Incorporation of Plant-Based Diet Surpasses Current Standards in Therapeutic Outcomes in Inflammatory Bowel Disease
Abstract
1. Introduction
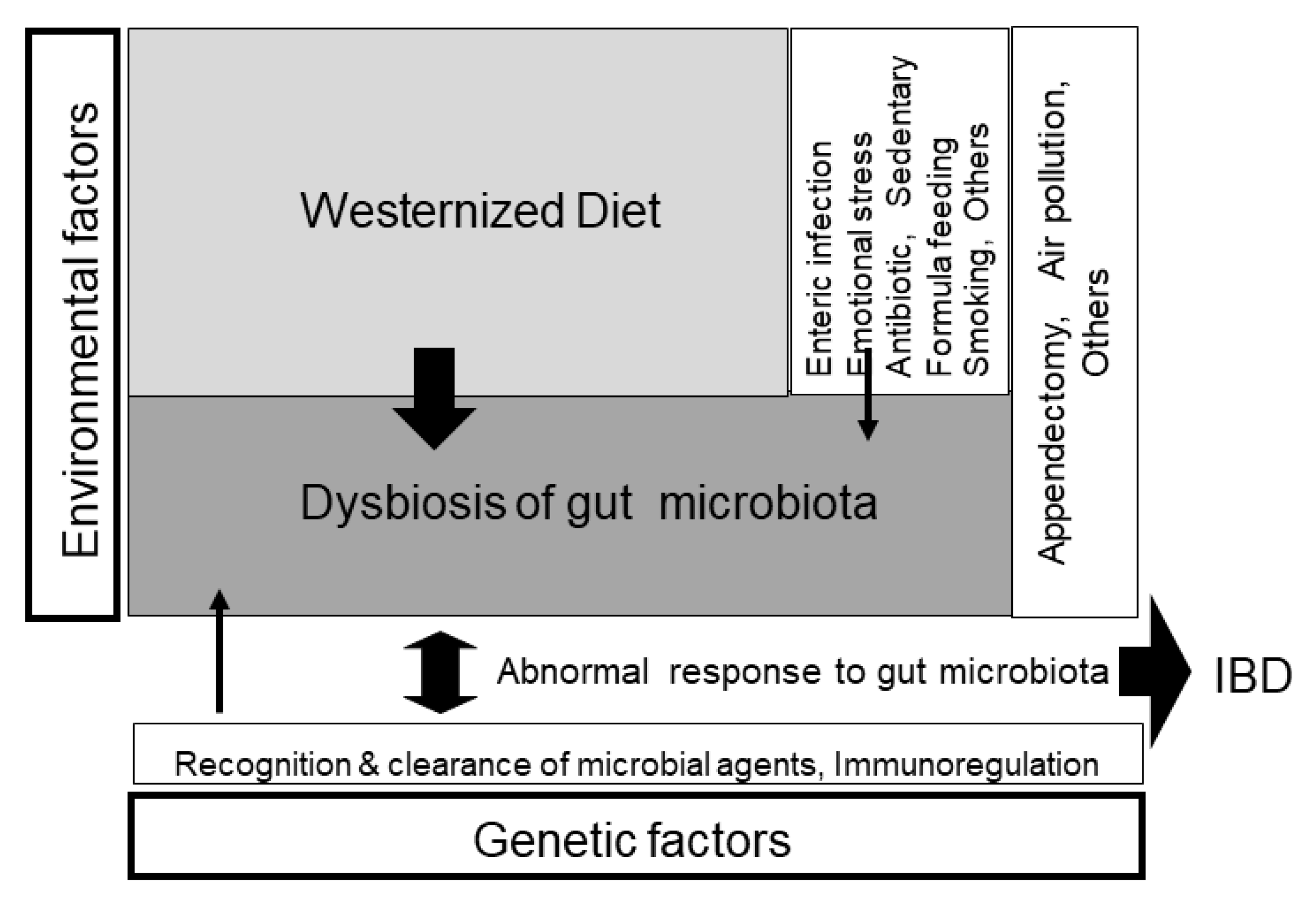
2. Our Current Westernized Diet Is the Ubiquitous Environmental Factor in IBD
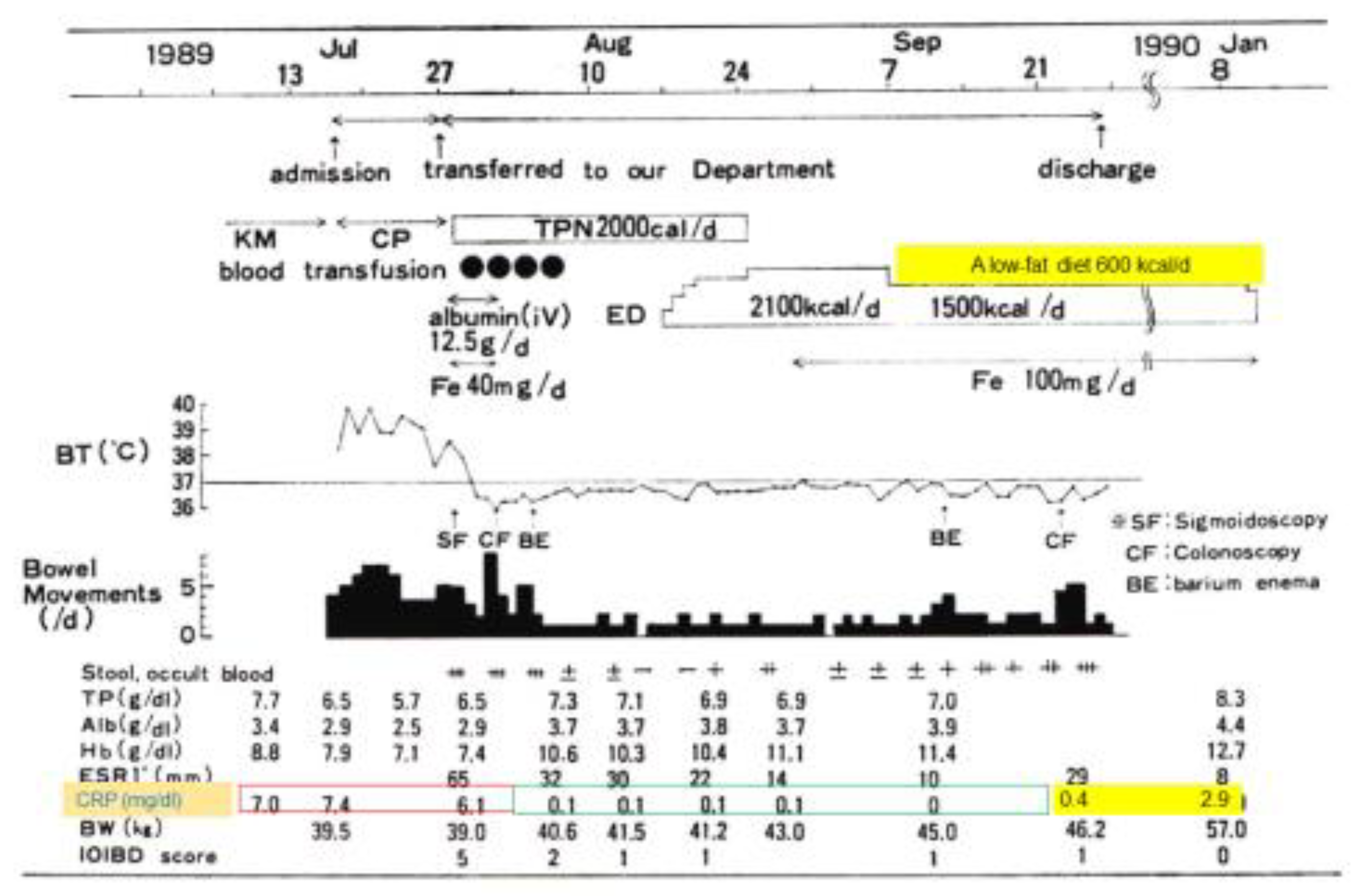
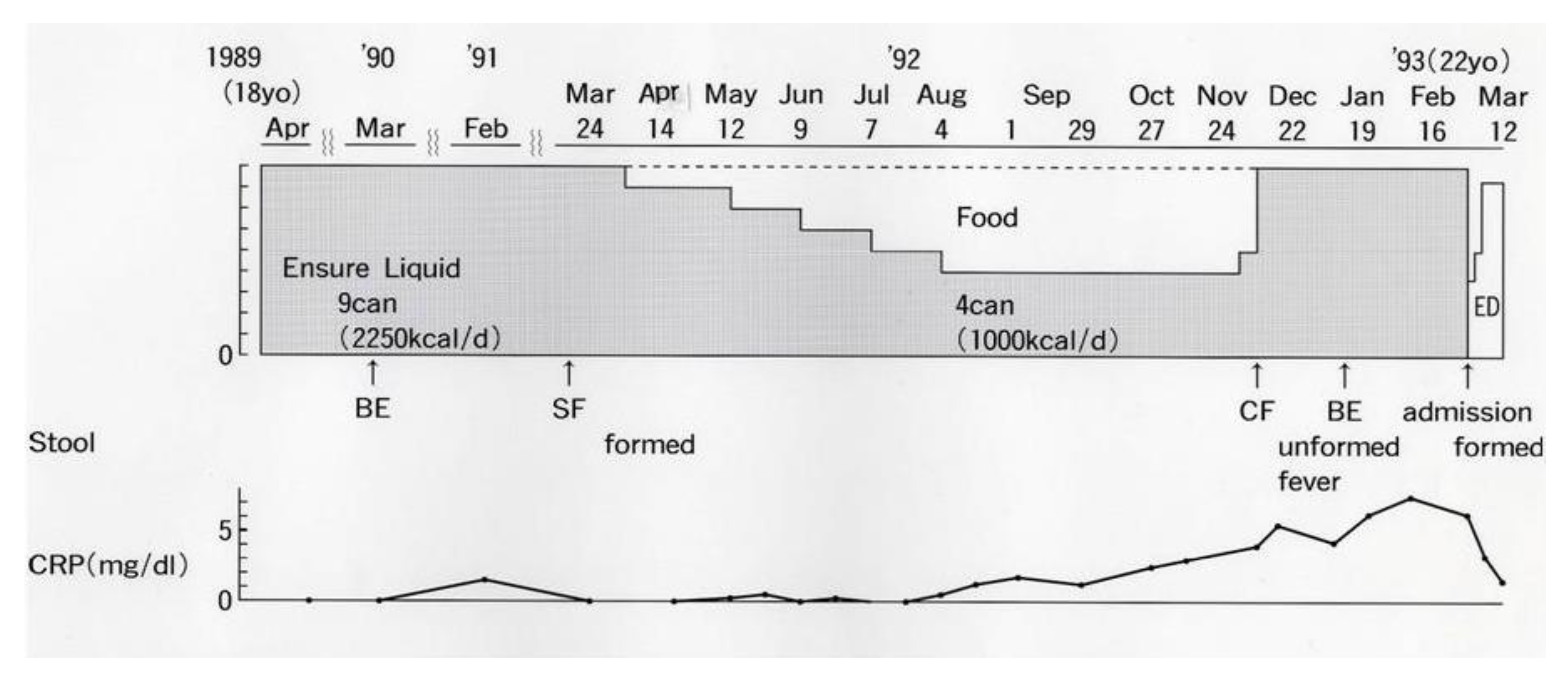
2.1. Bitter Experiences with Ordinary Food in Crohn’s Disease
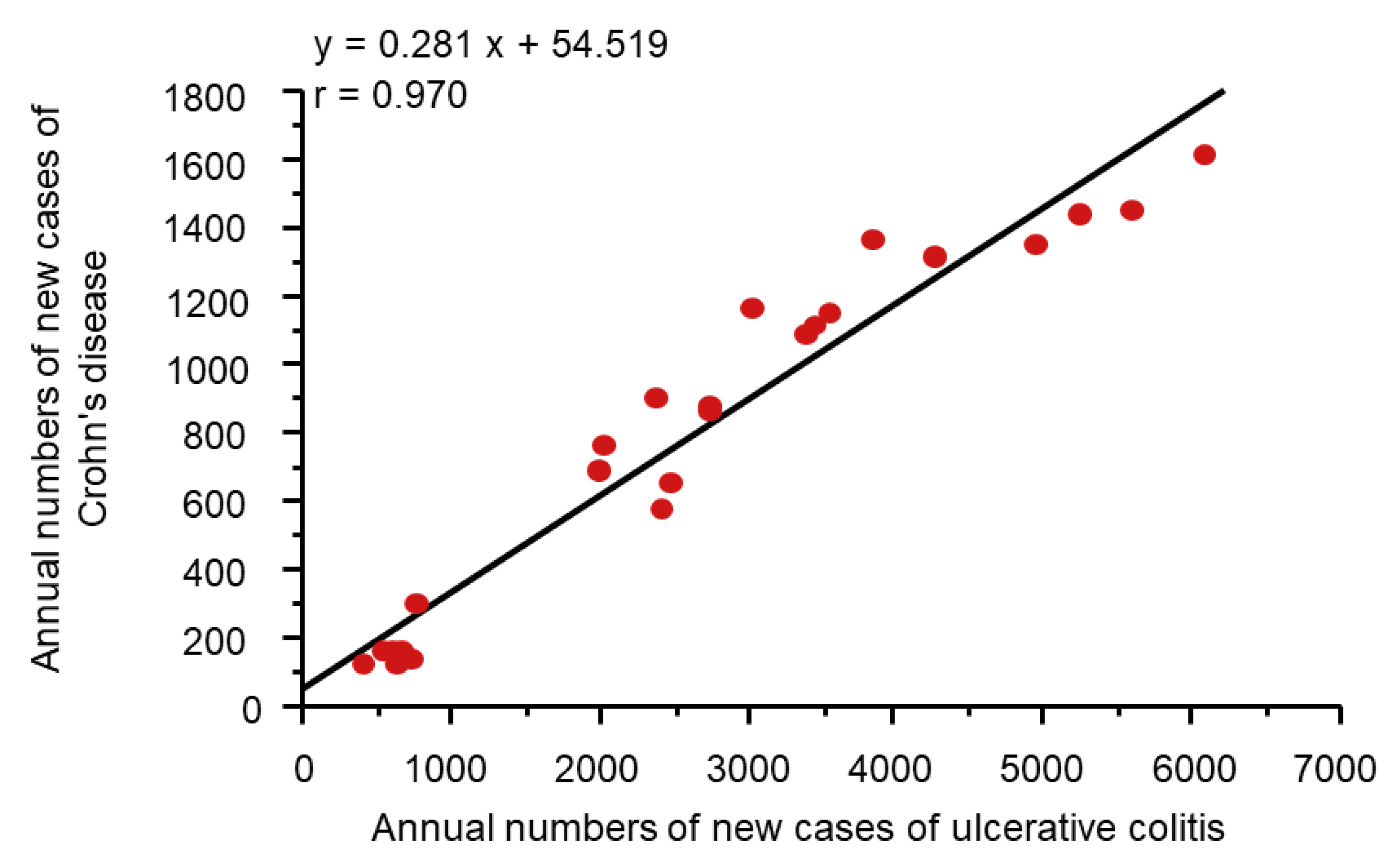
2.2. Environmental Factors in IBD
2.3. Epidemiologic Studies of the Relation between Diets and IBD
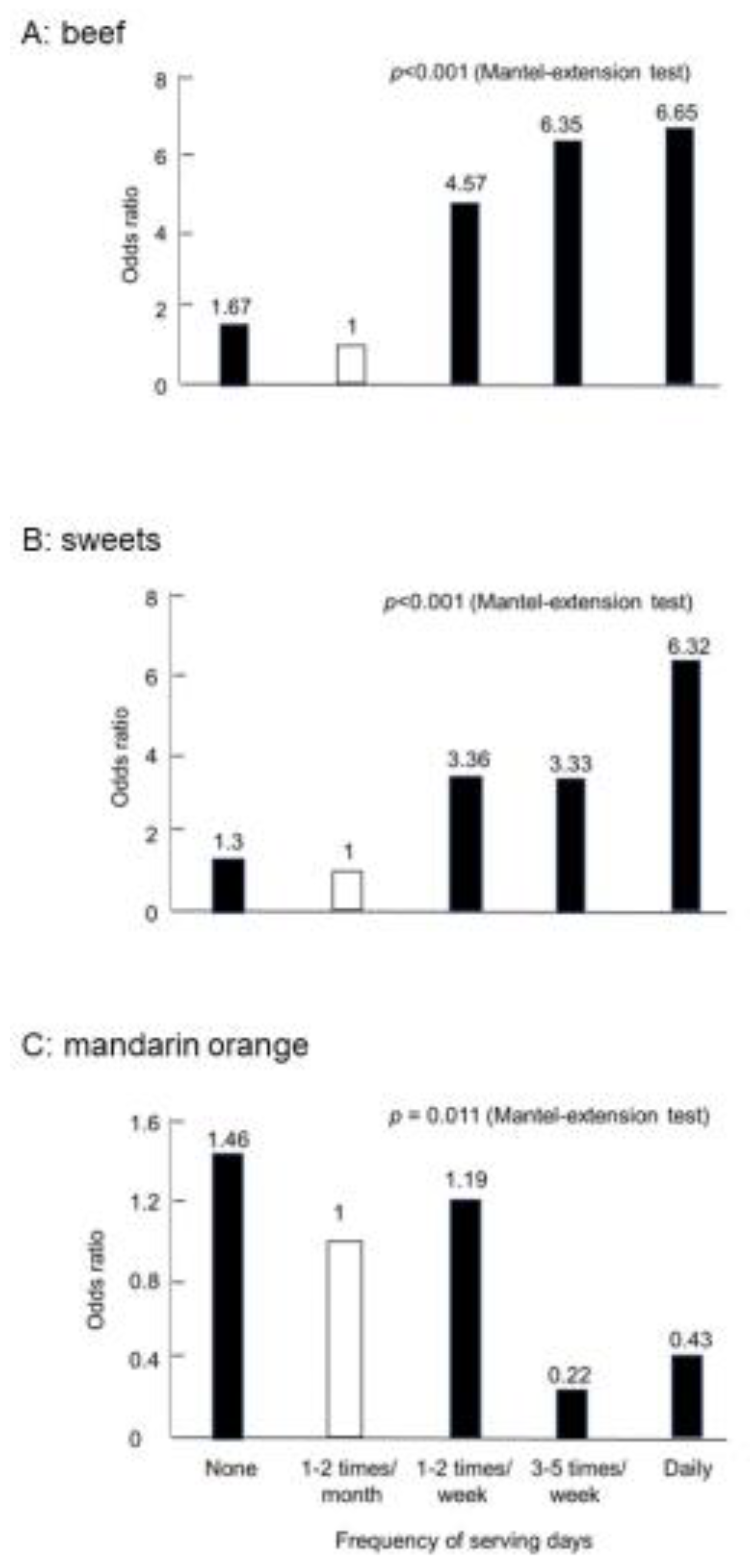
2.3.1. Risky or Preventive Foods for IBD
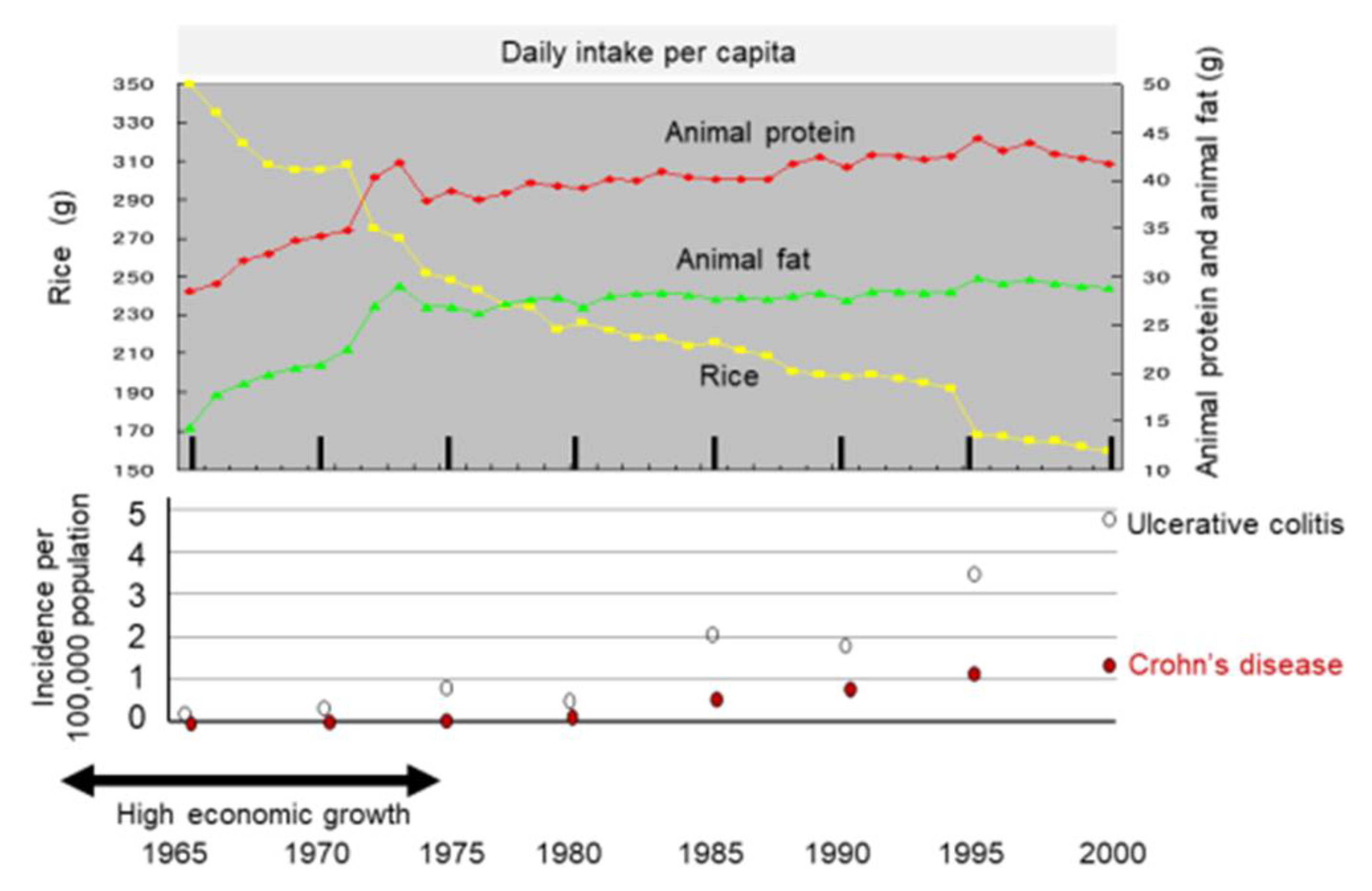
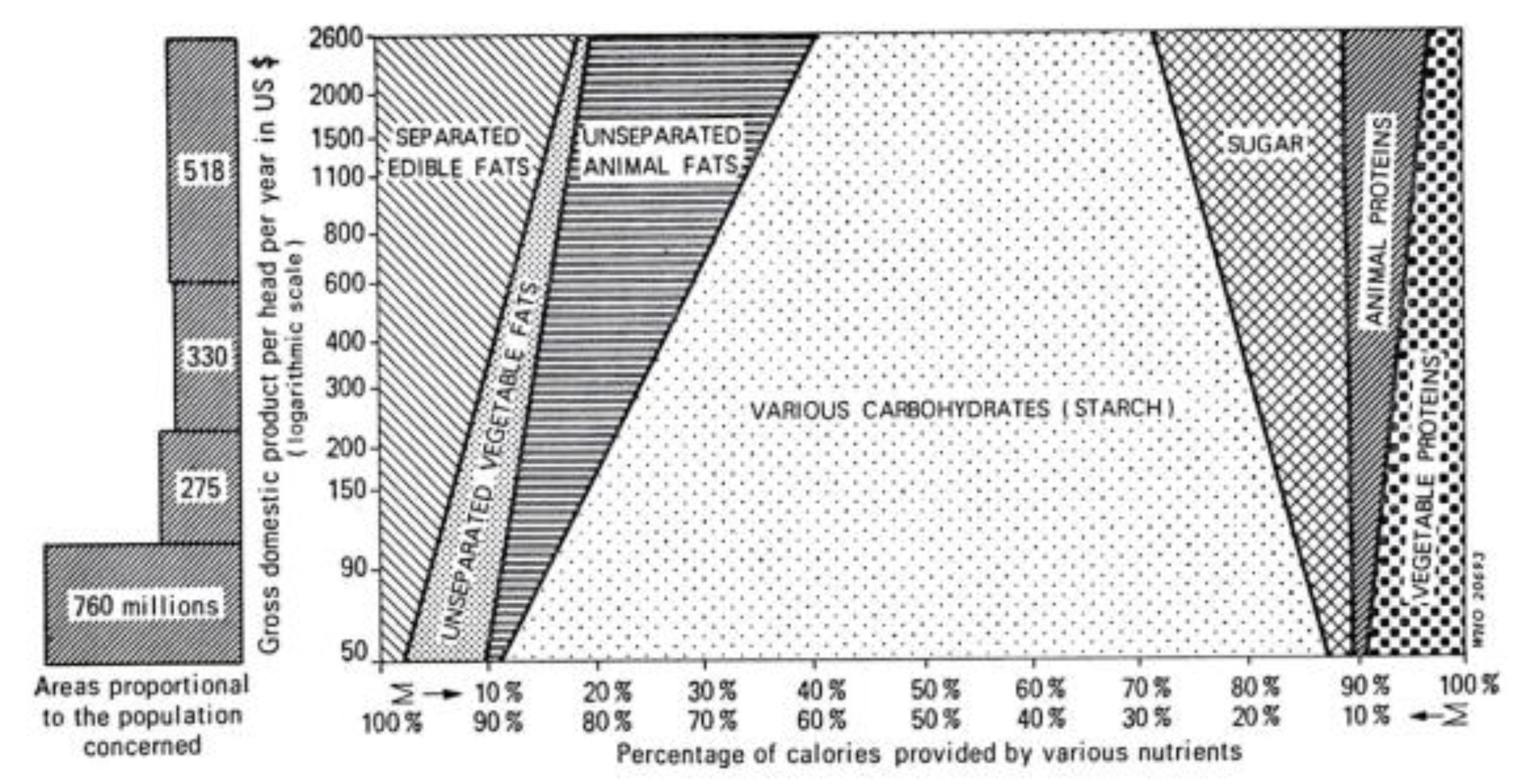
2.3.2. Dietary Transition from a Traditional Diet to a Westernized Diet
2.4. Onset of IBD during a Change in Dietary Habits towards Unhealthy Diets
2.5. Current Westernized Diets vs. PBDs
3. Plant-Based Diets for IBD
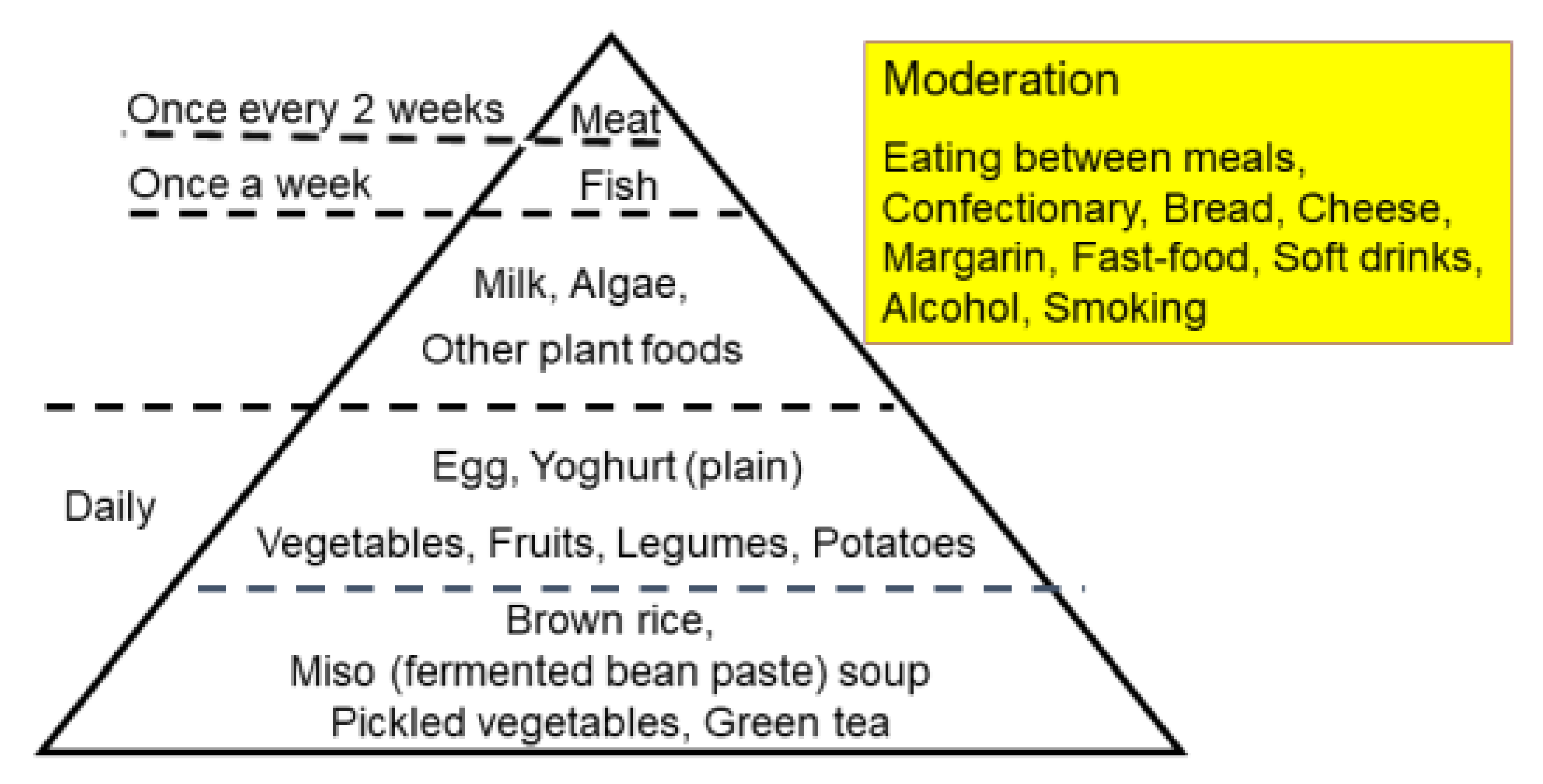
3.1. Development of Plant-Based Diet for IBD
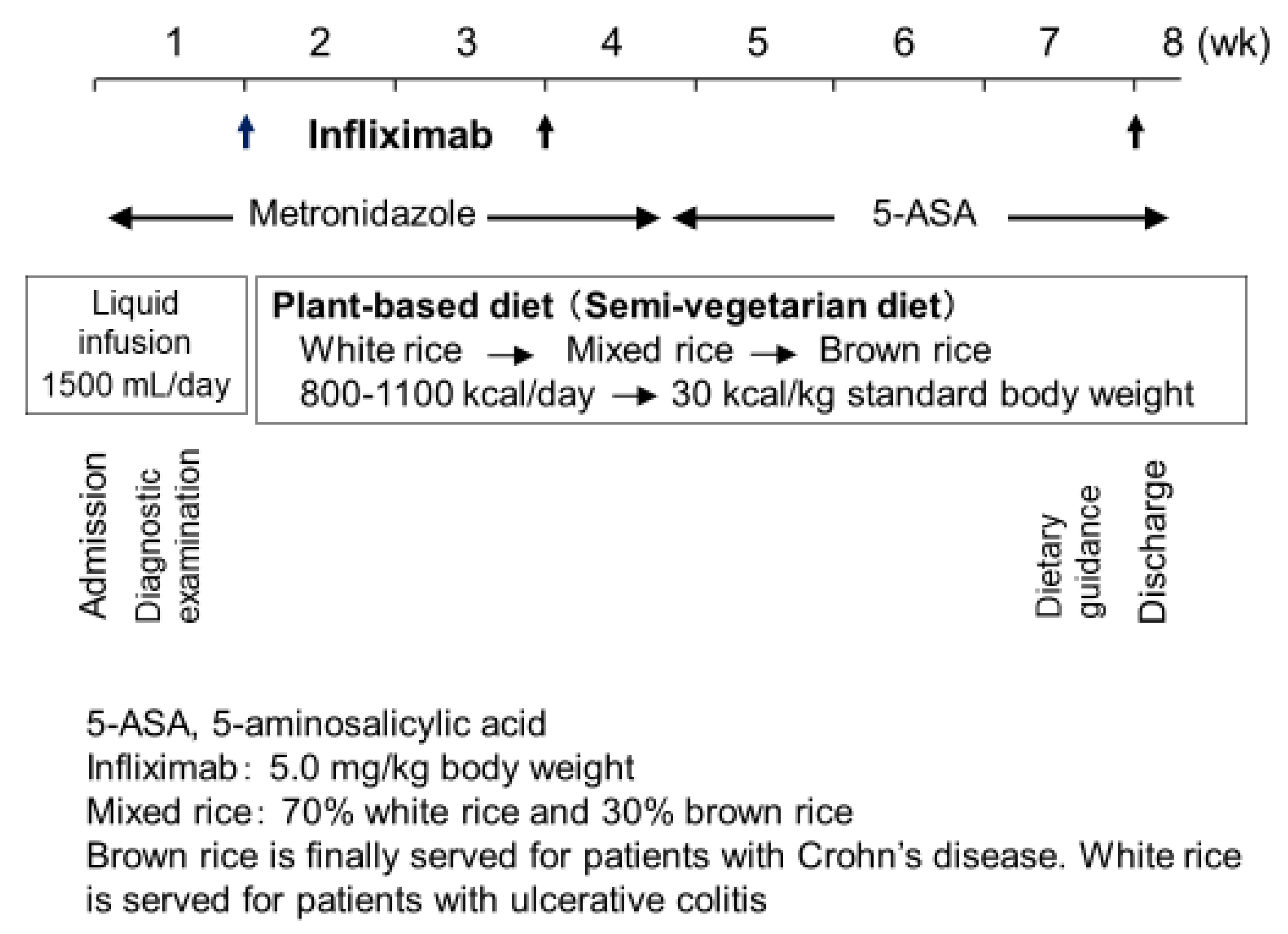
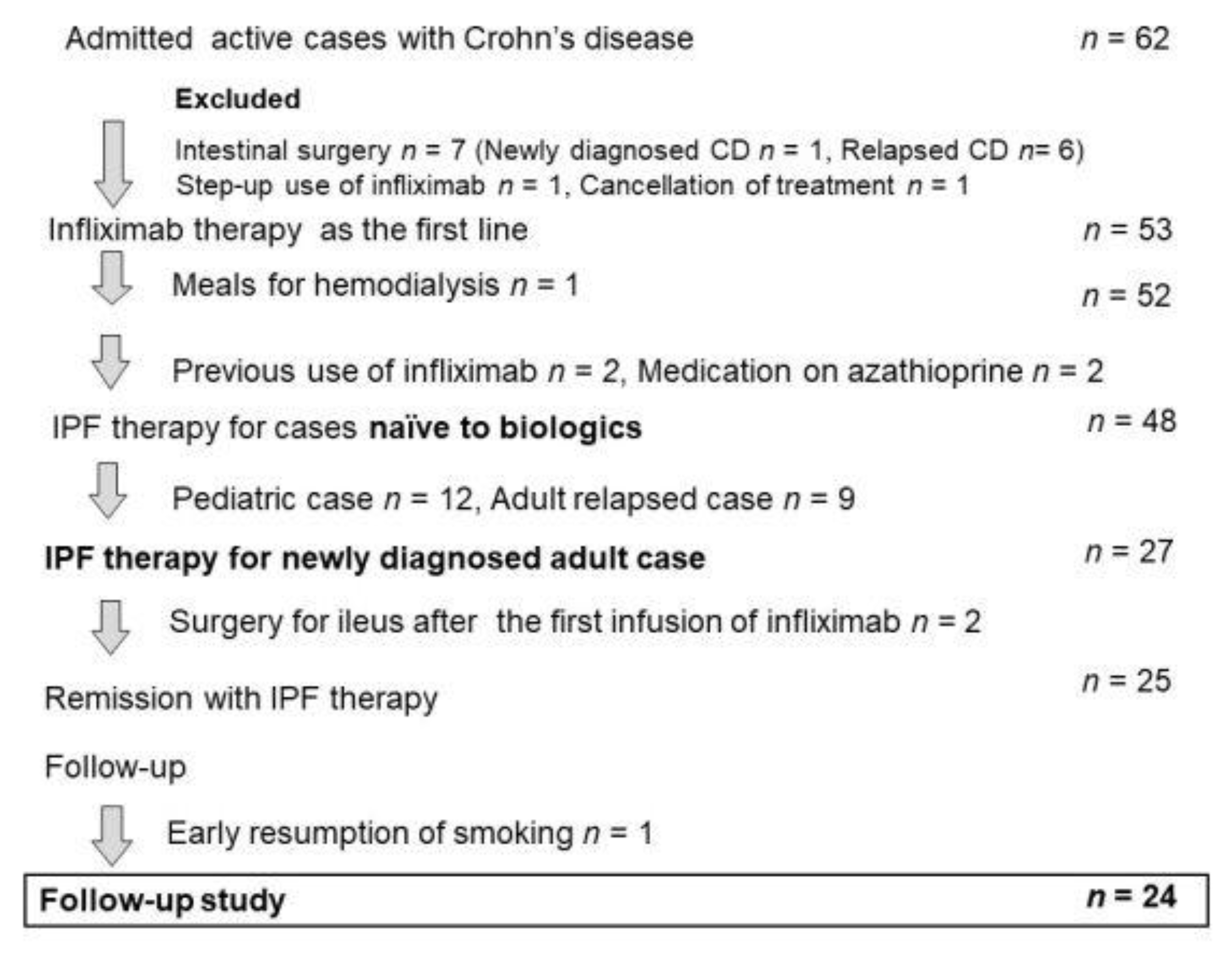
3.2. Indication of Infliximab and Plant-Based Diet as First-Line (IPF) Therapy
3.3. Protocol: IPF Therapy
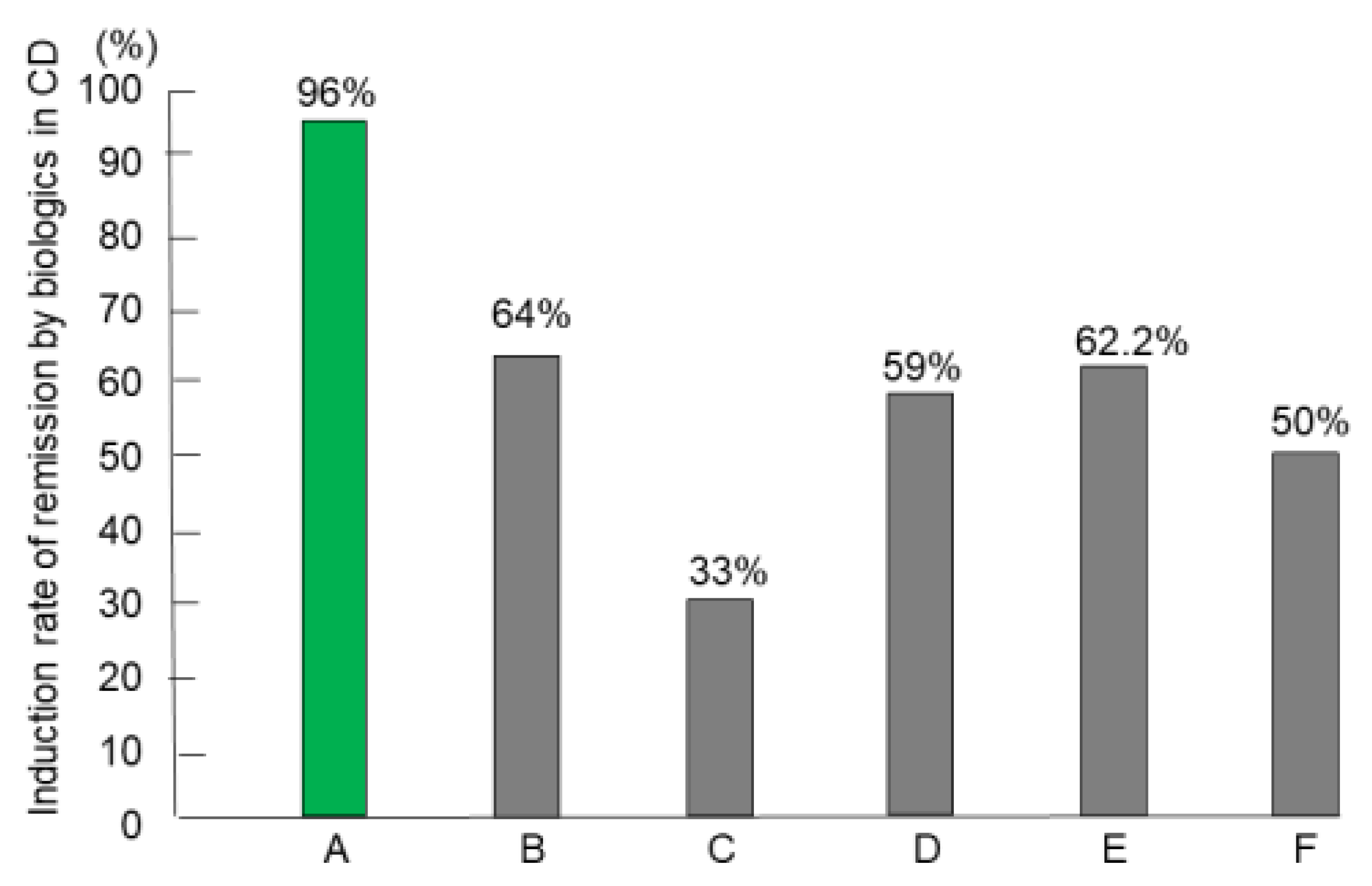
3.4. Remission (Induction) Rates of IPF Therapy in CD
3.5. Remission (Induction) Rates of IPF Therapy in Severe UC
3.6. Absence of Nonresponders to Infliximab with IPF Therapy
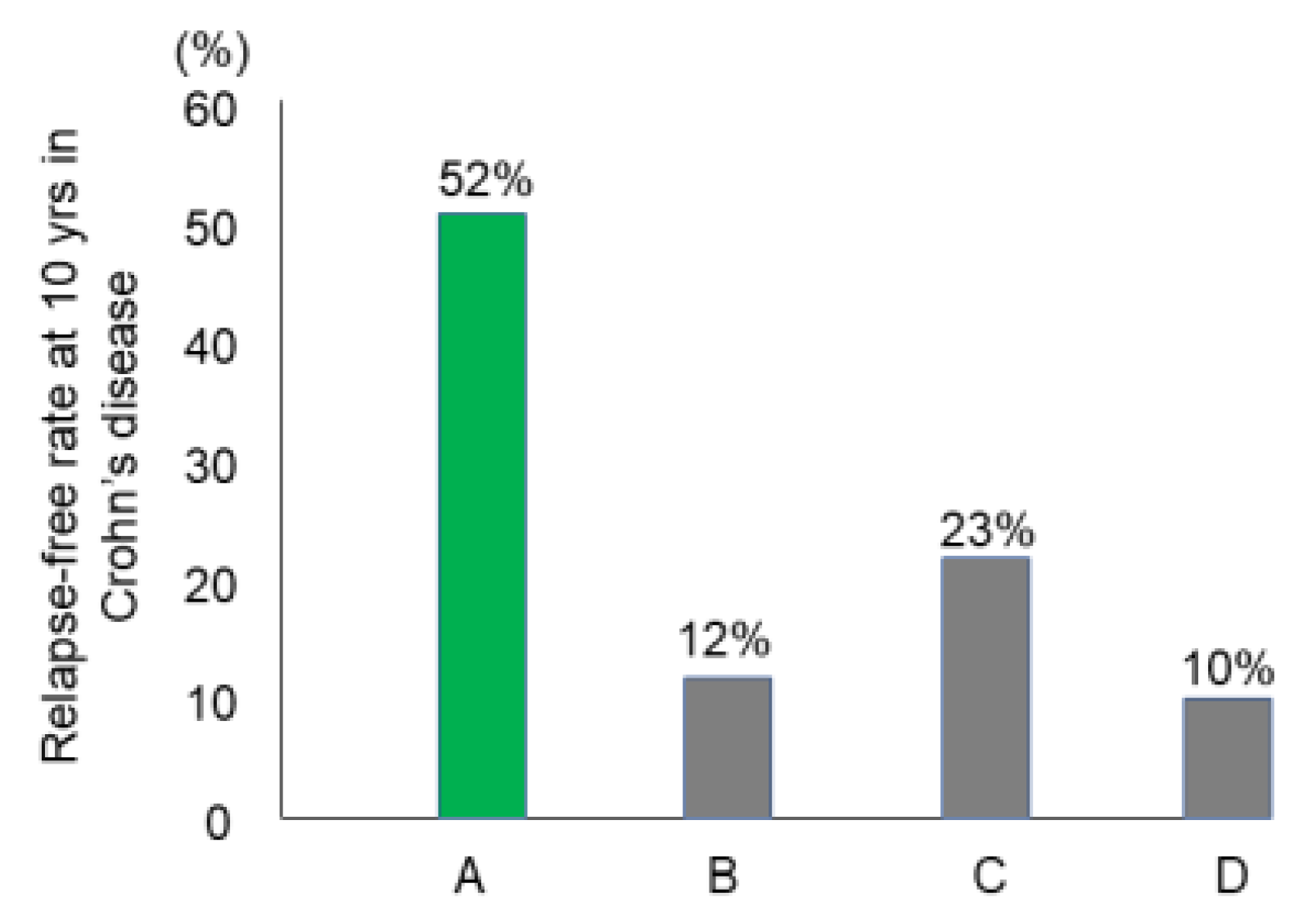
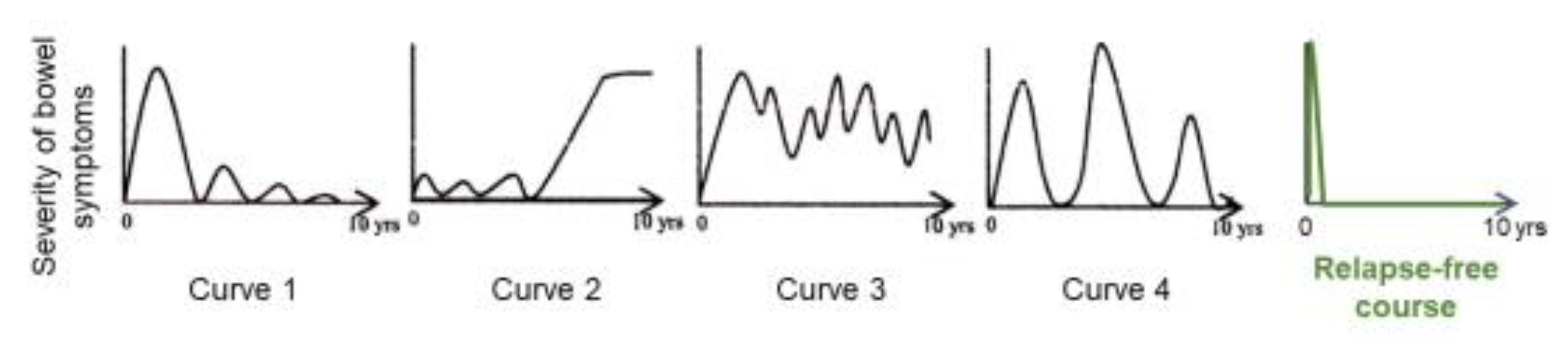
3.7. Relapse-Free Course in CD: Change of Natural History of CD
3.8. Dietary Intervention, Lifestyle Medicine, and Self-Management Skills
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| IBD | Inflammatory bowel disease |
| IPF | Infliximab and a plant-based diet as first-line |
| PBD | Plant-based diet |
| PBDS | Plant-based diet score |
| UC | Ulcerative colitis |
References
- Kaplan, G.G.; Ng, S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017, 152, 313–321. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, M.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuda, H.; Abe, T.; Sugawara, T.; Morikawa, Y. Missing environmental factor in inflammatory bowel disease: Diet-associated gut microflora. Inflamm. Bowel Dis. 2011, 17, E82–E83. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Fujiwara, K.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef]
- Hart, A.L.; Lomer, M.; Verjee, A.; Kemp, K.; Faiz, O.; Daly, A.; Solomon, J.; McLaughlin, J. What are the top 10 research questions in the treatment of inflammatory bowel disease? A priority setting partnership with the James Lind Alliance. J. Crohns Colitis 2017, 11, 204–211. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017, 152, 398–414. [Google Scholar] [CrossRef]
- Sasson, A.N.; Ananthakrishnan, A.N.; Raman, M. Diet in treatment of inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 425–435. [Google Scholar] [CrossRef]
- Chiba, M.; Suzuki, T.; Naganuma, H.; Masamune, O. A case of child Crohn’s disease markedly responded to TPN, ED, and HEEH treatment. J. Jpn. Soc. Colo-Proctol. 1991, 44, 235–242, (Abstract in English). [Google Scholar] [CrossRef]
- Boirivant, M.; Leoni, M.; Tariciotti, D.; Fais, S.; Squarcia, O.; Pallone, F. The clinical significance of serum C reactive protein levels in Crohn’s disease. Results of a prospective longitudinal study. J. Clin. Gastroenterol. 1988, 10, 401–405. [Google Scholar] [CrossRef]
