Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways
Abstract
1. Introduction
2. Study Design
3. Angiogenesis: Cellular Signaling Pathways
4. Targeting Tumor Cells with Anti-Angiogenic Agents: Recent Advances
5. Saponins
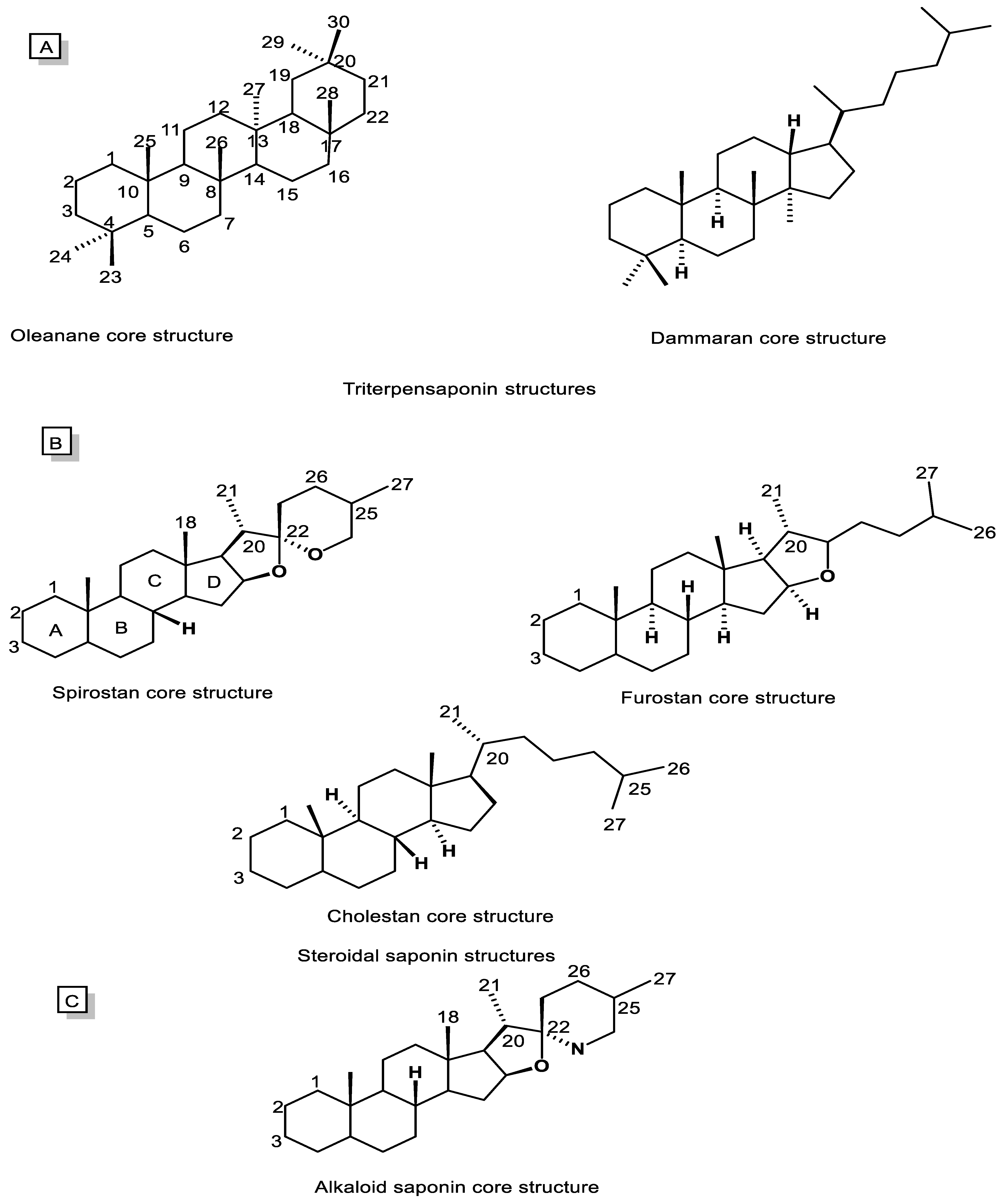
5.1. Chemistry, Biosynthesis, and Natural Sources
5.2. Pharmacological and Biological Activities
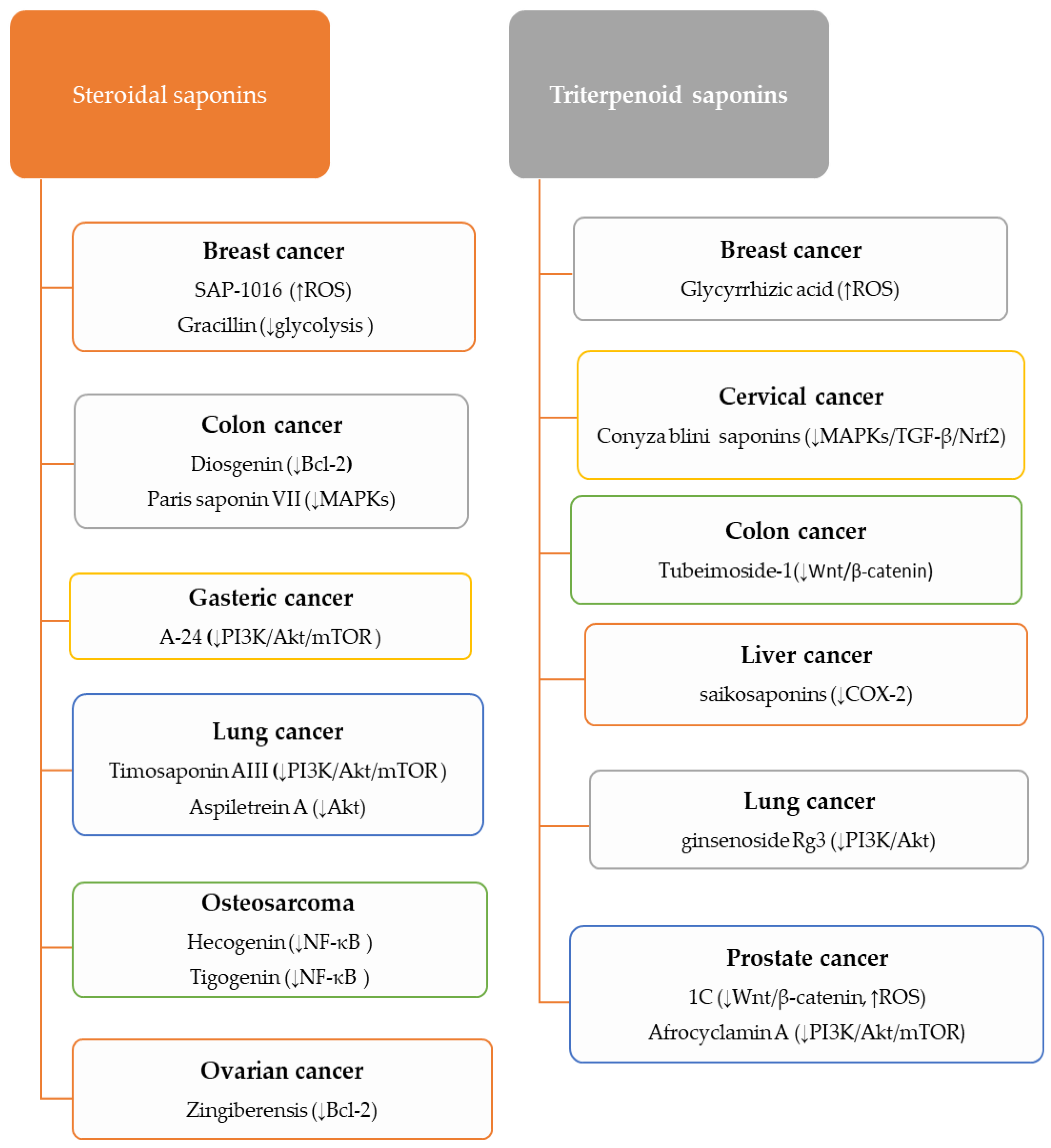
5.3. An Overview on the Anti-Cancer Mechanisms of Saponins
5.3.1. Anti-Cancer Activity of Steroidal Saponins
5.3.2. Anti-Cancer Activity of Triterpenoid Saponins
5.4. Anti-Angiogenic Potentials
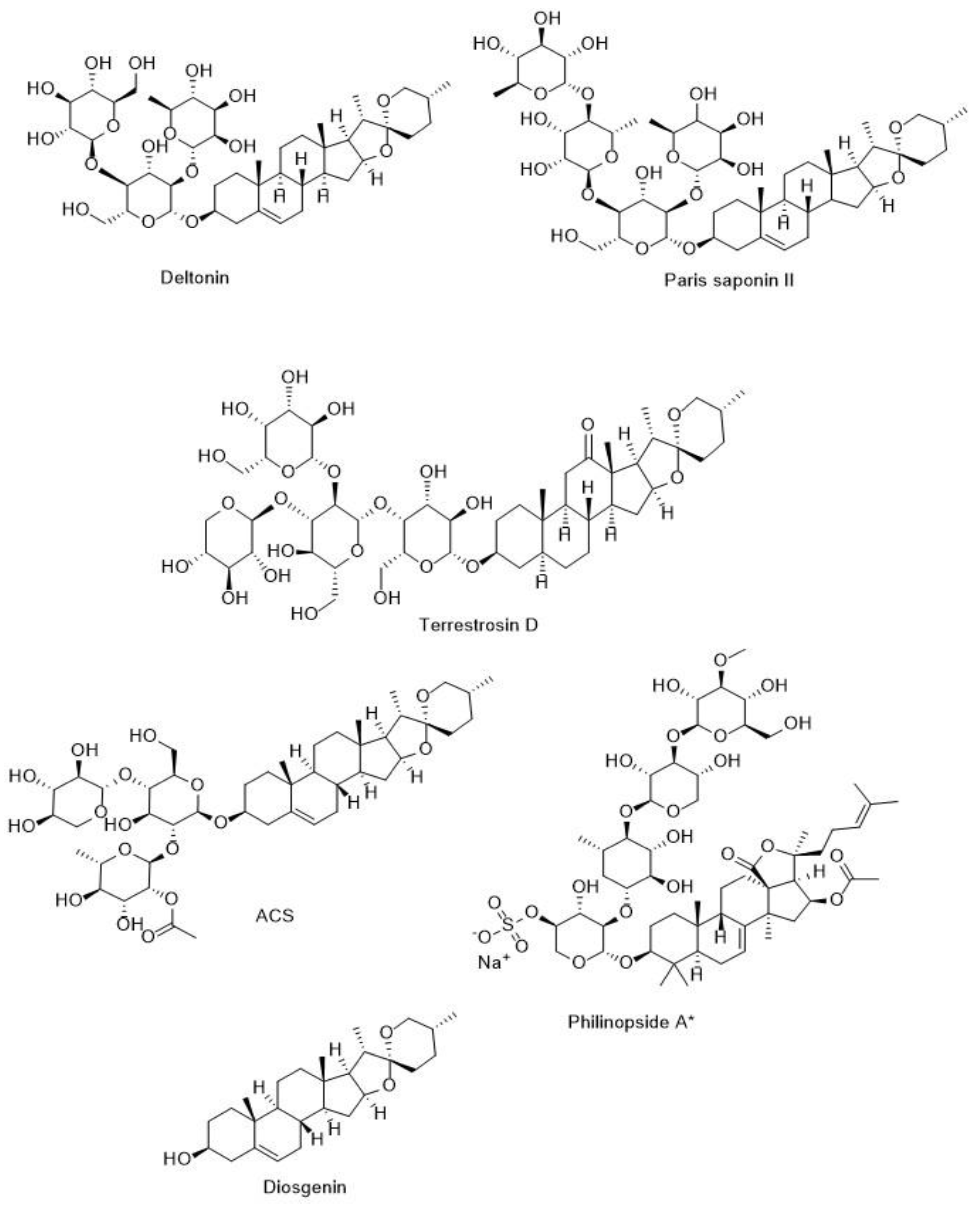
5.4.1. Anti-Angiogenic Potentials of Steroidal Saponins
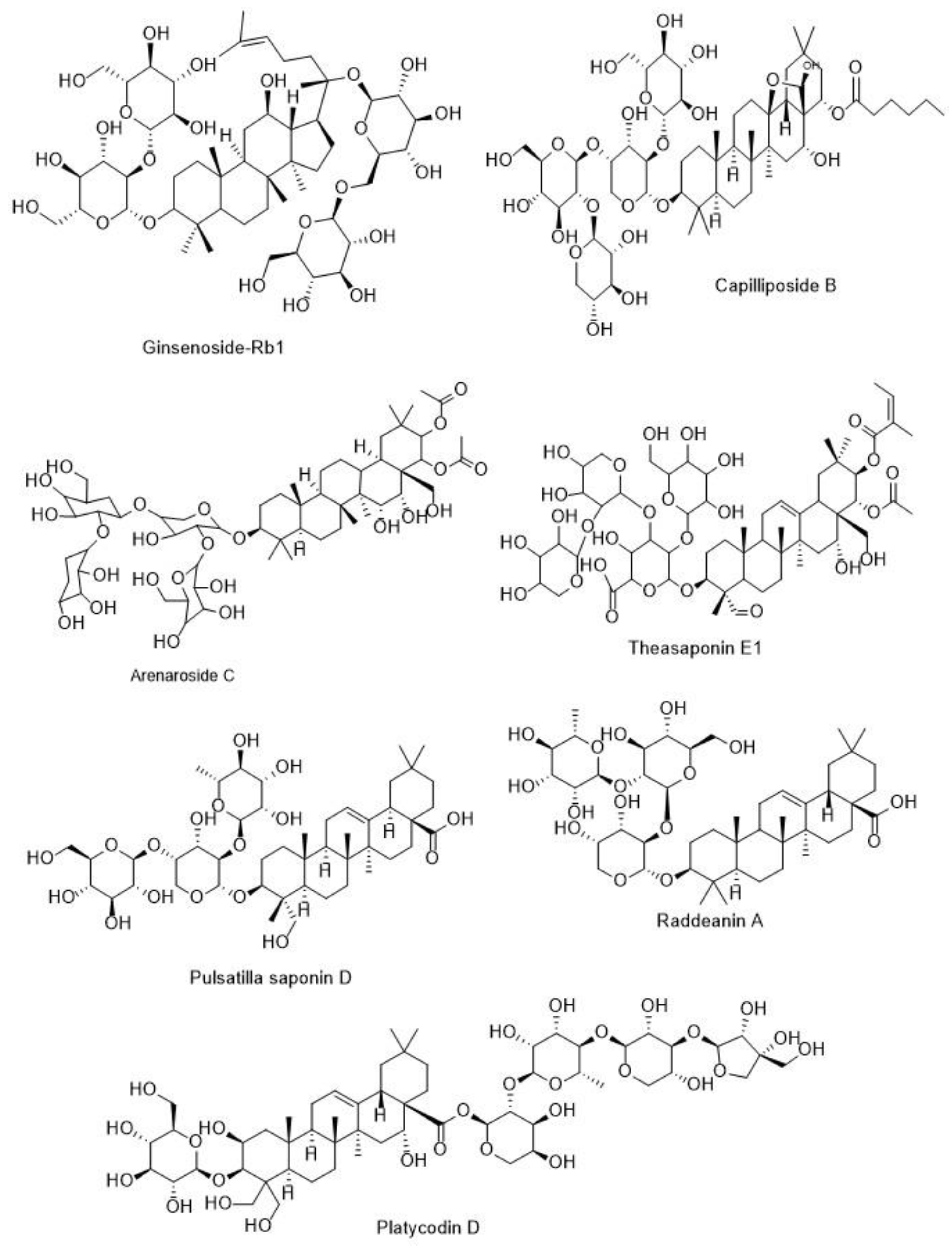
5.4.2. Anti-Angiogenic Potentials of Triterpenoid Saponins
5.4.3. Anti-Angiogenic Potentials of Marine Organism Saponins
5.4.4. Anti-Angiogenic Potentials of Total Saponin Extraction
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins—Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef]
- NajeebUllah, S.; Shinwari, Z.K.; Jan, S.A.; Khan, I.; Ali, M. Ethno medicinal and phytochemical properties of genus Allium: A review of recent advances. Pak. J. Bot. 2020, 53, 135–144. [Google Scholar] [CrossRef]
- Rao, A.V.; Gurfinkel, D.M. Dietary Saponins and Human Health. In Saponins in Food, Feedstuffs and Medicinal Plants; Oleszek, W., Marston, A., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 255–270. [Google Scholar]
- Vincken, J.-P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef]
- Seki, H.; Tamura, K.; Muranaka, T. P450s and UGTs: Key Players in the Structural Diversity of Triterpenoid Saponins. Plant Cell Physiol. 2015, 56, 1463–1471. [Google Scholar] [CrossRef]
- Upadhyay, S.; Jeena, G.S.; Shikha; Shukla, R.K. Recent advances in steroidal saponins biosynthesis and in vitro production. Planta 2018, 248, 519–544. [Google Scholar] [CrossRef]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.; Wagner, H. A review of the biological and pharmacological activities of saponins. Phytomedicine 1996, 2, 363–386. [Google Scholar] [CrossRef]
- Elekofehinti, O.; Iwaloye, O.; Olawale, F.; Ariyo, E. Saponins in Cancer Treatment: Current Progress and Future Prospects. Pathophysiology 2021, 28, 250–272. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Martins, F.; Pereira, S.; Serpa, J. Anti-Angiogenic Therapy: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 3765. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Lu, K.; Bhat, M.; Basu, S. Plants and their active compounds: Natural molecules to target angiogenesis. Angiogenesis 2016, 19, 287–295. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M.; Pourgholami, M.H. The effects of anticancer medicinal herbs on vascular endothelial growth factor based on pharmacological aspects: A review study. Nutr. Cancer 2019, 73, 1–15. [Google Scholar] [CrossRef]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef]
- Sobolewska, D.; Galanty, A.; Grabowska, K.; Makowska-Wąs, J.; Wróbel-Biedrawa, D.; Podolak, I. Saponins as cytotoxic agents: An update (2010–2018). Part I-steroidal saponins. Phytochem. Rev. 2020, 19. [Google Scholar] [CrossRef]
- Dimova, I.; Popivanov, G.; Djonov, V. Angiogenesis in cancer—General pathways and their therapeutic implications. JBUON 2014, 19, 15–21. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Mukherjee, A.; Basu, S.; Sarkar, N.; Ghosh, A. Advances in Cancer Therapy with Plant Based Natural Products. Curr. Med. Chem. 2001, 8, 1467–1486. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, X.; Cheng, X.; Cui, T.; Zhuo, Y.; Ma, W.; Zhao, X.; Zhao, P.; Liu, X.; Feng, W. Granulocyte-macrophage colony-stimulating factor increases tumor growth and angiogenesis directly by promoting endothelial cell function and indirectly by enhancing the mobilization and recruitment of proangiogenic granulocytes. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Fakhri, S.; Khodamorady, M.; Naseri, M.; Farzaei, M.H.; Khan, H. The ameliorating effects of anthocyanins on the cross-linked signaling pathways of cancer dysregulated metabolism. Pharmacol. Res. 2020, 159, 104895. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Xue, C.; Xie, J.; Zhao, D.; Lin, S.; Zhou, T.; Shi, S.; Shao, X.; Lin, Y.; Zhu, B.; Cai, X. The JAK/STAT3 signalling pathway regulated angiogenesis in an endothelial cell/adipose-derived stromal cell co-culture, 3D gel model. Cell Prolif. 2016, 50, e12307. [Google Scholar] [CrossRef]
- Tsao, S.-M.; Hsia, T.-C.; Yin, M.-C. Protocatechuic Acid Inhibits Lung Cancer Cells by Modulating FAK, MAPK, and NF-κB Pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef]
- de Bittencourt Pasquali, M.A.; Gelain, D.P.; Zeidán-Chuliá, F.; Pires, A.S.; Gasparotto, J.; Terra, S.R.; Moreira, J.C.F. Vitamin A (retinol) downregulates the receptor for advanced glycation endproducts (RAGE) by oxidant-dependent activation of p38 MAPK and NF-kB in human lung cancer A549 cells. Cell. Signal. 2013, 25, 939–954. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Zhang, M.; Li, R.; Li, Y.; Hu, X.; Wang, S.; Bao, Z. Characterization of three mitogen-activated protein kinases (MAPK) genes reveals involvement of ERK and JNK, not p38 in defense against bacterial infection in Yesso scallop Patinopecten yessoensis. Fish Shellfish. Immunol. 2016, 54, 507–515. [Google Scholar] [CrossRef]
- Chen, H.-X.; Xu, X.-X.; Tan, B.-Z.; Zhang, Z.; Zhou, X.-D. MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma. Cell. Physiol. Biochem. 2017, 41, 933–946. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; DeLiberto, L.K.; Bishayee, A. Cellular senescence signaling in cancer: A novel therapeutic target to combat human malignancies. Biochem. Pharmacol. 2022, 199, 114989. [Google Scholar] [CrossRef]
- Ahmed, A.M. The dual role of oxidative stress in lung cancer. In Oxidative Stress in Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–113. [Google Scholar]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Sakurai, T.; Kudo, M. Signaling Pathways Governing Tumor Angiogenesis. Oncology 2011, 81, 24–29. [Google Scholar] [CrossRef]
- Tiwari, A.; Mukherjee, B.; Dixit, M. MicroRNA Key to Angiogenesis Regulation: MiRNA Biology and Therapy. Curr. Cancer Drug Targets 2018, 18, 266–277. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef]
- Clarke, S.J.; Sharma, R. Angiogenesis inhibitors in cancer-mechanisms of action. Aust. Prescr. 2006, 29. [Google Scholar] [CrossRef]
- Al-Abd, A.M.; Alamoudi, A.J.; Abdel-Naim, A.B.; Neamatallah, T.A.; Ashour, O.M. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies—A review. J. Adv. Res. 2017, 8, 591–605. [Google Scholar] [CrossRef]
