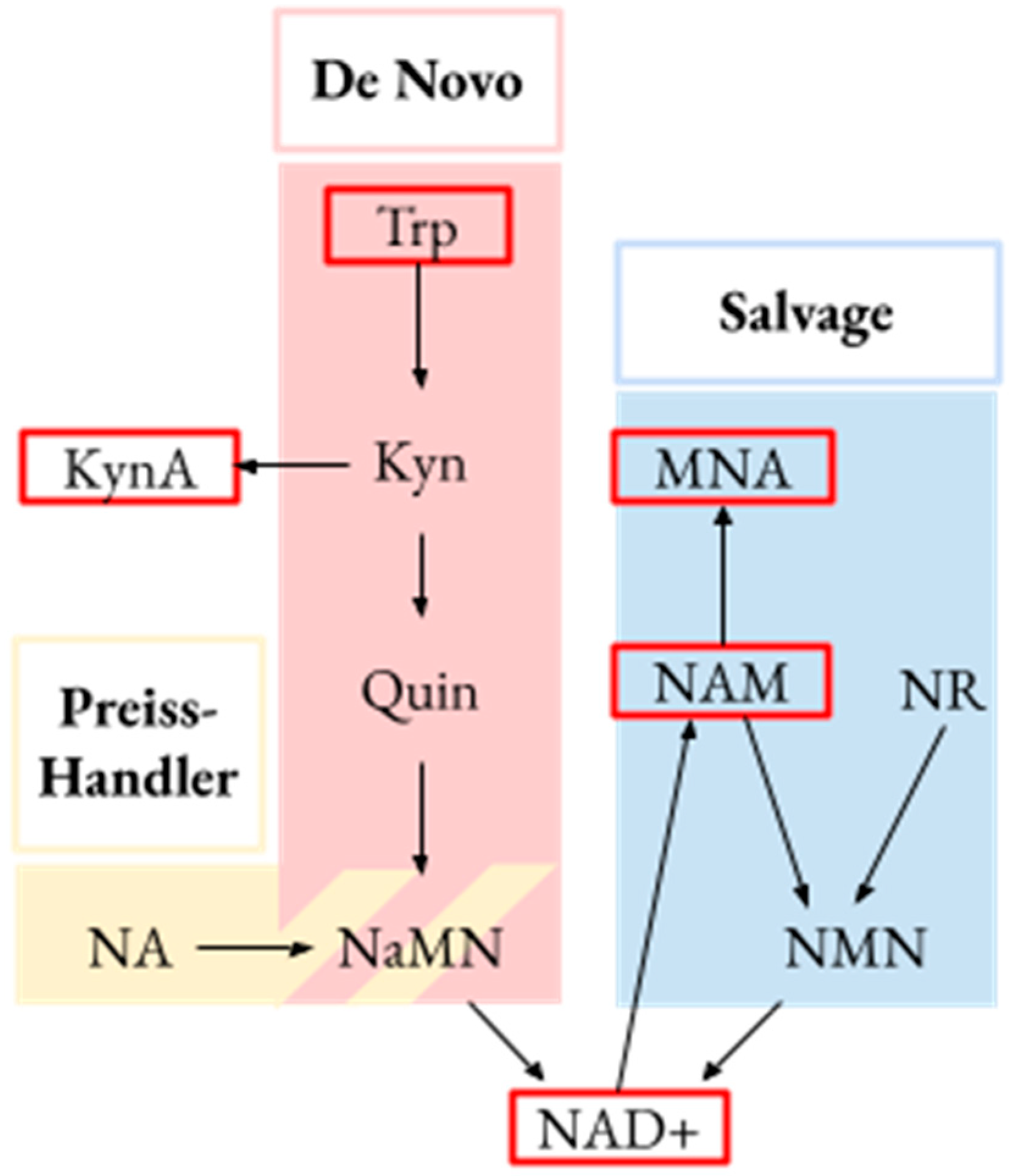
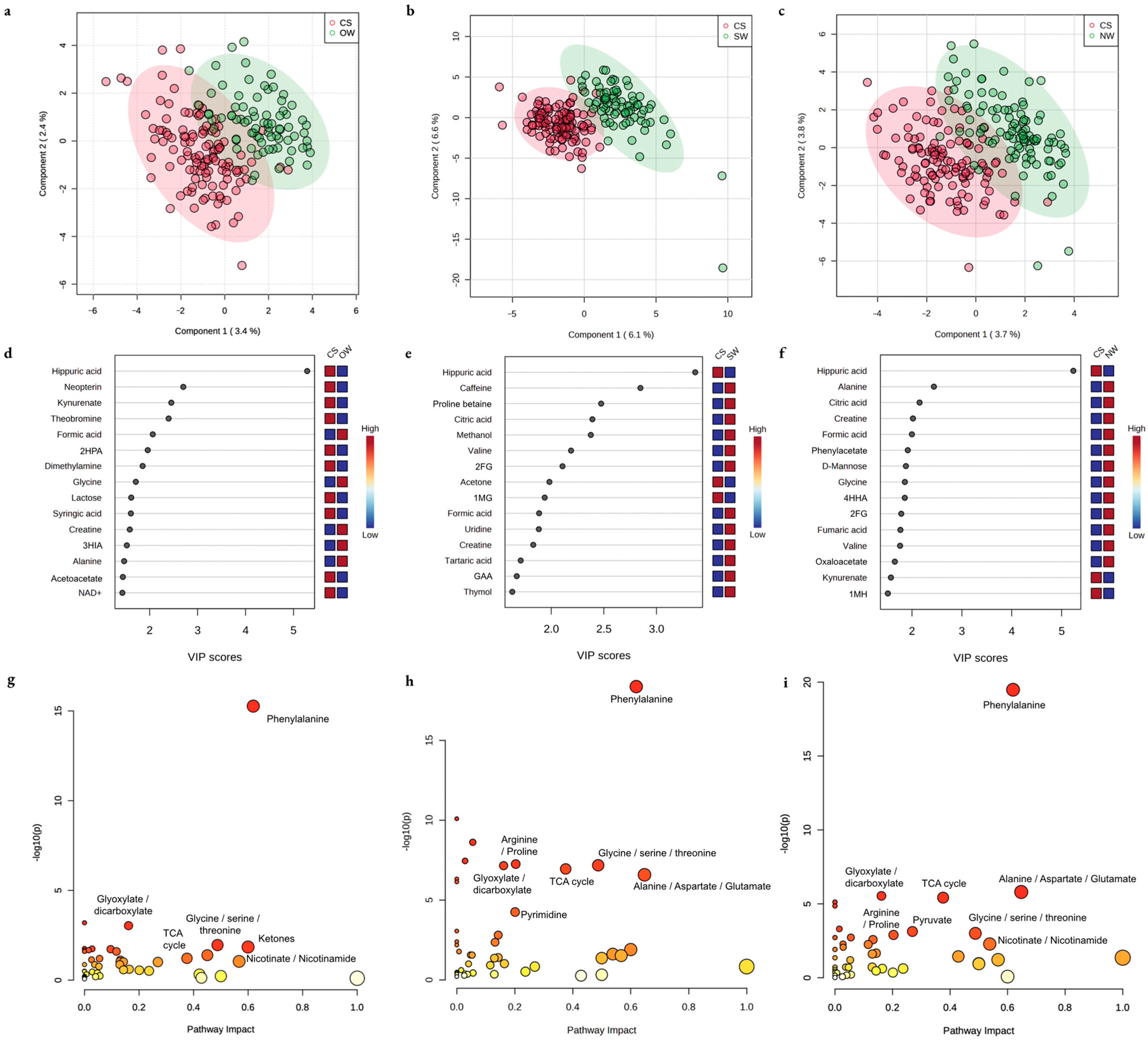

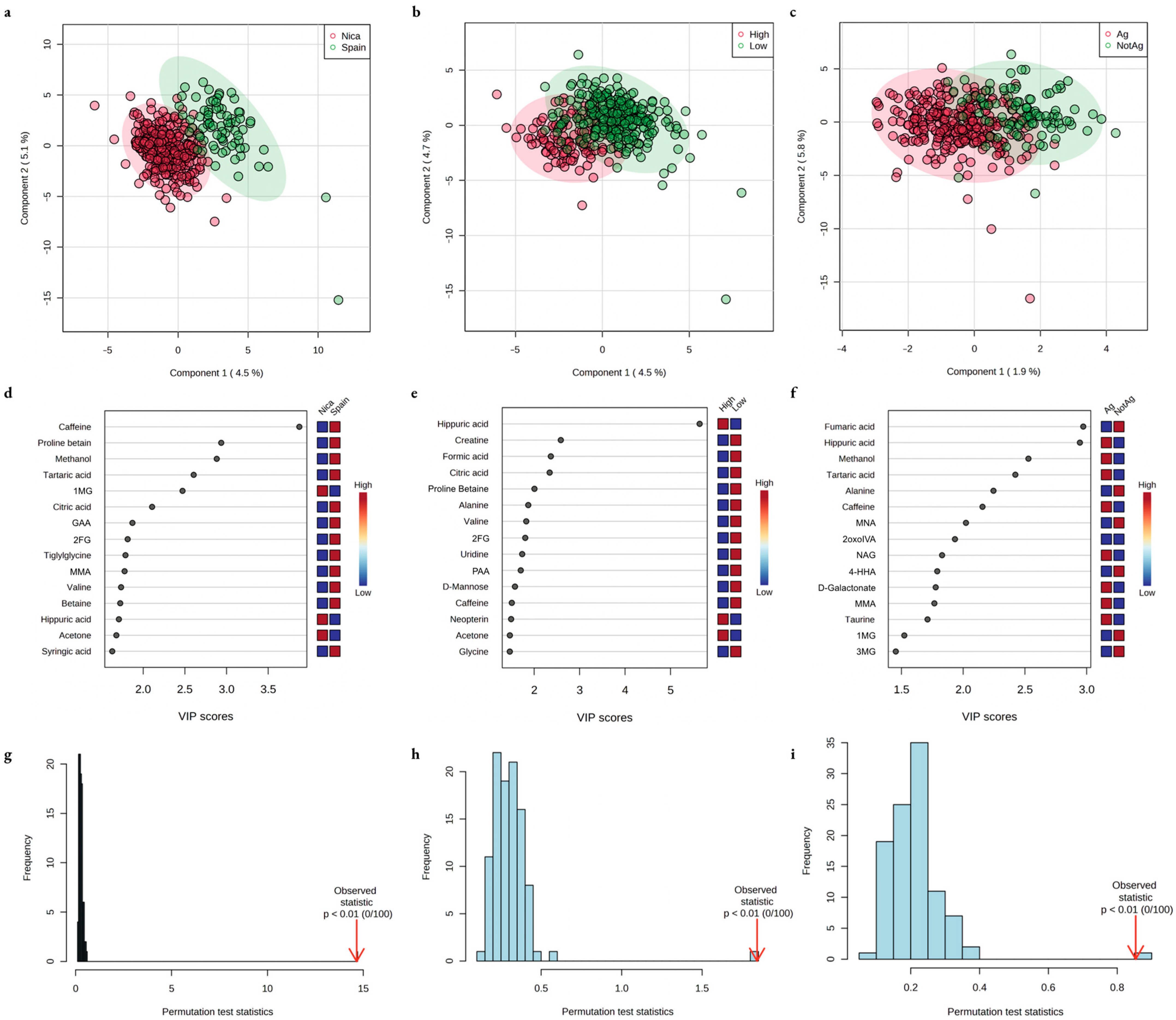

| Group | Residence in a High-Risk Region | Highest-Intensity Manual Labor | Agricultural Environment |
|---|---|---|---|
| Cane Harvest and Seed Cutters | + | + | + |
| Other Cane Workers | + | − | + |
| Spain Agricultural Workers | − | − | + |
| Non-Agricultural Workers | + | − | − |
| Variable | Cane Harvest and Seed Cutters n = 117 | Other Cane Workers n = 78 | Spain Agricultural Workers n = 78 | Non-Agricultural Workers n = 102 | p-Value |
|---|---|---|---|---|---|
| Age in years, mean (SD) | 35 (8) | 36 (9) | 42 (11) * | 48 (9) * | <0.0001 |
| Weight in kg, mean (SD) | 67 (11) | 75 (11) * | 80 (13) * | 79 (16) * | <0.0001 |
| Height in cm, mean (SD) | 168 (9) | 169 (7) | 172 (7) * | 167 (7) + | 0.0005 |
| BMI in kg/m2, mean (SD) | 25 (4) | 26 (4) | 27 (4) * | 25 (4) + | 0.006 |
| Serum creatinine in mg/dL, mean (SD) | 0.9 (0.1) | 0.8 (0.1) | 1.0 (0.1) * | 0.8 (0.1) | <0.0001 |
| eGFR, mL/min/1.73 m2, mean (SD) | 112 (12) | 114 (11) | 95 (13) * | 106 (12) * | <0.0001 |
| Urine creatinine in mmol/L, mean (SD) | 132 (86) | 126 (67) | 157 (67) | 140 (73) | 0.049 |