- Click, B.; Vargas, E.J.; Anderson, A.M.; Proksell, S.; Koutroubakis, I.E.; Rivers, C.R.; Hashash, J.G.; Regueiro, M.; Watson, A.; Dunn, M.A.; et al. Silent Crohn’s disease: Asymptomatic patients with elevated C-reactive protein are at risk for subsequent hospitalization. Inflamm. Bowel Dis. 2015, 21, 2254–2261. [Google Scholar]
- Chiba, M.; Iizuka, M.; Horie, Y.; Masamune, O.; Kinjo, F. Long remission in Crohn’s disease by polymeric enteral diet. Akita J. Med. 1996, 22, 131–138. [Google Scholar]
- Hirakawa, H.; Fukuda, Y.; Tanida, N.; Hosomi, M.; Shimoyama, T. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn’s disease. Gastroenterol. Jpn. 1993, 28, 379–384. [Google Scholar] [CrossRef]
- Chiba, M.; Morita, N.; Nakamura, A.; Tsuji, K.; Harashima, E. Increased incidence of inflammatory bowel disease in association with dietary transition (Westernization) in Japan. JMA J. 2021, 4, 347–357. [Google Scholar]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Moayyedi, P. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology 2020, 158, 930–946. [Google Scholar] [CrossRef]
- Amre, D.K.; D’Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalance in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef]
- Dolan, K.T.; Chang, E.B. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nut. Food Res. 2017, 61, 1600129. [Google Scholar] [CrossRef]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The role of diet in the etiopathogenesis of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. 3), S1–S106. [Google Scholar] [CrossRef]
- Morita, N.; Minoda, T.; Munekiyo, M.; Watanabe, Y.; Muto, T.; Yokoyama, T.; Tanaka, H.; Kawamura, T.; Morioka, S.; Hashimoto, T. Case-control study of ulcerative colitis in Japan. In Annual Epidemiology Report of the Intractable Diseases Research Committee; Ohno, Y., Ed.; The Ministry of Health and Welfare of Japan. Nagoya, The Department of Preventive Medicine, School of Medicine, Nagoya University: Nagoya, Japan, 1996; pp. 153–158, (Abstract in English). [Google Scholar]
- Morita, N.; Ohnaka, O.; Ando, S.; Watanabe, Y.; Takazoe, M.; Takagi, K.; Yokoyama, T.; Tanaka, H.; Kawamura, T.; Ohno, Y.; et al. Case-control study of Crohn’s disease in Japan. In Annual Epidemiology Report of the Intractable Diseases Research Committee; Ohno, Y., Ed.; The Ministry of Health and Welfare of Japan, Nagoya, The Department of Preventive Medicine, School of Medicine, Nagoya University: Nagoya, Japan, 1997; pp. 58–64, (Abstract in English). [Google Scholar]
- Fejfar, Z. Prevention against ischaemic heart disease: A critical review. In Modern Trends in Cardiology; Butterworth-Heinemann: Boston, FL, USA, 1974; pp. 465–499. [Google Scholar]
- Popkin, B.M. The nutrition transition in low-income countries: An emerging crisis. Nutr. Rev. 1994, 52, 285–298. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Dietary Guidelines for Americans 2015–2020, 8th ed; US Department of Health and Human Services: Washington, DC, USA, 2015; p. 35. [Google Scholar]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuda, S.; Komatsu, M.; Tozawa, H.; Takayama, Y. Onset of ulcerative colitis during low-carbohydrate weight-loss diet and its treatment with plant-based diet: A case report. Perm. J. 2016, 20, 80–84. [Google Scholar] [CrossRef]
