Ischemic Stroke Causes Disruptions in the Carnitine Shuttle System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Pneumatically Assisted (PA) Nano-DESI
2.3. Middle Cerebral Artery Occlusion Mouse Model
2.4. Mass Spectrometry Imaging
2.5. MS/MS of Carnitine Standards and Endogenous Carnitines
2.6. Data Processing and Statistical Analysis
3. Results
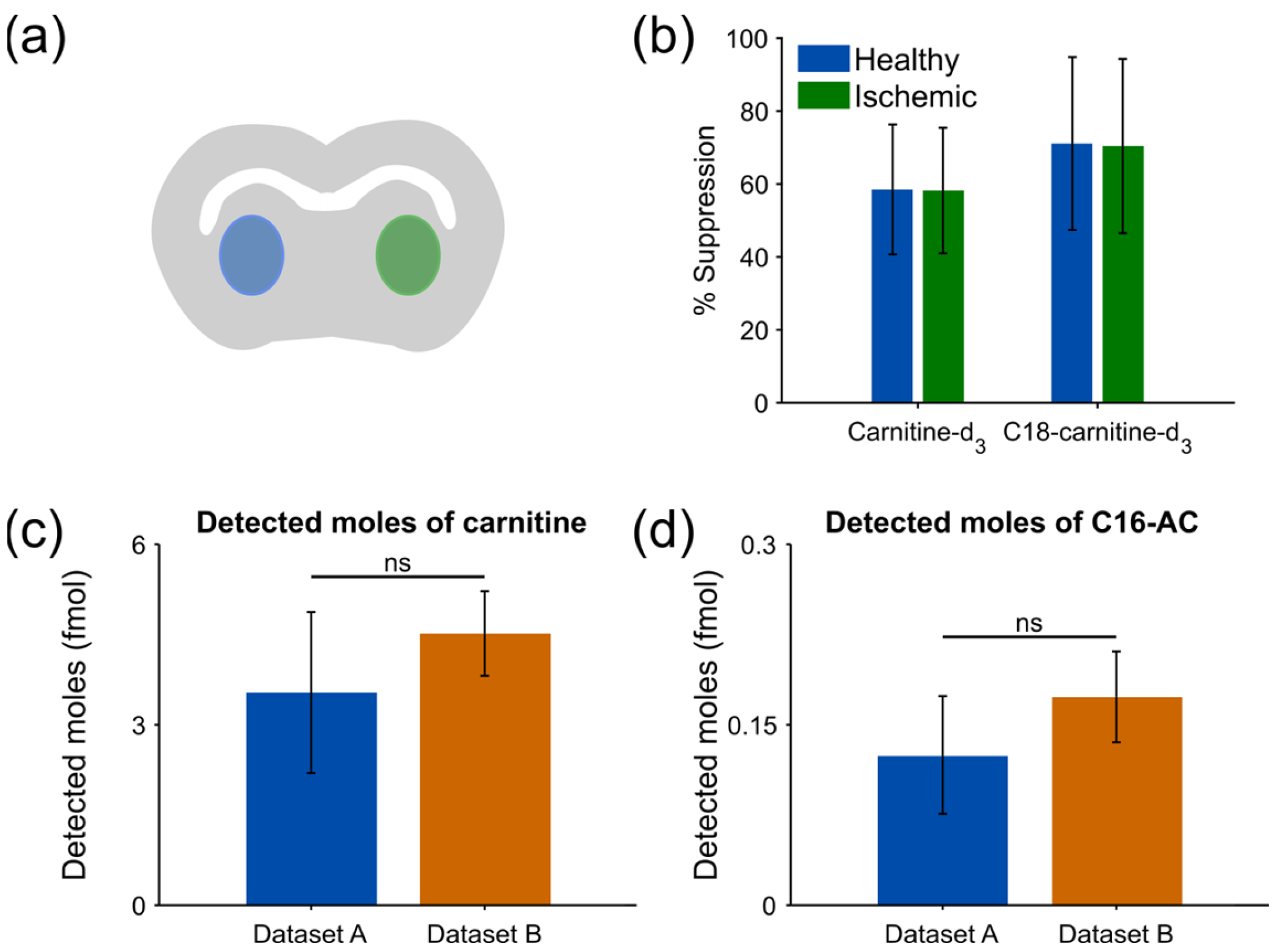
3.1. Matrix Effects between Healthy and Ischemic Region Are of Equal Magnitude
3.2. Use of Internal Standards Allows for Robust Relative Quantification of Endogenous Molecules
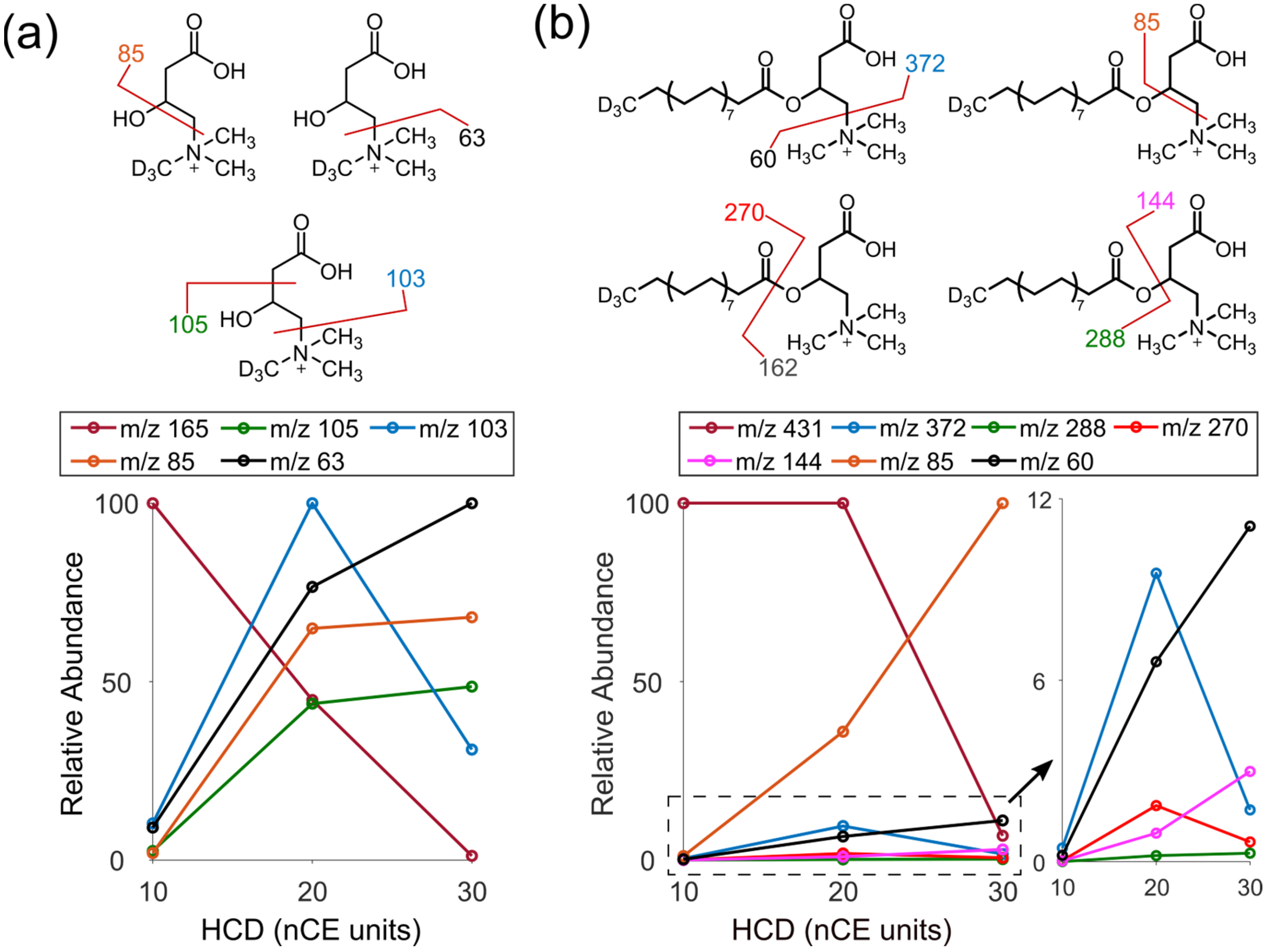
3.3. Identification of Endogenous Acylcarnitines through MS/MS
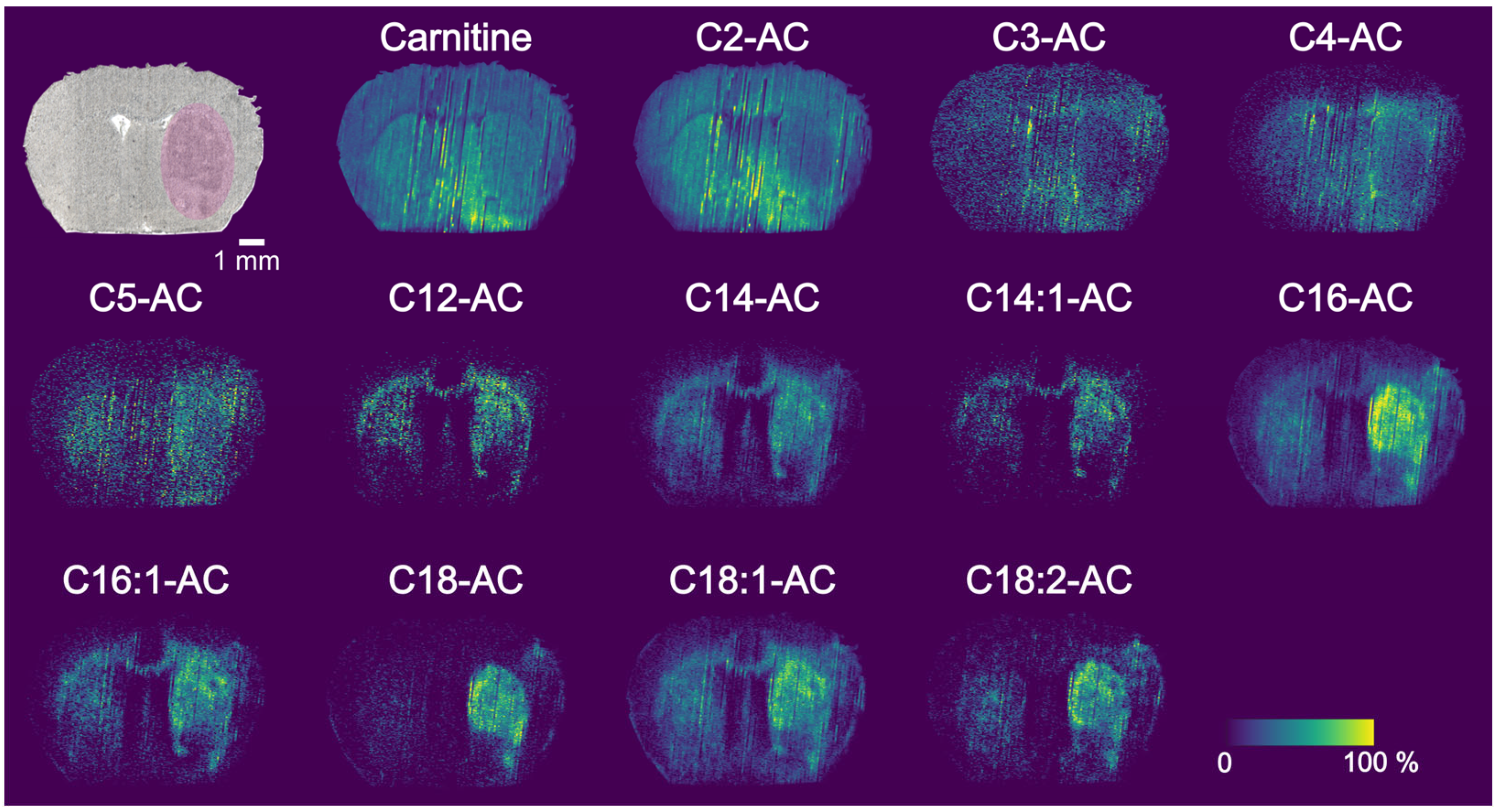
3.4. PA Nano-DESI MSI of MCAO Stroke Model
3.5. Region of Interest Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.J. Ischemic Stroke: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Doyle, K.P.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of Ischemic Brain Damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Reiser, G. Why Does Brain Metabolism Not Favor Burning of Fatty Acids to Provide Energy-Reflections on Disadvantages of the Use of Free Fatty Acids as Fuel for Brain. J. Cereb. Blood Flow Metab. 2013, 33, 1493–1499. [Google Scholar] [CrossRef]
- Ebert, D.; Haller, R.G.; Walton, M.E. Energy Contribution of Octanoate to Intact Rat Brain Metabolism Measured by 13C Nuclear Magnetic Resonance Spectroscopy. J. Neurosci. 2003, 23, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; De la Rosa, M.V.G.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines: Pharmacokinetic, Pharmacological and Clinical Aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Wu, T.; Zheng, X.; Yang, M.; Zhao, A.; Li, M.; Chen, T.; Panee, J.; Jia, W.; Ji, G. Serum Lipid Alterations Identified in Chronic Hepatitis B, Hepatitis B Virus-Associated Cirrhosis and Carcinoma Patients. Sci. Rep. 2017, 7, 42710. [Google Scholar] [CrossRef]
