Abstract
Rubi idaei fructus is a source of nutritionally important bioactive chemical compounds, mainly antioxidants, which strengthen the immune system and can be used in the prophylaxis and adjuvant therapies of many oxidative stress-induced diseases. There are no literature reports presenting a comprehensive comparative analysis of the antioxidant activity and nutritionally relevant metabolites contained in the fruits of repeat-fruiting raspberry cultivars, which are commonly grown in Europe. The aim of this study was to carry out a comparative analysis of the antioxidant potential (Folin–Ciocalteu, DPPH, FRAP), the content of selected primary and secondary metabolites, and the qualitative and quantitative composition of amino acids and fatty acids in the fruits of R. idaeus cv. ‘Pokusa’, ‘Polana’, and ‘Polka’. The fruits of the analyzed cultivars have a low caloric value (171–219 kcal/100 g); low content of available carbohydrates (6–6.6%) and total carbohydrates (3.4–4.8%); and high levels of dietary fiber (4.7–5.8%), vitamin C (22.8–27 mg/100 g), anthocyanins (25.1–29.6 mg/100 g), and flavonoids (0.5–2.6 mg/100 g). The fruits were found to contain valuable unsaturated fatty acids (35–60%), especially MUFAs with dominant oleic, elaidic, palmitic, and erucic acids and PUFAs (α-linolenic, eicosapentaenoic, and linoleic acids). MUFAs from the ω-9 group accounted for 12–18%, whereas the content of PUFAs from the ω-3 and ω-6 groups was in the range of 15–23 and 6–21%, respectively. Exogenous amino acids, accounting for 56–62%, were dominated by leucine, phenylalanine, and lysine. The following order of the total polyphenolic content was established in the fresh fruit juice from the analyzed cultivars: ‘Pokusa’ < ‘Polana’ < ‘Polka’. The different antioxidant capacity assays used in the study confirmed the high antioxidant potential of the fruits and fresh juice from the three R. idaeus cultivars. This indicates that raspberry fruits can serve as a source of nutrients and can be used as a valuable supplement in a healthy human diet and a raw material in the pharmaceutical and cosmetic industries.
1. Introduction
1.1. Pro-Health Antioxidant Activity of Rubus Fruits
Fruits of the genus Rubus are a rich source of bioactive chemical compounds, mainly antioxidants, including phenolic compounds. Several thousand polyphenolic molecules have been identified in the edible organs of various plants [1]. Secondary metabolites contained in R. idaeus fruits are represented by a number of antioxidants, e.g., flavonoids, vitamins, anthocyanins, terpenes, lipids, and amino acids, along with their derivatives, all strengthening the immune system [2,3,4]. The antioxidant phenolic compounds and other antioxidants contained in R. idaeus extracts serve many pharmacological functions [3,5]. The fruits of various Rubus species and other berries consumed as part of the diet can be used for the prophylaxis and adjuvant therapy of many diseases, mainly oxidative stress-induced conditions [6,7]. Rubus fruit phytochemicals exhibit a wide range of pharmacological activities, e.g., anti-obesity [8], anti-Alzheimer [9], antibacterial [6], anticancer [10], hepatoprotective [11], hypoglycemic [12], hypolipidemic [13], neuroprotective [14], against obesity [15], and skincare [16] effects.