- Chiba, M.; Sugawara, T.; Komatsu, M.; Tozawa, H. Onset of ulcerative colitis in the second trimester after emesis gravidarum: Treatment with plant-based diet. Inflamm. Bowel Dis. 2018, 24, e8–e9. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Komatsu, M.; Watanabe, H.; Takahashi, M. Ulcerative colitis in the postpartum period. Autops. Case Rep. 2020, 10, e2020187. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Tsuda, S.; Fujiwara, T.; Shindo, Y.; Tozawa, H. Relapse of ulcerative colitis in a patient with Takayasu arteritis treated with tocilizumab and tacrolimus—Successful induction with infliximab: A case report. Dig. Med. Res. 2022. [Google Scholar] [CrossRef]
- Mendeloff, A.L. The epidemiology of idiopathic inflammatory bowel disease. In Inflammatory Bowel Disease; Lea & Febiger: Philadelphia, PA, USA, 1975; pp. 3–19. [Google Scholar]
- Chiba, M.; Sugawara, T.; Morikawa, Y.; Tozawa, H.; Fujiwara, K.; Imai, H.; Sato, A. Onset of Crohn’s disease after moving to Tokyo –maintenance of remission by-vegetarian diet: A case report. Dig. Absorpt. 2006, 29, 92–96, (Abstract in English). [Google Scholar]
- Chiba, M.; Nakane, K.; Takayama, Y.; Sugawara, K.; Ohno, H.; Ischii, H.; Tsuda, S.; Tsuji, T.; Komatsu, M.; Sugawara, T. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm. J. 2016, 20, 62–68. [Google Scholar] [CrossRef]
- Wolfe, I.D.; Peterkin, B.B. Dietary guidelines: The USDA perspective. Food Technol. 1984, 38, 80–86. [Google Scholar]
- Drewnowski, A.; Popkin, B.M. The nutrition transition: New trends in the global diet. Nutr. Rev. 1997, 55, 31–43. [Google Scholar] [CrossRef]
- Crowley, J.; Ball, L.; Hiddink, G.J. Nutrition in medical education: A systematic review. Lancet Planet Health 2019, 3, e379–e389. [Google Scholar] [CrossRef]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional update for physicians: Plant-based diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef]
- Grant, J.D. Time for change Benefits of a plant-based diet. Can. Fam. Physician 2017, 63, 744–746. [Google Scholar]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, A.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain Fatty acid (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H. The impact of metabolites derived from the gut microbiota on immune regulation and diseases. Int. Immunol. 2020, 32, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Muir, G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Sandys, O.; Te Velde, A. Rising the alarm: Environmental factors in the onset and maintenance of chronic (low-grade) inflammation in the gastrointestinal tract. Dig. Dis. Sci. 2022, 67, 4355–4368. [Google Scholar] [CrossRef]
- Verdugo-Meza, A.; Ye, J.; Dadlani, H.; Ghosh, S.; Gibson, D.L. Connecting the dots between inflammatory bowel disease and metabolic syndrome: A focus on gut-derived metabolites. Nutrients 2020, 12, 1434. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Arnone, D.; Chabot, C.; Heba, A.C.; Kökten, T.; Caron, B.; Hansmannel, F.; Dreumont, N.; Ananthakrishnan, A.N.; Quilliot, D.; Peyrin-Biroulet, L. Sugars and gastrointestinal health. Clin. Gastroenterol. Hepatol. 2022, 20, 1912–1924. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized diet in the onset and progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef]
- Bernell, O.; Lapidus, A.; Hellers, G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann. Surg. 2000, 231, 38–45. [Google Scholar] [CrossRef]