- Saiki, S.; Hatano, T.; Fujimaki, M.; Ishikawa, K.I.; Mori, A.; Oji, Y.; Okuzumi, A.; Fukuhara, T.; Koinuma, T.; Imamichi, Y.; et al. Decreased Long-Chain Acylcarnitines from Insufficient β-Oxidation as Potential Early Diagnostic Markers for Parkinson’s Disease. Sci. Rep. 2017, 7, 7328. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; Delany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Chumachenko, M.S.; Waseem, T.V.; Fedorovich, S.V. Metabolomics and Metabolites in Ischemic Stroke. Rev. Neurosci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Sun, W.; Pei, L.L.; Tian, M.; Liang, J.; Liu, X.; Zhang, R.; Fang, H.; Wu, J.; et al. Changes of Metabolites in Acute Ischemic Stroke and Its Subtypes. Front. Neurosci. 2021, 14, 580929. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Wei, J.; Tian, X.Y.; Wang, B.; Chan, W.; Li, S.; Tang, Z.; Zhang, H.; Cheang, W.S.; Zhao, Q.; et al. Comprehensive Analysis of Acylcarnitine Species in Db/Db Mouse Using a Novel Method of High-Resolution Parallel Reaction Monitoring Reveals Widespread Metabolic Dysfunction Induced by Diabetes. Anal. Chem. 2017, 89, 10368–10375. [Google Scholar] [CrossRef]
- Spengler, B. Mass Spectrometry Imaging of Biomolecular Information. Anal. Chem. 2015, 87, 64–82. [Google Scholar] [CrossRef]
- Neumann, E.K.; Djambazova, K.V.; Caprioli, R.M.; Spraggins, J.M. Multimodal Imaging Mass Spectrometry: Next Generation Molecular Mapping in Biology and Medicine. J. Am. Soc. Mass Spectrom. 2020, 31, 2401–2415. [Google Scholar] [CrossRef]