- Qi, S.; Deng, S.; Lian, Z.; Yu, K. Novel Drugs with High Efficacy against Tumor Angiogenesis. Int. J. Mol. Sci. 2022, 23, 6934. [Google Scholar] [CrossRef]
- Rahman, R.; Smith, S.; Rahman, C.; Grundy, R. Antiangiogenic Therapy and Mechanisms of Tumor Resistance in Malignant Glioma. J. Oncol. 2010, 2010, 251231. [Google Scholar] [CrossRef]
- Oguntade, A.S.; Al-Amodi, F.; Alrumayh, A.; Alobaida, M.; Bwalya, M. Anti-angiogenesis in cancer therapeutics: The magic bullet. J. Egypt. Natl. Cancer Inst. 2021, 33, 1–11. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef]
- Eatock, M.; Tebbutt, N.; Bampton, C.; Strickland, A.; Valladares-Ayerbes, M.; Swieboda-Sadlej, A.; Van Cutsem, E.; Nanayakkara, N.; Sun, Y.-N.; Zhong, Z.; et al. Phase II randomized, double-blind, placebo-controlled study of AMG 386 (trebananib) in combination with cisplatin and capecitabine in patients with metastatic gastro-oesophageal cancer. Ann. Oncol. 2013, 24, 710–718. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Peng, C.; Peng, F. Co-Targeting Tumor Angiogenesis and Immunosuppressive Tumor Microenvironment: A Perspective in Ethnopharmacology. Front. Pharmacol. 2022, 13, 886198. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal Medicine as Inducers of Apoptosis in Cancer Treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Sobolewska, D.; Michalska, K.; Podolak, I.; Grabowska, K. Steroidal saponins from the genus Allium. Phytochem. Rev. 2014, 15, 1–35. [Google Scholar] [CrossRef]
- Weng, A.; Thakur, M.; Melzig, M.F.; Fuchs, H. Chemistry and pharmacology of saponins: Special focus on cytotoxic properties. Bot. Targets Ther. 2011, 1, 19–29. [Google Scholar] [CrossRef]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef]
- Ashour, A.S.; El Aziz, M.M.A.; Melad, A.S.G. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 7, 282–288. [Google Scholar] [CrossRef]
- Challinor, V.L.; De Voss, J.J. Open-chain steroidal glycosides, a diverse class of plant saponins. Nat. Prod. Rep. 2013, 30, 429–454. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Oleszek, W.; Hamed, A. Saponin-based surfactants. Surfactants Renew. Resour. 2010, 1, 239–251. [Google Scholar]
- Findlay, J.A.; Agarwal, V.K.; Moharir, Y.E. On the Saponins of the Starfish Asterias vulgaris. J. Nat. Prod. 1984, 47, 113–116. [Google Scholar] [CrossRef]
- Nicolas, G.; Oulad-Ali, A.; Guillaume, D.; Lobstein, A.; Weniger, B.; Anton, R. Triterpenoid saponins from the root of Sideroxylon foetidissimum. Phytochemistry 1995, 38, 225–228. [Google Scholar] [CrossRef]
- Woo, E.H.; Woo, W.S.; Chmurny, G.N.; Hilton, B.D. Melandrioside A, a Saponin from Melandrium firmum. J. Nat. Prod. 1992, 55, 786–794. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of Triterpenoid Saponins in Plants. In History and Trends in Bioprocessing and Biotransformation; Springer: Berlin/Heidelberg, Germany, 2002; Volume 75, pp. 31–49. [Google Scholar] [CrossRef]
- Broberg, S.; Nord, L.I.; Kenne, L. Oligosaccharide sequences inQuillaja saponins by electrospray ionization ion trap multiple-stage mass spectrometry. J. Mass Spectrom. 2004, 39, 691–701. [Google Scholar] [CrossRef]
- Üstündağ, G.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.; Qiu, D. Role of Saponins in Plant Defense against Specialist Herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2010, 10, 471–491. [Google Scholar] [CrossRef]
- Sautour, M.; Mitaine-Offer, A.-C.; Lacaille-Dubois, M.-A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, J.; Kong, L. Steroidal alkaloid saponins and steroidal saponins from Solanum surattense. Phytochemistry 2011, 72, 668–673. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Jahidin, A.H.; Lehmann, R.; Penman, K.; Kitching, W.; De Voss, J.J. Asparinins, asparosides, curillins, curillosides and shavatarins: Structural clarification with the isolation of shatavarin V, a new steroidal saponin from the root of Asparagus racemosus. Tetrahedron Lett. 2006, 47, 8683–8687. [Google Scholar] [CrossRef]
- Schmid, C.; Dawid, C.; Peters, V.; Hofmann, T. Saponins from European Licorice Roots (Glycyrrhiza glabra). J. Nat. Prod. 2018, 81, 1734–1744. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja Saponin Characteristics and Functional Properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef]
- Shin, B.-K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef]
- Peng, D.; Wang, H.; Qu, C.; Xie, L.; Wicks, S.M.; Xie, J. Ginsenoside Re: Its chemistry, metabolism and pharmacokinetics. Chin. Med. 2012, 7, 1–6. [Google Scholar] [CrossRef]
- Zheng, M.; Xin, Y.; Li, Y.; Xu, F.; Xi, X.; Guo, H.; Cui, X.; Cao, H.; Zhang, X.; Han, C. Ginsenosides: A Potential Neuroprotective Agent. BioMed Res. Int. 2018, 2018, 8174345. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Chataway, T.; Franco, C.M.M. Structural Elucidation of Novel Saponins in the Sea Cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 4439–4473. [Google Scholar] [CrossRef]
- Dahmoune, B.; Bachari-Houma, F.; Chibane, M.; Jéhan, P.; Guegan, J.-P.; Dahmoune, F.; Aissou-Akrour, C.; Mouni, L.; Ferrières, V.; Hauchard, D. Saponin contents in the starfish Echinaster sepositus: Chemical characterization, qualitative and quantitative distribution. Biochem. Syst. Ecol. 2021, 96, 104262. [Google Scholar] [CrossRef]
- Kun-Ya, W.; Ping-Lin, L.; Jing-Fan, S.; de Voogd, N.J.; Xu-Li, T.; Guo-Qiang, L. Four new polyhydroxylated steroids from the South Sea sponge Plakortis sp. Chin. J. Nat. Med. 2020, 18, 844–849. [Google Scholar]
- Bahrami, Y.; Zhang, W.; MM Franco, C. Distribution of Saponins in the Sea Cucumber Holothuria lessoni; the Body Wall Versus the Viscera, and Their Biological Activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef]
- Najjar-Tabrizi, R.; Javadi, A.; Sharifan, A.; Chew, K.W.; Lay, C.-H.; Show, P.L.; Jafarizadeh-Malmiri, H.; Berenjian, A. Hydrothermally extraction of saponin from Acanthophyllum glandulosum root—Physico-chemical characteristics and antibacterial activity evaluation. Biotechnol. Rep. 2020, 27, e00507. [Google Scholar] [CrossRef]
- Sonfack, G.; Fossi Tchinda, C.; Simo, I.K.; Bitchagno, G.T.M.; Nganou, B.K.; Çelik, İ.; Tene, M.; Funda Görkem, S.; Opatz, T.; Penlap Beng, V. Saponin with antibacterial activity from the roots of Albizia adianthifolia. Nat. Prod. Res. 2021, 35, 2831–2839. [Google Scholar] [CrossRef]
- Sharma, P.; Tyagi, A.; Bhansali, P.; Pareek, S.; Singh, V.; Ilyas, A.; Mishra, R.; Poddar, N.K. Saponins: Extraction, bio-medicinal properties and way forward to anti-viral representatives. Food Chem. Toxicol. 2021, 150, 112075. [Google Scholar] [CrossRef]