- Beaugerie, L.; Seksik, P.; Nion-Larmurier, I.; Gendre, J.P.; Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 2006, 130, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Pariente, B.; Cosnes, J.; Danese, S.; Sandborn, W.J.; Lewin, M.; Fletcher, J.G.; Chowers, Y.; D’Haens, G.; Feagan, B.G.; Hibi, T.; et al. Development of the Crohn’s Disease Digestive Damage Score, the Lémann score. Inflamm. Bowel Dis. 2011, 17, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, P.; Langholz, E.; Davidsen, M.; Binder, V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand. J. Gastroenterol. 1995, 30, 699–706. [Google Scholar] [CrossRef]
- Wolters, F.L.; Russel, M.G.; Sijbrandij, J.; Ambergen, T.; Odes, S.; Riis, L.; Langholz, E.; Politi, P.; Qasim, A.; Koutroubakis, I.; et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut 2006, 55, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Solberg, I.C.; Vatn, M.H.; Høie, O.; Stray, N.; Sauar, J.; Jahnsen, J.; Moum, B.; Lygren, I. IBSEN Study Group. Clinical course in Crohn’s disease: Result of a Norwegian population-based ten-year follow-up study. Clin. Gastroenterol. Hepatol. 2007, 5, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Hanauer, S.B.; Sandborn, W.J. Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am. J. Gastroenterol. 2009, 104, 465–483. [Google Scholar] [PubMed]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Komatsu, M.; Sugawara, T. Induction with infliximab and plant-based diet as first-line (IPF) therapy for Crohn disease: A single-group trial. Perm. J. 2017, 21, 17–009. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B. Infliximab in the treatment of Crohn’s disease: A user’s guide for clinicians. Am. J. Gastroenterol. 2002, 97, 2962–2972. [Google Scholar] [CrossRef]
- McClements, D.; Probert, C. Managing acute severe ulcerative colitis in the hospitalized setting. Frontline Gastroenterol. 2015, 6, 241–245. [Google Scholar] [CrossRef]
- Turner, D.; Walsh, C.M.; Steinhart, A.H.; Griffiths, A.M. Response to corticosteroids in severe ulcerative colitis: A systemic review of the literature and a meta-regression. Clin. Gastroenterol. Hepatol. 2007, 5, 103–110. [Google Scholar] [CrossRef]
- Choy, M.C.; Seah, D.; Faleck, D.M.; Shah, S.C.; Chao, C.; An, Y.; Radford-Smith, G.; Bessissow, T.; Dubinsky, M.C.; Ford, A.C.; et al. Systematic review and meta-analysis: Optimal salvage therapy in acute severe ulcerative colitis. Inflamm. Bowel Dis. 2019, 25, 1169–1186. [Google Scholar] [CrossRef]
- Faubion, W.A., Jr.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Sandborn, W.J. The natural history of corticosteroid therapy for inflammatory bowel disease: A population-based study. Gastroenterology 2001, 121, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Obara, Y.; Komatsu, M.; Sugawara, T. High remission rate with infliximab and plant-based diet as first-line (IPF) therapy for severe ulcerative colitis: Single-group trial. Perm. J. 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Tanaka, Y.; Ono, I. Early intestinal obstruction after infliximab therapy in Crohn’s disease. Autops. Case Rep. 2019, 9, e2018068. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Baert, F.; van Assche, G.; Caenepeel, P.; Vergauwe, P.; Tuynman, H.; De Vos, M.; van Deventer, S.; Stitt, L.; Donner, A.; et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomized trial. Lancet 2008, 371, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.M.E.; Aardoom, M.A.; Cozijnsen, M.A.; van Pieterson, M.; de Meij, T.; Groeneweg, M.; Norbruis, O.F.; Wolters, V.M.; van Wering, H.M.; Hojsak, I.; et al. First-line treatment with infliximab verses conventional treatment in children with newly diagnosed moderate-to-severe Crohn’s disease: An open-label multicenter randomized controlled trial. Gut 2022, 71, 34–42. [Google Scholar] [CrossRef]