1.1.1. Antidiabetic Properties
Oxidative stress may lead to the development of diabetes, metabolic syndrome, and obesity. In diabetic rats, ellagic acid decreases blood glucose levels and increases the levels of serum insulin, mitigates glucose intolerance, and stimulates insulin secretion [17]. High ellagic acid content, i.e., 207–244 mg kg−1 f.w., was detected in the R. idaeus fruits cv. ‘Autumn Bliss’, ‘Heritage’, ‘Rubi’, and ‘Zeva’, and 18 other common raspberry cultivars and R. occidentalis L. ‘Bristol’ contained 120–324 mg/100 g of this compound [18,19]. Oleanolic and ursolic acids contained in R. chingii fruits inhibit α-glucosidase and exert a beneficial effect on diabetes therapy. These acids can serve as therapeutic agents in the prophylaxis and treatment of metabolic diseases. Additionally, methanol extracts of R. chingii fruits inhibit protein tyrosine phosphatase 1B [20]. Polyphenols contained in R. chingii fruits alleviate hyperglycemia in mice with type 1 diabetes through the enhancement of the antioxidant reaction and inhibition of the expression of Atrogin-1 and Trim63 proteins. Polyphenols also protect pancreatic β cells from damage and stimulate insulin secretion and improve glucose tolerance through the regulation of glycogen metabolism and the inhibition of gluconeogenesis via activation of insulin signaling [17]. Procyanidins contained in R. amabilis fruits exert hypoglycemic effects, reduce the intracellular production of reactive oxygen species and MDA, and simultaneously increase SOD activity. Through the activation of PI3K/Akt/FoxO1 signaling, procyanidins protect cells against apoptosis. Therefore, they can be used as one of the components of antidiabetic herbal medicines. Three-month therapy with R. occidentalis extracts administered at a dose of 900 or 1800 mg/day regulates glycemia and has a positive effect on vascular inflammation markers and the function of pancreatic β cells in prediabetic patients [21].
1.1.2. Anti-Obesity Properties
Obesity is a cause of many diseases and metabolic dysfunctions, which are part of the so-called metabolic syndrome. The prophylaxis and adjuvant therapies for obesity necessitate the use of appropriate phytochemicals, e.g., vitamins and antioxidants [22,23]. Ellagic acid inhibits the expression of the gene responsible for white adipocytes in rat adipose tissue. It has a positive effect on obesity-induced dyslipidemia and on UCP1 gene expression in brown adipocytes [24]. Ellagic acid and ethanolic extracts from R. coreanus fruits exhibit hypocholesterolemic and anti-obesity effects and downregulate the expression of adipogenic and lipogenic genes in high-fat diets. R. coreanus extract suppresses lipid accumulation in white adipose tissue and induces thermogenesis in brown adipose tissue [25]. R. idaeus fruit extract reduces triglyceride and cholesterol levels, as well as oxidative stress and lipid metabolism, body weight, and body fat in hyperlipidemic mice. Additionally, it regulates the PPARa and Hmgcr genes, downregulates Hmgcr gene expression, and accelerates the conversion of triglycerides into fatty acids. It has a protective role against hypertriglyceridemia and mitigates lipid metabolism disorders [26]. Polysaccharide compounds from R. chingii fruits protect against palmitic acid-mediated lipotoxicity, a reduction in mitochondrial membrane potential, and a decrease in glutathione levels [27].
1.1.3. Anticancer Properties
Oxidative stress is one of the main factors in the pathogenesis of neoplastic cancer [28,29]. One of the causes of cancer development is the overproduction of reactive oxygen species leading to oxidative stress, cell metabolism disorders, damage to DNA and other metabolites, and pathological processes [30,31]. Ellagitannins, mainly sanguiin H-6 and lambertianin C, present in R. idaeus fruits exhibit a chemopreventive effect. They change the morphology of the nucleus and induce the apoptosis of Caco-2 cells; hence [32], R. idaeus extracts exhibit anticancer activity against human hepatocellular carcinoma HepG2 and mouse L20B cell lines. A strong cytotoxic and cytostatic effect on tumor cells has been noted in both lines [33]. Volatile substances present in berry fruit extracts inhibit the proliferation of non-small-cell lung cancer A549 cells via the induction of apoptosis [10]. Raspberry fruits used in the diet exert a chemopreventive effect on colorectal cancer in patients with non-specific inflammatory bowel disease. This disease, which is a chronic immune-mediated disease associated with an increased risk of colon dysplasia and colorectal cancer, is the third and fourth leading cause of cancer-related death in the United States and worldwide, respectively. Inflammation-induced carcinogenesis is associated with oxidative stress, genome instability, immune effectors, cytokine dysregulation, and the NFκB signaling pathway. Mainly anthocyanins were found to be involved in chemopreventive activity [34]. The bioactivity of blackberry fruit extracts is mainly related to the content of phenolic compounds and ursane-type triterpenes. The extracts have anticancer activity against colon cancer cells HT-29 and T-84 and anti-inflammatory effects through the inhibition of the secretion of pro-inflammatory cytokines IL-8 [6].