- Yang, C.-R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Li, X.-C. Antifungal Activity of C-27 Steroidal Saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef]
- Favel, A.; Steinmetz, M.; Regli, P.; Vidal-Ollivier, E.; Elias, R.; Balansard, G. In Vitro Antifungal Activity of Triterpenoid Saponins. Planta Med. 1994, 60, 50–53. [Google Scholar] [CrossRef]
- Germonprez, N.; Van Puyvelde, L.; Maes, L.; Van Tri, M.; De Kimpe, N. New pentacyclic triterpene saponins with strong anti-leishmanial activity from the leaves of Maesa balansae. Tetrahedron 2004, 60, 219–228. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, N.; Gajbhiye, R.L.; Mishra, S.; Jaisankar, P.; Datta, S.; Das Saha, K. Intracellular anti-leishmanial effect of Spergulin-A, a triterpenoid saponin of Glinus oppositifolius. Infect. Drug Resist. 2019, ume 12, 2933–2942. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, H.J.; Liu, S.M.; Jiang, X.H.; Wang, Q.Y.; Zhang, S.; Yu, D.H. Anti-inflammation effects of the total saponin fraction from Dioscorea nipponica Makino on rats with gouty arthritis by influencing MAPK signalling pathway. BMC Complement. Med. Ther. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, X.; Shi, Z.; Ren, G. Anti-inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated RAW 264.7 macrophages cells. J. Food Sci. 2014, 79, H1018–H1023. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, Y.; Huang, L.; Li, J.; Sun, H.; Zhao, Y.; Yang, J.; Zhou, W. Antioxidant activities of saponins extracted from Radix Trichosanthis: An in vivo and in vitro evaluation. BMC Complement. Altern. Med. 2014, 14, 86. [Google Scholar] [CrossRef]
- Zhou, N.; Tang, Y.; Keep, R.F.; Ma, X.; Xiang, J. Antioxidative effects of Panax notoginseng saponins in brain cells. Phytomedicine 2014, 21, 1189–1195. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhuri, P.K. Structural characteristics, bioavailability and cardioprotective potential of saponins. Integr. Med. Res. 2018, 7, 33–43. [Google Scholar] [CrossRef]
- Sun, A.; Xu, X.; Lin, J.; Cui, X.; Xu, R. Neuroprotection by Saponins. Phytother. Res. 2014, 29, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Raju, J.; Mehta, R. Cancer Chemopreventive and Therapeutic Effects of Diosgenin, a Food Saponin. Nutr. Cancer 2008, 61, 27–35. [Google Scholar] [CrossRef]
- Lee, K.J.; Choi, C.Y.; Chung, Y.C.; Kim, Y.S.; Ryu, S.Y.; Roh, S.H.; Jeong, H.G. Protective effect of saponins derived from roots of Platycodon grandiflorum on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Toxicol. Lett. 2004, 147, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.S.; Soni, K.K.; Saxena, R. Pharmacology and phytochemistry of saponin isolated from Aloe vera for wound healing activity. Asian J. Chem. 2009, 21, 1029–1032. [Google Scholar]
- Sevimli-Gür, C.; Onbaşılar, İ.; Atilla, P.; Genç, R.; Çakar, N.; Deliloğlu-Gürhan, I.; Bedir, E. In vitro growth stimulatory and in vivo wound healing studies on cycloartane-type saponins of Astragalus genus. J. Ethnopharmacol. 2011, 134, 844–850. [Google Scholar] [CrossRef]
- Wang, J.-R.; Zhou, H.; Jiang, Z.-H.; Wong, Y.F.; Liu, L. In Vivo Anti-inflammatory and Analgesic Activities of a Purified Saponin Fraction Derived from the Root of Ilex pubescens. Biol. Pharm. Bull. 2008, 31, 643–650. [Google Scholar] [CrossRef]
- Shu, P.-P.; Li, L.-X.; He, Q.-M.; Pan, J.; Li, X.-L.; Zhu, M.; Yang, Y.; Qu, Y. Identification and quantification of oleanane triterpenoid saponins and potential analgesic and anti-inflammatory activities from the roots and rhizomes of Panax stipuleanatus. J. Ginseng Res. 2020, 45, 305–315. [Google Scholar] [CrossRef]
- Meng, M.; Yue, Z.; Chang, L.; Liu, Y.; Hu, J.; Song, Z.; Tang, Z.; Zhou, R.; Wang, C. Anti-Rheumatoid Arthritic Effects of Paris Saponin VII in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes and Adjuvant-Induced Arthritis in Rats. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Choi, J.; Huh, K.; Kim, S.-H.; Lee, K.-T.; Park, H.-J.; Han, Y.N. Antinociceptive and anti-rheumatoidal effects of Kalopanax pictus extract and its saponin components in experimental animals. J. Ethnopharmacol. 2001, 79, 199–204. [Google Scholar] [CrossRef]
- Singh, D.; Singh, B.; Goel, R.K. Role of saponins for the anticonvulsant effect of adventitious roots of Ficus religiosa. Pharm. Biol. 2012, 50, 816–822. [Google Scholar] [CrossRef]
- Abaci, H.; Akagac, G.; Nalbantsoy, A.; Sarikahya, N.B. A hederagenin-type triterpene saponin, sumbulianoside a from Cephalaria sumbuliana and its potent immunomodulatory activity against seasonal flu virus H3N2. Nat. Prod. Res. 2021, 36, 2495–2503. [Google Scholar] [CrossRef]
- Sarikahya, N.B.; Nalbantsoy, A.; Top, H.; Gokturk, R.S.; Sumbul, H.; Kirmizigul, S. Immunomodulatory, hemolytic and cytotoxic activity potentials of triterpenoid saponins from eight Cephalaria species. Phytomedicine 2018, 38, 135–144. [Google Scholar] [CrossRef]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Sharma, R.; Palanisamy, A.; Dhama, K.; Mal, G.; Singh, B.; Singh, K.P. Exploring the possible use of saponin adjuvants in COVID-19 vaccine. Hum. Vaccines Immunother. 2020, 16, 2944–2953. [Google Scholar] [CrossRef]
- Waite, D.C.; Jacobson, E.W.; Ennis, F.A.; Edelman, R.; White, B.; Kammer, R.; Anderson, C.; Kensil, C.R. Three double-blind, randomized trials evaluating the safety and tolerance of different formulations of the saponin adjuvant QS-21. Vaccine 2001, 19, 3957–3967. [Google Scholar] [CrossRef]
- Khan, N.; Akhtar, M.S.; Khan, B.A.; Braga, V.D.A.; Reich, A. Antiobesity, hypolipidemic, antioxidant and hepatoprotective effects of Achyranthes aspera seed saponins in high cholesterol fed albino rats. Arch. Med. Sci. 2015, 6, 1261–1271. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Dong, P.; Li, Z.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea cucumber saponin liposomes ameliorate obesity-induced inflammation and insulin resistance in high-fat-diet-fed mice. Food Funct. 2017, 9, 861–870. [Google Scholar] [CrossRef]
- Lu, F.-r.; Shen, L.; Qin, Y.; Gao, L.; Li, H.; Dai, Y. Clinical observation on trigonella foenum-graecum L. total saponins in combination with sulfonylureas in the treatment of type 2 diabetes mellitus. Chin. J. Integr. Med. 2008, 14, 56–60. [Google Scholar] [CrossRef]
- Khatik, G.L.; Suttee, A. The Possible Role of Saponin in Type-II Diabetes—A Review. Curr. Diabetes Rev. 2021, 17, 107–121. [Google Scholar] [CrossRef]
- Oakenfull, D. Saponins in the treatment of hypercholesterolemia. In Handbook of Lipids in Human Nutrition; CRC Press: Boca Raton, FL, USA, 2020; pp. 107–112. [Google Scholar]
- Chen, M.; Long, Z.; Wang, Y.; Liu, J.; Pian, H.; Wang, L.; Chen, Z. Protective effects of saponin on a hypertension target organ in spontaneously hypertensive rats. Exp. Ther. Med. 2012, 5, 429–432. [Google Scholar] [CrossRef]