- Miyoshi, J.; Hisamatsu, T.; Matsuoka, K.; Naganuma, M.; Maruyama, Y.; Yoneno, K.; Mori, K.; Kiyohara, H.; Nanki, K.; Okamoto, S.; et al. Early intervention with adalimumab may contribute to favorable clinical efficacy in patients with Crohn’s disease. Digestion 2014, 90, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Irving, P.M.; Hoops, T.; Izanec, J.L.; Gao, L.L.; Gasink, C.; Greenspan, A.; Allez, M.; Danese, S.; Hanauer, S.B.; et al. on behalf of the SEAVUE Study Group. Ustekinumab versus adalimumab for induction and maintenance therapy in biologic-naïve patients with moderately to severely active Crohn’s disease: A multicentre, randomized, double-blind, parallel-group, phase 3b trial. Lancet 2022, 399, 2200–2211. [Google Scholar] [CrossRef]
- Lindgren, S.C.; Flood, L.M.; Kilander, A.F.; Löfberg, R.; Persson, T.B.; Sjödahl, R.I. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 1998, 10, 831–835. [Google Scholar] [CrossRef]
- Oshitani, N.; Matsumoto, T.; Jinno, Y.; Sawa, Y.; Hara, J.; Nakamura, S.; Arakawa, T.; Kitano, A.; Kuroki, T. Prediction of short-term outcome for patients with active ulcerative colitis. Dig. Dis. Sci. 2000, 45, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Altaras, J. Radiologic features of inflammatory diseases of the colon. In Radiologic Atlas of the Colon and Rectum; Altaras, J., Ed.; Urban & Schwarzenberg Inc.: Baltimore, MD, USA, 1984; pp. 101–180. [Google Scholar]
- Buhl, S.; Steenholdt, C.; Rasmussen, M.; Borghede, M.K.; Brynskov, J.; Thomsen, O.Ø.; Ainsworth, M.A. Outcomes after primary infliximab treatment failure in inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Aden, K.; Rehman, A.; Waschina, S.; Pan, W.H.; Walker, A.; Lucio, M.; Nunez, A.M.; Bharti, R.; Zimmerman, J.; Bethge, J.; et al. Metabolic functions of gut microbes associate with efficacy of tumor necrosis factor antagonists in patients with inflammatory bowel diseases. Gastroenterology 2019, 157, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, M.M.; Rocha, C.; Correia, L.; Lago, P.; Ministro, P.; Portela, F.; Trindade, E.; Afonso, J.; Peyrin-Biroulet, L.; Magro, F.; et al. Features of fecal and colon microbiomes associate with responses to biologic therapies for inflammatory bowel diseases: A systemic review. Clin. Gastroenterol. Hepatol. 2020, 18, 1054–1069. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.J.; Plichta, D.; Hogstrom, L.; Borren, N.Z.; Lau, H.; Gregory, S.M.; Tan, W.; Khalili, H.; Clish, C.; Vlamakis, H.; et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021, 29, 1294–1304. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Ishii, H.; Komatsu, M. How to avoid primary nonresponders to infliximab in Crohn’s disease. Inflamm. Bowel Dis. 2017, 23, E55–E56. [Google Scholar] [CrossRef][Green Version]
- Chiba, M.; Tsuji, T.; Komatsu, M. How to optimize effects of infliximab in inflammatory bowel disease: Incorporation of plant-based diet. Gastroenterology 2020, 158, 1512. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ohno, H.; Sugawara, K.; Komatsu, M.; Tozawa, H. Relapse-free course in nearly half of Crohn’s disease patients with infliximab and plant-based diet as first-line (IPF) therapy: Single-group trial. Perm. J. 2022, 26, 40–53. [Google Scholar] [CrossRef]