- Laskin, J.; Lanekoff, I. Ambient Mass Spectrometry Imaging Using Direct Liquid Extraction Techniques. Anal. Chem. 2016, 88, 52–73. [Google Scholar] [CrossRef]
- Roach, P.J.; Laskin, J.; Laskin, A. Nanospray Desorption Electrospray Ionization: An Ambient Method for Liquid-Extraction Surface Sampling in Mass Spectrometry. Analyst 2010, 135, 2233–2236. [Google Scholar] [CrossRef]
- Laskin, J.; Heath, B.S.; Roach, P.J.; Cazares, L.; Semmes, O.J. Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2012, 46, 141–148. [Google Scholar] [CrossRef]
- Duncan, K.D.; Bergman, H.M.; Lanekoff, I. A Pneumatically Assisted Nanospray Desorption Electrospray Ionization Source for Increased Solvent Versatility and Enhanced Metabolite Detection from Tissue. Analyst 2017, 142, 3424–3431. [Google Scholar] [CrossRef]
- Lanekoff, I.; Stevens, S.L.; Stenzel-Poore, M.P.; Laskin, J. Matrix Effects in Biological Mass Spectrometry Imaging: Identification and Compensation. Analyst 2014, 139, 3528–3532. [Google Scholar] [CrossRef] [PubMed]
- Lanekoff, I.; Thomas, M.; Laskin, J. Shotgun Approach for Quantitative Imaging of Phospholipids Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2014, 86, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Mavroudakis, L.; Duncan, K.D.; Lanekoff, I. Host-Guest Chemistry for Simultaneous Imaging of Endogenous Alkali Metals and Metabolites with Mass Spectrometry. Anal. Chem. 2022, 94, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.D.; Fang, R.; Yuan, J.; Chu, R.K.; Dey, S.K.; Burnum-Johnson, K.E.; Lanekoff, I. Quantitative Mass Spectrometry Imaging of Prostaglandins as Silver Ion Adducts with Nanospray Desorption Electrospray Ionization. Anal. Chem. 2018, 90, 7246–7252. [Google Scholar] [CrossRef]
