Baicalin Exhibits a Protective Effect against Cisplatin-Induced Cytotoxic Damage in Canine Renal Tubular Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Materials
2.2. Cell Culture
2.3. Drug Treatment
2.4. Apoptosis Detection
2.5. Oxidative and Antioxidant Function Assessment
2.6. Detection of Cellular Inflammatory Cytokine Levels
2.7. Western Blot Analysis
2.8. Data Analysis
3. Results
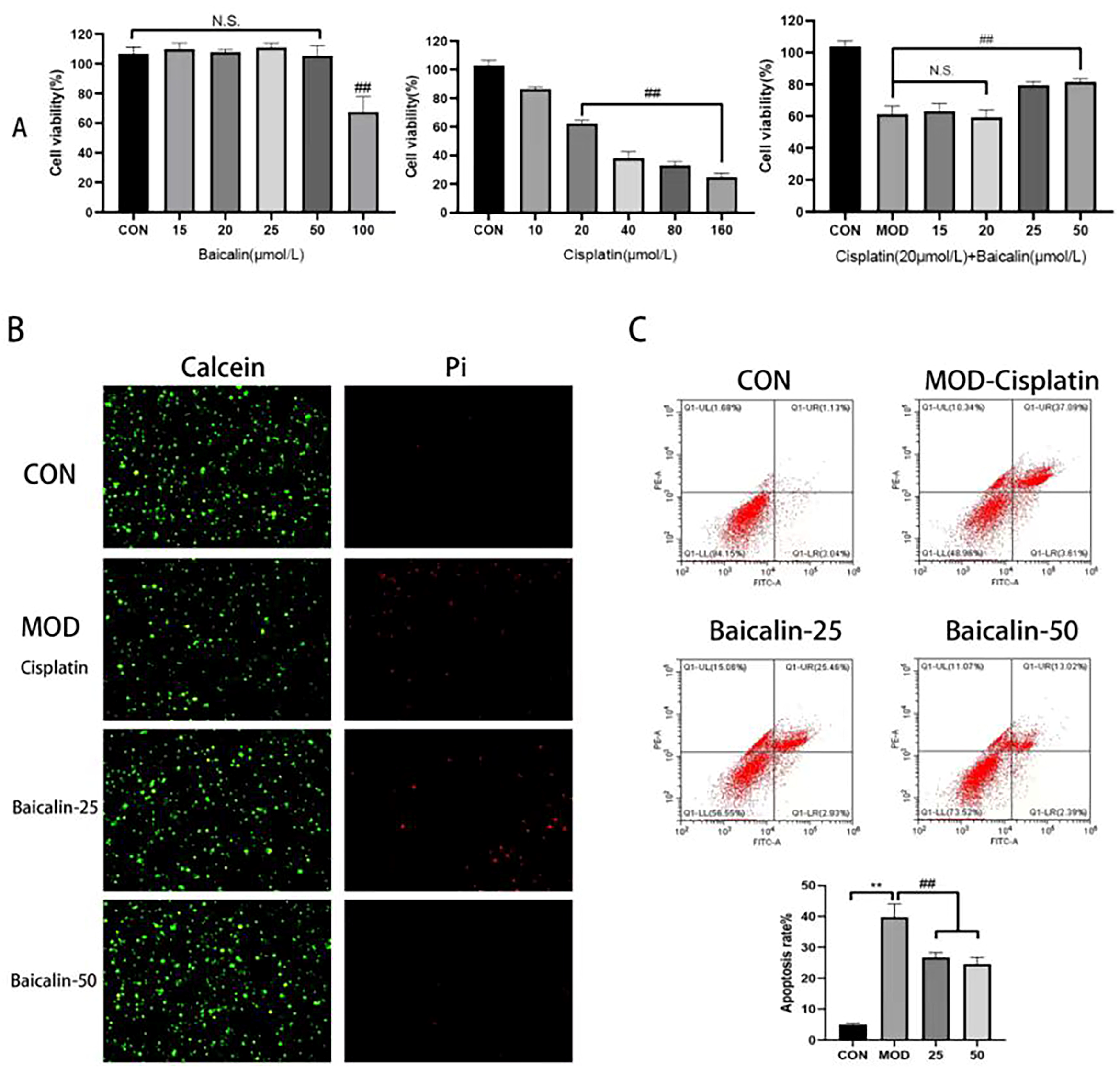
3.1. Modeling Concentration and Safe Concentration of Baicalin
3.2. Baicalin Improved Cisplatin-Mediated MDCK Cell Apoptosis
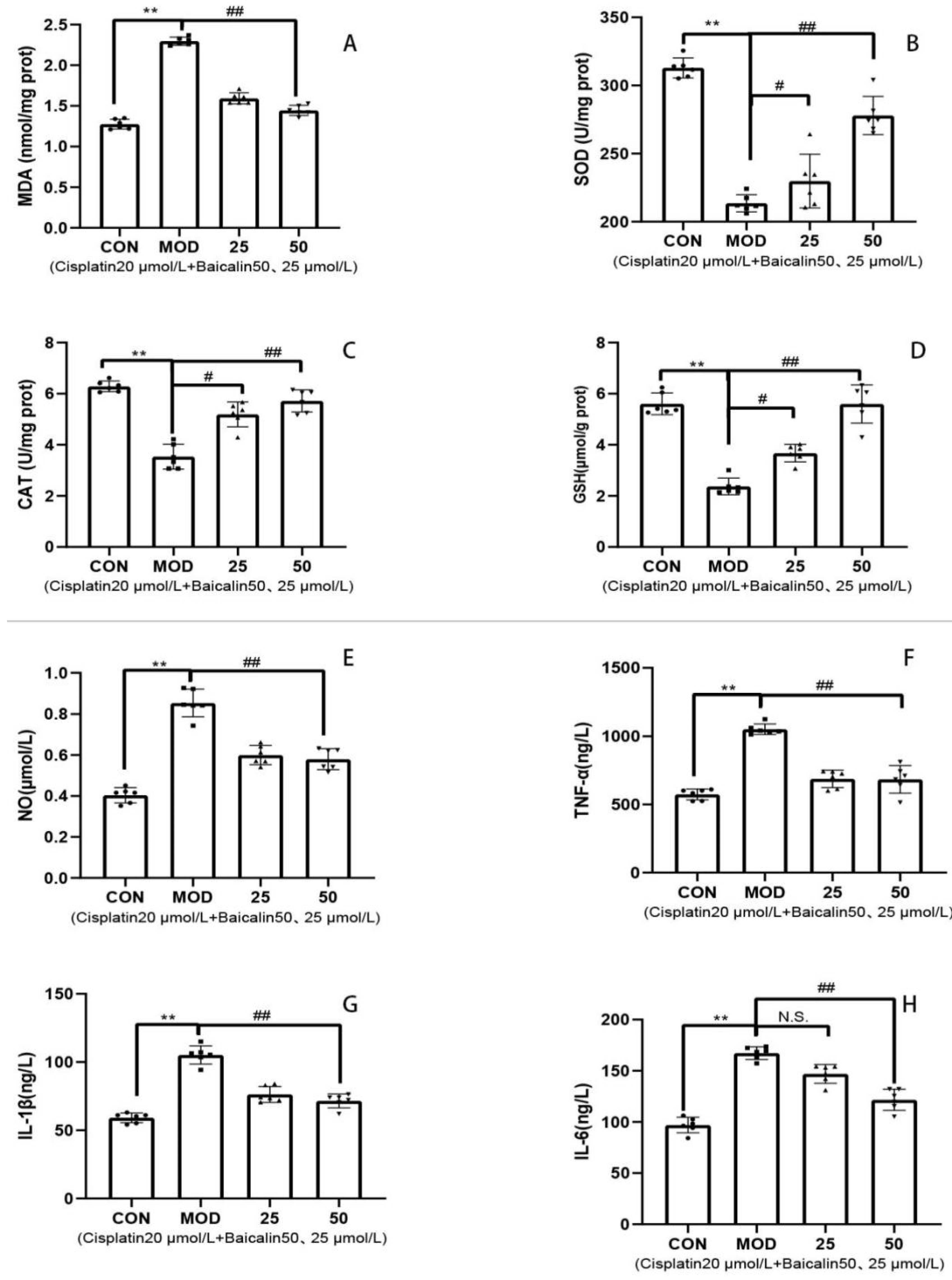
3.3. Baicalin Alleviated Cisplatin-Mediated Oxidative Stress and Inflammation of MDCK Cells
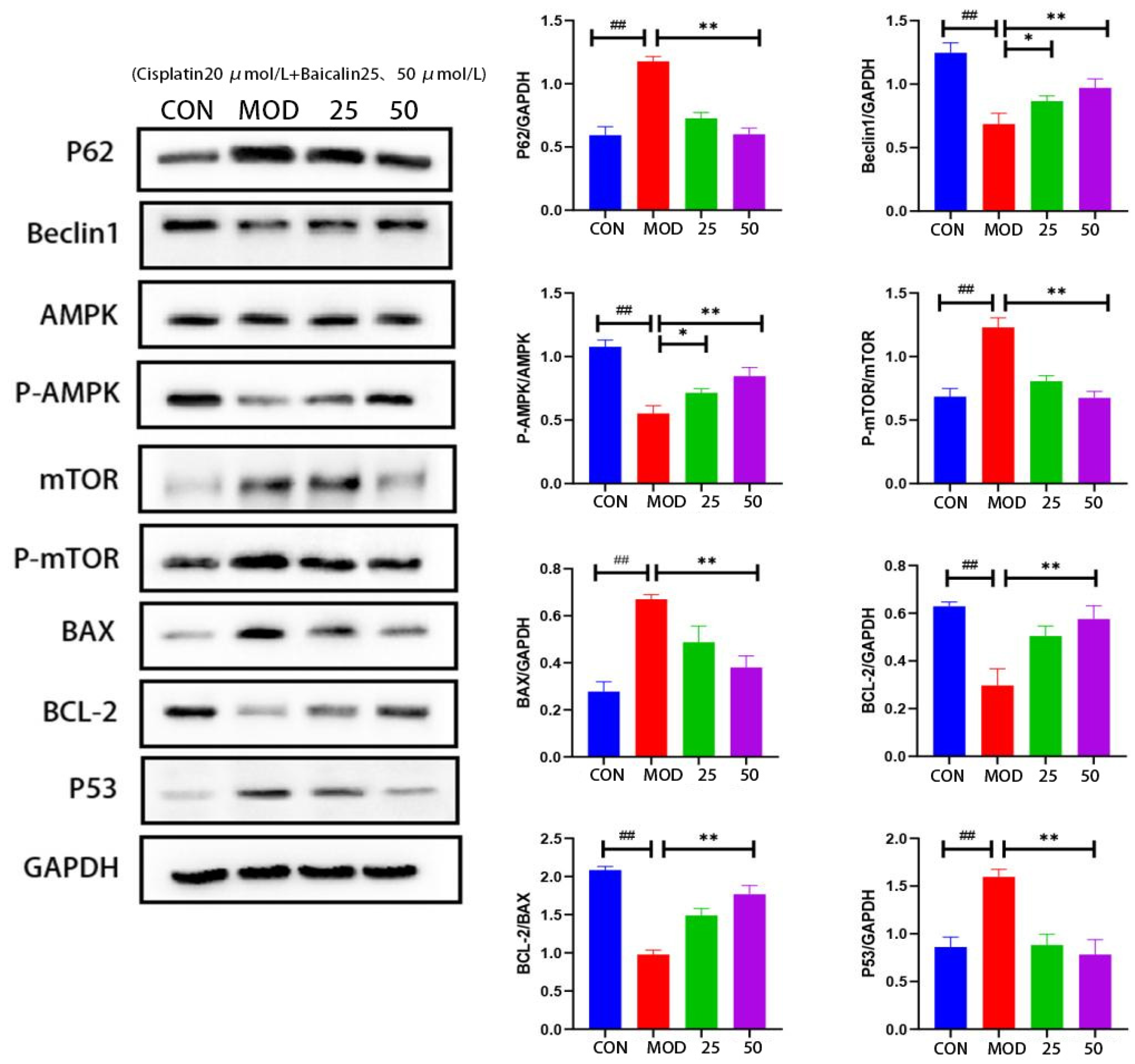
3.4. Baicalin Effectively Counteracted Cisplatin-Mediated MDCK Cells Apoptosis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedmann, E.; Gee, N.R.; Simonsick, E.M.; Studenski, S.; Resnick, B.; Barr, E.; Kitner-Triolo, M.; Hackney, A. Pet Ownership Patterns and Successful Aging Outcomes in Community Dwelling Older Adults. Front. Vet. Sci. 2020, 7, 293. [Google Scholar] [CrossRef]
- Moon, D.C.; Choi, J.H.; Boby, N.; Kim, S.J.; Song, H.J.; Park, H.S.; Gil, M.C.; Yoon, S.S.; Lim, S.K. Prevalence of Bacterial Species in Skin, Urine, Diarrheal Stool, and Respiratory Samples in Cats. Pathogens 2022, 11, 324. [Google Scholar] [CrossRef]
- Stokes, J.E.; Forrester, S.D. New and unusual causes of acute renal failure in dogs and cats. Vet. Clin. N. Am.-Small 2004, 34, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, G.B.D.C.K.D. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Banerji, B.; Pramanik, S.K. Binding studies of creatinine and urea on iron-nanoparticle. Springerplus 2015, 4, 708. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Tabassum, H.; Bhardwaj, M.; Parvez, S. Ameliorative efficacy of quercetin against cisplatin-induced mitochondrial dysfunction: Study on isolated rat liver mitochondria. Mol. Med. Rep. 2017, 16, 2939–2945. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Wang, L.; Li, M.L.; Gong, Z.C.; Du, J. Role of myo-inositol in acute kidney injury induced by cisplatin. Toxicology 2023, 499, 153653. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Yingrui, W.; Zheng, L.; Guoyan, L.; Hongjie, W. Research progress of active ingredients of Scutellaria baicalensis in the treatment of type 2 diabetes and its complications. Biomed. Pharmacother. 2022, 148, 112690. [Google Scholar] [CrossRef]
- Hu, F.M.; Bi, Y.L.; Zheng, X.L.; Lu, M.; Diao, Q.Y.; Tu, Y. Effect of baicalin supplementation on the growth, health, antioxidant and anti-inflammatory capacity, and immune function of preweaned calves. Anim. Feed. Sci. Tech. 2023, 298, 115598. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mortazavi, Z.; Mehri, S.; Hosseinzadeh, H. Scutellaria baicalensis and its constituents baicalin and baicalein as antidotes or protective agents against chemical toxicities: A comprehensive review. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1297–1329. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review. Int. J. Mol. Sci. 2023, 24, 1954. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Wang, C.Y.; Jin, L. Baicalin downregulates Porphyromonas gingivalis lipopolysaccharide-upregulated IL-6 and IL-8 expression in human oral keratinocytes by negative regulation of TLR signaling. PLoS ONE 2012, 7, e51008. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Guo, M.; Jiang, H.; Cao, Y.; Zhang, N. The Protective Effect of Baicalin Against Lead-Induced Renal Oxidative Damage in Mice. Biol. Trace Elem. Res. 2017, 175, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, L.; Li, L.; Pokhrel, G.; Qi, G.; Rong, R.; Zhu, T. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement. Altern. Med. 2014, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; He, Z.; Pei, H.; Zhai, L.; Guan, Q.; Wang, G. Aurantiamide promotes M2 polarization of microglial cells to improve the cognitive ability of mice with Alzheimer’s disease. Phytother. Res. 2023, 37, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, H.; Hou, Y.Y.; Hu, R.Y.; Zhang, J.J.; Chen, X.; Wang, S.; Hu, J.N.; Wang, Z.; Li, W. Arginyl-fructosyl-glucose, a major Maillard reaction product of red ginseng mitigates cisplatin-evoked intestinal toxicity in vivo and in vitro. Food Funct. 