Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review
Abstract
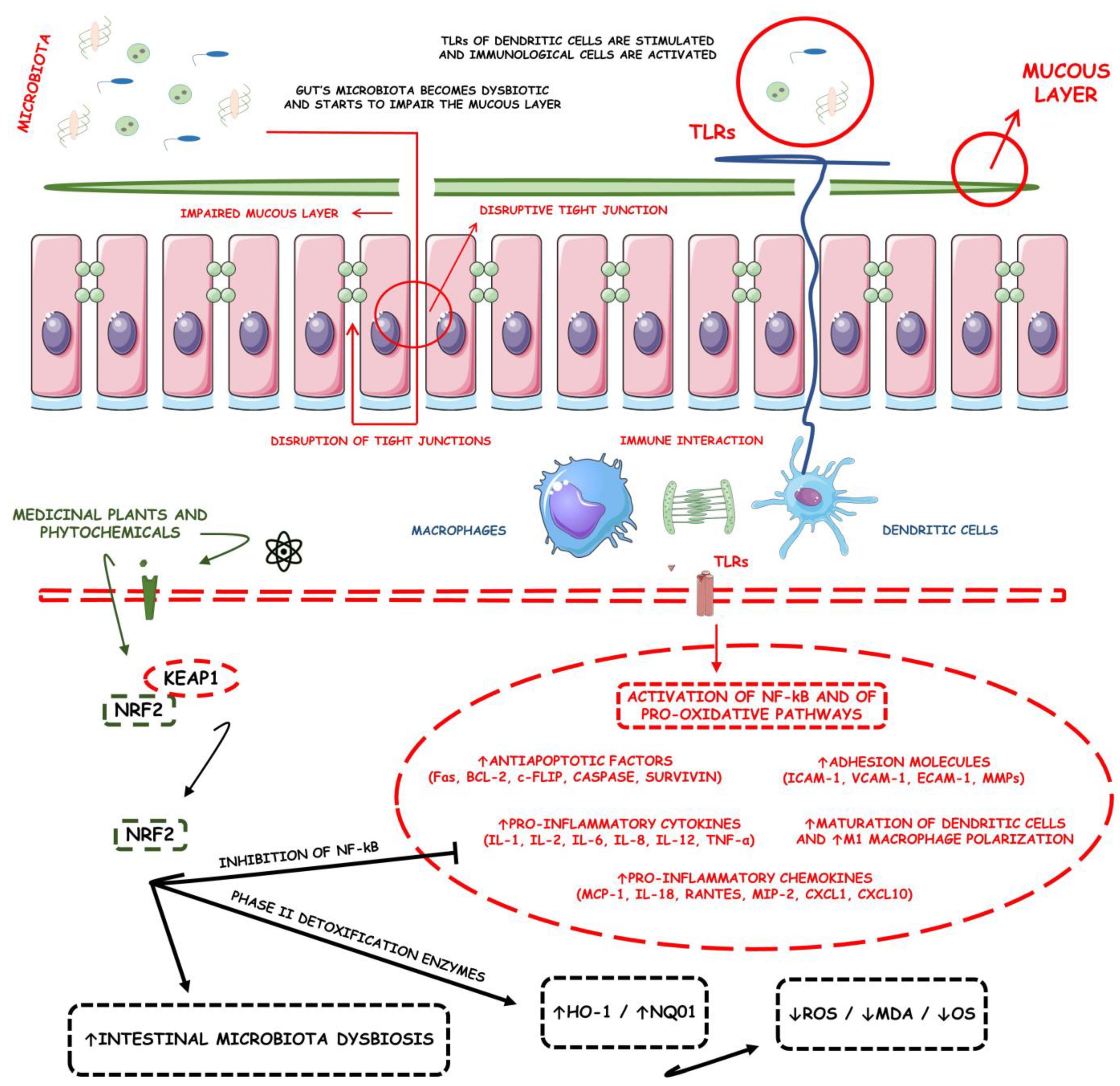
1. Introduction
2. Physiopathology of CD
2.1. Definition and General Aspects
2.2. Genetics Influencing the Pathogenesis of CD
2.3. Immune Response in CD
3. Physiopathology of UC
3.1. Definition and General Aspects
3.2. Genetics Influencing the Pathogenesis of Ulcerative Colitis
3.3. Genetics Influencing the Pathogenesis of Ulcerative Colitis
4. Nrf2 Signaling Pathway
4.1. Historical Perspective of Nrf2
4.2. Nrf2 Regulation, Signaling Pathways, and Repercussions
4.3. Nrf2 Regulation by Phytochemicals
4.4. Nrf2 Signaling Pathway: Implications for IBDs
5. Medicinal Plants That Target Nrf2 Pathways
5.1. Cynara cardunculus L.
5.2. Panax ginseng
5.3. Rose odorata sweet var. gigantean
5.4. Ficus pandurata Hance
5.5. Moringa oleifera Lam
5.6. Aucklandia lappa Decne
5.7. Vaccinium myrtillus and Ribes nigrum
5.8. Mesua assamica (King&Prain) Kosterm
5.9. Vaccinium myrtillus
5.10. Vitis vinifera
5.11. Acanthopanax senticosus
5.12. Forsythia suspensa
5.13. Artemisia argyi
5.14. Dendrobium fimbriatum
5.15. Pisum sativum L.
5.16. Tetrastigma hemsleyanum
5.17. Rhus chinensis Mill
5.18. Crocus sativus
5.19. Prunus mahaleb
5.20. Quercus ilex L.
5.21. Ziziphus spina-christi
5.22. Perilla frutescens
6. Phytochemicals Targeting Nrf2 Pathways
6.1. Bioactive 6-Shogaol
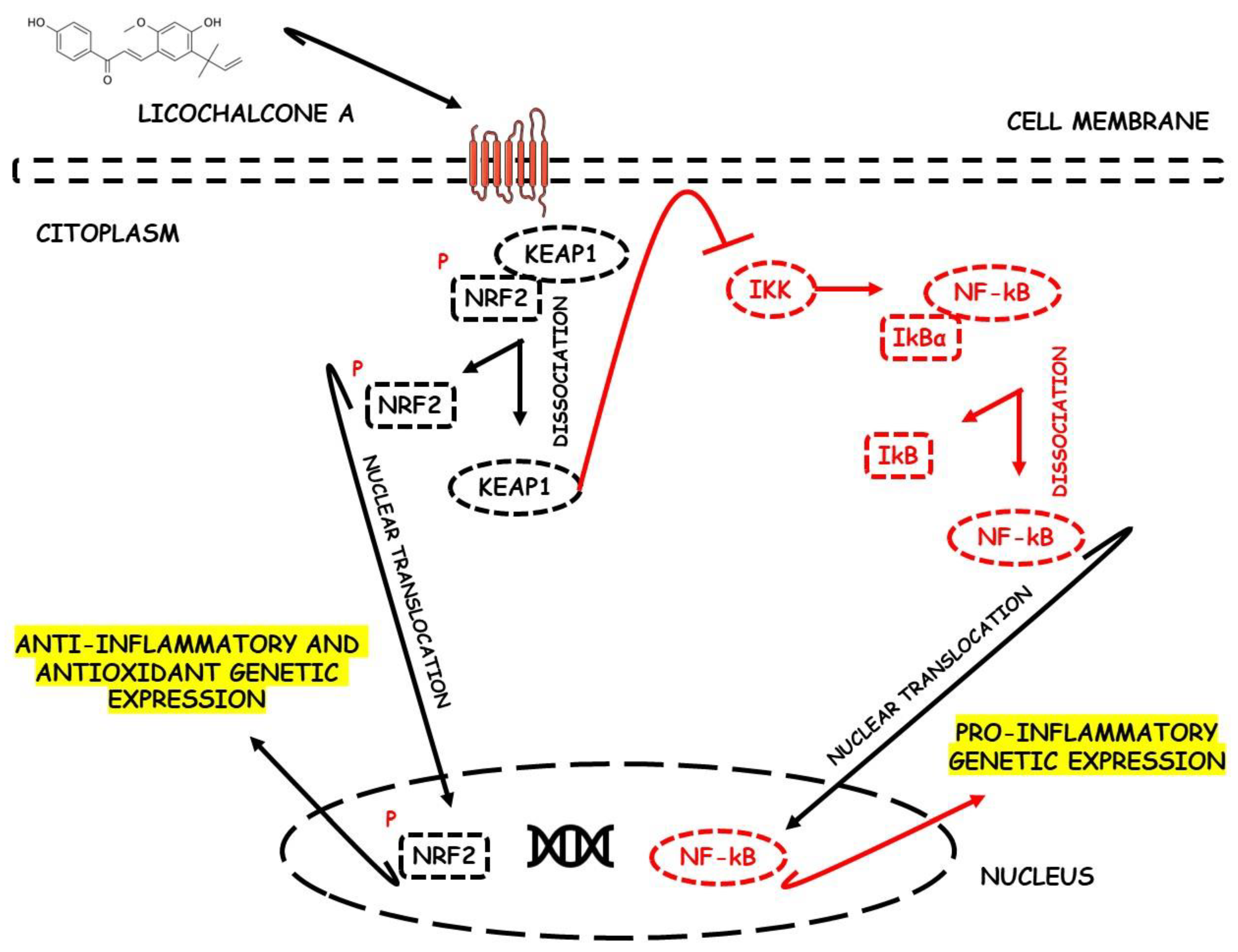
6.2. Licochalcone A
6.3. Oligonol
6.4. Cyanidin-3-O-Glucoside
6.5. Luteolin
6.6. Alpinetin
6.7. Cardamonin
6.8. Puerarin
6.9. Gallic Acid
6.10. Sulforaphane
6.11. Asperuloside
6.12. Syringin
6.13. Paeoniflorin
6.14. Dehydrocostus Lactone
6.15. Leonurine
6.16. Crocin
6.17. Quercetin
6.18. OPAL, 8-Oxypalmatine
6.19. PMID, 3-(3-Pyridylmethylidene)-2-Indolinone
6.20. Ruscogenins
6.21. Caffeic Acid
6.22. Schisandrin B
6.23. GB1a
6.24. Diosmetin
6.25. Atractylenolide III
6.26. Polydatin
6.27. Rosmarinic Acid
6.28. Imperatorin
6.29. Berberine
6.30. Curcumin
6.31. Sesamin
6.32. Toosendanin
6.33. Galangin
6.34. Apocynin
6.35. Hesperidin
6.36. Norisoboldine
6.37. Hyperoside
6.38. Glyceollins
6.39. Carnosic Acid
6.40. Protocatechuic Acid
7. Medicinal Plants and Phytochemicals Targeting the Nrf2 Signaling Pathway during IBD-Related Colorectal Cancer Models
7.1. Procyanidin B2
7.2. Resveratrol
7.3. Digitoflavone (Dietary)
7.4. Theobroma cacao
7.5. Tussilagone
7.6. Glucosinolates
7.7. Crocin
7.8. Peracetylated (−)-Epigallocatechin-3-Gallate (AcEGCG)
7.9. Pterostilbene
7.10. Nobiletin
7.11. Wogonin
7.12. Cinnamaldehyde
8. Future Perspectives
8.1. Algae-Derived Constituents in Regulating Nrf2 in IBD Models
8.2. Fungus-Derived Constituents Regulating Nrf2 in IBD Models
8.3. Bee Pollen Regulating Nrf2 in IBD Models
8.4. Thai Royal Jelly Regulating Nrf2 in IBD Models
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.Y.; Kim, K.D.; Yoo, J.; Lee, J.H.; Hwangbo, C. Phytochemicals Targeting JAK-STAT Pathways in Inflammatory Bowel Disease: Insights from Animal Models. Molecules 2021, 26, 2824. [Google Scholar] [CrossRef]
- Dai, W.; Long, L.; Wang, X.; Li, S.; Xu, H. Phytochemicals targeting Toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin. Med. 2022, 17, 53. [Google Scholar] [CrossRef]
- Goulart, R.A.; Barbalho, S.M. Can vitamin D induce remission in patients with inflammatory bowel disease? Ann. Gastroenterol. 2022, 35, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Matias, J.N.; Lima, V.M.; Nutels, G.S.; Laurindo, L.F.; Barbalho, S.M.; de Alvares Goulart, R.; Araújo, A.C.; Suzuki, R.B.; Guiguer, E.L. The use of vitamin D for patients with inflammatory bowel diseases. Int. J. Vitam. Nutr. Res. 2022. [Google Scholar] [CrossRef]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.A.; Araújo, A.C.; Bechara, M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Matias, J.N.; Flato, U.A.P.; Pilon, J.P.G.; Bitelli, P.; Pagani, M.A., Jr.; de Carvalho, A.C.A.; Haber, J.; Reis, C.H.B.; Goulart, R.A. What Do Influenza and COVID-19 Represent for Patients With Inflammatory Bowel Disease? Gastroenterol. Res. 2021, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goulart, R.A.; Barbalho, S.M.; Rubira, C.J.; Araújo, A.C.; Lima, V.M.; Rogerio Leoni, B.; Guiguer, E.L. Curcumin therapy for ulcerative colitis remission: Systematic review and meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Swierczynski, M.; Fichna, J.; Piechota-Polanczyk, A. The Nrf2 in the pathophysiology of the intestine: Molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021, 163, 105243. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Huang, C.; Chen, J.; Zhi, F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Aparna, J.S.; Babu, A.; Paul, A.M.; Lankadasari, M.B.; Athira, S.R.; Kumar, S.S.; Vijayan, Y.; Namitha, N.N.; Mohammed, S.; et al. Cardamonin Attenuates Experimental Colitis and Associated Colorectal Cancer. Biomolecules 2021, 11, 661. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Nouri, Z.; Cao, H.; Wang, H.; Khan, H.; Xiao, J. Modulation of integrin receptor by polyphenols: Downstream Nrf2-Keap1/ARE and associated cross-talk mediators in cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2022, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and anti-inflammation effects of dietary phytochemicals: The Nrf2/NF-κB signalling pathway and upstream factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef]
- Speciale, A.; Muscarà, C.; Molonia, M.S.; Toscano, G.; Cimino, F.; Saija, A. In Vitro Protective Effects of a Standardized Extract From Cynara cardunculus L. Leaves Against TNF-α-Induced Intestinal Inflammation. Front. Pharmacol. 2022, 13, 809938. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, F.; Lu, S.; Ren, L.; Bian, S.; Liu, M.; Zhao, D.; Wang, S.; Wang, J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 2022, 283, 114739. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Zhao, Y.; Gao, N.; Jin, X.; Gao, X.; Li, T.; Liu, D. The extract from the roots of Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils attenuates DSS-induced ulcerative colitis by regulating the Nrf2/NF-κB signaling pathways. RSC Adv. 2020, 10, 9450–9461. [Google Scholar] [CrossRef]
- Dai, W.; Zhan, X.; Peng, W.; Liu, X.; Peng, W.; Mei, Q.; Hu, X. Ficus pandurata Hance Inhibits Ulcerative Colitis and Colitis-Associated Secondary Liver Damage of Mice by Enhancing Antioxidation Activity. Oxidative Med. Cell. Longev. 2021, 2021, 2617881. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wu, A.G.; Jaja-Chimedza, A.; Graf, B.L.; Waterman, C.; Verzi, M.P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS ONE 2017, 12, e0184709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miao, Z.; Sheng, X.; Li, X.; Ma, J.; Xu, X.; Li, H.; Kang, A. Sesquiterpene lactones-rich fraction from Aucklandia lappa Decne. alleviates dextran sulfate sodium induced ulcerative colitis through co-regulating MAPK and Nrf2/Hmox-1 signaling pathway. J. Ethnopharmacol. 2022, 295, 115401. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Bashllari, R.; Muscarà, C.; Molonia, M.S.; Saija, A.; Saha, S.; Wilde, P.J.; Cimino, F. Anti-Inflammatory Activity of an In Vitro Digested Anthocyanin-Rich Extract on Intestinal Epithelial Cells Exposed to TNF-α. Molecules 2022, 27, 5368. [Google Scholar] [CrossRef] [PubMed]
- Puppala, E.R.; Yalamarthi, S.S.; Aochenlar, S.L.; Prasad, N.; Np, S.; Singh, M.; Nanjappan, S.K.; Ravichandiran, V.; Tripathi, D.M.; Gangasani, J.K.; et al. Mesua assamica (King&Prain) kosterm. Bark ethanolic extract attenuates chronic restraint stress aggravated DSS-induced ulcerative colitis in mice via inhibition of NF-κB/STAT3 and activation of HO-1/Nrf2/SIRT1 signaling pathways. J. Ethnopharmacol. 2022, 301, 115765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gu, Y.; Dai, X. Protective Effect of Bilberry Anthocyanin Extracts on Dextran Sulfate Sodium-Induced Intestinal Damage in Drosophila melanogaster. Nutrients 2022, 14, 2875. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quan, S.; Li, J.; Liu, Y.; Sun, H.; Zhang, J.; Liu, D. Protective Effects of Grape Seed Proanthocyanidin Extract in Preventing DSS Induced Ulcerative Colitis Based on Pharmacodynamic, Pharmacokinetic and Tissue Distribution. Curr. Drug Metab. 2022, 23, 496–505. [Google Scholar] [CrossRef]
- Su, J.; Zhang, X.; Kan, Q.; Chu, X. Antioxidant Activity of Acanthopanax senticosus Flavonoids in H2O2-Induced RAW 264.7 Cells and DSS-Induced Colitis in Mice. Molecules 2022, 27, 2872. [Google Scholar] [CrossRef]