- Sudheendra, A.T.; Shenoy, R.; Taranalli, A.D. Evaluation of saponin rich fraction of Trigonella foenum graecum for antihypertensive activity. Pharmacol. Online 2009, 1, 1229–1233. [Google Scholar]
- Siddiqi, M.H.; Ahn, S.; Kang, S.; Kim, Y.-J.; Sathishkumar, N.; Yang, D.-U.; Yang, D.-C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng Res. 2013, 37, 261–268. [Google Scholar] [CrossRef]
- Dutta, A.; Ghoshal, A.; Mandal, D.; Mondal, N.B.; Banerjee, S.; Sahu, N.P.; Mandal, C. Racemoside A, an anti-leishmanial, water-soluble, natural steroidal saponin, induces programmed cell death in Leishmania donovani. J. Med. Microbiol. 2007, 56, 1196–1204. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef]
- Sur, P.; Chaudhuri, T.; Vedasiromoni, J.; Gomes, A.; Ganguly, D. Antiinflammatory and antioxidant property of saponins of tea [Camellia sinensis (L.) O. Kuntze] root extract. Phytother. Res. 2001, 15, 174–176. [Google Scholar] [CrossRef]
- Mostafa, A.; Sudisha, J.; El-Sayed, M.; Ito, S.-I.; Ikeda, T.; Yamauchi, N.; Shigyo, M. Aginoside saponin, a potent antifungal compound, and secondary metabolite analyses from Allium nigrum L. Phytochem. Lett. 2013, 6, 274–280. [Google Scholar] [CrossRef]
- Li, H.-X.; Han, S.-Y.; Ma, X.; Zhang, K.; Wang, L.; Ma, Z.-Z.; Tu, P.-F. The saponin of red ginseng protects the cardiac myocytes against ischemic injury in vitro and in vivo. Phytomedicine 2012, 19, 477–483. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yu, S.; Chen, N.; Xue, W.; Hu, J.; Zhang, D. Triterpenoid Saponins with Neuroprotective Effects from the Roots of Polygala tenuifolia. Planta Med. 2008, 74, 133–141. [Google Scholar] [CrossRef]
- Meng, J.; Hu, X.; Zhang, T.; Dong, P.; Li, Z.; Xue, C.; Chang, Y.; Wang, Y. Saponin from sea cucumber exhibited more significant effects than ginsenoside on ameliorating high fat diet-induced obesity in C57BL/6 mice. MedChemComm 2018, 9, 725–734. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Nesil, T.; Yılmaz-Dilsiz, Ö.; Aksu, G.; Khan, S.; Bedir, E. Evaluation of the immunomodulatory properties in mice and in vitro anti-inflammatory activity of cycloartane type saponins from Astragalus species. J. Ethnopharmacol. 2012, 139, 574–581. [Google Scholar] [CrossRef]
- Pal, D.; Sannigrahi, S.; Mazumder, U.K. Analgesic and anticonvulsant effects of saponin isolated from the leaves of Clerodendrum infortunatum Linn. in mice. Experiment 2009, 47. [Google Scholar]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological Properties of Allium cepa, preclinical and clinical evidences; a review. Iran. J. Pharm. Res. 2021, 20, 107. [Google Scholar]
- Steels, E.; Steele, M.; Harold, M.; Coulson, S. Efficacy of a proprietary Trigonella foenum-graecum L. de-husked seed extract in reducing menopausal symptoms in otherwise healthy women: A double-blind, randomized, placebo-controlled study. Phytother. Res. 2017, 31, 1316–1322. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Zhou, J.; Wu, D.; Ye, J.; Sun, G.; Sun, X. Saponins in Chinese Herbal Medicine Exerts Protection in Myocardial Ischemia–Reperfusion Injury: Possible Mechanism and Target Analysis. Front. Pharmacol. 2021, 11, 570867. [Google Scholar] [CrossRef]
- Verma, N.; Usman, K.; Patel, N.; Jain, A.; Dhakre, S.; Swaroop, A.; Bagchi, M.; Kumar, P.; Preuss, H.G.; Bagchi, D. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food Nutr. Res. 2016, 60, 32382. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Lv, Y.; Chen, J.; Yin, F.; Huang, J.; Zheng, Q. A Review of Ginseng Clinical Trials Registered in the WHO International Clinical Trials Registry Platform. BioMed Res. Int. 2018, 2018, 1843142. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Bin Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef]
- Song, X.-Y.; Han, F.-Y.; Chen, J.-J.; Wang, W.; Zhang, Y.; Yao, G.-D.; Song, S.-J. Timosaponin AIII, a steroidal saponin, exhibits anti-tumor effect on taxol-resistant cells in vitro and in vivo. Steroids 2019, 146, 57–64. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Nguyen, H.T.; Seephan, S.; Do, H.B.; Nguyen, H.T.; Ho, D.V.; Pongrakhananon, V. Antitumor activities of Aspiletrein A, a steroidal saponin from Aspidistra letreae, on non-small cell lung cancer cells. BMC Complement. Med. Ther. 2021, 21, 87. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, J.-J.; Zhou, S.-J.; Chen, J.; Hu, Q.; Pu, J.-X.; Lu, J.-L. Diosgenin inhibits the expression of NEDD4 in prostate cancer cells. Am. J. Transl. Res. 2019, 11, 3461–3471. [Google Scholar]
- Wang, W.; Chen, Z.; Chen, X.; Ni, S.; Jia, Y.; Fan, L.; Ma, L. DG-8d, a novel diosgenin derivative, decreases the proliferation and induces the apoptosis of A549 cells by inhibiting the PI3k/Akt signaling pathway. Steroids 2021, 174, 108898. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Lin, X.; Wang, Y.; He, X. A steroidal saponin isolated from Allium chinense simultaneously induces apoptosis and autophagy by modulating the PI3K/Akt/mTOR signaling pathway in human gastric adenocarcinoma. Steroids 2020, 161, 108672. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef]
- Kunac, N.; Filipović, N.; Kostić, S.; Vukojević, K. The Expression Pattern of Bcl-2 and Bax in the Tumor and Stromal Cells in Colorectal Carcinoma. Medicina 2022, 58, 1135. [Google Scholar] [CrossRef]
- Tong, Q.-Y.; He, Y.; Zhao, Q.-B.; Qing, Y.; Huang, W.; Wu, X.-H. Cytotoxicity and apoptosis-inducing effect of steroidal saponins from Dioscorea zingiberensis Wright against cancer cells. Steroids 2012, 77, 1219–1227. [Google Scholar] [CrossRef]
- Raju, J.; Patlolla, J.M.; Swamy, M.V.; Rao, C.V. Diosgenin, a Steroid Saponin of Trigonella foenum graecum (Fenugreek), Inhibits Azoxymethane-Induced Aberrant Crypt Foci Formation in F344 Rats and Induces Apoptosis in HT-29 Human Colon Cancer Cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1392–1398. [Google Scholar] [CrossRef]
- Kooshki, L.; Mahdavi, P.; Fakhri, S.; Akkol, E.K.; Khan, H. Targeting lactate metabolism and glycolytic pathways in the tumor microenvironment by natural products: A promising strategy in combating cancer. Biofactors 2021, 48, 359–383. [Google Scholar] [CrossRef]
- Solaini, G.; Sgarbi, G.; Baracca, A. Oxidative phosphorylation in cancer cells. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 534–542. [Google Scholar] [CrossRef]