2022, 13, 11283–11297. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, W.; Zong, Y.; Shi, K.; Li, J.; Zeng, F.; He, Z.; Du, R. Cognitive-enhancing effects of fibrauretine on Aβ(1-42)-induced Alzheimer’s disease by compatibilization with ginsenosides. Neuropeptides 2020, 82, 102020. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Ding, C.; Peng, X.; Chen, H.; Dong, L.; Zhang, Y.; Chen, X.; Liu, W.; Luo, Y. Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine 2022, 105, 154365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wang, J.Q.; Xu, X.Y.; Yang, J.Y.; Wang, Z.; Jiang, S.; Wang, Y.P.; Zhang, J.; Zhang, R.; Li, W. Red ginseng protects against cisplatin-induced intestinal toxicity by inhibiting apoptosis and autophagy via the PI3K/AKT and MAPK signaling pathways. Food Funct. 2020, 11, 4236–4248. [Google Scholar] [CrossRef] [PubMed]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: Standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Atale, N.; Gupta, S.; Yadav, U.C.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.M.; Armstrong, P.J.; Kirk, C.A.; Kolar, L.M.; Klausner, J.S. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J. Am. Vet. Med. Assoc. 1999, 214, 1336–1341. [Google Scholar]
- Stevens, K.B.; O’Neill, D.; Jepson, R.; Holm, L.P.; Walker, D.J.; Cardwell, J.M. Signalment risk factors for cutaneous and renal glomerular vasculopathy (Alabama rot) in dogs in the UK. Vet. Rec. 2018, 183, 448. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Elliott, J.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J. Vet. Intern. Med. 2013, 27, 814–821. [Google Scholar] [CrossRef]
- Martínez-Hernández, S.L.; Muñoz-Ortega, M.H.; Ávila-Blanco, M.E.; Medina-Pizaño, M.Y.; Ventura-Juárez, J. Novel Approaches in Chronic Renal Failure without Renal Replacement Therapy: A Review. Biomedicines 2023, 11, 2828. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Guastalla, J.P.; Ferrero, J.M.; Curé, H.; Weber, B.; Winckel, P.; Lortholary, A.; Mayer, F.; Paraiso, D.; Magherini, E.; et al. A multicenter phase II study of cisplatin and docetaxel (Taxotere) in the first-line treatment of advanced ovarian cancer: A GINECO study. Cancer Chemother. Pharmacol. 2004, 53, 489–495. [Google Scholar] [CrossRef]