| Urine dipstick specific gravity, median (IQR) | 1.015 (1.010 to 1.020) | 1.015 (1.010 to 1.020) | 1.030 (1.020 to 1.030) * | 1.020 (1.015 to 1.020) + | <0.0001 |
| Urine dipstick protein, n (%) | |||||
| 0–30 mg/dL | 117 (100) | 78 (100) | 68 (87) | 100 (98) | <0.0001 |
| >30–<300 mg/dL | 0 (0) | 0 (0) | 5 (6) | 2 (2) | |
| ≥300 mg/dL | 0 (0) | 0 (0) | 5 (6) | 0 (0) | |
| Urine dipstick leukocyte esterase > trace, n (%) | 12 (10) | 4 (5) | 5 (6) | 1 (1) | 0.02 |
| Hypertension, n (%) | 8 (7) | 2 (3) | 16 (21) | 10 (10) | 0.002 |
| Diabetes, n (%) | 0 (0) | 0 (0) | 3 (4) | 0 (0) | 0.02 |
| Current use of ACEi or ARB, n (%) | 0 (0) | 0 (0) | 5 (6) | 0 (0) | 0.0007 |
| NSAID use category, n (%) | |||||
| <1×/month | 76 (65) | 51 (65) | 44 (56) | 70 (69) | <0.0001 |
| 1×/month to 1×/week | 21 (18) | 12 (15) | 30 (38) | 25 (25) | |
| >1×/week | 20 (17) | 15 (19) | 4 (5) | 7 (7) | |
| Tobacco use category, n (%) | |||||
| Current smoker | 51 (44) | 23 (29) | 27 (35) | 38 (37) | 0.54 |
| Former smoker | 21 (18) | 20 (26) | 19 (24) | 25 (25) | |
| Never smoker | 45 (38) | 35 (45) | 32 (41) | 39 (38) | |
| Alcohol > 1×/month, n (%) | 52 (47) | 26 (34) | 36 (46) | 15 (68) + | 0.04 |
| Years working in occupational category, mean (SD) | 12 (5) | 15 (6) | 14 (11) | 14 (11) | 0.11 |
| Cane Harvest and Seed Cutters (n = 117) | Other Cane Workers (n = 78) | Spain Agricultural Workers (n = 78) | Non-Agriculture (n = 102) | p | |