1.2. Rationale behind the Study
These investigations were undertaken given the increase in the commercial production of the R. idaeus repeat-fruiting cultivars ‘Polka’, ‘Pokusa’, and ‘Polana’. The demand for these fruits suggests the need for comparative studies of bioactive chemical compounds contained in Rubi fructus, which is increasingly being recommended as a superfood with health-promoting properties. The literature does not provide comprehensive comparative analyses of the antioxidant activity, selected antioxidants, or other nutritionally important metabolites in the fruits of raspberry cultivars grown in southeastern Poland. This issue is of paramount importance for dietetics and the potential use of raspberries in the production of functional food mainly for their rich antioxidant and dietary fiber content. It is also important for molecular biology to elucidate the molecular basis of the activity of bioactive food ingredients that can enhance, weaken, or modify the physiological and metabolic functions of the organism. Additionally, they can be used in research strategies: genomics, proteomics, and metabolomics, the development of tools for the acquisition of knowledge of active phytochemicals used as food ingredients, and the elucidation of the molecular basis of the biological activity of these compounds.
The aim of this study was to compare the antioxidant activity, determine the total content of selected primary and secondary metabolites (proteins, lipids, total sugars, available sugars, dietary fiber, polyphenols, flavonoids, anthocyanins, and vitamin C), and analyze the qualitative and quantitative composition of amino acids and fatty acids in the fruits of the Rubus idaeus L. repeat-fruiting cultivars ‘Pokusa’, ‘Polana’, and ‘Polka’, which are commonly cultivated in Europe and have been listed in the National Register of Horticultural Plant Varieties.
2. Materials and Methods
2.1. Study Material
Fruits of three Rubus idaeus repeat-fruiting cultivars, ‘Polka’, ‘Polana’, and ‘Pokusa’ (Figure 1A–C), which are commonly cultivated in commercial production in Poland and Europe and have been listed in the National Register of Horticultural Plant Varieties, were collected in a commercial plantation located in Blinów II, Szastarka Commune, Lublin Province (50°52′53″ N 22°23′05″ E). Fertilization and plant protection agents were applied in accordance with the recommended raspberry cultivation calendar. The fruits of the three cultivars were harvested at the full maturity stage in September.

Figure 1.
(A–C). R. idaeus ‘Pokusa’ (A), ‘Polana’ (B), and ‘Polka’ (C) fruits.
Origin and Lineage of the Analyzed Cultivars
Most commercial cultivars derive from R. idaeus. The Polish repeat-fruiting cultivars ‘Polana’, ‘Polka’, and ‘Pokusa’ are commonly grown in commercial production. ‘Polana’ is a cross between the American cultivar ‘Heritage’ and the English cultivar ‘Zeva Herbsternte’. It was entered into the National Register of Horticultural Plant Varieties on 13 February 1991 as the first repeat-fruiting raspberry cultivar. In turn, the cultivar ‘Polka’ was obtained by crossing the English cultivars ‘Autumn Bliss’ and ‘Lloyd George’ with R. coreanus. Tribes P 93563 and P 89141 are part of the lineage of this cultivar. ‘Polka’ was entered into the National Register of Horticultural Plant Varieties in 2003. Both R. idaeus cultivars have gained worldwide recognition and have contributed to the establishment of fruit quality standards. The lineage of ‘Pokusa’ includes two English cultivars: ‘Autumn Bliss’ and ‘Heritage’ and several species of the genus Rubus: R. idaeus, R. strigosus, R. odoratus, and R. occidentalis [35].
2.2. Determination of Antioxidant Activity
The antioxidant activity and total polyphenol content were determined in fresh raspberry fruit and juice. Samples of blended fruit and juice from the analyzed cultivars were subjected to extraction with the use of 100 mL of water at 100 °C. The fruit extract was filtered, and its appropriate amount was used in the subsequent analyses. The following methods were employed to determine the antioxidant potential: Folin–Ciocalteu (FC), ferric reducing antioxidant power (FRAP), and 2,2-diphenyl-1-picrylhydrazyl (DPPH). Total polyphenols were determined with the spectrophotometric method using the Folin–Ciocalteu (FC) reagent.