- Min, H.-Y.; Pei, H.; Hyun, S.Y.; Boo, H.-J.; Jang, H.-J.; Cho, J.; Kim, J.H.; Son, J.; Lee, H.-Y. Potent Anticancer Effect of the Natural Steroidal Saponin Gracillin Is Produced by Inhibiting Glycolysis and Oxidative Phosphorylation-Mediated Bioenergetics. Cancers 2020, 12, 913. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Beit-Yannai, E.; Ben-Shabat, S.; Goldschmidt, N.; Chapagain, B.P.; Liu, R.H.; Wiesman, Z. Antiproliferative activity of steroidal saponins from Balanites aegyptiaca—An in vitro study. Phytochem. Lett. 2011, 4, 43–47. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Q.; Jiang, S.; Li, M.; Wang, X. Anti-colorectal cancer activity of macrostemonoside A mediated by reactive oxygen species. Biochem. Biophys. Res. Commun. 2013, 441, 825–830. [Google Scholar] [CrossRef]
- Ha, D.T.; Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Popov, R.S.; Malyarenko, O.S.; Ermakova, S.P.; Thuy, T.T.T.; Long, P.Q.; Ivanchina, N.V. Asterosaponins from the tropical starfish Acanthaster planci and their cytotoxic and anticancer activities in vitro. Nat. Prod. Res. 2019, 35, 548–555. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Fan, L.; Zhang, F.; Meng, J.; Han, J.; Guo, X.; Zhang, D.; Zhang, R.; Yue, Z.; et al. Paris saponin VII inhibits growth of colorectal cancer cells through Ras signaling pathway. Biochem. Pharmacol. 2014, 88, 150–157. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Yu, R.; Kim, J.-S.; Kim, Y.-K.; Sung, M.-K. Antiproliferative crude soy saponin extract modulates the expression of IκBα, protein kinase C, and cyclooxygenase-2 in human colon cancer cells. Cancer Lett. 2004, 210, 1–6. [Google Scholar] [CrossRef]
- Hu, K.; Yao, X. The cytotoxicity of methyl protoneogracillin (NSC-698793) and gracillin (NSC-698787), two steroidal saponins from the rhizomes of Dioscorea collettii var. hypoglauca, against human cancer cells in vitro. Phytother. Res. 2003, 17, 620–626. [Google Scholar] [CrossRef]
- Corbiere, C.; Liagre, B.; Bianchi, A.; Bordji, K.; Dauça, M.; Netter, P.; Beneytout, J.-L. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int. J. Oncol. 2003, 22, 899–905. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, Y.; Zhang, J.; Xiang, Z.; Liu, Y.; Miao, T.; Liu, G.; Liu, B.; Liu, X.; Shen, L.; et al. Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am. J. Transl. Res. 2019, 11, 2580–2589. [Google Scholar] [PubMed]
- Sachan, R.; Kundu, A.; Jeon, Y.; Choi, W.S.; Yoon, K.; Kim, I.S.; Kwak, J.H.; Kim, H.S. Afrocyclamin A, a triterpene saponin, induces apoptosis and autophagic cell death via the PI3K/Akt/mTOR pathway in human prostate cancer cells. Phytomedicine 2018, 51, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wen, H.; Zhang, Q.; Zhou, W.; Lin, X.; Xie, D.; Liu, Y. Inhibiting PI3K-AKt signaling pathway is involved in antitumor effects of ginsenoside Rg3 in lung cancer cell. Biomed. Pharmacother. 2017, 85, 16–21. [Google Scholar] [CrossRef]
- Peng, Z.; Wu, W.W.; Yi, P. The Efficacy of Ginsenoside Rg3 Combined with First-line Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 11. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, M.; Jiang, Z.; Zhao, F.; Xi, B.; Zhang, X.; Fu, H.; Zhou, K. Platycodin-D Induced Autophagy in Non-Small Cell Lung Cancer Cells via PI3K/Akt/mTOR and MAPK Signaling Pathways. J. Cancer 2015, 6, 623–631. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Peng, L.; Hu, C.; Zhang, C.; Lu, Y.; Man, S.; Ma, L. Anti-cancer activity of Conyza blinii saponin against cervical carcinoma through MAPK/TGF-β/Nrf2 signaling pathways. J. Ethnopharmacol. 2020, 251, 112503. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- De Wang, X.; Su, G.Y.; Zhao, C.; Qu, F.Z.; Wang, P.; Zhao, Y.Q. Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J. Ginseng Res. 2016, 42, 133–143. [Google Scholar] [CrossRef]
- Zafar, M.; Sarfraz, I.; Rasul, A.; Jabeen, F.; Samiullah, K.; Hussain, G.; Riaz, A.; Ali, M. Tubeimoside-1, Triterpenoid Saponin, as a Potential Natural Cancer Killer. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Bian, Q.; Liu, P.; Gu, J.; Song, B. Tubeimoside-1 inhibits the growth and invasion of colorectal cancer cells through the Wnt/β-catenin signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 12517–12524. [Google Scholar]
- Lin, S.-C.; Chu, P.-Y.; Liao, W.-T.; Wu, M.-Y.; Tsui, K.-H.; Lin, L.-T.; Huang, C.-H.; Chen, L.-L.; Li, C.-J. Glycyrrhizic acid induces human MDA-MB-231 breast cancer cell death and autophagy via the ROS-mitochondrial pathway. Oncol. Rep. 2017, 39, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.X.; Vien, L.T.; Hanh, T.T.H.; Thao, N.P.; Thao, D.T.; Van Thanh, N.; Nam, N.H.; Thung, D.C.; Van Kiem, P.; Van Minh, C. Cytotoxic triterpene saponins from Cercodemas anceps. Bioorganic Med. Chem. Lett. 2015, 25, 3151–3156. [Google Scholar] [CrossRef]
- Fotso, W.G.; Na-Iya, J.; Mbaveng, T.A.; Ango Yves, P.; Demirtas, I.; Kuete, V.; Samuel, Y.; Ngameni, B.; Efferth, T.; Ngadjui, T.B. Polyacanthoside A, a new oleanane-type triterpenoid saponin with cytotoxic effects from the leaves of Acacia polyacantha (Fabaceae). Nat. Prod. Res. 2019, 33, 3521–3526. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, L.; Wu, J.; Zhou, X.; Li, X.; Sun, X.; Zhu, L.; Xia, T.; Ding, Q. A hederagenin saponin isolated from Clematis ganpiniana induces apoptosis in breast cancer cells via the mitochondrial pathway. Oncol. Lett. 2017, 15, 1737–1743. [Google Scholar] [CrossRef]
- Ren, M.; McGowan, E.; Li, Y.; Zhu, X.; Lu, X.; Zhu, Z.; Lin, Y.; He, S. Saikosaponin-d Suppresses COX2 Through p-STAT3/C/EBPβ Signaling Pathway in Liver Cancer: A Novel Mechanism of Action. Front. Pharmacol. 2019, 10, 623. [Google Scholar] [CrossRef]
- El-Kenawi, A.E.; El-Remessy, A.B. Angiogenesis inhibitors in cancer therapy: Mechanistic perspective on classification and treatment rationales. Br. J. Pharmacol. 2013, 170, 712–729. [Google Scholar] [CrossRef]
- Munir, S.; Shah, A.A.; Shahid, M.; Ahmed, M.S.; Shahid, A.; Rajoka, M.S.; Akash, M.S.H.; Akram, M.; Khurshid, M. Anti-angiogenesis Potential of Phytochemicals for the Therapeutic Management of Tumors. Curr. Pharm. Des. 2020, 26, 265–278. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Nico, B.; De Falco, G.; Montaldo, P.G.; Ponzoni, M. Angiogenesis and anti-angiogenesis in neuroblastoma. Eur. J. Cancer 2002, 38, 750–757. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Liu, H.-Y.; Xu, S.-F.; Han, R. Anti-invasion and anti-angiogenesis effect of taxol and camptothecin on melanoma cells. J. Asian Nat. Prod. Res. 2003, 5, 121–129. [Google Scholar] [CrossRef]
- Subbaraj, G.K.; Kumar, Y.S.; Kulanthaivel, L. Antiangiogenic role of natural flavonoids and their molecular mechanism: An update. Egypt. J. Intern. Med. 2021, 33, 29. [Google Scholar] [CrossRef]