- Wintjens, D.; Bergey, F.; Saccenti, E.; Jeuring, S.; van den Heuvel, T.; Romberg-Camps, M.; Oostenbrug, L.; Masclee, A.; dos Santos, V.M.; Jonkers, D.; et al. Disease activity patterns of Crohn’s disease in the first ten years after diagnosis in the population-based IBD South Limburg cohort. J. Crohn Colitis 2021, 15, 391–400. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Nugent, Z.; Shaw, S.; Bernstein, C.N. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011, 141, 90–97. [Google Scholar] [CrossRef]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; Debruyn, J.; Jette, M.; Fiest, K.N.; Frolkis, T.; Barkema, H.W.; Rioux, K.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systemic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Rungoe, C.; Langholz, E.; Andersson, M.; Basit, S.; Nielsen, N.M.; Wohlfahrt, J.; Jess, T. Changes in medical treatment and surgery rates in inflammatory bowel disease: A nationwide cohort study 1979–2011. Gut 2014, 63, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, O.; Studd, C.; Hair, C.; Wilson, J.; Ding, N.S.; Heerasing, N.; Ting, A.; McNeill, J.; Knight, R.; Santamaria, J.; et al. Prospective population-based cohort of inflammatory bowel disease in the biologic era: Disease course and predictors of severity. J. Gastroenterol. Hepatol. 2015, 30, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hann, H.J.; Hong, S.N.; Kim, K.H.; Ahn, I.M.; Song, J.Y.; Lee, S.H.; Ahn, H.S. Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: A nation-wide population-based study. Inflamm. Bowel Dis. 2015, 21, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Sakurai, T.; Yao, T.; Iida, M.; Okabe, N.; Maeda, K.; Matsui, T.; Fuchigami, T.; Yoshinaga, K.; Imamura, K. Clinical course and long-term prognosis of Crohn’s disease in Japan. J. Gastroenterol. 1994, 29, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.A.; Ornish, D.; Roizen, M. Lifestyle medicine: Treating causes of disease. Altern. Ther. Health Med. 2009, 15, 12–14. [Google Scholar] [PubMed]
- Bodai, B.I.; Nakata, T.E.; Wong, W.T.; Clark, D.R.; Lawenda, S.; Tsou, C.; Liu, R.; Shiue, L.; Cooper, N.; Rehbein, M.; et al. Lifestyle medicine: A brief review of its dramatic impact on health and survival. Perm. J. 2018, 22, 17–025. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Desroches, S.; Lapointe, A.; Ratté, S.; Gravel, K.; Légaré, F.; Turcotte, S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst. Rev. 2013, 2, CD008722. [Google Scholar] [CrossRef]
- Atallah, R.; Filion, K.B.; Wakil, S.M.; Genest, J.; Joseph, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: A systematic review of randomized controlled trials. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 815–827. [Google Scholar] [CrossRef]
- Breslow, L.; Enstrom, J.E. Persistence of health habits and their relationship to mortality. Prev. Med. 1980, 9, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.; Brensinger, C.M.; Wu, Q.; Gilroy, E.; Kappelman, M.D.; Sandler, R.S.; Lewis, J.D. A diet low in red and processed meat does not reduce rate of Crohn’s disease flares. Gastroenterology 2019, 157, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Peyrin-Biroulet, L.; the SPIRIT-IOIBD study group. Selecting end points for disease-modification trials in inflammatory bowel disease: The SPIRIT consensus from the IOIBD. Gastroenterology 2021, 160, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Nakane, K.; Tsuji, T.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Ito, M.; Komatsu, M.; Yamada, K.; et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm. J. 2018, 22, 17–167. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Tsuji, T.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Obara, Y.; Komatsu, M.; Sugawara, T. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: A single group trial. Perm. J. 2019, 23, 18–220. [Google Scholar] [CrossRef]