- Lanekoff, I.; Thomas, M.; Carson, J.P.; Smith, J.N.; Timchalk, C.; Laskin, J. Imaging Nicotine in Rat Brain Tissue by Use of Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2013, 85, 882–889. [Google Scholar] [CrossRef]
- Lanekoff, I.; Heath, B.S.; Liyu, A.; Thomas, M.; Carson, J.P.; Laskin, J. Automated Platform for High-Resolution Tissue Imaging Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2012, 84, 8351–8356. [Google Scholar] [CrossRef]
- Duncan, K.D.; Lanekoff, I. Oversampling to Improve Spatial Resolution for Liquid Extraction Mass Spectrometry Imaging. Anal. Chem. 2018, 90, 2451–2455. [Google Scholar] [CrossRef]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open Source Software for Rapid Proteomics Tools Development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Mavroudakis, L.; Stevens, S.L.; Duncan, K.D.; Stenzel-poore, M.P.; Laskin, J.; Lanekoff, I. CpG Preconditioning Reduces Accumulation of Lysophosphatidylcholine in Ischemic Brain Tissue after Middle Cerebral Artery Occlusion. Anal. Bioanal. Chem. 2021, 413, 2735–2745. [Google Scholar] [CrossRef]
- Koizumi, S.; Yamamoto, S.; Hayasaka, T.; Konishi, Y.; Yamaguchi-Okada, M.; Goto-Inoue, N.; Sugiura, Y.; Setou, M.; Namba, H. Imaging Mass Spectrometry Revealed the Production of Lyso-Phosphatidylcholine in the Injured Ischemic Rat Brain. Neuroscience 2010, 168, 291–325. [Google Scholar] [CrossRef]
- Lanekoff, I.; Laskin, J. Quantitative Mass Spectrometry Imaging of Molecules in Biological Systems. In Advances in Chromatography; Grinberg, N., Grushka, E., Eds.; Taylor & Francis: Abingdon, UK, 2017; p. 30. ISBN 9781351652971. [Google Scholar]