- Chao, L.; Lin, J.; Zhou, J.; Du, H.; Chen, X.; Liu, M.; Qu, Q.; Lv, W.; Guo, S. Polyphenol Rich Forsythia suspensa Extract Alleviates DSS-Induced Ulcerative Colitis in Mice through the Nrf2-NLRP3 Pathway. Antioxidants 2022, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Son, Y.J.; Ha, I.J.; Erdenebileg, S.; Jung, D.S.; Song, D.G.; Kim, Y.S.; Kim, S.M.; Nho, C.W. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement. Med. Ther. 2022, 22, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct 2022, 13, 143–160. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green Pea (Pisum sativum L.) Hull Polyphenol Extracts Ameliorate DSS-Induced Colitis through Keap1/Nrf2 Pathway and Gut Microbiota Modulation. Foods 2021, 10, 2765. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, X.; Xiong, H.; Deng, Z.; Peng, X.; Xiao, L.; Jiang, L.; Sun, Y. Bioactives and their metabolites from Tetrastigma hemsleyanum leaves ameliorate DSS-induced colitis via protecting the intestinal barrier, mitigating oxidative stress and regulating the gut microbiota. Food Funct. 2021, 12, 11760–11776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The preventive effect and underlying mechanism of Rhus chinensis Mill. fruits on dextran sulphate sodium-induced ulcerative colitis in mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Haileselassie, Y.; Ji, A.R.; Maecker, H.T.; Sinha, S.R.; Brim, H.; Habtezion, A.; Ashktorab, H. Protective Effect of Saffron in Mouse Colitis Models Through Immune Modulation. Dig. Dis. Sci. 2022, 67, 2922–2935. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Aufiero, V.R.; Mazzarella, G.; Maurano, F.; Gerardi, C.; Rossi, M.; Zara, V.; Mita, G.; et al. Prunus Mahaleb Fruit Extract Prevents Chemically Induced Colitis and Enhances Mitochondrial Oxidative Metabolism via the Activation of the Nrf2 Pathway. Mol. Nutr. Food Res. 2019, 63, e1900350. [Google Scholar] [CrossRef]
- Castejón, M.L.; Rosillo, M.; Villegas, I.; Sánchez-Hidalgo, M.; Hadidi, L.; Zaidi, F.; Alarcón-de-la-Lastra, C. Quercus ilex Extract Ameliorates Acute TNBS-Induced Colitis in Rats. Planta Med. 2019, 85, 670–677. [Google Scholar] [CrossRef]
- Almeer, R.S.; Mahmoud, S.M.; Amin, H.K.; Abdel Moneim, A.E. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol 2018, 115, 49–62. [Google Scholar] [CrossRef]
- Park, D.D.; Yum, H.W.; Zhong, X.; Kim, S.H.; Kim, S.H.; Kim, D.H.; Kim, S.J.; Na, H.K.; Sato, A.; Miura, T.; et al. Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-κB, STAT3, and Nrf2 as Putative Targets. Front. Pharmacol. 2017, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohns Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef]
- Liu, D.; Huo, X.; Gao, L.; Zhang, J.; Ni, H.; Cao, L. NF-κB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed. Pharmacother. 2018, 102, 922–929. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, J.M.; Lee, J.S.; Kim, Y.S.; Kangwan, N.; Han, Y.M.; Kang, E.A.; An, J.M.; Park, Y.K.; Hahm, K.B. Oligonol prevented the relapse of dextran sulfate sodium-ulcerative colitis through enhancing NRF2-mediated antioxidative defense mechanism. J. Physiol. Pharmacol. 2018, 69, 359–371. [Google Scholar] [CrossRef]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-kB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Luo, H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zheng, C. Effects of Alpinetin on Intestinal Barrier Function, Inflammation and Oxidative Stress in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice. Am. J. Med. Sci. 2018, 355, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Dev. Ther. 2015, 9, 3923–3934. [Google Scholar] [CrossRef] [PubMed]
- Alattar, A.; Alshaman, R.; Al-Gayyar, M.M.H. Therapeutic effects of sulforaphane in ulcerative colitis: Effect on antioxidant activity, mitochondrial biogenesis and DNA polymerization. Redox. Rep. 2022, 27, 128–138. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Gao, M.; Zhang, X.; Lei, P.; Yang, H.; Qing, Y.; Zhang, L. The Protective Effect of Sulforaphane on Dextran Sulfate Sodium-Induced Colitis Depends on Gut Microbial and Nrf2-Related Mechanism. Front. Nutr. 2022, 9, 893344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Xu, S.J.; Lu, Y.Y.; Chen, S.X.; Du, X.H.; Hou, S.Z.; Huang, H.Y.; Liang, J. Asperuloside suppressing oxidative stress and inflammation in DSS-induced chronic colitis and RAW 264.7 macrophages via Nrf2/HO-1 and NF-κB pathways. Chem. Biol. Interact. 2021, 344, 109512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, H.; Jia, Q.; Zhao, Y.; Li, H.; Shen, S.; Liu, X.; Wang, G.; Shi, Q. Syringin protects against colitis by ameliorating inflammation. Arch. Biochem. Biophys. 2020, 680, 108242. [Google Scholar] [CrossRef]
- Wu, X.X.; Huang, X.L.; Chen, R.R.; Li, T.; Ye, H.J.; Xie, W.; Huang, Z.M.; Cao, G.Z. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, Q.; Liu, L.; Xie, F.; Yang, L.; Li, Y.; Zhang, C.; Chen, H.; Tang, J.; Shen, X. Dehydrocostus Lactone Suppresses Dextran Sulfate Sodium-Induced Colitis by Targeting the IKKα/β-NF-κB and Keap1-Nrf2 Signalling Pathways. Front. Pharmacol. 2022, 13, 817596. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Chen, X.; Pan, Y.; Zha, Z.; Tang, M.; Shi, C.; Yang, B.; Wang, H. Leonurine exerts a protective effect in dextran sodium sulfate-induced experimental inflammatory bowel disease mice model. Gen. Physiol. Biophys. 2022, 41, 43–51. [Google Scholar] [CrossRef]
- Khodir, A.E.; Said, E.; Atif, H.; ElKashef, H.A.; Salem, H.A. Targeting Nrf2/HO-1 signaling by crocin: Role in attenuation of AA-induced ulcerative colitis in rats. Biomed. Pharmacother. 2019, 110, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Khater, S.I.; Lotfy, M.M.; Alandiyjany, M.N.; Alqahtani, L.S.; Zaglool, A.W.; Althobaiti, F.; Ismail, T.A.; Soliman, M.M.; Saad, S.; Ibrahim, D. Therapeutic Potential of Quercetin Loaded Nanoparticles: Novel Insights in Alleviating Colitis in an Experimental DSS Induced Colitis Model. Biomedicines 2022, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ma, X.; Zhang, H.; Wu, X.; Li, M.; Ai, G.; Zhan, R.; Xie, J.; Su, Z.; Huang, X. 8-Oxypalmatine, a novel oxidative metabolite of palmatine, exhibits superior anti-colitis effect via regulating Nrf2 and NLRP3 inflammasome. Biomed. Pharmacother. 2022, 153, 113335. [Google Scholar] [CrossRef]
- Wang, K.P.; Zhang, C.; Zhang, S.G.; Liu, E.D.; Dong, L.; Kong, X.Z.; Cao, P.; Hu, C.P.; Zhao, K.; Zhan, Y.Q.; et al. 3-(3-pyridylmethylidene)-2-indolinone reduces the severity of colonic injury in a murine model of experimental colitis. Oxidative Med. Cell. Longev. 2015, 2015, 959253. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhang, X.; Li, Q.; Huang, J.; Liu, G.; Zhao, J.; Liu, Y.; Shen, L.; Li, Y.; Yang, K.; et al. Ruscogenins Improve CD-Like Enteritis by Inhibiting Apoptosis of Intestinal Epithelial Cells and Activating Nrf2/NQO1 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 4877275. [Google Scholar] [CrossRef]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Shen, C.; Wang, X.; Pu, Z.; Yin, Q. Network pharmacology for systematic understanding of Schisandrin B reduces the epithelial cells injury of colitis through regulating pyroptosis by AMPK/Nrf2/NLRP3 inflammasome. Aging (Albany NY) 2021, 13, 23193–23209. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, C.; Lu, X.; Deng, C.; Xu, Q.; Guo, W.; Wu, Q.; Wang, Q.; Liu, C.; Huang, X.; et al. GB1a Ameliorates Ulcerative Colitis via Regulation of the NF-κB and Nrf2 Signaling Pathways in an Experimental Model. Front. Med. (Lausanne) 2021, 8, 654867. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wei, Y.Y.; Li, X.H.; Zhang, S.S.; Zhang, R.T.; Li, J.H.; Ma, B.W.; Shao, S.B.; Lv, Z.W.; Ruan, H.; et al. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol. Sin. 2022, 43, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, W.; Luo, C.; Zhang, X.; Huang, M. Atractylenolide III Ameliorates TNBS-Induced Intestinal Inflammation in Mice by Reducing Oxidative Stress and Regulating Intestinal Flora. Chem. Biodivers. 2021, 18, e2001001. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wang, J.; Wu, C.; Dong, L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immun. Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef]
- Peritore, A.F.; D’Amico, R.; Cordaro, M.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. PEA/Polydatin: Anti-Inflammatory and Antioxidant Approach to Counteract DNBS-Induced Colitis. Antioxidants 2021, 10, 464. [Google Scholar] [CrossRef]
- Marinho, S.; Illanes, M.; Ávila-Román, J.; Motilva, V.; Talero, E. Anti-Inflammatory Effects of Rosmarinic Acid-Loaded Nanovesicles in Acute Colitis through Modulation of NLRP3 Inflammasome. Biomolecules 2021, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Luo, Y. Imperatorin Relieved Ulcerative Colitis by Regulating the Nrf-2/ARE/HO-1 Pathway in Rats. Inflammation 2021, 44, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xue, K.; Liu, J.; Habotta, O.A.; Hu, L.; Abdel Moneim, A.E. Anticolitic Effect of Berberine in Rat Experimental Model: Impact of PGE2/p38 MAPK Pathways. Mediat. Inflamm. 2020, 2020, 9419085. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, X.; Peng, B.; Zou, H.; Li, S.; Wang, J.; Cao, J. Curcumin improves necrotising microscopic colitis and cell pyroptosis by activating SIRT1/NRF2 and inhibiting the TLR4 signalling pathway in newborn rats. Innate. Immun. 2020, 26, 609–617. [Google Scholar] [CrossRef]