2.2.1. FRAP Method (Ferric Reducing Antioxidant Power Assay)
The antioxidant activity of the Rubi idaei fructus raw material was assessed using the ferric reducing antioxidant power (FRAP) assay [36]. The standard curve was plotted for iron sulfate. The absorbance of the solution was measured at a wavelength of λ = 593 nm using a Hitachi U-2900 spectrophotometer (Hitachi, Tokyo, Japan). The absorbance values were converted into the FRAP unit, which expresses the amount of antioxidant compounds per 1 g of raw material (µmol/g) capable of reducing 1 mole of iron (III) to iron (II).
2.2.2. DPPH Method
The antioxidant activity of the Rubi fructus raw material was determined as in Bondet et al. [37] with the use of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. A methanol solution of the DPPH radical (3.9 mL) with a concentration of 6 × 10−5 mol/L was added to a specified amount of the extract. After 30 min, a decrease in the absorbance of the solution was measured at a wavelength of λ = 515 nm. The absorbance result was converted into a Trolox unit based on the standard curve.
2.3. Chemical Analyses
The energy value and the content of selected bioactive compounds were determined in fresh R. idaeus fruits.
2.3.1. Available Carbohydrates
The total sugar and available carbohydrate content in the analyzed fruits was determined with the Luff–Schoorl method [38]. The method is based on the reduction of Cu2+ ions contained in Luff’s solution by sugars present in the sample at pH = 9.5. The difference between the sodium thiosulfate (VI) utilized in the blank sample and in the reaction with copper (II) reduced by saccharides in the sample corresponds to the amount of copper reduced directly by the reducing sugars.
2.3.2. Total Fiber
The amount of fiber in the fruits was determined using the enzymatic method described by Asp et al. [39]. The sample was treated with α-amylase, pepsin, and pancreatin enzymes. The amount of undigested insoluble and soluble fiber precipitated from the filtrate was determined using the gravimetric method.
2.3.3. Total Protein
The total nitrogen content in the fruit samples (n = 3) was determined with the Kjeldahl method [40] using a FOSS Kjeltec 2300 analyzer (Foss Tecator, Höganäs, Sweden). The total protein content was estimated following the Polish standard (PN-75 A-04018:2002) [41] by multiplying the nitrogen content by the protein factor: 5.6 [42].
2.3.4. Amino Acids
The qualitative and quantitative composition of amino acids in the fruits was determined as in Davies and Thomas [43]. A 5 g aliquot was placed in an INGOS hydrolyzer thimble (INGOS, Prague, Czech Republic) and flooded with 6 M of HCl. The solution was saturated with nitrogen, hydrolyzed at 110 °C for 20 h, cooled, and filtered. The hydrolyzate was evaporated in an RVO 400 SD vacuum evaporator (Hamburg, Boeco, Germany) at 50 °C, washed with 1 mL of distilled water, and evaporated again. The dry residue remaining in the vacuum flask was dissolved in 5 mL of citrate buffer, pH 2.2. The sample was applied onto a 35 cm long column with a 5 mm diameter filled with ion exchange resin. Amino acids were separated using an AAA 400 amino acid analyzer (Ingos, Prague, Czech Republic) at temperatures of T1 = 60 °C and T2 = 63 °C. The amino acids were derivatized into colored amino acid–ninhydrin complexes and identified using a photometric detector at 570 nm and 440 nm for proline. The chromatogram was read in the Chromulan computer program.
2.3.5. Total Fat Content
The total fat content was estimated in accordance with the Polish standard (PN-EN ISO 5508:1996) [44]. The Soxhlet method, based on the acid hydrolysis of 4 mol/dm3 HCl, was used in this analysis.