- Mallah, K.; Quanico, J.; Ra, A.; Cardon, T.; Aboulouard, S.; Devos, D.; Kobeissy, F.; Zibara, K.; Salzet, M.; Fournier, I.; et al. Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging of Lipids in Experimental Model of Traumatic Brain Injury Detecting Acylcarnitines as Injury Related Markers. Anal. Chem. 2019, 91, 11879–11887. [Google Scholar] [CrossRef]
- Mulder, I.A.; Ogrinc Potočnik, N.; Broos, L.A.M.; Prop, A.; Wermer, M.J.H.; Heeren, R.M.A.; van den Maagdenberg, A.M.J.M. Distinguishing Core from Penumbra by Lipid Profiles Using Mass Spectrometry Imaging in a Transgenic Mouse Model of Ischemic Stroke. Sci. Rep. 2019, 9, 1090. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gao, D.; Jiang, Y. Function, Detection and Alteration of Acylcarnitine Metabolism in Hepatocellular Carcinoma. Metabolites 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Kanekar, S.G.; Zacharia, T.; Roller, R. Imaging of Stroke: Part 2, Pathophysiology at the Molecular and Cellular Levels and Corresponding Imaging Changes. Am. J. Roentgenol. 2012, 198, 63–74. [Google Scholar] [CrossRef] [PubMed]
- López-Suárez, O.; Concheiro-Guisán, A.; Sánchez-Pintos, P.; Cocho, J.A.; Fernández Lorenzo, J.R.; Couce, M.L. Acylcarnitine Profile in Neonatal Hypoxic-Ischemic Encephalopathy. Medicine 2019, 98, e15221. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B.; Bischoff, C.; Christensen, E.; Simonsen, H.; Lund, A.M.; Young, S.P.; Koeberl, D.D.; Millington, D.S.; Roe, C.R.; Roe, D.S.; et al. Variations in IBD (ACAD8) in Children with Elevated C4-Carnitine Detected by Tandem Mass Spectrometry Newborn Screening. Pediatr. Res. 2006, 60, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in Brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Lin, C.