|---|---|---|---|---|---|
| NAD+, mmol/mmol Cr | |||||
| median | 0.0156 | 0.0126 ** | 0.0122 ** | 0.0146 | 0.0004 |
| IQR | 0.0120, 0.0197 | 0.0094, 0.0164 | 0.0093, 0.0181 | 0.0124, 0.0185 | |
| Tryptophan, mmol/mmol Cr | |||||
| median | 0.0056 | 0.0055 | 0.0061 | 0.0068 ** | 0.01 |
| IQR | 0.0038, 0.0072 | 0.0042, 0.0075 | 0.0043, 0.0094 | 0.0043, 0.0093 | |
| Kynurenic acid, mmol/mmol Cr | |||||
| median | 0.0200 | 0.0160 ** | 0.0184 | 0.0188 * | <0.0001 |
| IQR | 0.0166, 0.0249 | 0.0140, 0.0189 | 0.0160, 0.0255 | 0.0152, 0.0217 | |
| Nicotinamide, mmol/mmol Cr | |||||
| median | 0.0141 | 0.0130 | 0.0139 | 0.0126 | 0.22 |
| IQR | 0.0111, 0.0181 | 0.0112, 0.0182 | 0.0104, 0.0260 | 0.0101, 0.0167 | |
| Methylnicotinamide, mmol/mmol Cr | |||||
| median | 0.0040 | 0.0039 | 0.0035 | 0.0048 * | 0.0004 |
| IQR | 0.0030, 0.0055 | 0.0031, 0.0057 | 0.0027, 0.0047 | 0.0034, 0.0069 | |
| DE NOVO PATHWAY | |||||
| NAD+ to Tryptophan Ratio, mmol/mmol | |||||
| median | 2.92 | 2.32 * | 2.12 * | 2.06 ** | 0.006 |
| IQR | 1.99, 5.23 | 1.53, 4.24 | 1.32, 4.89 | 1.43, 4.49 | |
| Kynurenic Acid to Tryptophan Ratio, mmol/mmol | |||||
| median | 3.48 | 2.89 * | 3.06 | 2.66 ** | 0.001 |