- Bai, X.; Gou, X.; Cai, P.; Xu, C.; Cao, L.; Zhao, Z.; Huang, M.; Jin, J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxidative Med. Cell. Longev. 2019, 2019, 2432416. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Chen, W.; Zhu, J.; Zhang, J.; Peng, S. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflammasome and Nrf2/HO-1 signaling. Int. Immunopharmacol. 2019, 76, 105909. [Google Scholar] [CrossRef] [PubMed]
- Sangaraju, R.; Nalban, N.; Alavala, S.; Rajendran, V.; Jerald, M.K.; Sistla, R. Protective effect of galangin against dextran sulfate sodium (DSS)-induced ulcerative colitis in Balb/c mice. Inflamm. Res. 2019, 68, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Nam, S.J.; Chun, W.; Kim, S.I.; Park, S.C.; Kang, C.D.; Lee, S.J. Anti-inflammatory effects of apocynin on dextran sulfate sodium-induced mouse colitis model. PLoS ONE 2019, 14, e0217642. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Ren, J.; Gu, G.; Wang, G.; Gong, W.; Wu, X.; Ren, H.; Hong, Z.; Li, J. Hesperidin Protects Against Intestinal Inflammation by Restoring Intestinal Barrier Function and Up-Regulating Treg Cells. Mol. Nutr. Food Res. 2019, 63, e1800975. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, K.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Norisoboldine, a natural aryl hydrocarbon receptor agonist, alleviates TNBS-induced colitis in mice, by inhibiting the activation of NLRP3 inflammasome. Chin. J. Nat. Med. 2018, 16, 161–174. [Google Scholar] [CrossRef]
- Yang, L.; Shen, L.; Li, Y.; Li, Y.; Yu, S.; Wang, S. Hyperoside attenuates dextran sulfate sodium-induced colitis in mice possibly via activation of the Nrf2 signalling pathway. J. Inflamm. 2017, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Oh, J.; Hahn, D.; Kwon, C.S.; Lee, J.S.; Kim, J.S. Protective Effect of Glyceollins in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Med. Food 2017, 20, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xia, Z.; Shao, N.; Li, B.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 11036. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; San-Miguel, B.; Mauriz, J.L.; Ortiz de Urbina, J.J.; Almar, M.; Tuñón, M.J.; González-Gallego, J. Protective Effect of Protocatechuic Acid on TNBS-Induced Colitis in Mice Is Associated with Modulation of the SphK/S1P Signaling Pathway. Nutrients 2017, 9, 288. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Cotran Pathologic Basis of Disease; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Caparrós, E.; Wiest, R.; Scharl, M.; Rogler, G.; Gutiérrez Casbas, A.; Yilmaz, B.; Wawrzyniak, M.; Francés, R. Dysbiotic microbiota interactions in Crohn’s disease. Gut Microbes 2021, 13, 1949096. [Google Scholar] [CrossRef] [PubMed]
- Cucchiara, S.; D’Arcangelo, G.; Isoldi, S.; Aloi, M.; Stronati, L. Mucosal healing in Crohn’s disease: New insights. Expert. Rev. Gastroenterol. Hepatol. 2020, 14, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ponce, C.; Navarro Quiroz, R.; Díaz Perez, A.; Aroca Martinez, G.; Cadena Bonfanti, A.; Acosta Hoyos, A.; Gómez Escorcia, L.; Hernández Agudelo, S.; Orozco Sánchez, C.; Villarreal Camacho, J.; et al. MicroRNAs overexpressed in Crohn’s disease and their interactions with mechanisms of epigenetic regulation explain novel aspects of Crohn’s disease pathogenesis. Clin. Epigenetics 2021, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Hammer, G.D.; McPhee, S.J. Pathophysiology of Disease: An. Introduction to Clinical Medicine 8E; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Lightner, A.L.; Ashburn, J.H.; Brar, M.S.; Carvello, M.; Chandrasinghe, P.; van Overstraeten, A.d.B.; Fleshner, P.R.; Gallo, G.; Kotze, P.G.; Holubar, S.D.; et al. Fistulizing Crohn’s disease. Curr. Probl. Surg. 2020, 57, 100808. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Chien Ng, S.; Kotze, P.G.; Argollo, M.; Panaccione, R.; Spinelli, A.; Kaser, A.; Peyrin-Biroulet, L.; Danese, S. Crohn’s disease. Nat. Rev. Dis. Prim. 2020, 6, 22. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ikeya, K.; Bamba, S.; Andoh, A.; Yamasaki, H.; Mitsuyama, K.; Nasuno, M.; Tanaka, H.; Matsuura, A.; Kato, M.; et al. Highly Bioavailable Curcumin Derivative Ameliorates Crohn’s Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J. Crohns Colitis 2020, 14, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Neurath, M.F. Cytokines in inflammatory bowel diseases—Update 2020. Pharmacol. Res. 2020, 158, 104835. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. North Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Carvalho, A.C.A.; Souza, G.A.; Marqui, S.V.; Guiguer, É.L.; Araújo, A.C.; Rubira, C.J.; Goulart, R.A.; Flato, U.A.P.; Bueno, P.; Buchaim, R.L.; et al. Cannabis and Canabidinoids on the Inflammatory Bowel Diseases: Going Beyond Misuse. Int. J. Mol. Sci. 2020, 21, 2940. [Google Scholar] [CrossRef]
- Marton, L.T.; Goulart, R.A.; Carvalho, A.C.A.; Barbalho, S.M. Omega Fatty Acids and Inflammatory Bowel Diseases: An Overview. Int. J. Mol. Sci. 2019, 20, 4851. [Google Scholar] [CrossRef]
- He, J.; Zhang, S.; Qiu, Y.; Liu, F.; Liu, Z.; Tan, J.; Hu, F.; Wu, X.; Wang, Y.; Zhou, L.; et al. Ulcerative colitis increases risk of hypertension in a UK biobank cohort study. United Eur. Gastroenterol. J. 2022, 11, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kaenkumchorn, T.; Wahbeh, G. Ulcerative Colitis: Making the Diagnosis. Gastroenterol. Clin. North Am. 2020, 49, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Huang, Y.H. Dose-Intensified Infliximab Rescue Therapy on Short-term Colectomy Rates in patients with Severe Ulcerative Colitis. Dis. Colon Rectum 2022. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Koletzko, S.; Kannengiesser, K.; Dignass, A. Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Dtsch. Arztebl. Int. 2020, 117, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Affendi, N.; Ooi, C.J.; Hilmi, I.N. Accelerated ustekinumab dosing as rescue therapy in acute severe ulcerative colitis. J. Gastrointest. Liver Dis. JGLD 2022, 31, 478–479. [Google Scholar] [CrossRef]
- Voitalov, I.; Zhang, L.; Kilpatrick, C.; Withers, J.B.; Saleh, A.; Akmaev, V.R.; Ghiassian, S.D. The module triad: A novel network biology approach to utilize patients’ multi-omics data for target discovery in ulcerative colitis. Sci. Rep. 2022, 12, 21685. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Geng, C.; Wang, C.; Gu, R.; Zhu, Z. A variant rs6214 within IGF-1 confers risk for ulcerative colitis in Chinese Han populations. Funct. Integr. Genom. 2022, 23, 1. [Google Scholar] [CrossRef]
- Beaudoin, M.; Goyette, P.; Boucher, G.; Lo, K.S.; Rivas, M.A.; Stevens, C.; Alikashani, A.; Ladouceur, M.; Ellinghaus, D.; Törkvist, L.; et al. Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. PLoS Genet. 2013, 9, e1003723. [Google Scholar] [CrossRef]
- Dubuquoy, L.; Jansson, E.A.; Deeb, S.; Rakotobe, S.; Karoui, M.; Colombel, J.F.; Auwerx, J.; Pettersson, S.; Desreumaux, P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology 2003, 124, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Grisham, M.; Hodge, J.; Telliez, J.B. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: A hub for multiple inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G155–G162. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Wattenberg, L.W. Inhibitors of Chemical Carcinogenesis. In Advances in Cancer Research; Klein, G., Weinhouse, S., Eds.; Academic Press: Cambridge, MA, USA, 1978; Volume 26, pp. 197–226. [Google Scholar]
- Benson, A.M.; Batzinger, R.P.; Ou, S.Y.; Bueding, E.; Cha, Y.N.; Talalay, P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978, 38, 4486–4495. [Google Scholar] [PubMed]
- Rushmore, T.H.; Pickett, C.B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 1990, 265, 14648–14653. [Google Scholar] [CrossRef] [PubMed]
- Prestera, T.; Holtzclaw, W.D.; Zhang, Y.; Talalay, P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc. Natl. Acad. Sci. USA 1993, 90, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Mignotte, V.; Eleouet, J.F.; Raich, N.; Romeo, P.H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc. Natl. Acad. Sci. USA 1989, 86, 6548–6552. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, L.; Liu, J. Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review). Exp. Ther. Med. 2021, 22, 678. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, H.; Wang, M.; Wang, R.; Yi, G.; Zhang, M.; Chen, R. Role of STAT3 and NRF2 in Tumors: Potential Targets for Antitumor Therapy. Molecules 2022, 27, 8768. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.; Jiang, T.; Wu, T.; Tao, S.; Rojo de la Vega, M.; Tian, W.; Chapman, E.; Zhang, D.D. Molecular mechanisms of Nrf2 regulation and how these influence chemical modulation for disease intervention. Biochem. Soc. Trans. 2015, 43, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Tucci, P.; Lattanzi, R.; Severini, C.; Saso, L. Nrf2 Pathway in Huntington’s Disease (HD): What Is Its Role? Int. J. Mol. Sci. 2022, 23, 5272. [Google Scholar] [CrossRef]