- Khathayer, F.; Ray, S.K. Diosgenin as a Novel Alternative Therapy for Inhibition of Growth, Invasion, and Angiogenesis Abilities of Different Glioblastoma Cell Lines. Neurochem. Res. 2020, 45, 2336–2351. [Google Scholar] [CrossRef]
- Tong, Q.; Zhao, Q.; Qing, Y.; Hu, X.; Jiang, L.; Wu, X. Deltonin inhibits angiogenesis by regulating VEGFR2 and subsequent signaling pathways in endothelial cells. Steroids 2015, 96, 30–36. [Google Scholar] [CrossRef]
- Tong, Q.; Qing, Y.; Wu, Y.; Hu, X.; Jiang, L.; Wu, X. Dioscin inhibits colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol. Appl. Pharmacol. 2014, 281, 166–173. [Google Scholar] [CrossRef]
- Wei, S.; Fukuhara, H.; Chen, G.; Kawada, C.; Kurabayashi, A.; Furihata, M.; Inoue, K.; Shuin, T. Terrestrosin D, a Steroidal Saponin from Tribulus terrestris L., Inhibits Growth and Angiogenesis of Human Prostate Cancer in vitro and in vivo. Pathobiology 2014, 81, 123–132. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; You, L.; Sun, M.; Qu, C.; Dong, X.; Yin, X.; Ni, J. Inhibitory effects of Paris saponin I, II, Ⅵ and Ⅶ on HUVEC cells through regulation of VEGFR2, PI3K/AKT/mTOR, Src/eNOS, PLCγ/ERK/MERK, and JAK2-STAT3 pathways. Biomed. Pharmacother. 2020, 131, 110750. [Google Scholar] [CrossRef]
- Chan, J.; Koon, J.C.-M.; Liu, X.; Detmar, M.; Yu, B.; Kong, S.-K.; Fung, K.P. Polyphyllin D, a steroidal saponin from Paris polyphylla, inhibits endothelial cell functions in vitro and angiogenesis in zebrafish embryos in vivo. J. Ethnopharmacol. 2011, 137, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yang, M.; Xiao, J.; Zou, J.; Huang, Q.; Yang, K.; Zhang, B.; Yang, F.; Liu, S.; Wang, H.; et al. Paris Saponin II suppresses the growth of human ovarian cancer xenografts via modulating VEGF-mediated angiogenesis and tumor cell migration. Cancer Chemother. Pharmacol. 2014, 73, 807–818. [Google Scholar] [CrossRef]
- Yang, M.; Zou, J.; Zhu, H.; Liu, S.; Wang, H.; Bai, P.; Xiao, X. Paris saponin II inhibits human ovarian cancer cell-induced angiogenesis by modulating NF-κB signaling. Oncol. Rep. 2015, 33, 2190–2198. [Google Scholar] [CrossRef]
- Zeng, K.W.; Song, F.J.; Li, N.; Dong, X.; Jiang, Y.; Tu, P.F. ASC, a Bioactive Steroidal Saponin from Ophitopogin japonicas, Inhibits Angiogenesis through Interruption of Src Tyrosine Kinase-dependent Matrix Metalloproteinase Pathway. Basic Clin. Pharmacol. Toxicol. 2015, 116, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Sun, L.; Lin, S.; Bai, X.; Yu, B.; Yuan, S.; Zhang, L. The saponin monomer of dwarf lilyturf tuber, DT-13, inhibits angiogenesis under hypoxia and normoxia via multi-targeting activity. Oncol. Rep. 2013, 29, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Nartowska, J.; Sommer, E.; Pastewka, K.; Sommer, S.; Skopińska-Rózewska, E. Anti-angiogenic activity of convallamaroside, the steroidal saponin isolated from the rhizomes and roots of Convallaria majalis L. Acta Pol. Pharm. -Drug Res. 2004, 61. [Google Scholar]
- Mu, L.-H.; Wang, L.-H.; Wang, Y.-N.; Liu, P.; Yan, C. Antiangiogenic effects of AG36, a triterpenoid saponin from Ardisia gigantifolia stapf. J. Nat. Med. 2020, 74, 732–740. [Google Scholar] [CrossRef]
- Nguyen, N.-L.; Vo, T.-H.; Lin, Y.-C.; Liaw, C.-C.; Lu, M.-K.; Cheng, J.-J.; Chen, M.-C.; Kuo, Y.-H. Arenarosides A–G, Polyhydroxylated Oleanane-Type Saponins from Polycarpaea arenaria and their Cytotoxic and Antiangiogenic Activities. J. Nat. Prod. 2021, 84, 259–267. [Google Scholar] [CrossRef]
- Mochizuki, M.; YOO, Y.; Matsuzawa, K.; Sato, K.; Saiki, I.; Tonooka, S.; Samukawa, K.; Azuma, I. Inhibitory Effect of Tumor Metastasis in Mice by Saponins, Ginsenoside-Rb2, 20(R)- and 20(S)-Ginsenoside-Rg3, of Red ginseng. Biol. Pharm. Bull. 1995, 18, 1197–1202. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.; Weng, Y.; Yu, Y.; Zhang, D.; Fan, W.; Dai, R.; Hu, Z. Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts. Life Sci. 2004, 74, 2467–2478. [Google Scholar] [CrossRef]
- Leung, K.; Cheung, L.; Pon, Y.; Wong, R.; Mak, N.; Fan, T.P.; Au, S.; Tombran-Tink, J.; Wong, A. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen β receptor. Br. J. Pharmacol. 2007, 152, 207–215. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, X.; Kwok, H.-H.; Dong, M.; Liu, Z.; Poon, P.-Y.; Luan, X.; Wong, R.N.-S. Ginsenoside-Rb1-Mediated Anti-angiogenesis via Regulating PEDF and miR-33a through the Activation of PPAR-γ Pathway. Front. Pharmacol. 2017, 8, 783. [Google Scholar] [CrossRef]
- Sato, K.; Mochizuki, M.; Saiki, I.; YOO, Y.; Samukawa, K.; Azuma, I. Inhibition of Tumor Angiogenesis and Metastasis by a Saponin of Panax ginseng, Ginsenoside-Rb2. Biol. Pharm. Bull. 1994, 17, 635–639. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.-J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Shin, K.-O.; Seo, C.-H.; Cho, H.-H.; Oh, S.; Hong, S.-P.; Yoo, H.-S.; Hong, J.-T.; Oh, K.-W.; Lee, Y.-M. Ginsenoside compound K inhibits angiogenesis via regulation of sphingosine kinase-1 in human umbilical vein endothelial cells. Arch. Pharmacal Res. 2014, 37, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-G.; Huang, Y.; Cui, D.-D.; Huang, X.-B.; Mao, S.-H.; Ji, L.-L.; Song, H.-B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009, 9, 250. [Google Scholar] [CrossRef]
- Arai, M.; Hayashi, A.; Sobou, M.; Ishida, S.; Kawachi, T.; Kotoku, N.; Kobayashi, M. Anti-angiogenic effect of triterpenoidal saponins from Polygala senega. J. Nat. Med. 2010, 65, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zuo, Y.; Hou, L.; Dong, L.; Sun, X. Oldhamianoside inhibits the growth of ovarian cancer both in vitro and in vivo via adjusting inflammation and angiogenesis signals. OncoTargets Ther. 2018, ume 11, 6031–6037. [Google Scholar] [CrossRef]
- Wang, F.-L.; Sun, J.-Y.; Wang, Y.; Mu, Y.-L.; Liang, Y.-J.; Chong, Z.-Z.; Qin, S.-H.; Yao, Q.-Q. Oldhamianoside II, a New Triterpenoid Saponin, Prevents Tumor Growth via Inducing Cell Apoptosis and Inhibiting Angiogenesis. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2013, 20, 369–376. [Google Scholar] [CrossRef]
- Kim, J.-D.; Chaudhary, N.; Seo, H.-J.; Kim, M.-Y.; Shin, T.-S. Theasaponin E1 as an effective ingredient for anti-angiogenesis and anti-obesity effects. Biosci. Biotechnol. Biochem. 2014, 78, 279–287. [Google Scholar] [CrossRef]
- Li, B.; Tong, T.; Ren, N.; Rankin, G.; Rojanasakul, Y.; Tu, Y.; Chen, Y. Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis. Molecules 2021, 26, 1681. [Google Scholar] [CrossRef]
- Bian, X.; Zhao, Y.; Guo, X.; Zhang, L.; Li, P.; Fu, T.; Wang, W.; Yin, Y.; Chen, G.; Liu, J. Chiisanoside, a triterpenoid saponin, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. RSC Adv. 2017, 7, 41640–41650. [Google Scholar] [CrossRef]