- Chiba, M.; Ishii, H.; Komatsu, M. Recommendation of plant-based diet for inflammatory bowel disease. Transl. Pediatr. 2019, 8, 23–27. [Google Scholar] [CrossRef]
- Chiba, M.; Hosoba, M.; Yamada, K. Plant-based diet recommended for inflammatory bowel disease. Inflamm. Bowel Dis. 2023, 29. [Google Scholar] [CrossRef]

| No | Content | Our Comment |
|---|---|---|
| 1 | What is the optimal treatment strategy considering efficacy, safety and cost effectiveness in IBD management? | IPF therapy is partly applcable. |
| 2 | What are the optimal markers combinations for stratification of patients? | None. |
| 3 | What role does diet have in the management of mildly active or inactive UC or CD to achieve normal daily activities and symptom control? | Diet plays a critical role. |
| 4 | How can pain be most effectively managed in people with IBD? | Relapse prevention by PBD particularly in CD. |
| 5 | What is an optimal treatment strategy for perianal CD? | IPF therapy is highly effective. |
| 6 | What is the best treatment for controlling diarrhoea and/or in continence symptoms? | Relapse prevention in cooperation with PBD. |
| 7 | What is the optimal dietary therapy? | PBD particularly in CD. |
| 8 | What is the association between IBD and fatigue and how should it be managed? | Relapse prevention in cooperation with PBD. |
| 9 | Does early surgery or later surgery for ileal CD result in better outcomes? | None. |
| 10 | Does influencing the gut microbiota influence the course of IBD? | Definitely indicated by literature. |
| Environmental Factor | Role in IBD | Exposure to the Majority of IBD Patients | Relevance to Gut Microbiota | ||
|---|---|---|---|---|---|
| UC | CD | Mode of Role | |||
| Lifestyle | |||||
| Smoking | P | R | Divergent in IBD | No | Yes |
| Diet | Yes | Yes | |||
| Animal protein | R | R | Identical in IBD | Yes | Yes |
| Dietary fiber | N | P | Yes | Yes | |
| Tea or coffee | P | P | Identical in IBD | Yes | Unknown |
| Low levels of vitamin D | R | R | Identical in IBD | No | Unknown |
| Breast feeding | P | P | Identical in IBD | No | Yes |
| Pharmacological agents | |||||
| NSAID | R | R | Identical in IBD | No | Unknown |
| Antibiotics in childhood | Divergent among ethnic groups | No | Yes | ||
| Oral contraceptives | R | R | Identical in IBD | No | Unknown |
| Dipeptidyl peptidase-4 inhibitors | R | N | No | Unknown | |
| Vaccination | N | N | Yes | Unknown | |
| Appendectomy | P | R | Divergent in IBD | No | Unknown |
| Air pollution | R | R | Identical in IBD | No | Unknown |
| Crohn’s Disease (CD) | Ulcerative Colitis (UC) | |
|---|---|---|
| Risky foods | Beef | |
| Excessive consumption | Greasy foods | |
| Cheese | Cheese | |
| Butter | ||
| Sweets | Sweets | |
| Yoghurt (plain) | Yoghurt (plain) | |
| Natural fruit juice | ||
| Preventive foods | Chinese cabbage | Liver |
| Shortage of consumption | Edible wild plants | Edible wild plants |
| Tomato | Other fruits | |
| Mandarin orange | Mandarin orange | |
| Pickles | Pickles | |
| Green tea | Green tea | |
| Dried fishes | ||
| Identified to be neither risk | Salty foods, Pork, Chicken, Ham, Egg, Milk, Margarine, Fried food, Cabbage, | |
| nor preventive foods | Potato, Sauteed vegetables, Fresh fish, Mushroom, Processed fish paste, | |