- Poornashree, M.; Kumar, H.; Ajmeer, R.; Jain, R.; Jain, V. Dual role of Nrf2 in cancer: Molecular mechanisms, cellular functions and therapeutic interventions. Mol. Biol. Rep. 2022, 50, 1871–1883. [Google Scholar] [CrossRef] [PubMed]
- Mathis, B.J.; Kato, H.; Hiramatsu, Y. Induction of Cardiac Pathology: Endogenous versus Exogenous Nrf2 Upregulation. Cells 2022, 11, 3855. [Google Scholar] [CrossRef]
- Corradi, D.; Callegari, S.; Maestri, R.; Benussi, S.; Alfieri, O. Structural remodeling in atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Kobayashi, A.; Katsuoka, F.; Yamamoto, M. Two-site substrate recognition model for the Keap1-Nrf2 system: A hinge and latch mechanism. Biol. Chem. 2006, 387, 1311–1320. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Dashwood, R.H. Epigenetic Regulation of NRF2/KEAP1 by Phytochemicals. Antioxidants 2020, 9, 865. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci. 2020, 244, 117329. [Google Scholar] [CrossRef]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E.; Bonacasa, B.; Ishii, T.; Siow, R.C. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: Protection afforded by dietary isoflavones. Curr. Opin. Pharmacol. 2009, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Lossow, K.; Kopp, J.F.; Schwerdtle, T.; Kipp, A.P. Crosstalk of Nrf2 with the Trace Elements Selenium, Iron, Zinc, and Copper. Nutrients 2019, 11, 2112. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, A.; Suzuki, T.; Ono, M.; Kobayashi, T.; Yamamoto, M. Roles of Keap1-Nrf2 system in upper aerodigestive tract carcinogenesis. Cancer Prev. Res. 2013, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Third universal definition of myocardial infarction. Nat. Rev. Cardiol. 2012, 9, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Gao, Z.W.; Hou, J.; Zhou, Q.; Ma, W.; Dai, Y.H.; She, W.D. Nuclear Factor Erythroid 2-Related Factor 2-Histone Deacetylase 2 Pathway in the Pathogenesis of Refractory Sudden Sensorineural Hearing Loss and Glucocorticoid Resistance. ORL J. Otorhinolaryngol. Relat. Spec. 2021, 83, 227–233. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, F. Targeting Transcription Factor Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) for the Intervention of Vascular Cognitive Impairment and Dementia. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Johnston, L.J.; Wang, F.; Ma, X. Triggers for the Nrf2/ARE Signaling Pathway and Its Nutritional Regulation: Potential Therapeutic Applications of Ulcerative Colitis. Int. J. Mol. Sci. 2021, 22, 1411. [Google Scholar] [CrossRef]
- Lifei, L.; Zhang, J.; Li, X.; Zhu, Y.; Wang, Y.; Liu, D. Sericic acid ameliorates DSS-induced ulcerative colitis in mice by modulating the NF-κB and Nrf2 pathways. Curr. Mol. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Ostadal, B.; Drahota, Z.; Houstek, J.; Milerova, M.; Ostadalova, I.; Hlavackova, M.; Kolar, F. Developmental and sex differences in cardiac tolerance to ischemia-reperfusion injury: The role of mitochondria. Can. J. Physiol. Pharmacol. 2019, 97, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Zhang, L.; Xi, S.; Cheng, Y.; Jiang, H.; Hu, R. Nrf2 Activation Induced by Sirt1 Ameliorates Acute Lung Injury After Intestinal Ischemia/Reperfusion Through NOX4-Mediated Gene Regulation. Cell. Physiol. Biochem. 2018, 46, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.G.; Yong, Y.Y.; Pan, Y.R.; Zhang, L.; Wu, J.M.; Zhang, Y.; Tang, Y.; Wei, J.; Yu, L.; Law, B.Y.; et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxidative Med. Cell. Longev. 2022, 2022, 1015791. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Khan, H.; Alsharif, K.F.; Hayat Khan, A.; Aschner, M.; Saso, L. The Therapeutic Potential of Kaemferol and Other Naturally Occurring Polyphenols Might Be Modulated by Nrf2-ARE Signaling Pathway: Current Status and Future Direction. Molecules 2022, 27, 4145. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Ghosh, S.; Bishayee, A.; Sinha, D. Flexion of Nrf2 by tea phytochemicals: A review on the chemopreventive and chemotherapeutic implications. Pharmacol. Res. 2022, 182, 106319. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Liu, Y.; Zhou, Y. Role of NRF2 in Colorectal Cancer Prevention and Treatment. Technol. Cancer Res. Treat. 2022, 21, 15330338221105736. [Google Scholar] [CrossRef]
- Gerstgrasser, A.; Melhem, H.; Leonardi, I.; Atrott, K.; Schäfer, M.; Werner, S.; Rogler, G.; Frey-Wagner, I. Cell-specific Activation of the Nrf2 Antioxidant Pathway Increases Mucosal Inflammation in Acute but Not in Chronic Colitis. J. Crohns Colitis 2017, 11, 485–499. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, H.; Ding, Q.; Xu, X.; Yu, B.; Huang, Z. Nuclear Factor Erythroid 2-related Factor 2 Deficiency Exacerbates Lupus Nephritis in B6/lpr mice by Regulating Th17 Cell Function. Sci. Rep. 2016, 6, 38619. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Zhang, M.; Fields, P.E.; Klaassen, C.D. Th2 skewing by activation of Nrf2 in CD4(+) T cells. J. Immunol. 2012, 188, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.; Martina, M.N.; Bandapalle, S.; Racusen, L.C.; Potteti, H.R.; Hamad, A.R.; Reddy, S.P.; Rabb, H. T Lymphocyte-Specific Activation of Nrf2 Protects from AKI. J. Am. Soc. Nephrol 2015, 26, 2989–3000. [Google Scholar] [CrossRef]
- Beury, D.W.; Carter, K.A.; Nelson, C.; Sinha, P.; Hanson, E.; Nyandjo, M.; Fitzgerald, P.J.; Majeed, A.; Wali, N.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cell Survival and Function Are Regulated by the Transcription Factor Nrf2. J. Immunol. 2016, 196, 3470. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.K.; Sha, W.; Kuchler, L.; Daiber, A.; Giegerich, A.K.; Weigert, A.; Knape, T.; Snodgrass, R.; Schröder, K.; Brandes, R.P.; et al. Loss of Nrf2 in bone marrow-derived macrophages impairs antigen-driven CD8+ T cell function by limiting GSH and Cys availability. Free Radic. Biol. Med. 2015, 83, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, P.; Xin, S.; Wang, Z.; Li, J. Nrf2 suppresses the function of dendritic cells to facilitate the immune escape of glioma cells. Exp. Cell Res. 2017, 360, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Qu, J.; Shen, X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription factors NRF2 and NF-κB are coordinated effectors of the Rho family, GTP-binding protein RAC1 during inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2016, 116, 984–995. [Google Scholar] [CrossRef]
- Luo, J.-F.; Shen, X.-Y.; Lio, C.K.; Dai, Y.; Cheng, C.-S.; Liu, J.-X.; Yao, Y.-D.; Yu, Y.; Xie, Y.; Luo, P.; et al. Activation of Nrf2/HO-1 Pathway by Nardochinoid C Inhibits Inflammation and Oxidative Stress in Lipopolysaccharide-Stimulated Macrophages. Front. Pharmacol. 2018, 9, 911. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lin, Z.; Hu, S.; Shi, Y.; Jiang, Z.; Zhao, J.; Zhou, Y.; Wu, Y.; Tian, N.; Sun, L.; et al. Itaconate attenuates osteoarthritis by inhibiting STING/NF-κB axis in chondrocytes and promoting M2 polarization in macrophages. Biochem. Pharmacol. 2022, 198, 114935. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Scollick, C.; Traore, K.; Yates, M.; Trush, M.A.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006, 351, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Innamorato, N.G.; Martín-Moreno, A.M.; De Ceballos, M.L.; Yamamoto, M.; Cuadrado, A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson’s disease. Glia 2010, 58, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wu, R.T.; Wu, T.; Khor, T.-O.; Wang, H.; Kong, A.-N. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem. Pharmacol. 2008, 76, 967–973. [Google Scholar] [CrossRef]
- Song, Y.; Hao, D.; Jiang, H.; Huang, M.; Du, Q.; Lin, Y.; Liu, F.; Chen, B. Nrf2 Regulates CHI3L1 to Suppress Inflammation and Improve Post-Traumatic Osteoarthritis. J. Inflamm. Res. 2021, 14, 4079–4088. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, A.; Mendonca, P.; Russell, T.D.; Soliman, K.F.A. The Protective Effects of Flavonoids in Cataract Formation through the Activation of Nrf2 and the Inhibition of MMP-9. Nutrients 2020, 12, 3651. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, F.; Fu, R.; Li, X.; French, R.; Mose, E.; Pu, X.; Trinh, B.; Kumar, A.; Liu, J.; et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 2021, 39, 678–693. [Google Scholar] [CrossRef]