- Son, M.K.; Jung, K.H.; Hong, S.-W.; Lee, H.-S.; Zheng, H.-M.; Choi, M.-J.; Seo, J.H.; Suh, J.-K.; Hong, S.-S. SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway. Food Chem. 2013, 136, 26–33. [Google Scholar] [CrossRef]
- Son, M.K.; Jung, K.H.; Lee, H.-S.; Lee, H.; Kim, S.J.; Yan, H.H.; Ryu, Y.-L.; Hong, S.-S. SB365, Pulsatilla saponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells. Oncol. Rep. 2013, 30, 801–808. [Google Scholar] [CrossRef]
- Hong, S.W.; Jung, K.H.; Lee, H.S.; Choi, M.J.; Son, M.K.; Zheng, H.M.; Hong, S.S. SB 365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI 3 K/A kt/mTOR signaling pathway. Cancer Sci. 2012, 103, 1929–1937. [Google Scholar] [CrossRef]
- Hong, S.-W.; Jung, K.H.; Lee, H.-S.; Son, M.K.; Yan, H.H.; Kang, N.S.; Lee, J.; Hong, S.-S. SB365, Pulsatilla saponin D, targets c-Met and exerts antiangiogenic and antitumor activities. Carcinogenesis 2013, 34, 2156–2169. [Google Scholar] [CrossRef]
- Tong, X.; Han, L.; Duan, H.; Cui, Y.; Feng, Y.; Zhu, Y.; Chen, Z.; Yang, S. The derivatives of Pulsatilla saponin A, a bioactive compound from Pulsatilla chinensis: Their synthesis, cytotoxicity, haemolytic toxicity and mechanism of action. Eur. J. Med. Chem. 2017, 129, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Son, J.-A.; Chung, W.-Y.; Kim, Y.-S.; Ha, Y.-W.; Park, K.-K. Platycodin D, a triterpene saponin from Platycodon grandiflorum, inhibits vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Cancer Res. 2008, 68, 477. [Google Scholar]
- Luan, X.; Gao, Y.-G.; Guan, Y.-Y.; Xu, J.-R.; Lu, Q.; Zhao, M.; Liu, Y.-R.; Liu, H.-J.; Fang, C.; Chen, H.-Z. Platycodin D inhibits tumor growth by antiangiogenic activity via blocking VEGFR2-mediated signaling pathway. Toxicol. Appl. Pharmacol. 2014, 281, 118–124. [Google Scholar] [CrossRef]
- Hua, H.; Feng, L.; Zhang, X.-P.; Zhang, L.-F.; Jin, J. Anti-angiogenic activity of julibroside J8, a natural product isolated from Albizia julibrissin. Phytomedicine 2009, 16, 703–711. [Google Scholar] [CrossRef]
- Foubert, K.; Breynaert, A.; Theunis, M.; Bossche, R.V.D.; De Meyer, G.; Van Daele, A.; Faizal, A.; Goossens, A.; Geelen, D.; Conway, E.; et al. Evaluation of the Anti-angiogenic Activity of Saponins from Maesa lanceolata by Different Assays. Nat. Prod. Commun. 2012, 7, 1149–1154. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, D.; Zang, W.; Yin, G.; Dai, J.; Sun, Y.U.; Yang, Z.; Hoffman, R.M.; Guo, X. Synergistic Inhibitory Effect of Traditional Chinese Medicine Astragaloside IV and Curcumin on Tumor Growth and Angiogenesis in an Orthotopic Nude-Mouse Model of Human Hepatocellular Carcinoma. Anticancer Res. 2017, 37, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, Y.; Wu, Z.; Liu, B.; Dong, L.; Deng, H.; Tian, J.; Lei, H. Capilliposide B blocks VEGF-induced angiogenesis in vitro in primary human retinal microvascular endothelial cells. Biomed. Pharmacother. 2020, 133, 110999. [Google Scholar] [CrossRef]
- Guan, Y.-Y.; Liu, H.-J.; Luan, X.; Xu, J.-R.; Lu, Q.; Liu, Y.-R.; Gao, Y.-G.; Zhao, M.; Chen, H.-Z.; Fang, C. Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine 2015, 22, 103–110. [Google Scholar] [CrossRef]
- Wu, B.; Zhu, J.; Dai, X.; Ye, L.; Wang, B.; Cheng, H.; Wang, W. Raddeanin A inhibited epithelial-mesenchymal transition (EMT) and angiogenesis in glioblastoma by downregulating β-catenin expression. Int. J. Med Sci. 2021, 18, 1609–1617. [Google Scholar] [CrossRef]
- Lu, D.; Xia, Y.; Tong, B.; Zhang, C.; Pan, R.; Xu, H.; Yang, X.; Dai, Y. In vitro Anti-Angiogenesis Effects and Active Constituents of the Saponin Fraction from Gleditsia sinensis. Integr. Cancer Ther. 2012, 13, 446–457. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Miao, Z.-H. Marine-Derived Angiogenesis Inhibitors for Cancer Therapy. Mar. Drugs 2013, 11, 903–933. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, X.; Tong, Y.; Yi, Y.; Zhang, S.; Liping, L.; Sun, P.; Lin, L.; Ding, J. PE, a new sulfated saponin from sea cucumber, Exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biol. Ther. 2005, 4, 874–882. [Google Scholar] [CrossRef]
- Tian, F.; Zhu, C.-h.; Zhang, X.-w.; Xie, X.; Xin, X.-l.; Yi, Y.-h.; Lin, L.-p.; Geng, M.-y.; Ding, J. Philinopside E, a new sulfated saponin from sea cucumber, blocks the interaction between kinase insert domain-containing receptor (KDR) and αvβ3 integrin via binding to the extracellular domain of KDR. Mol. Pharmacol. 2007, 72, 545–552. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, X.; Tian, F.; Yi, Y.; Xu, Q.; Li, L.; Tong, L.; Lin, L.; Ding, J. Philinopside a, a novel marine-derived compound possessing dual anti-angiogenic and anti-tumor effects. Int. J. Cancer 2005, 114, 843–853. [Google Scholar] [CrossRef]
- Attoub, S.; Arafat, K.; Gélaude, A.; Al Sultan, M.A.; Bracke, M.; Collin, P.; Takahashi, T.; Adrian, T.E.; De Wever, O. Frondoside A Suppressive Effects on Lung Cancer Survival, Tumor Growth, Angiogenesis, Invasion, and Metastasis. PLoS ONE 2013, 8, e53087. [Google Scholar] [CrossRef]
- Soltani, M.; Parivar, K.; Baharara, J.; Kerachian, M.A.; Asili, J. Transcriptional analysis of VEGF-D and TGFβ genes in MCF7 cells exposed to saponin isolated from Holothuria leucospilota (sea cucumber). Rep. Biochem. Mol. Biol. 2015, 4, 25–31. [Google Scholar]
- Ahmad, S.; Ullah, F.; Ayaz, M.; Zeb, A.; Ullah, F.; Sadiq, A. Antitumor and anti-angiogenic potentials of isolated crude saponins and various fractions of Rumexhastatus D. Don. Biol. Res. 2016, 49, 1–9. [Google Scholar] [CrossRef]
- Law, P.-C.; Auyeung, K.K.; Chan, L.-Y.; Ko, J.K. Astragalus saponins downregulate vascular endothelial growth factor under cobalt chloride-stimulated hypoxia in colon cancer cells. BMC Complement. Altern. Med. 2012, 12, 160. [Google Scholar] [CrossRef]
- Auyeung, K.; Woo, P.; Law, P.; Ko, J. Astragalus saponins modulate cell invasiveness and angiogenesis in human gastric adenocarcinoma cells. J. Ethnopharmacol. 2012, 141, 635–641. [Google Scholar] [CrossRef]
- Zheng, G.-Y.; Xin, H.-L.; Xu, Y.-F.; Li, B.; Zhai, X.-F.; Zhang, Y.-H.; Ling, C.-Q. Total saponin from root of actinidia valvata Dunn inhibits hepatoma 22 growth and metastasis in vivo by suppression angiogenesis. Evid.-Based Complement. Altern. Med. 2012, 2012, 432814. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.H.; Joshi, S.V. Anticancer activity of saponin isolated from Albizia lebbeck using various in vitro models. J. Ethnopharmacol. 2018, 231, 494–502. [Google Scholar] [CrossRef]
- Oakenfull, D. Saponins in food—A review. Food Chem. 1981, 7, 19–40. [Google Scholar] [CrossRef]