| IQR | 2.61, 6.34 | 2.12, 3.91 | 2.15, 4.88 | 1.95, 4.19 | |
| NAD+ to Kynurenic Acid Ratio, mmol/mmol | |||||
| median | 0.727 | 0.698 | 0.594 | 0.758 | 0.01 |
| IQR | 0.542, 1.057 | 0.561, 0.896 | 0.462, 1.138 | 0.610, 0.990 | |
| SALVAGE PATHWAY | |||||
| NAD+ to Nicotinamide Ratio, mmol/mmol | |||||
| median | 1.022 | 0.939 | 0.924 | 1.193 | 0.07 |
| IQR | 0.765, 1.597 | 0.684, 1.260 | 0.351, 1.796 | 0.773, 1.627 | |
| Methylnicotinamide to Nicotinamide Ratio, mmol/mmol | |||||
| median | 0.304 | 0.301 | 0.236 | 0.374 * | <0.0001 |
| IQR | 0.204, 0.407 | 0.193, 0.500 | 0.110, 0.346 | 0.248, 0.575 | |
| NAD+ to Methylnicotinamide Ratio, mmol/mmol | |||||
| median | 3.68 | 2.72 ** | 3.52 | 3.01 | 0.007 |
| IQR | 2.72, 5.89 | 2.17, 3.93 | 2.47, 5.31 | 2.07, 4.90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raines, N.H.; Leone, D.A.; O’Callaghan-Gordo, C.; Ramirez-Rubio, O.; Amador, J.J.; Lopez Pilarte, D.; Delgado, I.S.; Leibler, J.H.; Embade, N.; Gil-Redondo, R.; et al. Metabolic Features of Increased Gut Permeability, Inflammation, and Altered Energy Metabolism Distinguish Agricultural Workers at Risk for Mesoamerican Nephropathy. Metabolites 2023, 13, 325. https://doi.org/10.3390/metabo13030325