- Schmidlin, C.J.; Shakya, A.; Dodson, M.; Chapman, E.; Zhang, D.D. The intricacies of NRF2 regulation in cancer. Semin. Cancer Biol. 2021, 76, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Motohashi, H. NRF2 addiction in cancer cells. Cancer Sci. 2018, 109, 900–911. [Google Scholar] [CrossRef]
- Song, C.-H.; Kim, N.; Nam, R.H.; Choi, S.I.; Kang, C.; Jang, J.Y.; Nho, H.; Shin, E.; Lee, H.-N. Nuclear Factor Erythroid 2-related Factor 2 Knockout Suppresses the Development of Aggressive Colorectal Cancer Formation Induced by Azoxymethane/Dextran Sulfate Sodium-Treatment in Female Mice. J. Cancer Prev. 2021, 26, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Huang, M.-T.; Prawan, A.; Liu, Y.; Hao, X.; Yu, S.; Cheung, W.K.L.; Chan, J.Y.; Reddy, B.S.; Yang, C.S.; et al. Increased Susceptibility of Nrf2 Knockout Mice to Colitis-Associated Colorectal Cancer. Cancer Prev. Res. 2008, 1, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, X.; Yang, M.; Yu, Y.; Zhou, Y.; Gao, Y.; Zhang, L.; Li, Z.; Xiao, Y.; Moses, R.E.; et al. Procyanidin B2 Promotes Intestinal Injury Repair and Attenuates Colitis-Associated Tumorigenesis via Suppression of Oxidative Stress in Mice. Antioxid. Redox Signal. 2021, 35, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chen, Y.; Huang, J.; Deng, H.; Tang, X.; Wang, X.J. Mkp-1 is required for chemopreventive activity of butylated hydroxyanisole and resveratrol against colitis-associated colon tumorigenesis. Food Chem. Toxicol. 2019, 127, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, X.; Yang, J.; Sun, X.; Hu, C.; Yan, Z.; Xu, X.; Lu, W.; Wang, X.; Cao, P. Chemoprevention of dietary digitoflavone on colitis-associated colon tumorigenesis through inducing Nrf2 signaling pathway and inhibition of inflammation. Mol. Cancer 2014, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Saadatdoust, Z.; Esa, N.M.; Hamzah, H.; Ismail, A. Dietary cocoa protects against colitis-associated cancer by activating the Nrf2/Keap1 pathway. Biofactors 2015, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Kim, J.K. Tussilagone Reduces Tumorigenesis by Diminishing Inflammation in Experimental Colitis-Associated Colon Cancer. Biomedicines 2020, 8, 86. [Google Scholar] [CrossRef]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Wiesner, M.; Schreiner, M.; Glatt, H.; Brigelius-Flohé, R.; et al. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar] [CrossRef]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, T.; Tanaka, T. Dietary Crocin Inhibits Colitis and Colitis-Associated Colorectal Carcinogenesis in Male ICR Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 820415. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Ma, N.J.; Sang, S.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J. Agric. Food Chem. 2012, 60, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Tsai, M.L.; Nagabhushanam, K.; Wang, Y.J.; Wu, C.H.; Ho, C.T.; Pan, M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, M.; Gao, Z.; Sun, Y.; Wang, M.; Li, F.; Zheng, J.; Xiao, H. Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J. Nutr. Biochem. 2017, 42, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhao, L.; Zhao, Q.; Zhao, Y.; Sun, Y.; Zhang, Y.; Miao, H.; You, Q.D.; Hu, R.; Guo, Q.L. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014, 5, e1283. [Google Scholar] [CrossRef]
- Long, M.; Tao, S.; Rojo de la Vega, M.; Jiang, T.; Wen, Q.; Park, S.L.; Zhang, D.D.; Wondrak, G.T. Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev. Res. 2015, 8, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Bagalagel, A.; Diri, R.; Noor, A.; Almasri, D.; Bakhsh, H.T.; Kutbi, H.I.; Al-Gayyar, M.M.H. Curative effects of fucoidan on acetic acid induced ulcerative colitis in rats via modulating aryl hydrocarbon receptor and phosphodiesterase-4. BMC Complement. Med. Ther. 2022, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Filippone, A.; Mannino, D.; Scuderi, S.A.; Casili, G.; Lanza, M.; Cucinotta, L.; Campolo, M.; Esposito, E. Ulva pertusa, a Marine Green Alga, Attenuates DNBS-Induced Colitis Damage via NF-κB/Nrf2/SIRT1 Signaling Pathways. J. Clin. Med. 2022, 11, 4301. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Fusco, R.; Genovese, T.; Cordaro, M.; D’Amico, R.; Trovato Salinaro, A.; Ontario, M.L.; Modafferi, S.; Cuzzocrea, S.; Di Paola, R.; et al. Coriolus Versicolor Downregulates TLR4/NF-κB Signaling Cascade in Dinitrobenzenesulfonic Acid-Treated Mice: A Possible Mechanism for the Anti-Colitis Effect. Antioxidants 2022, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, Y.; Sun, J.; Xu, H.; Wang, M.; Zuo, X.; Fu, Q.; Guo, Y.; Chen, Z.; Zhang, P.; et al. Saccharomyces boulardii Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Regulating NF-κB and Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 1622375. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhang, Y.; Zhang, Y.; Liu, S.; Zhang, N.; Li, Y.; Wang, D. Protective effect of Gloeostereum incarnatum on ulcerative colitis via modulation of Nrf2/NF-κB signaling in C57BL/6 mice. Mol. Med. Rep. 2020, 22, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liang, X.; Guo, N.; Hu, L.; Prasad, E.M.; Wu, Y.; Xue, X.; Wu, L.; Wang, K. Protective effects of Bee pollen extract on the Caco-2 intestinal barrier dysfunctions induced by dextran sulfate sodium. Biomed. Pharmacother. 2019, 117, 109200. [Google Scholar] [CrossRef] [PubMed]
- Jenkhetkan, W.; Thitiorul, S.; Jansom, C.; Ratanavalachai, T. Molecular and cytogenetic effects of Thai royal jelly: Modulation through c-MYC, h-TERT, NRF2, HO-1, BCL2, BAX and cyclins in human lymphocytes in vitro. Mutagenesis 2017, 32, 525–531. [Google Scholar] [CrossRef] [PubMed]

| Medicinal Plants | In Vivo/In Vitro Models | Plant Parts Used or Extracts | Effective Doses/Concentrations | Related Clinical Features of IBD | Nrf2-Related Dysregulation Indicators | Related Molecular Mechanisms in Nrf2 Regulation in IBD | Ref. |
|---|---|---|---|---|---|---|---|
| Cynara cardunculus L. Asteraceae | TNF-α-induced intestinal Caco-2 epithelial cells model of colitis | Leaves extract rich in chlorogenic acid and luteolin | 5, 10, and 15 μg/mL | ↑Intestinal inflammation and ↑OS | ↑NF-κB activation, ↑ROS intracellular levels, ↓TAA, and ↓GSH | ↑Nrf2 nuclear translocation, ↑GCLC mRNA, and ↑NQO1 mRNA | [17] |
| Panax ginseng Araliaceae | DSS-induced C57BL/6J mice model of colitis and LPS-treated RAW264.7 cells | Root extract rich in ginsenosides | 32.5, 125, and 500 μg/mL | ↑Intestinal inflammation and ↑OS | ↑NO, ↑TNF-α, ↑IL-6, ↑IL-1β, ↓IL-10, ↑ROS, and ↑mitochondrial dysfunction | ↑Nrf2 nuclear translocation, ↑p62 phosphorylation, and ↑Akt-mTOR signaling | [18] |
| Rose odorata sweet var. gigantean (Coll. et Hemsl.) Rehd. et Wils Rosaceae | DSS-induced C57BL/6 mice model of colitis | Root extract rich in total tannins and triterpenoids | 125, 250 and 500 mg/kg | ↑DAI, ↑OS, ↑loss of epithelial and goblet cells, ↑crypt aberrations, and ↑prominent transmural inflammatory cells infiltration | ↑NOS, ↑MDA, ↑MPO, ↑TNF-α, ↑IL-6, and ↑IL-1β | ↑HO-1, ↑SOD, ↓number of nuclear NF-κB-p65 positive cells, ↓p-NF-κB-p65, ↓p-IKKα/β, and ↓Keap1 | [19] |
| Ficus pandurata Hance Moraceae | DSS-induced C57BL/6 mice model of colitis | Crude extract | 24-48 g/kg | ↑DAI, ↑intestinal inflammation, and ↑OS | ↑NF-κB activation, ↓intestinal barrier integrity, ↑MPO, ↓DAO, ↓T-SOD, ↓GSH-Px, ↑MDA, and ↓HO-1 | ↓TLR4/MyD88/NF-κB, ↑DAO, ↑T-SOD, ↓Keap1, ↓NOX2, ↓p22-phox, and ↑Nrf2 translocation, ↑HO-1, and ↑NQO1 | [20] |
| Moringa oleifera Lam. Moringaceae | DSS-induced C57BL/6 mice model of colitis | Isothiocyanate-enriched seed extract | 150 mg/kg | ↑DAI, ↓intestinal integrity, ↑intestinal inflammation, and ↑OS | ↑TNF-α, ↑IL-1β, ↑IL-6, ↑NO, ↑iNOS, and ↑MPO | ↓Pro-inflammatory genes expression, ↑GSTP1, ↑NQO1, ↑HO-1, and ↓NF-κB-dependent pro-inflammatory signals | [21] |
| Aucklandia lappa Decne. Asteraceae | DSS-induced C57BL/6 mice model of colitis and LPS-treated RAW264.7 cells | Aqueous and SLRF (rich in COS and DHL) ALDE extracts | 1.82 g/kg for aqueous or 0.91 g/kg-1.82 g/kg for SLRF extracts in mice and 2, 4, or 8 μM for COS or 1.25, 2.5, or 5 μM for DHL in treated cells | ↑DAI and ↑intestinal inflammation | ↑TNF-α, ↑IL-1β, ↑IL-6, ↑M1 macrophage polarization, ↑iNOS, ↑COX-2, ↑NF-κB-related proteins, ↓HMOX-1, ↑p38 MAPK phosphorylation, ↑NF-κB p65 phosphorylation, ↑Erk phosphorylation, and ↑JNK phosphorylation | ↓IL-1β mRNA, ↓IL-6 mRNA, ↓TNF-α mRNA, ↓MAPK expression, ↓NF-κB expression, ↑HMOX-1 mRNA expression, ↑Nrf2 mRNA expression in mice, and ↓p38MAPK phosphorylation, ↓p-NF-κB-p65 phosphorylation, ↓Erk phosphorylation, ↓JNK phosphorylation, ↑Nrf2 expression, and ↑HMOX-1 expression in treated cells | [22] |
| Vaccinium myrtillus L. Ericaceae and Ribes nigrum L. Grossulariacae | Caco-2 human intestinal epithelial cells exposed to TNF-α | ACN-rich purified and standardized Vaccinium myrtillus and Ribes nigrum extract | 0.18, 0.37, 0.75, and 1.5 µg/mL | ↑Intestinal inflammation | ↑TNF-α-induced nuclear translocation of NF-κB-p65, ↑IL-8 mRNA, and ↑IL-6 mRNA, | ↓NF-κB-p65 subunit translocation, ↑Nrf2 nuclear translocation, and ↑NQO1 mRNA | [23] |