- de Godoy Torso, N.; Visacri, M.B.; Quintanilha, J.C.F.; Cursino, M.A.; Pincinato, E.C.; Moriel, P. Assessment of Renal Function in Head and Neck Cancer Patients Treated with Cisplatin: Different Biomarkers and Acute Kidney Injury Classifications. Int. J. Mol. Sci. 2022, 24, 141. [Google Scholar] [CrossRef]
- Ueno, M.; Morizane, C.; Okusaka, T.; Mizusawa, J.; Kataoka, T.; Ikeda, M.; Ozaka, M.; Okano, N.; Sugimori, K.; Todaka, A.; et al. Comparison of gemcitabine-based chemotherapies for advanced biliary tract cancers by renal function: An exploratory analysis of JCOG1113. Sci. Rep. 2021, 11, 12885. [Google Scholar] [CrossRef]
- Cavallo, F.; Feldman, D.R.; Barchi, M. Revisiting DNA damage repair, p53-mediated apoptosis and cisplatin sensitivity in germ cell tumors. Int. J. Dev. Biol. 2013, 57, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; He, B.; Fang, N.; Lu, S.; Liao, Y.Q.; Wan, Y.Y. Autophagy inhibition sensitizes cisplatin cytotoxicity in human gastric cancer cell line SGC7901. Asian Pac. J. Cancer Prev. 2013, 14, 4685–4688. [Google Scholar] [CrossRef] [PubMed]
- Llambi, F.; Wang, Y.M.; Victor, B.; Yang, M.; Schneider, D.M.; Gingras, S.; Parsons, M.J.; Zheng, J.H.; Brown, S.A.; Pelletier, S.; et al. BOK Is a Non-canonical BCL-2 Family Effector of Apoptosis Regulated by ER-Associated Degradation. Cell 2016, 165, 421–433. [Google Scholar] [CrossRef]
- Huang, T.H.; Wu, T.H.; Guo, Y.H.; Li, T.L.; Chan, Y.L.; Wu, C.J. The concurrent treatment of Scutellaria baicalensis Georgi enhances the therapeutic efficacy of cisplatin but also attenuates chemotherapy-induced cachexia and acute kidney injury. J. Ethnopharmacol. 2019, 243, 112075. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, L.; Zhang, Y.; Zheng, L.; Xu, M.; Rong, R.; Zhu, T. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int. J. Mol. Sci. 2014, 15, 12507–12522. [Google Scholar] [CrossRef]