2.3.6. Qualitative and Quantitative Composition of Fatty Acids
The quantitative and qualitative analysis of fatty acids was performed in accordance with the Polish standard (PN-EN ISO 12966-1:2014) [45]. The saponification process was carried out using a methanolic solution of potassium hydroxide. Next, esterification was performed by adding a methanolic boron trifluoride solution, and separation was carried out using hexane and a saturated sodium chloride solution. The hexane layer was collected into a glass vial and dried over anhydrous sodium sulfate. The chromatographic analysis was performed using a Varian 450-GC gas chromatograph (Temecula, CA, USA) equipped with an 1177 Split/Splitless injector at a temperature of 250 °C with a Select™ Biodiesel for FAME capillary column (30 m; 0.32 mm; 0.25 μm). The stationary phase included Select Biodiesel for FAME Fused Silica, a column furnace with a starting temperature of 100 °C and a final temperature of 240 °C, and a FID detector (temperature of 270 °C). The carrier gas (helium) flow rate was 1.5 mL/min. The Galaxie™ Chromatography Data System Autosampler Varian CP-8400 software (Version 3.3.5.) was used for the collection, integration, and calculation of results. (Version 1.9.302.530) from Bruker Daltonics (BrukerCorporation, Fremont, CA, USA)
2.3.7. Folin–Ciocalteu Method
The total phenolic compound content was determined with the Folin–Ciocalteu method as in Prior [46]. Absorbance at λ = 765 nm was measured after 30 min with a Hitachi U-2900 spectrophotometer (Hitachi, Tokyo, Japan). The results were expressed in mg of phenolic compounds per 1 g of dry material as caffeic acid equivalents.
2.3.8. Anthocyanins
The accumulation of anthocyanins in the fruits was determined using the modified Martínez and Favret method [47]. Anthocyanins were extracted via the maceration of fresh samples in a methanol:HCl solution (99:1, v/v) at 4 °C for 24 h. The extracts were centrifuged at 10,000× g rpm for 10 min at 4 °C, and their absorbance was read at 527 nm and 652 nm (Cecil CE 9500, Cecil Instruments, Cambridge, UK). The concentration of anthocyanins was calculated using the molar extinction coefficient (ε = 29,600 M−1 cm−1) for cyanidin 3-glycoside (C3G).
2.3.9. Vitamin C
The vitamin C content was determined using HPLC according to the Polish standard (PN-EN 14130:2004) [48]. The extraction was carried out with the use of a metaphosphoric acid solution. In this process, dehydro-L(+) ascorbic acid is transformed into L(+) ascorbic acid. A 3 g fruit sample was treated with 80 mL of metaphosphoric acid and shaken; after filtration, a 100 mL flask was filled with the acid. To reduce the resulting solution (20 mL), 10 mL of an L-cysteine solution was added, trisodium phosphate was used to achieve pH = 7.0–7.2, and metaphosphoric acid was added. The vitamin C content was determined against the standard curve with the use of HPLC.
2.3.10. Flavonoids
The total flavonoid content was determined with the method proposed by Lamaison and Carnet [49]. A raw material aliquot was transferred to a flask, and 20 mL of acetone, 2 mL of hydrochloric acid, and 1 mL of methenamine solution were added. The mixture was kept under reflux in a boiling water bath for 30 min. The hydrolyzate was filtered into a volumetric flask (100 mL) and supplemented with acetone. After transferring 20 mL of the solution into a separatory funnel, 20 mL of water was added, and the mixture was extracted with ethyl acetate in 15 mL portions and 3 times in 10 mL portions. The combined organic layers were washed with water twice (40 mL each), filtered into a volumetric flask (50 mL), and supplemented with ethyl acetate. The solution intended for the analyses was prepared by adding 2 mL of an aluminum chloride solution (20 g/L) to 10 mL of the stock solution followed by the addition of an acetic acid:methanol mixture (1:19) to a volume of 25 mL. Next, the reference solution was prepared by supplementing 10 mL of the stock solution with an acetic acid:methanol mixture (1:19) to 25 mL. After 45 min, the absorption of the solutions at 425 nm was measured and compared with the reference solution. The percentage of flavonoids was expressed as quercetin equivalents.
2.3.11. Energy Value
The energy value of the analyzed fruits was determined according to EU Commission Delegated Regulation No. 78/2014 [50].
2.4. Statistical Analysis
The means of the measurements and the standard deviation were calculated. The significance of differences was analyzed statistically using the Statistica 6.0 software (StatSoft, Tulsa, OK, USA). Differences between selected traits were assessed with the use of one-way ANOVA. Statistical inference was performed at a significance level of p < 0.05.
