Integrating Metabolomics and Gene Expression Underlying Potential Biomarkers Compounds Associated with Antioxidant Activity in Southern Grape Seeds
Abstract
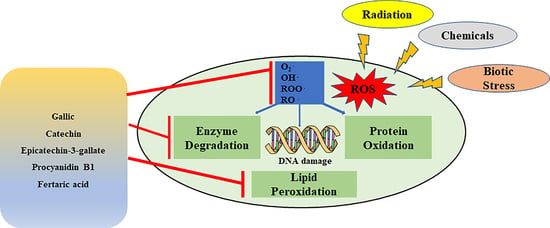
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Muscadine Grape Materials
2.3. Muscadine Extracts
2.4. Analysis of Antioxidant Activities
2.4.1. DPPH Radical Scavenging Activity
2.4.2. Ferric Reducing Antioxidant Potential (FRAP) Assay
2.4.3. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) Radical Cation-Based Assays (ABTS) Assay
2.4.4. Cupric Ion-Reducing Antioxidant Capacity (CUPRAC) Assay
2.5. Untargeted Liquid Chromatography-Mass Spectrum (LC-MS) Metabolome Analysis
2.6. Nucleic Acid Extraction and qPCR Analysis
2.7. Metabolite Identification
2.8. Statistical Analysis
3. Results
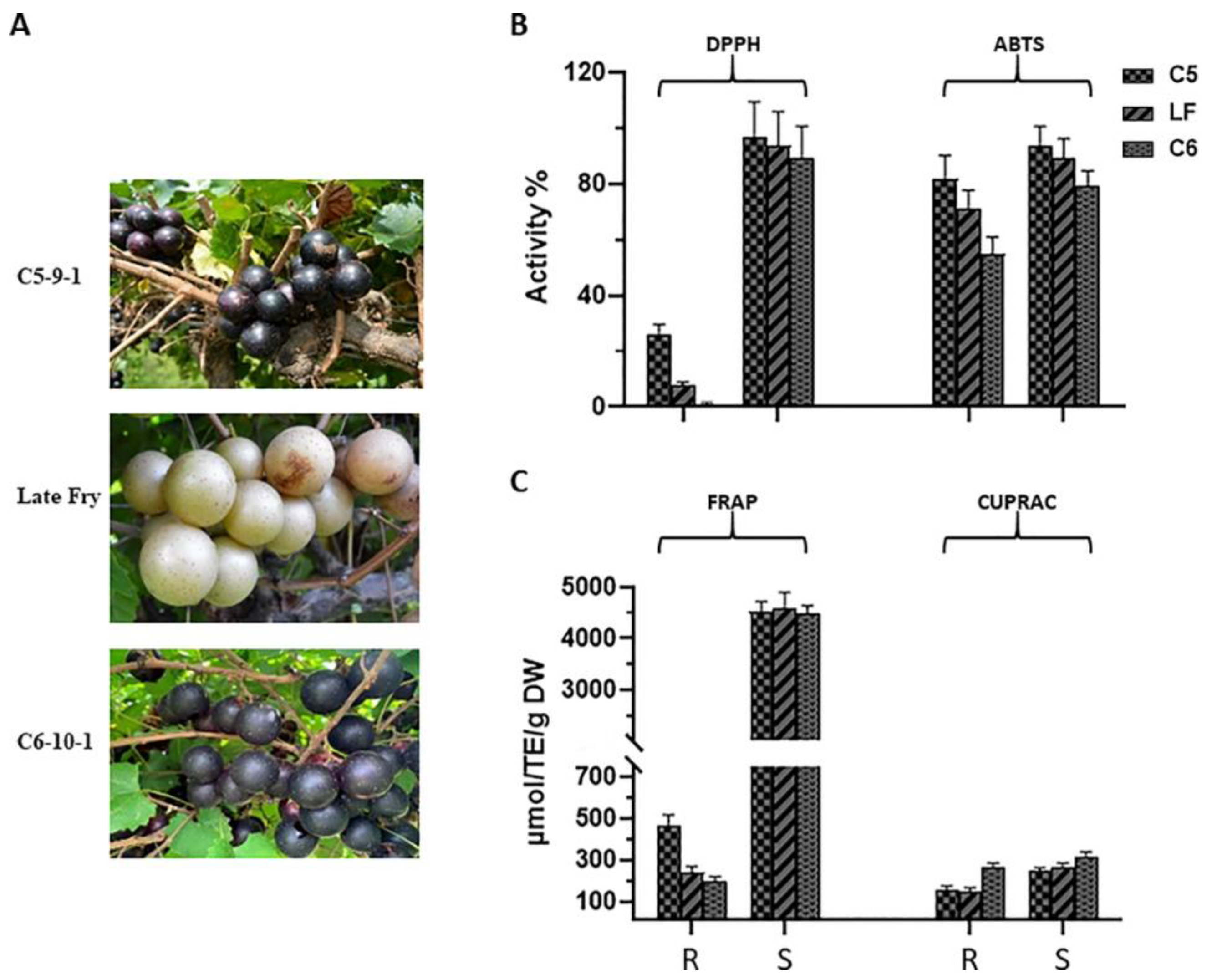
3.1. Antioxidant Activities
3.2. Metabolite Profiling of Muscadine Skin and Seed Tissues
3.3. Multivariate Analysis of Candidate Metabolites and Antioxidant Activities of Muscadine
3.4. Expression of Genes Underlying Biomarker Compounds Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The Study of Antioxidant Components in Grape Seeds. Molecules. 2020, 15, 3736. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2021, 73, 285–290. [Google Scholar] [CrossRef]
- Grases, F.; Prieto, R.M.; Fernández-Cabot, R.A. Effect of consuming a grape seed supplement with abundant phenolic compounds on the oxidative status of healthy human volunteers. Nutr. J. 2015, 14, 94. [Google Scholar] [CrossRef] [Green Version]
- Grimplet, J.; Deluc, L.G.; Tillett, R.L.; Wheatley, M.D.; Schlauch, K.A.; Cramer, G.R.; Cushman, J.C. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genom. 2007, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Castellari, M.; Versari, A.; Spinabelli, U.; Galassi, S.; Amati, A. An improved hplc method for the analysis of organic acids, carbohydrates, and alcohols in grape musts and wines. J. Liq. Chromatogr. Relat. 2000, 13, 2047–2056. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Redman, E.K. Mediterranean diet and cardioprotection: The role of nitrite, polyunsaturated fatty acids, and polyphenols. Nutrition 2011, 27, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content, and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Prasain, J.K.; Carlson, S.H.; Wyss, J.M. Flavonoids, and age-related disease: Risk, benefits, and critical windows. Maturitas 2010, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Vislocky, L.M.; Fernandez, M.L. Biomedical effects of grape products. Nutr. Rev. 2010, 68, 656–670. [Google Scholar] [CrossRef]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F.A. The anticancer and antioxidant effects of muscadine grape extracts on racially different triple-negative breast cancer cells. Anticancer Res. 2019, 39, 4043–4053. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (muscadine grapes) as determined by HPLC-DAD-ESI-Msn. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds—Biochemistry and functionality. J. Med. Food. 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef]