- Singh, J.; Chaudhari, B.P.; Kakkar, P. Baicalin and chrysin mixture imparts cyto-protection against methylglyoxal induced cytotoxicity and diabetic tubular injury by modulating RAGE, oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2017, 50, 67–75. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.E.; Wang, N.; Liu, X.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; et al. Chemical Composition and Antioxidant Activities of Polysaccharides from Yingshan Cloud Mist Tea. Oxid. Med. Cell Longev. 2019, 2019, 1915967. [Google Scholar] [CrossRef]
- Yang, C.; Li, L.; Ma, Z.; Zhong, Y.; Pang, W.; Xiong, M.; Fang, S.; Li, Y. Hepatoprotective effect of methyl ferulic acid against carbon tetrachloride-induced acute liver injury in rats. Exp. Ther. Med. 2018, 15, 2228–2238. [Google Scholar] [CrossRef]
- Shao, D.; Wu, Z.; Bai, S.; Fu, G.; Zou, Z. The function of miRNA-153 against isoflurane-induced neurotoxicity via Nrf2/ARE cytoprotection. Mol. Med. Rep. 2019, 19, 4001–4010. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; Xu, P.; Yin, G. Effects of dietary baicalin supplementation on growth performance, antioxidative status and protection against oxidative stress-induced liver injury in GIFT tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 240, 108914. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.Y.; Sun, Y.; Wang, H.; Shan, H.; Wang, S. Baicalin protects LPS-induced blood-brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharmacol. 2021, 96, 107725. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, B.; Zhang, Y.; Liu, S.; Duan, Z.; Chen, Y.; Zhang, X. Baicalin Attenuates Oxidative Stress in a Tissue-Engineered Liver Model of NAFLD by Scavenging Reactive Oxygen Species. Nutrients 2022, 14, 541. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, G.; Yu, Q.; Wang, L.; Wu, L.; Tao, Z.; Ding, J.; Lin, D. Baicalin promotes random-pattern skin flap survival by inducing autophagy via AMPK-regulated TFEB nuclear transcription. Phytother. Res. 2023, 37, 3926–3938. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liang, M.; Sun, X.; Mohyuddin, S.G.; Chen, S.; Wen, J.; Yong, Y.; Ma, X.; Yu, Z.; Ju, X.; et al. Baicalin Alleviates LPS-Induced Oxidative Stress via NF-κB and Nrf2-HO1 Signaling Pathways in IPEC-J2 Cells. Front. Vet. Sci. 2021, 8, 808233. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cong, X.; Yu, W.; Jiang, Z.; Fu, K.; Cao, R.; Tian, W.; Feng, Y. Baicalin inhibits oxidative injures of mouse uterine tissue induced by acute heat stress through activating the Keap1/Nrf2 signaling pathway. Res. Vet. Sci. 2022, 152, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, H.; Wang, D.; Yang, Z.; Zhuang, L.; Yang, N.; Dong, J. Weicao capsule ameliorates renal injury through increasing autophagy and NLRP3 degradation in UAN rats. Int. J. Biochem. Cell Biol. 2018, 96, 1–8. [Google Scholar] [CrossRef]
- Gu, X.; Ma, Z.; Fang, J.; Cai, D.; Zuo, Z.; Liang, S.; Cui, H.; Deng, J.; Ma, X.; Ren, Z.; et al. Obesity Enhances Antioxidant Capacity and Reduces Cytokine Levels of the Spleen in Mice to Resist Splenic Injury Challenged by Escherichia coli. J. Immunol. Res. 2020, 2020, 5948256. [Google Scholar] [CrossRef]
- Li, L.; Dong, J.M.; Ye, H.H.; Jiang, M.J.; Yang, H.H.; Liang, L.P.; Ning, L.J.; Wu, Y. Baicalin promotes antiviral IFNs production and alleviates type I IFN-induced neutrophil inflammation. J. Nat. Med. 2023, 77, 677–687. [Google Scholar] [CrossRef]
- Wang, X.; Xie, L.; Long, J.; Liu, K.; Lu, J.; Liang, Y.; Cao, Y.; Dai, X.; Li, X. Therapeutic effect of baicalin on inflammatory bowel disease: A review. J. Ethnopharmacol. 2022, 283, 114749. [Google Scholar] [CrossRef]
- Dlamini, N.Z.; Somboro, A.M.; Amoako, D.G.; Arhin, I.; Khumalo, H.M.; Khan, R.B. Toxicogenicity and mechanistic pathways of aflatoxin B1 induced renal injury. Environ. Toxicol. 2021, 36, 1857–1872. [Google Scholar] [CrossRef]