- Shu, Y.; Cao, M.; Yin, Z.-Q.; Li, P.; Li, T.-Q.; Long, X.-F.; Zhu, L.-F.; Jia, R.-Y.; Dai, S.-J.; Zhao, J. The reproductive toxicity of saponins isolated from Cortex Albiziae in female mice. Chin. J. Nat. Med. 2015, 13, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.; Kerem, Z.; Makkar, H.P.S.; Becker, K. The biological action of saponins in animal systems: A review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Wisløff, H.; Uhlig, S.; Scheie, E.; Loader, J.; Wilkins, A.; Flåøyen, A. Toxicity testing of saponin-containing Yucca schidigera Roetzl. juice in relation to hepato-and nephrotoxicity of Narthecium ossifragum (L.) Huds. Toxicon 2008, 51, 140–150. [Google Scholar] [CrossRef]

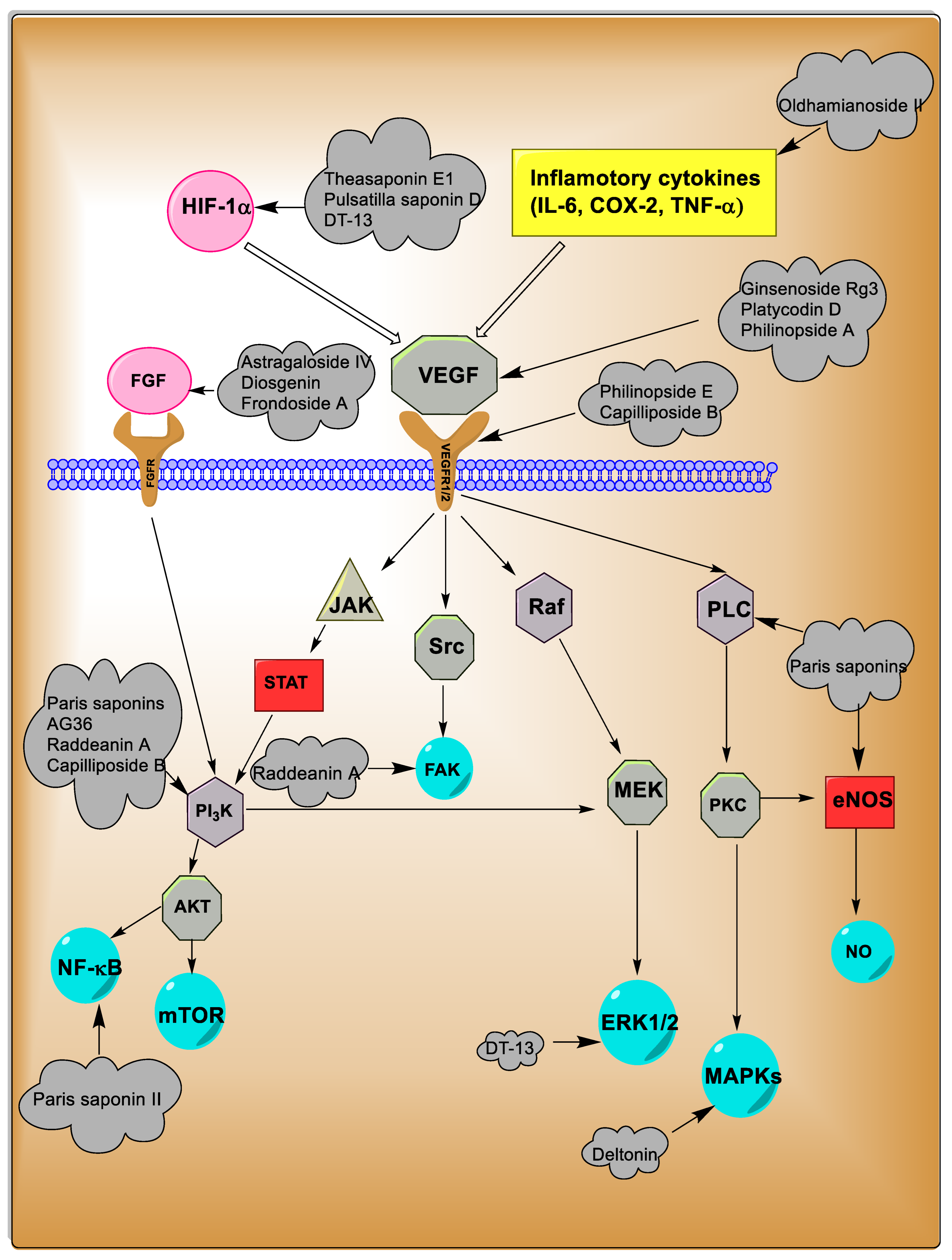
| Saponins | Anti-Angiogenesis Mechanisms | Administration Rout/Dosage | Natural Sources | References |
|---|---|---|---|---|
| Terpenoid saponin | ||||
| AG36 | ↓ VEGF and FAK/PI3K/Akt gene expression | In vivo study; 75 and 1.5 mg/kg/day/i.p. in vitro study; 20 5, 10, and 20 μM | Ardisia gigantifolia (Primulaceae) | [188] |
| Arenaroside C, D, E, and G | NR | In vitro study; <5 μM | Polycarpaea arenaria (Caryophyllaceae) | [189] |
| Ginsenoside-Rb1 | ↑ PEDF and PPAR-γ gene expression | In vitro study; 100–500 nM | Panax spp. (Araliaceae) | [192,193] |
| Ginsenoside-Rb2 | NR | In vivo study; 100 µg/mouse, i.v. | Panax spp. (Araliaceae) | [194] |
| Ginsenoside K | ↓ SPHK1 activities | In vitro study; 10 µg/mL | Panax ginseng (Araliaceae) | [196] |
| Ginsenoside Rg3 | ↓ VEGF gene expression | In vivo study; 20 mg/kg | Panax spp. (Araliaceae) | [197] |
| senegin III | ↑ PEDF, inhibition VEGF activities | In vitro study; 0.1, 1, and 10 μM | Polygala senega (Polygalaceae) | [198] |
| Theasaponin E1 | ↓ VEGF, Akt, and HIF-1α gene expression | In vitro study; 2 μM and 10 μg/mL | Camellia sinensis (Theaceae) | [201,202] |
| Oldhamianoside II | ↓ VEGF and VEGFR2, COX-2, bFGF gene expression, ↓ pro-inflammatory cytokines activities including TNF-α and IL-6 | In vivo study; 5, 10, and 20 mg/kg, i.p. | Gypsophila oldhamiana (Caryophyllaceae) | [170,200] |
| Platycodin D | ↓ VEGFR2, PLCγ1, JAK2, FAK, Src, and Akt gene expression | In vitro study; 0.3, 1, 3, 10, and 30 μM | Platycodon grandiflorus (Campanulaceae) | [209,210] |
| Julibroside J8 | NR | In vivo study; 0.5, 1.5, and 3 mg/kg, p.o. In vitro study; 0.5, 1, 2, and 4 μg/mL. Ex vivo study 30 and 50 μg/egg | Albizia julibrissin (Fabaceae) | [211] |
| Maesasaponins (I-VII.1) | 25 and 50 μg/mL for in vivo (zebrafish model) and in vitro studies. Ex vivo study 1 µg/pellet. | Maesa lanceolata (Myrsinaceae) | [212] | |
| Astragaloside IV | ↓ VEGF and FGF2 gene expression | In vivo study; 20 mg/kg, p.o. | Astragalus membranaceus (Fabaceae) | [213] |
| Capilliposide B | ↓ VEGF, Erk, VEGFR2, and Akt gene expression | In vitro study; 0.25, 0.5, 1 μM | Lysimachia capillipes (Primulaceae) | [214] |
| Chiisanoside | ↓ VEGF gene expression | In vivo study; 60, 120, and 240 mg/kg, i.p. | Acanthopanax sessiliflorus (Araliaceae) | [203] |
| Pulsatilla saponin D | ↓ VEGF and HIF-1α gene expression | In vitro study; 10 μM | Pulsatilla koreana (Ranunculaceae) | [204,205,206,207] |
| Gleditsiosides B | ↓ VEGF and bFGF gene expression | In vitro study; 1 μM | Gleditsia sinensis (Fabaceae) | [217] |
| Raddeanin A | ↓ PLCγ 1, JAK2, FAK, Src, and Akt and VEGFR2 activities and ↓ Wnt/β-catenin gene expression | In vivo study; 100 mg/kg/day, i.p. in vitro study; 100, 200 nM, 0.1, 0.3, 1, and 3 μM. Ex vivo study; 0.3, 1, 3, and 10 μM. | Anemone raddeana (Ranunculaceae) | [215,216] |
| Philinopside E * | ↓ (KDR)/VEGFR2 gene expression | In vivo study; 2 mg/kg and 3 mg/kg, i.v. In vitro study; 1.25, 2.5, and 5 μM. Ex vivo; 2.5, 5, 10, and 10 nM/egg | pentacta quadrangularis (Cucumariidae) | [219,220] |
| Philinopside A * | ↓ VEGF, bFGF and PDGF gene expression | In vivo study; 1, 2, and 4 mg/kg, i.v. In vitro study; 50 μM. Ex vivo study, 10 nM/egg | pentacta quadrangularis (Cucumariidae) | [221] |
| Frondoside A * | ↓ bFGF activities | In vivo study; 1 and 0.01 mg/kg/day, i.p. Ex vivo study 100 and 500 nM/egg | Cucumaria frondose (Cucumariidae) | [222] |
| Steroidal saponin | ||||
| Diosgenin | ↓ VEGF and bFGF gene expression | In vitro study; 5, 15, and 25 µM. | Dioscorea spp. (Dioscoreaceae), Trigonella foenum-graecum (Fabaceae) | [177] |
| Deltonin | ↓ VEGF, MAPK/AKT pathway activities | In vitro study; 1 and 4 μM | Dioscorea zingiberensis (Dioscoreaceae) | [178] |
| Terrestrosin D | Direct inhibition of vascular endothelial cell proliferation | In vivo study; 20 mg/kg, i.p. | Tribulus terrestris (Zygophyllaceae) | [180] |
| Paris saponin II | ↓ NF-κB gene expression | In vivo study; 25 mg/kg, i.p. In vitro study 2.5 μM. | Paris polyphylla (Melanthiaceae) | [183,184] |
| Paris saponin I, II, Ⅵ and Ⅶ | ↓ PI3K/AKT/mTOR/S6K1, SRC/eNOS, PLCγ/ERK and JAK2/STAT3 and R2 gene expression | In vitro study, 2 and 4 μM | Paris polyphylla (Melanthiaceae) | [181] |
| Polyphyllin D | Direct inhibition of vascular endothelial cell proliferation | In vivo study; 0.313 μM and 0.156 μM, zebrafish model. In vitro study, 200, 300, and 400 nM | Paris polyphylla (Melanthiaceae) | [182] |
| ACS | ↓ MMP 2 and 9 gene expression | In vivo study; 5 μM, i.p. In vitro study 1.25, 2.5, and 5 μM | Ophiopogon japonicas (Asparagaceae) | [185] |
| DT-13 | ↓ ERK1/2, HIF-1α and Akt gene expression | In vitro study, 0.01, 0.1, and 1 μM. Ex vivo study; 100, 10, and 1 μmol/egg. | Ophiopogon japonicas (Asparagaceae) | [186] |
| Convallamaroside | NR | In vivo study; 5, 10, 20, 50, and 100 μg/mL, p.o. In vitro study; 10 and 50 μg/mL | Convallaria majalis (Asparagaceae) | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majnooni, M.B.; Fakhri, S.; Ghanadian, S.M.; Bahrami, G.; Mansouri, K.; Iranpanah, A.; Farzaei, M.H.; Mojarrab, M. Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways. Metabolites 2023, 13, 323. https://doi.org/10.3390/metabo13030323
Majnooni MB, Fakhri S, Ghanadian SM, Bahrami G, Mansouri K, Iranpanah A, Farzaei MH, Mojarrab M. Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways. Metabolites. 2023; 13(3):323. https://doi.org/10.3390/metabo13030323
Chicago/Turabian StyleMajnooni, Mohammad Bagher, Sajad Fakhri, Syed Mustafa Ghanadian, Gholamreza Bahrami, Kamran Mansouri, Amin Iranpanah, Mohammad Hosein Farzaei, and Mahdi Mojarrab. 2023. "Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways" Metabolites 13, no. 3: 323. https://doi.org/10.3390/metabo13030323
APA StyleMajnooni, M. B., Fakhri, S., Ghanadian, S. M., Bahrami, G., Mansouri, K., Iranpanah, A., Farzaei, M. H., & Mojarrab, M. (2023). Inhibiting Angiogenesis by Anti-Cancer Saponins: From Phytochemistry to Cellular Signaling Pathways. Metabolites, 13(3), 323. https://doi.org/10.3390/metabo13030323