- Palma, M.; Taylor, L.R. Extraction of polyphenolic compounds from grape seeds with near critical carbon dioxide. J. Chromatogr. A 1999, 849, 117–124. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Abdou, H.M.; Wahby, M.M. Neuroprotection of grape seed extract and pyridoxine against triton-induced neurotoxicity. Oxid. Med. Cell Longev. 2016, 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Cádiz-Gurrea, M.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef] [Green Version]
- Lotito, S.B.; Fraga, C.G. (+)-Catechin prevents human plasma oxidation. Free Radic. Biol. Med. 1997, 24, 435–441. [Google Scholar] [CrossRef]
- Moini, H.; Guo, Q.; Packer, L. Enzyme inhibition and protein-binding action of the procyanidin-rich french maritime pine bark extract, pycnogenol: Effect on xanthine oxidase. J. Agric. Food Chem. 2000, 48, 5630–5639. [Google Scholar] [CrossRef]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 199447635. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free Radic. Biol. Med. 1999, 27, 1413–1426. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Mehrdad, J.; Masoumeh, K.; Asra, M. Naringin Chelates Excessive Iron and Prevents the Formation of Amyloid-Beta Plaques in the Hippocampus of Iron-Overloaded Mice. Front. Pharmacol. 2021, 12, 651156. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.P.; Zhou, B.; Yang, L.; Liu, Z.L. Antioxidative effects of green tea polyphenols on free radical initiated and photosensitized peroxidation of human low-density lipoprotein. Chem. Phys. Lipids. 2000, 106, 53–63. [Google Scholar] [CrossRef]
- Nakao, M.; Takio, S.; Ono, K. Alkyl peroxyl radical-scavenging activity of catechins. Phytochemistry 1998, 49, 2379–2382. [Google Scholar] [CrossRef]
- Sato, M.; Bagchi, D.; Tosaki, A.; Das, D.K. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic. Biol. Med. 2001, 31, 729–737. [Google Scholar] [CrossRef]
- Bouhamidi, R.; Prevost, V.; Nouvelot, A. High protection by grape seed proanthocyanidins (GSPC) of polyunsaturated fatty acids against UV-C induced peroxidation. Life Sci. 1998, 321, 31–38. [Google Scholar] [CrossRef]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Bagchi, D.J.; Balmoori, J.; Stohs, S.J. Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen. Pharmacol. 1998, 30, 771–776. [Google Scholar] [CrossRef]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. The antioxidant activity of phloretin: The disclosure of a new antioxidant pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 2002, 295, 9–13. [Google Scholar] [CrossRef]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar]