- Wei, X.; Mao, T.; Li, S.; He, J.; Hou, X.; Li, H.; Zhan, M.; Yang, X.; Li, R.; Xiao, J.; et al. DT-13 inhibited the proliferation of colorectal cancer via glycolytic metabolism and AMPK/mTOR signaling pathway. Phytomedicine 2019, 54, 120–131. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, T.; Luo, W.; Lv, Y.; Zhang, L.; Wang, C.; Li, M.; Wu, W.; Shi, S. Metformin induces apoptotic cytotoxicity depending on AMPK/PKA/GSK-3β-mediated c-FLIP(L) degradation in non-small cell lung cancer. Cancer Manag. Res. 2019, 11, 681–689. [Google Scholar] [CrossRef]
- Wang, C.; Mei, X.; Wu, Y.; Yang, Y.; Zeng, Z. Cinobufagin alleviates lipopolysaccharide-induced acute lung injury by regulating autophagy through activation of the p53/mTOR pathway. Front. Pharmacol. 2022, 13, 994625. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Yan, X.; Cheng, M.; Jiang, X.; Xu, L.; Shen, L.; Yu, H.; Sun, L. p62 Promotes the Mitochondrial Localization of p53 through Its UBA Domain and Participates in Regulating the Sensitivity of Ovarian Cancer Cells to Cisplatin. Int. J. Mol. Sci. 2022, 23, 3290. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, E.; Maiuri, M.C.; Galluzzi, L.; Vitale, I.; Djavaheri-Mergny, M.; D’Amelio, M.; Criollo, A.; Morselli, E.; Zhu, C.; Harper, F.; et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008, 10, 676–687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, X.; Yan, C.; Xie, L.; Yang, Y. Baicalin Exhibits a Protective Effect against Cisplatin-Induced Cytotoxic Damage in Canine Renal Tubular Epithelial Cells. Metabolites 2023, 13, 1173. https://doi.org/10.3390/metabo13121173
Wang Y, Li X, Yan C, Xie L, Yang Y. Baicalin Exhibits a Protective Effect against Cisplatin-Induced Cytotoxic Damage in Canine Renal Tubular Epithelial Cells. Metabolites. 2023; 13(12):1173. https://doi.org/10.3390/metabo13121173
Chicago/Turabian StyleWang, Yao, Xiao Li, Chuanguo Yan, Liuwei Xie, and Yang Yang. 2023. "Baicalin Exhibits a Protective Effect against Cisplatin-Induced Cytotoxic Damage in Canine Renal Tubular Epithelial Cells" Metabolites 13, no. 12: 1173. https://doi.org/10.3390/metabo13121173
APA StyleWang, Y., Li, X., Yan, C., Xie, L., & Yang, Y. (2023). Baicalin Exhibits a Protective Effect against Cisplatin-Induced Cytotoxic Damage in Canine Renal Tubular Epithelial Cells. Metabolites, 13(12), 1173. https://doi.org/10.3390/metabo13121173