| Mesua assamica (King&Prain) Kosterm Calophyllacae | NF-κB-RE-luc2P HEK 293 TNF-α-stimulated cells and DSS-induced C57BL/6 mice model of colitis | Bark ethanolic extract | 100 and 200 mg/kg | ↑Intestinal inflammation and ↑OS in vitro and ↑DAI, ↓intestinal integrity, splenomegaly, and ↓colon length in vivo | ↑MDA, ↑MPO, ↑nitrite levels, ↓GSH, ↑inflammatory cells accumulation, ↑IL-6, ↑IL-1β, and ↑TNF-α, | ↓NF-κB-related proteins expression, ↓STAT3 signaling cascades, ↓IKBα phosphorylation, ↑SOD2, ↑HO-1, and ↑SIRT1 | [24] |
| Vaccinium myrtillus L. Ericaceae | DSS-induced Drosophila melanogaster model of intestinal inflammatory damage | Berry anthocyanin extracts | 0.1 mg/mL | ↓Intestinal integrity, ↓intestinal length, ↑intestinal inflammation, and ↑intestinal OS | ↑ROS content and ↑pro-inflammatory cytokines levels | ↑Nrf2-related protein genetic expression (↑GCL, ↑GSTS, and ↑SOD) | [25] |
| Vitis vinifera L. Vitaceae | DSS-induced C57BL/6 mice model of colitis | Seed proanthocyanidin extract | - | ↓Body weight, ↑diarrhea, and ↑bloody stool | ↑IL-1β, ↑IL-6, ↑TNF-α, ↑NO, ↑MDA, ↓SOD, ↑NF-κB-related proteins expression, and ↓HO-1 | ↑HO-1 and other Nrf2-related protein’s genetic expression, and ↓NF-κB-related proteins expression | [26] |
| Acanthopanax senticosus (Rupr. et Maxim) Araliaceae | H2O2-Induced RAW 264.7 Cells and DSS-induced C57BL/6 mice model of colitis | Flavonoid extract | 15, 30, 60, 90, 120, and 150 mg/L in treated cells and 50, 100, and 200 g/L in treated mice | ↑Intestinal inflammation and ↑OS in vitro and ↑DAI in vivo | ↑MDA, ↓CAT, ↓SOD, ↓GPx, ↑Keap1, and ↓HO-1 | ↓Keap1 and ↑HO-1 | [27] |
| Forsythia suspensa (Thunb.) Vahl Oleracea | DSS-induced C57BL/6 mice model of colitis | Polyphenol-rich extract | 0.1, 0.2, and 0.4 g/mL | ↑DAI, ↓body weight, ↑intestinal villi degeneration, ↑necrosis, ↑proliferation and infiltration of inflammatory cells in the intestine | ↑IL-1β, ↑MDA, ↓SOD, and ↑MPO | ↓MDA, ↑SOD, ↓MPO, ↑Nrf2 activity, ↑HO-1, ↑NQO1, ↓caspase-1 expression, ↓IL-1β expression, ↓GSDMD expression, ↓NLRP3 expression, ↓ROS levels, ↓pyroptosis, and ↓ASC | [28] |
| Artemisia argyi H. Lév. & Vaniot Asteraceae | DSS-induced C57BL/6 mice model of colitis | Ethanolic extract | 200 mg/kg | ↑DAI, ↑colonic dysplasia, ↑colon barrier disruption, and ↓body weight | ↑AST, ↑ALT, ↑IL-6, ↑IL-1β, ↑TNF-α, and ↑COX-2 | ↓IL-6, ↓IL-1β and ↓TNF-α expressions, ↓p-IκBα, ↓p-NF-kB, ↓COX-2 expression, ↓ICAM-1 expression, ↓MCP1 expression, ↓iNOS expression, ↑Nrf2 activity, and ↑HO-1 | [29] |
| Dendrobium fimbriatum L. Orchidaceae | DSS-induced mice model of colitis | Polysaccharide extract | - | ↑DAI, ↑colon barrier disruption, and ↓body weight | ↑NF-κB signaling and ↑Th17/regulatory T homeostasis disruption | ↓NF-κB signaling activation, ↓Th17/regulatory T homeostasis disruption, and ↑Nrf2 signaling | [30] |
| Pisum sativum L. Fabaceae | DSS-induced C57BL/6 mice model of colitis | Hull polyphenol extracts | 100 and 600 mg/kg | ↑DAI, ↑diarrhea, blood in the stool, ↓colon length, and ↓body weight | ↑MPO, ↓claudin-1, ↓occludin, ↓ZO-1, ↑MDA, ↓SOD, ↓CAT, ↓T-AOC, ↑TNF-α, ↑IL-1β, ↑IL-6, ↓IL-10, ↑Keap1, ↓Nrf2 expression, ↓GCLC, ↓HO-1, and ↓NQO1 | ↓MPO expression, ↑claudin-1 expression, ↑occludin expression, ↑ZO-1 expression, ↓MDA, ↑SOD, ↑CAT, ↑T-AOC, ↓TNF-α expression, ↓IL-1β expression, ↓IL-6 expression, ↑IL-10 expression, ↓Keap1, ↑Nrf2 expression, ↑Nrf2 nuclear translocation, ↑GCLC, ↑HO-1, and ↑NQO1 | [31] |
| Tetrastigma hemsleyanum Diels & Gilg Vitaceae | DSS-induced mice model of colitis | Leaves bioactive and their metabolites | - | ↓Colon length and ↓body weight | ↓Claudin-1, ↓occludin, ↓ZO-1, ↑IL-1β, ↑IL-6, ↑TNF-α, ↓IL-10, ↓Nrf2 nuclear translocation, ↓SOD, ↓CAT, ↓HO-1, ↓NQO1, ↓GCLC, ↑MPO, and ↑MDA | ↑Claudin-1, occludin and ZO-1 expressions, ↓IL-1β, IL-6 and TNF-α expressions, ↑IL-10 expression, ↑Nrf2 nuclear translocation, ↑SOD, ↑CAT, ↑HO-1, ↑NQO1 and ↑GCLC expression, ↓MPO, and ↓MDA expressions | [32] |
| Rhus chinensis Mill. Anacardiaceae | DSS-induced mice model of colitis | Fruits ethanolic extrat | - | ↑DAI, ↑colon barrier disruption, and ↓colon length | ↑MPO, ↑MDA, ↑IL-1β, ↑IL-6, ↑TNF-α, ↑p-NF-κB, ↑p-IκB, ↑COX-2, ↑iNOS, ↑p-P38, ↑p-Erk1/2, ↑p-JNK, ↓SOD, ↓GSH, ↓claudin-1, ↓occludin, ↓ZO-1, ↓Nrf2 activation, ↓NQO1, and ↓HO-1 | ↓MPO, ↓MDA, ↓IL-1β, ↓IL-6, ↓TNF-α, ↓p-NF-κB, ↓p-IκB, ↓COX-2, ↓iNOS, ↓p-P38, ↓p-Erk1/2 and ↓p-JNK expressions, ↑SOD, ↑GSH, ↑claudin-1, ↑occludin and ↑ZO-1 expressions, ↑Nrf2 activation and ↑NQO1, and ↑HO-1 expressions | [33] |
| Crocus sativus L. Iridaceae | DSS-induced C57BL/6 mice model of colitis | Aqueous extract | 7.5, 15, 20, and 25 mg/kg | ↑DAI, ↓colon length, and ↓body weight | ↓HO-1, ↓GPX-2, ↓Nrf2 expression and activity, ↑TNF-α, and ↑IFN-γ | ↑HO-1, ↑GPX-2, ↑Nrf2 expression and activity, ↓TNF-α and ↓IFN-γ expressions | [34] |
| Prunus mahaleb L. Rosaceae | DSS-induced BALB/c mice model of colitis | Concentrated fruit extract | 1300 mg/Kg | ↑Intestinal inflammation and ↑OS | ↓Nrf2 expression and activity, ↓GSH, ↓GSR, and ↓G6PD | ↑Nrf2 expression and activity, ↑GSH, ↑GSR, and ↑G6PD | [35] |
| Quercus ilex L. Fagaceae | TNBS-induced Wistar mice model of colitis | Leaves polyphenolic extract | 250 and 500 mg/kg | ↑Anorexia, ↓body weight, ↑intestinal adhesions to adjacent organs, ↑weight/length ratio of the colon, ↑transmural necrosis, ↑edema, ↑diffuse inflammatory cells infiltration, ↑ulceration, and ↑crypt distortion | ↑MPO, ↑TNF-α, ↑IL-1β, ↑COX-2, ↑iNOS, ↑IκB-α degradation, ↑p65 nuclear translocation, and ↑p50 nuclear translocation, ↑NF-κB-mediated transcriptional protein activation, ↑MAPK (JNK, p38, and ERK1/2) protein activation, ↓HO-1, and ↓Nrf2-mediated transcriptional protein activation | ↓MPO, ↓TNF-α, ↓IL-1β, ↓COX-2 and ↓iNOS expressions, ↓IκB-α degradation, ↓p65 and ↓p50 nuclear translocation, ↓NF-κB-mediated transcriptional protein activation, ↓MAPK (JNK, p38, and ERK1/2) protein activation, ↑HO-1 expression, and ↑Nrf2-mediated transcriptional protein activation | [36] |
| Ziziphus spina-christi L. Rhaminaceae | AcOH-induced Wistar mice model of colitis | Fruits extract | 100, 200, and 400 mg/kg | ↑Weight/length ratio of the colon, ↓mucin concentration, ↑necrosis ↑ulceration, ↑corrosion, ↑hemorrhagic diarrhea, ↑intestinal focal infiltration of inflammatory cells, and ↑crypt distortion | ↑MPO, ↑LPO, ↑NO, ↓GSH, ↓SOD, ↓CAT, ↓GSR, ↓GPx, ↑TNF-α, ↑IL-1β, ↑iNOS, ↑COX-2, ↑NF-κB activation and signaling, and ↓Nrf2/HO-1 activation and signaling | ↓p38, ↓MAPK, and ↓VEGF-A expressions, ↓Bax and ↓caspase-3-mediated apoptosis, ↑Bcl-2 expression, ↓MPO, ↓LPO, and ↓NO expressions, ↑GSH, ↑SOD, ↑CAT, ↑GSR, and ↑GPx expressions, ↓TNF-α, ↓IL-1β, ↓iNOS, ↓COX-2 expressions, ↓NF-κB activation, and signaling and ↑Nrf2/HO-1 activation, and signaling | [37] |
| Perilla frutescens L. Laminaceae | DSS-induced ICR mice model of colitis | Leaf extracts | 20 and 100 mg/kg | ↓Body weight, ↑DAI, ↑diarrhea, ↑bloody stool, ↓colon length, and ↑intestinal infiltration of inflammatory cells | ↑COX-2, ↑iNOS, ↑cyclin D1, ↑NF-κB activation and translocation, ↑IκBα phosphorylation and degradation, ↑p65 nuclear translocation, ↑STAT3 signaling, ↑TLR4, ↑IRF3, ↑IFN-α, ↑IFN-γ, ↓Nrf2/HO-1 activation and signaling, and ↓HO-1 | ↓COX-2, ↓iNOS and ↓cyclin D1 expressions, ↓NF-κB activation and translocation, ↓IκBα phosphorylation and degradation, ↓p65 nuclear translocation, ↓STAT3 signaling, ↓TLR4, ↓IRF3, ↓IFN-α, and ↓IFN-γ expressions, ↑Nrf2/HO-1 activation and signaling, and ↑HO-1 | [38] |
| Compounds/Phytochemicals | In Vivo/In Vitro Model | Effective Doses/Concentrations | Related Clinical Features of IBD | Nrf2-Related Dysregulation Indicators | Related Molecular Mechanisms in the Regulation of Nrf2 | Ref. |
|---|---|---|---|---|---|---|
6-shogaol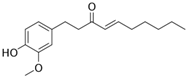 | Raw 264.7 macrophage and colon-26 in vitro LPS-activated cells and DSS-induced FVB/NJ mice model of colitis in vivo | 50, 100, 200, 500, and 1000 µg/mL in vitro and 15 mg/kg in vivo | ↑Intestinal inflammation and ↑OS both in vitro and in vivo and ↓intestinal integrity and ↑intestinal leukocyte invasion in vivo | ↑Lcn-2, ↑TNF-α, ↑IL-6, and ↑IL-1β | ↑HO-1, ↓TNF-α mRNA, ↓IL-6 mRNA, ↓IL-1β mRNA, and ↓iNOS mRNA, | [39] |
Licochalcone A 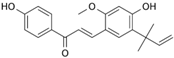 | DSS-induced C57BL/6 mice model of colitis | 20, 40, and 80 mg/kg | ↓Colon length, ↓stool consistency, ↑colonic necrosis, ↑inflammatory cells infiltration, and thickening of muscular mucosa | ↑MPO, ↑TNF-α, ↑IL-6, ↑IL-1β, ↑COX-2, ↑NF-κB p65, ↑IKKα, ↑p-IκB, ↑NO, ↓SOD, ↓GSH, and ↑Keap1 | ↑HO-1 and ↑GCL expressions and ↓Keap1, ↓NF-κB p65, ↓IKKα, and ↓p-IκB expressions | [40] |