-T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher-Wellman, K.; Ellis, J.M. Skeletal Muscle Undergoes Fiber Type Metabolic Switch without Myosin Heavy Chain Switch in Response to Defective Fatty Acid Oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines--Old Actors Auditioning for New Roles in Metabolic Physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty Acid Oxidation and Carnitine Palmitoyltransferase I: Emerging Therapeutic Targets in Cancer. Cell Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Brindle, N.P.; Zammit, V.A.; Pogson, C.I. Regulation of Carnitine Palmitoyltransferase Activity by Malonyl-CoA in Mitochondria from Sheep Liver, a Tissue with a Low Capacity for Fatty Acid Synthesis. Biochem. J. 1985, 232, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elheiga, L.; Matzuk, M.M.; Abo-Hashema, K.A.H.; Wakil, S.J. Continuous Fatty Acid Oxidation and Reduced Fat Storage in Mice Lacking Acetyl-CoA Carboxylase 2. Science 2001, 291, 2613–2616. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Dean, D. Malonyl CoA, Long Chain Fatty Acyl CoA and Insulin Resistance in Skeletal Muscle. J. Basic Clin. Physiol. Pharmacol. 1998, 9, 295–308. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudakis, L.; Lanekoff, I. Ischemic Stroke Causes Disruptions in the Carnitine Shuttle System. Metabolites 2023, 13, 278. https://doi.org/10.3390/metabo13020278
Mavroudakis L, Lanekoff I. Ischemic Stroke Causes Disruptions in the Carnitine Shuttle System. Metabolites. 2023; 13(2):278. https://doi.org/10.3390/metabo13020278
Chicago/Turabian StyleMavroudakis, Leonidas, and Ingela Lanekoff. 2023. "Ischemic Stroke Causes Disruptions in the Carnitine Shuttle System" Metabolites 13, no. 2: 278. https://doi.org/10.3390/metabo13020278
APA StyleMavroudakis, L., & Lanekoff, I. (2023). Ischemic Stroke Causes Disruptions in the Carnitine Shuttle System. Metabolites, 13(2), 278. https://doi.org/10.3390/metabo13020278