- Cui, X.; Zhang, M.; Guan, X.; Yin, L.; Sun, Y.; Fawcett, J.P.; Gu, J. LC-MS–MS determination of troxerutin in plasma and its application to a pharmacokinetic study. Chromatographia 2011, 73, 165–169. [Google Scholar] [CrossRef]
- Nakano, E.; Higgins, J.A.; Powers, H.J. Folate protects against oxidative modification of human LDL. Br. J. Nutr. 2001, 86, 637–639. [Google Scholar] [CrossRef] [Green Version]
- Thorsten, M.; Solveigh, S.; Dietmar, K.; Nicolai, B.; Jürgen, C.; Uwe, B.; Reinhold, C.; Andreas, S. Isolation of hydroxycinnamoyltartaric acids from grape pomace by high-speed counter-current chromatography. J. Chromato. A 2006, 1128, 61–67. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Duszenko, M.; Gospodaryov, D.V.; Garaschuk, O. Oxidative Stress and Energy Metabolism in the Brain: Midlife as a Turning Point. Antioxidants 2021, 10, 1715. [Google Scholar] [CrossRef]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Gomez-Plaza, E.; Gil-Munoz, R.; Lopez-Roca, J.M.; Martinez-Cutillas, A.; Fernandez-Fernandez, J.I. Phenolic compounds and color stability of red wines: Effect of skin maceration time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Duan, S.; Wu, Y.; Fu, R.; Wang, L.; Chen, Y.; Xu, W.; Zhang, C.; Ma, C.; Shi, J.; Wang, S. Comparative metabolic profiling of grape skin tissue along grapevine berry developmental stages reveals systematic influences of root restriction on skin metabolome. Int. J. Mol. Sci. 2019, 20, 534. [Google Scholar] [CrossRef] [Green Version]
- Darwish, A.G.; Das, P.R.; Ismail, A.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Sherif, S.M.; El-Sharkawy, I. Untargeted metabolomics and antioxidant capacities of muscadine grape genotypes during berry development. Antioxidants 2021, 10, 914. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. 2021, 27, 100149. [Google Scholar] [CrossRef]
- Lomolino, G.; Zocca, F.; Spettoli, P.; Zanin, G.; Lante, A. A preliminary study on changes in phenolic content during Bianchetta Trevigiana winemaking. J. Food Compos. Anal. 2010, 23, 575–579. [Google Scholar] [CrossRef]
- Darwish, A.G.; Samy, M.N.; Sugimoto, S.; Otsuka, H.; Abdel-Salam, H.; Matsunami, K. Effects of hepatoprotective compounds from the leaves of Lumnitzera racemosa on acetaminophen-induced liver damage in vitro. Chem. Pharm. Bull. 2016, 64, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef] [Green Version]
- Zheleva-Dimitrova, D.Z.H. Antioxidant and acetylcholinesterase inhibition properties of Amorpha fruticosa L. and Phytolacca americana L. Pharmacogn. Mag. 2013, 9, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Gambino, G.; Perrone, I.; Gribaudo, I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. Metlin: A metabolite mass spectral database. Ther. Drug Monit. 2005, 6, 747–751. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human metabolome database. Nucleic Acids Res. 2007, 35, 521–526. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Aharoni, A.; Willmitzer, L.; Stitt, M.; Tohge, T.; Kopka, J.; Carroll, A.J.; Saito, K.; Frase, P.D.; DeLuca, V. Recommendations for reporting metabolite data. Plant Cell 2011, 23, 2477–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, I.A.; Rogero, M.M.; Junqueira, R.M.; Carrapeiro, M.M. Free radical scavenger and antioxidant capacity correlation of alpha-tocopherol and Trolox measured by three in vitro methodologies. Int. J. Food Sci. Nutr. 2006, 57, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Darwish, A.G.; Park, M.; Gajjar, P.; Tsolova, V.; Soliman, K.F.A.; El-Sharkawy, I. Transcriptome profiling during muscadine berry development reveals the dynamic of polyphenols metabolism. Front. Plant Sci. 2022, 12, 818071. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 20, 965. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Ye, J.; Leonard, S.; Ding, M.; Vallyathan, V.; Castranova, V.; Rojanasakul, Y.; Dong, Z. Antioxidant properties of (-)-epicatechin-3-gallate and its inhibition of Cr (VI)-induced DNA damage and Cr (IV)-or TPA-stimulated NF-κB activation. Mol. Cell. Biochem. 2002, 206, 125–132. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, A.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postepy. Hig. Med. Dosw. 2013, 67, 958. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. Biomed. Res. Int. 2018, 12, 8584136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrelia, S.; Angeloni, C. New Mechanisms of Action of Natural Antioxidants in Health and Disease. Antioxidants 2020, 23, 344. [Google Scholar] [CrossRef] [Green Version]
- Alicja, U.; Jacek, K.; Kornelia, C.; Malgorzata, S. Antioxidant properties of several caffeic acid derivatives: A theoretical study. Comptes. Rendus. Chimie. 2017, 20, 1072–1082. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, A.G.; Moniruzzaman, M.; Tsolova, V.; El-Sharkawy, I. Integrating Metabolomics and Gene Expression Underlying Potential Biomarkers Compounds Associated with Antioxidant Activity in Southern Grape Seeds. Metabolites 2023, 13, 210. https://doi.org/10.3390/metabo13020210
Darwish AG, Moniruzzaman M, Tsolova V, El-Sharkawy I. Integrating Metabolomics and Gene Expression Underlying Potential Biomarkers Compounds Associated with Antioxidant Activity in Southern Grape Seeds. Metabolites. 2023; 13(2):210. https://doi.org/10.3390/metabo13020210
Chicago/Turabian StyleDarwish, Ahmed G., Md Moniruzzaman, Violeta Tsolova, and Islam El-Sharkawy. 2023. "Integrating Metabolomics and Gene Expression Underlying Potential Biomarkers Compounds Associated with Antioxidant Activity in Southern Grape Seeds" Metabolites 13, no. 2: 210. https://doi.org/10.3390/metabo13020210
APA StyleDarwish, A. G., Moniruzzaman, M., Tsolova, V., & El-Sharkawy, I. (2023). Integrating Metabolomics and Gene Expression Underlying Potential Biomarkers Compounds Associated with Antioxidant Activity in Southern Grape Seeds. Metabolites, 13(2), 210. https://doi.org/10.3390/metabo13020210