| DSS-Induced C57BL/6 mice model colitis | 20, 40, and 80 mg/kg | ↑Intestinal inflammation and ↑OS | ↑TNF-α, ↑IL-6, ↑IL-1β, ↑COX-2, and ↑ROS | ↓p-NF-κB-p65, ↓pIkB kinase α, ↑HO-1, ↑GCLC, ↓Keap1, and ↑Nrf2 translocation and signaling | [40] | |
Oligonol | DSS-induced C57BL/6 mice model colitis | 10, 50, and 100 mg/kg | ↑Intestinal inflammation, ↑OS, ↑destruction of the mucosal barrier, and ↓colon length | ↑TNF-α, ↑IL-6, ↑IL1β, ↑COX- 2, ↑iNOS, ↑ROS, ↑JNK, and ↓HO-1 | ↓NF-κB-p65, ↑Nrf2 transcription and signaling, ↑HO-1, ↑AKRs, and ↑ GSTs | [41] |
Cyanidin-3-O-glucoside | TNF-α-induced intestinal Caco-2 epithelial cells | 20-40 µM | ↑Intestinal inflammation, ↑OS, and ↑cellular damage | ↑TNF-α, ↑IL-6, ↑COX- 2, ↑PGE2, ↑thromboxane A2, ↑leukotrienes, ↑ROS, and ↓ GSH | ↓NF-κB-p65, ↓NF-κB-p50, ↑Nrf2 expression and signaling, ↑NQO1, and ↑HO-1 | [42] |
Luteolin | DSS-induced C57BL/6 mice model colitis | 20 and 50 mg/kg | ↑Intestinal inflammation, ↑OS, ↑glands destruction, and ↑colon length | ↑TNF-α, ↑IL-6, ↑iNOS, and ↑MDA | ↓NF-κB-p65, ↑Nrf2 expression and signaling, ↑NQO1, ↑HO-1 and ↑SOD, and ↑CAT expressions | [43] |
Alpinetin  | DSS-induced C57BL/6 mice model colitis | 25, 50, and 100 mg/kg | ↑DAI, ↑intestinal inflammation, ↑OS, ↓colon length, ↑loss of epithelial and goblet cells, and ↑crypt aberrations | ↓SOD, ↓Nrf2 expression, and ↓HO-1 | ↑SOD expression, ↑Nrf2 expression and signaling, and ↑HO-1 | [44] |
Cardamonin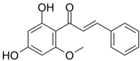 | DSS-induced C57BL/6 mice model of colitis and TNBS-induced BALB/c mice model of colitis | 15, 30, 60 mg/kg (DSS), and 200mg/kg (TNBS) | ↑DAI, ↑OS, ↑distorted crypts, ↑loss of goblet cells, and ↑mucosal damage | ↑TNF-α, ↑IL-6, ↑IL1β, ↑ NLRP3, ↑ROS, and ↑cleaved caspase-1 | ↑ Nrf2 transcription and signaling, ↑NQO1, ↑SOD expression, and↑ HO-1 | [45] |
Puerarin 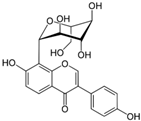 | DSS-induced BALB/c mice model of colitis | 10 and 50 mg/kg | ↑DAI, ↑intestinal inflammation, ↑OS, ↑ colon length, ↑ spleen index, and ↑crypt aberrations | ↑MPO, ↑TNF-α, ↑IL-6, ↑ IFN- γ, ↑IL-1β, ↑ROS, ↑COX-2, ↑PGE2 ↑iNOS, ↑MDA, ↓GSH, and ↓ ZO-1 | ↓p-NF-κB, ↑Nrf2 transcription, ↑NQO1, ↑ HO-1, ↑SOD, and ↑CAT expressions | [46] |
Gallic acid  | DSS-induced BALB/c mice model of colitis | 10 mg/kg | ↑OS, ↑intestinal inflammation, ↑DAI and ↑colon length, and ↑gland distortion | ↑IL-23, ↑IL-21, and ↑MDA | ↑SOD, ↑CAT, ↑Nrf2 expression and signaling, ↑NQO1, and ↑UDP-GT | [47] |
Sulforaphane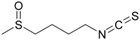 | DSS-induced mice model of colitis | 10, 15, and 20 mg/kg | ↑Intestinal inflammation, ↑OS, and ↑barrier injury | ↑TFAM expression, ↑mTOR expression, and ↑cyclin D1 expression | ↑Nrf2 expression and signaling and ↑HO-1 | [48] |
| DSS-induced Cc57bl/6 mice model of colitis | 2.5, 5, 10, and 20 mg/kg | ↑OS, ↑intestinal inflammation, ↑DAI, and ↑ colon length | ↑TNF-α, ↑IFN- γ, ↑ IL-1β, and ↑COX-2 | ↑Nrf2 expression and signaling | [49] | |
Asperuloside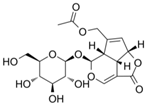 | DSS-induced KM mice model of colitis and LPS-treated RAW264.7 cells | 125 and 500 μg/kg in vivo and 5, 10, 20 μM in vitro | ↑DAI, ↑inflammatory cell infiltration ↑OS, ↑colon length, ↑loss of epithelial and ↓goblet cells in vivo and ↑inflammation and ↑OS in vitro | ↑MPO, ↓GSH, ↑ MDA, ↑TNF-α, ↑IL-6, ↓ IL-10, and ↑ROS | ↓p-NF-ΚB-p65, ↑SOD, ↑Nrf2 expression, ↑NQO1 mRNA, and ↑HO-1 mRNA | [50] |
Syringin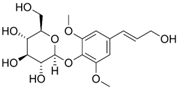 | DSS-induced BALB/c mice model of colitis | 100 mg/kg | ↑Inflammatory cell infiltration, ↑OS, and ↑colon length | ↑TNF-α, ↑IL-6, ↑IL1β, ↑ COX-2, ↑iNOS, ↓ZO-1, and ↓occludin | ↓p-NF-κB, ↓IκBα, ↓p-IκBα, ↑Nrf2 expression, and ↑HO-1 | [51] |
Paeoniflorin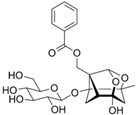 | LPS-treated Caco-2 cells | 10, 50, 100, and 150 μM | ↑Intestinal inflammation, ↑OS, and ↑loss of mucosal barrier proteins | ↑TNF-α, ↑IL-6, ↑COX-2, ↑iNOS, ↓ZO-1, and ↓occludin | ↓p-NF-κB, ↑Nrf2 mRNA expression, and ↑HO-1 | [52] |
Dehydrocostus lactone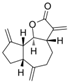 | DSS-induced ICR mice model of colitis and LPS-treated RAW264.7 cells | 5–15 mg/kg in vivo and 2–9 μM in vitro | ↑Intestinal inflammation, ↑OS, ↑barrier injury, and ↓goblet cells in vivo and ↑Intestinal inflammation and ↑OS in vitro | ↑MPO, ↑TNF-α, ↑IL-6, ↑ COX-2, and ↑ROS | ↓p-NF-κB p-65, ↑IKKα/β, ↑Nrf2, ↑ HO-1, ↓Keap1, and ↓MAPK | [53] |
Leonurine 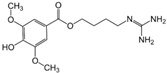 | DSS-induced C57BL mice model of colitis | 7, 5–15, and 30 mg/kg | ↑DAI, ↑inflammatory cell infiltration, ↑OS, and ↓colon length | ↑TNF-α, ↑IL-6, ↑IL-1β, and ↓GSH | ↑Nrf2 expression, ↑ HO-1, ↓p-NF-κB, ↓TLR4, and ↑SOD | [54] |
Crocin | AA-induced Sprague Dawley mice model of colitis | 20 mg/kg | ↑DAI, ↑stromal edema, ↑ulcerated mucosa, ↑infiltration of inflammatory cells, and ↑lymphoid follicles | ↓TAC, ↓CAT, ↓SOD, ↓GSH, ↑MDA, ↑TNF-α, ↓Nrf2, ↓ HO-1, ↑ROS, and ↑apoptosis | ↓TNF-α expression, ↓ROS production, ↓caspase-3 expression, ↓MDA, ↑Nrf2, ↑HO-1, ↑, ↑CAT, ↑SOD, and ↑GSH expression | [55] |
Quercetin | DSS-induced Wistar mice model of colitis | 10, 15, and 20 mg/kg | ↑DAI, ↑coagulative epithelial necrosis, ↑intestinal inflammation, ↑inflammatory cell infiltration, ↓colonic crypts, ↑ulceration in epithelial tissues, ↓mucin, and ↓goblet cells | ↑MPO, ↑IL-6, ↑TNF-α, ↑ IFN-γ, ↑IL-10, ↑COX-2, ↑MDA, ↑ROS, ↑NF-κB, ↑CD-4, ↑CD-8, ↑TLR-4, ↑inflammatory cytokines, ↑proteolytic enzymes, ↓MUC2 genetic expression, ↓occludin, ↓plasma antioxidants, ↓GSH, ↓SOD, and ↓CAT | ↑Nrf2 and HO-1 genetic expression, ↑occludin, ↑MUC2 genetic expression, ↓NF-κB activation, and signaling | [56] |
8-Oxypalmatine | DSS-induced Balb/c mice model of colitis | 12, 5, 25, and 50 mg/kg | ↑ DAI, ↑markers of OS, ↑inflammatory mediators ↓body weight, ↑colon shortening, ↑massive infiltration of inflammatory cells, ↑excessive crypt damage, ↑epithelial cell destruction, and ↑mucosal thickening | ↑NLRP3, ↑MPO, ↑TNF-α, ↑IL-1β, ↑IFN-γ, ↑IL-17, ↑IL-6, ↑NO, ↑MDA, ↑ASC, ↑caspase-1, ↓SOD, ↓GSH, ↓CAT, ↓GSH-Px, ↓Nrf2 expression, ↓HO-1, and ↓IL-10 | ↓NLRP3 activation and signaling, ↓ASC expression, ↓caspase-1, ↑Nrf2 expression and ↑HO-1, ↓pro-inflammatory cytokines expression, and ↑anti-inflammatory cytokines expression | [57] |
| 3-(3-pyridylmethylidene)-2-indolinone | DSS-induced C57BL/6J and ICR mice model of colitis | 2.2, 11, and 22 mg/kg | ↑DAI, ↓length of the colon, ↑infiltration of inflammatory cells, ↑loss of colonic crypts, and ↑epithelial cell necrosis | ↑ IL-6 mRNA, ↑TNFα mRNA, ↑ IFN-γ mRNA,↑ NF-κB mRNA, ↑p65, ↑MCP-1, ↓Nrf2 activation and signaling, and ↓Nrf2 target genes activation and expression | ↓NF-κB signaling and activation, ↓p65, ↓TNF-α mRNA, ↓IFN-y mRNA, ↓IL-6 mRNA, ↑Nrf2 activation and signaling, ↑NQO1 mRNA, ↑HO-1 mRNA and Nrf2 target genes activation and expression | [58] |
Ruscogenins | DSS-induced C57BL/6J mice model of colitis in vivo and LPS-induced inflammation in colonic organoids in vitro | 0.5, 1, and 2 mg/kg in vivo and 50 μg/mL in vitro | ↑DAI, ↑apoptosis, ↑intestinal barrier dysfunction, ↑inflammation in the intestinal epithelium, ↑bacterial translocation, ↑abscesses and ↑crypt ulcers in vivo and ↑inflammatory burden lesions in vitro | ↑TNF-α, ↑ IFN-γ, ↑NLRP3, ↑caspase1, ↑caspase-11, ↑GSDMD, ↑apoptosis, ↑Bax, and ↑caspase-3 in vivo and ↑TNF-a, ↑IFN-y, ↑factors related to macrophage migration (G-CSF, RANTES, and MCP1), ↑apoptosis, ↑Bax, ↑caspase-3 expression, and ↓Bcl-2 in vitro | ↑Nrf2/NQO1, ↑expression of proteins associated with the Nrf2/NQO1 pathway, ↑Bcl-2 protein and ↓Bax/c-caspase-3 signaling pathway in vivo and ↑Nrf2, ↑NQO1, ↓TNF-α, ↓IFN-γ, and ↓expression of factors related to macrophage migration in vitro | [59] |
Caffeic acid | DSS-induced ICR mice model of colitis | 251 mg/kg | ↑DAI, ↑intestinal permeability and ↑intestinal infiltration, ↓goblet cells, ↑weight loss, ↓colon length, and ↑intestinal microbiota dysbiosis | ↑IL-6, ↑TNF-α, ↑IL-1β, ↑IL-12, ↑MDA, ↑ROS, ↓ZO-1, ↓occludin, ↓IL-10, ↓GSH-Px, ↓SOD, and ↓ CAT | ↓IL-1β mRNA, ↓IL-6 mRNA and ↓TNF-α mRNA, ↑SOD, ↑GPX1, ↑GPX2, ↑CAT, ↑IL-10 mRNA, ↑Nrf2 mRNA, ↑HO-1 mRNA, ↑NQO1, and ↑occludin mRNA | [60] |
Schisandrin B | DSS-induced C57BL/6 mice model of colitis in vivo and HCT-116 cells induced by LPS in vitro | 10 mg/kg in vivo and 40 uM in vitro | ↑DAI, ↑intestinal ulceration, ↑intestinal permeability, ↑bacterial translocation, ↑immune cell infiltration, and ↓body weight in vivo and ↑intestinal inflammation and ↑intestinal OS in vitro | ↑TNF-α, ↑IL-6, ↑IL-18, and ↑IL-1β in vivo and ↑TNF-α, ↑IL-6, ↑IL-18, ↑IL-1β, ↑NLRP3, ↑ ROS, ↑GSDMD protein, ↑LDH activity, ↑cellular apoptosis, and ↑mitochondrial damage in vitro | ↑AMPK/Nrf2, ↑ NRF2, ↑ pAMPK, ↓ TNF-α, ↓ IL-6, ↓ IL-18, ↓ IL-1β, ↓ NLRP3 inflammasome, and ↓GSDMD protein expression in vivo and ↓TNF-α, ↓IL-6, ↓IL-18, ↓IL-1β, ↓NLRP3 protein expression, ↓NLRP3 activity, ↓GSDMD protein expression, ↑p-AMPK protein expression, and ↑Nrf2 activity in vitro | [61] |