| for both CD & UC | Spinach, Carrot, Tsukudani*, Cooked beans, Marine alga, Bean curd, Coffee | |
| Not identified to be risk or | Liver, Dried fishes, Other fruits | Beef, Greasy foods, Butter, |
| preventive factor for either | Natural fruit juice, Chinese cabbage, | |
| CD or UC | Tomato | |
| PBDS Scoring | PBDS in the Present Case | |||||||
|---|---|---|---|---|---|---|---|---|
| Food Groups | Frequency of Consumption | |||||||
| Daily | 3–5 times | 1–2 times | Rarely | A | B | C | D | |
| /wk | /wk | |||||||
| Vegetables | 5 | 3 | 1 | 0 | 5 | 5 | 5 | 5 |
| Fruits | 5 | 3 | 1 | 0 | 1 | 0 | 5 | 0 |
| Pulses | 5 | 3 | 1 | 0 | 5 | 3 | 5 | 5 |
| Potatoes/starches | 5 | 3 | 1 | 0 | 3 | 3 | 5 | 5 |
| Rice | 5 | 3 | 1 | 0 | 5 | 5 | 5 | 5 |
| Miso soup | 5 | 3 | 1 | 0 | 5 | 5 | 5 | 5 |
| Green tea | 5 | 3 | 1 | 0 | 0 | 0 | 0 * | 3 |
| Yoghurt (plain) | 5 | 3 | 1 | 0 | 1 | 0 | 5 | 1 |
| Meat | −5 | −3 | −1 | 0 | −1 | −1 | 0 | −1 |
| Minced or processed meat | −5 | −3 | −1 | 0 | −1 | −1 | 0 | 0 |
| Cheese/butter/margarine | −5 | −3 | −1 | 0 | 0 | 0 | 0 | 0 |
| Sweets/ice cream/milk shake | −5 | −3 | −1 | 0 | −3 | −5 | 0 | −1 |
| Soft drinks (cola/ carbonated beverages/juice) | −5 | −3 | −1 | 0 | −5 | −5 | 0 | −1 |
| Alcohol | −5 | −3 | −1 | 0 | 0 | 0 | 0 | 0 |
| Bread | −5 | −3 | −1 | 0 | −1 | −3 | 0 | 0 |
| Fish | −2 | −1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Plant-based diet score (PBDS) | 14 | 6 | 35 | 26 | ||||
| Author | Country | Subjects & Year | Number of Cases | Cumulative Relapse-Free Rate | Cumulative Surgical Rate | ||
|---|---|---|---|---|---|---|---|
| 5 Years | 10 Years | 5 Years | 10 Years | ||||
| Munkholm et al, 1995 [58] | Denmark | PBIC 1962–1987 | 373 | 22% | 12% | n.d. | n.d. |
| Wolters et al, 2006 [59] | European countries | PBIC 1991–1993 | 358 | 31% | 23% | 21% | 29% |
| Solberg et al, 2007 [60] | Norway | PBIC 1990–1994 | 237 | 15% | 10% | 27% | 38% |
| Nguyen et al, 2011 [86] | Canada | 2001–2008 | 1119 | 18% | n.a. | ||
| Frolkis et al, 2013 [87] | Meta-analysis | 1990– | 3239 | 28% | 39% | ||
| 2000– | 1193 | 24% | n.a. | ||||
| Rungoe et al, 2014 [88] | Denmark | 1995–2002 | 3718 | 27% | 31% | ||
| 2003–2011 | 5552 | 20% | 23% | ||||
| Niewiadomski et al, 2015 [89] | Australia | PBIC 2007–2013 | 146 | 26% | n.a. | ||
| Kim et al, 2015 [90] | Korea | PBIC 2006–2012 | 11,267 | 9% | n.a. | ||
| Okada et al, 1994 [91] | Japan | 1973–1988 | 58 | 29% | 46% | ||
| Chiba et al, 2022 [84] | Japan | 2003–2017 | 26 | 52% | 52% | 12% | 19% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiba, M.; Morita, N. Incorporation of Plant-Based Diet Surpasses Current Standards in Therapeutic Outcomes in Inflammatory Bowel Disease. Metabolites 2023, 13, 332. https://doi.org/10.3390/metabo13030332
Chiba M, Morita N. Incorporation of Plant-Based Diet Surpasses Current Standards in Therapeutic Outcomes in Inflammatory Bowel Disease. Metabolites. 2023; 13(3):332. https://doi.org/10.3390/metabo13030332
Chicago/Turabian StyleChiba, Mitsuro, and Norikazu Morita. 2023. "Incorporation of Plant-Based Diet Surpasses Current Standards in Therapeutic Outcomes in Inflammatory Bowel Disease" Metabolites 13, no. 3: 332. https://doi.org/10.3390/metabo13030332
APA StyleChiba, M., & Morita, N. (2023). Incorporation of Plant-Based Diet Surpasses Current Standards in Therapeutic Outcomes in Inflammatory Bowel Disease. Metabolites, 13(3), 332. https://doi.org/10.3390/metabo13030332