Raines NH, Leone DA, O’Callaghan-Gordo C, Ramirez-Rubio O, Amador JJ, Lopez Pilarte D, Delgado IS, Leibler JH, Embade N, Gil-Redondo R, et al. Metabolic Features of Increased Gut Permeability, Inflammation, and Altered Energy Metabolism Distinguish Agricultural Workers at Risk for Mesoamerican Nephropathy. Metabolites. 2023; 13(3):325. https://doi.org/10.3390/metabo13030325
Chicago/Turabian StyleRaines, Nathan H., Dominick A. Leone, Cristina O’Callaghan-Gordo, Oriana Ramirez-Rubio, Juan José Amador, Damaris Lopez Pilarte, Iris S. Delgado, Jessica H. Leibler, Nieves Embade, Rubén Gil-Redondo, and et al. 2023. "Metabolic Features of Increased Gut Permeability, Inflammation, and Altered Energy Metabolism Distinguish Agricultural Workers at Risk for Mesoamerican Nephropathy" Metabolites 13, no. 3: 325. https://doi.org/10.3390/metabo13030325
APA StyleRaines, N. H., Leone, D. A., O’Callaghan-Gordo, C., Ramirez-Rubio, O., Amador, J. J., Lopez Pilarte, D., Delgado, I. S., Leibler, J. H., Embade, N., Gil-Redondo, R., Bruzzone, C., Bizkarguenaga, M., Scammell, M. K., Parikh, S. M., Millet, O., Brooks, D. R., & Friedman, D. J. (2023). Metabolic Features of Increased Gut Permeability, Inflammation, and Altered Energy Metabolism Distinguish Agricultural Workers at Risk for Mesoamerican Nephropathy. Metabolites, 13(3), 325. https://doi.org/10.3390/metabo13030325