| GB1a | DSS-induced C57BL/6 mice model of colitis | 25, 50, and 100 mg/kg | Weight loss, ↑DAI, ↓number of crypts, and ↑infiltration of inflammatory cells in the mucosa and submucosa | ↑MPO mRNA, ↑IL-6 mRNA, ↑TNF-α mRNA, ↑CCL5, ↑CCL20, ↑CXCL1, ↑NF-κBp65, ↑NF-κBp65 translocation to the nucleus, ↑MDA, ↓GSH, ↓SOD, ↓HO-1, ↓Nrf2 translocation to the nucleus and Nrf2-related proteins expression, ↓ZO-1 mRNA, and ↓occludin mRNA | ↓MPO mRNA, ↓IL-6 mRNA, ↓TNF-α mRNA, ↓CCL5, ↓CCL20, ↓CXCL1, ↓NF-κBp65, ↓NF-κBp65 translocation to the nucleus, ↓MDA, ↑GSH, ↑SOD, ↑HO-1, ↑Nrf2 translocation to the nucleus and Nrf2-related proteins expression, ↑ZO-1 mRNA, and ↑occludin mRNA | [62] |
Diosmetin | DSS-induced C57BL/6 mice model of colitis in vivo and Caco-2 and IEC-6 treated with LPS in vitro | 25 and 50 mg/kg in vivo and 0–400 μM in vitro | ↑DAI, ↓body weight, ↓colon length, ↑inflammatory infiltration, ↑intestinal permeability, and ↑intestinal epithelial barrier dysfunction in vivo and ↑intestinal inflammation and ↑intestinal OS in vitro | ↑ROS, ↑IL-1β, ↑IL-6, ↑TNF-α, ↑COX-2, ↑MDA ↓occludin, ↓claudin-1, ↓GSH, ↓GSH-Px and ↓SOD in vivo and ↓occludin, ↓claudin and ↑ROS in vitro | ↑GSH-Px, ↑SOD, ↑GSH, ↑Sirt1, ↑Nrf2 activation, ↑HO-1, ↑ZO-1, ↓MDA, ↓NF-κB signaling, ↓IL-1β mRNA, ↓IL-6 mRNA, and ↓COX-2 mRNA in vivo and ↓ROS, ↓NF-κB signaling, ↑claudin-1, ↑occludin, ↑Sirt1 expression, ↑Nrf2 expression and signaling, and ↑HO-1, ↑ZO-1 in vitro | [63] |
Atractylenolide III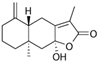 | TNBS-induced C57BL/6 mice model of colitis | 5, 10, or 20 mg/kg | ↑DAI, ↑inflammation, ↑weight loss, ↓colon length, ↑inflammatory cell infiltration, ↑OS, ↑submucosal necrosis, ↑structural mucosal distortion, and ↑ulcerated areas | ↑MPO, ↑IL-1β, ↑TNF-α, ↑MDA, ↑ROS, ↑FPR1, ↑NOX1, ↑phosphorylated (p)-Nrf2, ↓Nrf2 activation and signaling, ↓SOD, ↓GSHPx, and ↓CAT | ↑Nrf2 activation and signaling, ↑CAT, ↑SOD, ↑GSH-Px, ↓mRNA of IL-1β and TNF-α, ↓MDA, ↓FPR1, ↓NOX1, ↓ (p)-Nrf2 phosphorylated, and ↓MPO | [64] |
Polydatin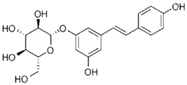 | DSS-induced C57BL/6 mice model of colitis and LPS-treated RAW264.7 cells | Not reported in vivo and 100 μM, 200 μM, 300 μM and 400 μM in vitro | ↑DAI, ↓colon length, ↑intestinal inflammation, and ↑intestinal OS in vivo and ↑inflammation and ↑OS in vitro | ↑TNF-α, ↑IL-4, ↑IL-6, ↓IL-10, ↑JNK phosphorylation, and ↑COX-2 | ↑Nrf2 nuclear translocation and phosphorylation, ↓Erk1/2 phosphorylation, ↓JNK1/2 phosphorylation, ↑HO-1, ↑NQO1, ↑Akt phosphorylation, and ↑IL-10 | [65] |
| DNBS-induced CD1 mice model of colitis | 10 mg/kg | ↑Intestinal inflammation and ↑intestinal OS, ↑prominent transmural inflammatory cells infiltration, and ↑ulcer formation | ↑IL-1β, ↑TNF-α, ↑prostaglandins, ↑NO, ↓HO-1, and ↑NF-κB-p65 phosphorylation | ↓NF-κB translocation and ↓IkBα degradation, ↑SIRT1, ↑Nrf2, ↑HO-1, ↓pro-inflammatory genes expression, and ↓OS genes expression | [66] | |
Rosmarinic Acid | DSS-induced C57BL/6 mice model of colitis | 5, 10, and 20 mg/kg | ↑DAI, ↑intestinal inflammation, and ↑prominent transmural inflammatory cells infiltration | ↑TNF-α, ↑IL-1β, ↑MPO, and ↑ inflammasome-related proteins | ↑Nrf2 expression, ↑HO-1, ↓pro-inflammatory genes expression, and ↓OS genes expression | [67] |
Imperatorin | TNBS-induced SD mice model of colitis | 15, 30, and 60 mg/kg | ↑DAI, ↑intestinal inflammation, and ↑intestinal OS | ↑ROS, ↑TNF-α and ↑IL-6 | ↑Nrf-2 expression, ↑ARE, ↑HO-1, and ↓OS genes expression | [68] |
Berberine  | AcOH-induced Wistar mice model of colitis | 25 and 50 mg/kg | ↑Intestinal inflammation, ↑prominent transmural inflammatory cells infiltration, ↑mucosal edema, and ↑necrosis | ↑IL-1β, ↑IL-6, ↑TNF-α, ↑MPO, and ↑PGE2 | ↑p38MAPK mRNA, ↓OS genetic expression, ↓apoptotic activity, and ↑Nrf2/HO-1 signaling pathway activity | [69] |
Curcumin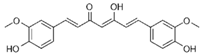 | Hypoxia and hypothermia-induced NEC mice model of colitis | 20 and 50 mg/kg | Intestinal inflammation | ↑IL-1 β, ↑IL-6, ↑IL-18, and ↑TNF-α | ↑SIRT1/NRF2 pathway, ↓TLR4 expression, and ↓pro-inflammatory genes expression | [70] |
Sesamin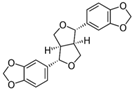 | Caco-2 human intestinal epithelial cells exposed to H2O2 | 10, 20, 40, 80, 160, and 320 mM | ↑Intestinal inflammation, ↑intestinal OS, and ↑intestinal cytotoxicity | ↑ROS, ↓GSH, ↑IL-1β, ↑IL-6, ↑TNF-α, and ↑M1 macrophages polarization | ↓Keap1, ↑Nrf-2/ARE expression, ↑p-AKT/AKT, ↑GCLC, ↑GCLM, ↑NQO1, and ↑HO-1 | [71] |
Toosendanin | DSS-induced C57BL/6 mice model of colitis | 0.5 mg/kg and 1 mg/kg | ↑DAI, ↑intestinal inflammation, and ↑intestinal OS | ↑IL-1β, ↑IL-6, ↑TNF-α, ↑RONS, and ↓GSH | ↑Nrf2 expression, ↑HO-1, ↓pro-inflammatory genes expression, and ↓OS genes expression | [72] |
Galangin | LPS-treated RAW264.7 cells and DSS-induced Balb/c mice model of colitis | 0.39 and 0.78 µg/mL for LPS-treated cells and 20 and 40 mg/kg for mice | ↑DAI, ↑loss of epithelial and goblet cells, ↑crypt aberrations in vivo and ↑inflammation and ↑OS in vitro | ↑Nitrites, ↑IL-6, ↑TNF-α, ↓SOD, ↓GSH, ↑NF-κB activation, ↑iNOS, and ↑COX-2 | ↑Nrf2 signaling pathway activation, ↑HO-1, ↓pro-inflammatory genetic expression, ↓JNK phosphorylation, and ↓p-IKKα/β | [73] |
Apocynin | DSS-induced Balb/c mice model of colitis | 400 mg/kg | ↑Intestinal inflammation and ↑intestinal OS | ↑iNOS, ↑COX-2, ↑TNF-α, ↑IL-1β, ↑IL-6, and ↑MCP-1 | ↑Nrf2 signaling pathway activation, ↑HO-1, and ↓pro-inflammatory genetic expression expression | [74] |
Hesperidin | DSS-induced C57BL/6 mice model of colitis and TNF-α/IFN-γ treated Caco-2 cells | 10, 20, or 40 mg/kg in vivo and 10, 20 or 40 μg/mL in vitro | ↑Intestinal inflammation | ↑TNF-α, ↑IL-6, ↑IL-10, and ↑MPO | ↑Nrf2 antioxidant signaling pathways activation, ↑NQO1, ↑HO-1, ↑regulatory T cells expression, ↓MPO ↓TNF-α expression, ↓IL-6 expression, and ↑IL-10 expression | [75] |
Norisoboldine | TNBS-induced Balb/c rats model of colitis | 20 and 40 mg/kg | ↑Intestinal inflammation and ↑intestinal OS | ↑TNF-α, ↑IL-1β, and ↑IL-6 | ↓NLRP3 inflammasome, ↑Nrf2 signaling pathway activation, and ↑Nrf2 expression | [76] |
Hyperoside | DSS-induced C57BL/6 mice model of colitis | 80 and 120 mg/kg | ↑DAI, ↑intestinal inflammation ↑intestinal OS, and ↓colon length | ↑TNFα, ↑IL-4, ↑IL-6, and ↑ROS | ↑Nrf2 signaling pathway activation, ↑HO-1, ↑SOD mRNA, ↓pro-inflammatory genetic expression, and ↓NF-κB expression | [77] |
Glyceollins   | DSS-induced Balb/c rats model of colitis | 4 and 10 mg/kg | ↑DAI, ↑intestinal inflammation, ↑intestinal OS, ↑prominent transmural inflammatory cells infiltration, and ↑mucosal necrosis | ↑TNFα, ↑IL-6, ↑iNOS, and ↑COX-2 | ↓NF-κB expression, ↑Nrf2 signaling pathway activation, and ↓pro-inflammatory genetic expression | [78] |
Carnosic acid | DSS-induced mice model of colitis | 50 and 100mg/kg | ↑DAI, ↑intestinal inflammation, ↑intestinal OS, and ↑prominent transmural inflammatory cells infiltration | ↑TNF-α, ↑IL-17A, ↑IL-6, ↑IFN-γ, ↑IL-1β, ↑IL-18, and ↑NF-κB activation | ↑Nrf2 expression, ↓Nrf2 ubiquitination and degradation, ↓NLRP3 inflammasome, ↑GCLM, ↑NQO1, ↑HO-1, ↓OS genes expression, and ↓Keap1 | [79] |
Protocatechuic acid | TNBS-induced Balb/c mice model of colitis | 30 and 60 mg/kg | ↑Intestinal inflammation and ↑intestinal OS | ↑IL-6, ↑TNF-α, ↑IL-1β, and ↑COX-2 | ↑Nrf2 expression, ↓COX-2 expression, and ↓NF-κB expression | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; de Maio, M.C.; Minniti, G.; de Góes Corrêa, N.; Barbalho, S.M.; Quesada, K.; Guiguer, E.L.; Sloan, K.P.; Detregiachi, C.R.P.; Araújo, A.C.; et al. Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review. Metabolites 2023, 13, 243. https://doi.org/10.3390/metabo13020243
Laurindo LF, de Maio MC, Minniti G, de Góes Corrêa N, Barbalho SM, Quesada K, Guiguer EL, Sloan KP, Detregiachi CRP, Araújo AC, et al. Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review. Metabolites. 2023; 13(2):243. https://doi.org/10.3390/metabo13020243
Chicago/Turabian StyleLaurindo, Lucas Fornari, Mariana Canevari de Maio, Giulia Minniti, Natália de Góes Corrêa, Sandra Maria Barbalho, Karina Quesada, Elen Landgraf Guiguer, Kátia Portero Sloan, Claudia R. P. Detregiachi, Adriano Cressoni Araújo, and et al. 2023. "Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review" Metabolites 13, no. 2: 243. https://doi.org/10.3390/metabo13020243
APA StyleLaurindo, L. F., de Maio, M. C., Minniti, G., de Góes Corrêa, N., Barbalho, S. M., Quesada, K., Guiguer, E. L., Sloan, K. P., Detregiachi, C. R. P., Araújo, A. C., & de Alvares Goulart, R. (2023). Effects of Medicinal Plants and Phytochemicals in Nrf2 Pathways during Inflammatory Bowel Diseases and Related Colorectal Cancer: A Comprehensive Review. Metabolites, 13(2), 243. https://doi.org/10.3390/metabo13020243