3. Results
3.1. Selected Nutrients and Energy Value
The total fiber content in the fruits of the analyzed ‘Pokusa’, ‘Polana’, and ‘Polka’ cultivars was 4.7, 5.6, and 5.8 g/100 g f.w., respectively. In turn, the content of total sugars and available carbohydrates ranged from 6.1 in ‘Polka’ to 6.7 g/100 g f.w. in ‘Polana’. The protein content ranged from 0.95 in ‘Pokusa’ and ‘Polana’ to 1.15 g/100 g f.w. in ‘Polka’. The amount of fat was estimated at approximately 0.05 mg/100 g f.w. in ‘Pokusa’ and ‘Polka’ and 0.63 mg/100 g f.w. in ‘Polana’. The levels of ash ranged from 0.32 (‘Pokusa’) to 0.42 mg/100 g f.w. (‘Polka’ and ‘Polana’). Therefore, the ‘Pokusa’ fruits had lower fiber and ash content than ‘Polka’ and ‘Polana’, but ‘Polka’ had a higher level of protein and lower amounts of available carbohydrates than ‘Pokusa’ and ‘Polana’ (Figure 2A–C).
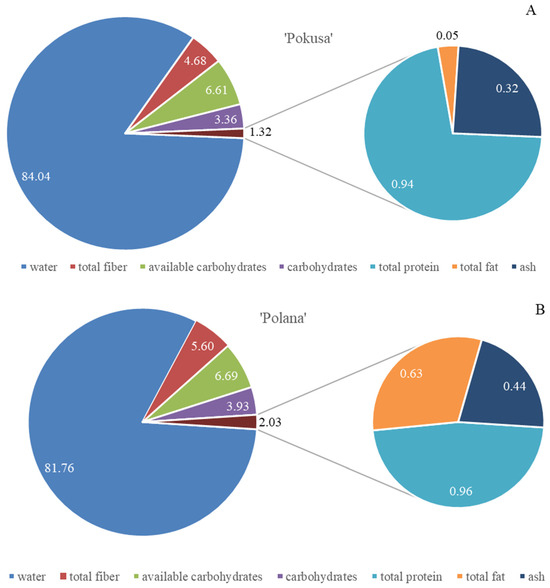
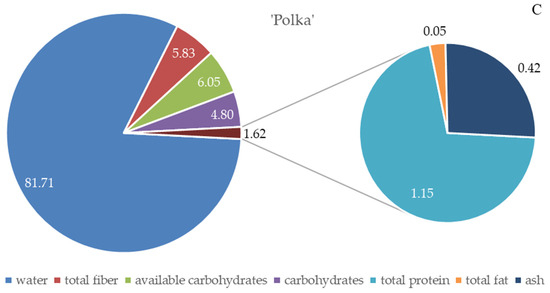
Figure 2.
(A–C) Total water, protein, dietary fiber, sugar, available carbohydrate (% f.w.), fat, and ash (mg/100 g f.w.) content in the fruits of the repeat-fruiting R. idaeus cultivars: (A) ‘Pokusa’, (B) ‘Polana’, (C) ‘Polka’.
The energy value of the fruits of the analyzed R. idaeus cultivars ranged from 171 in ‘Pokusa’ to 219 kJ/100 g in ‘Polka’. This parameter, converted into kcal/100 g, was in a range of 41–53. The value of this parameter in ‘Polana’ was significantly lower than in ‘Polka’ and markedly higher than in ‘Pokusa’ (Figure 3.)
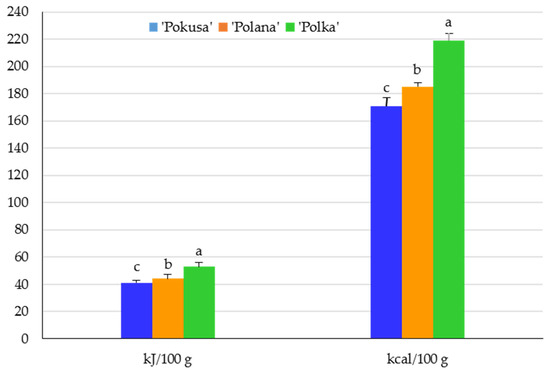
Figure 3.
Energy value of the fruits of the repeat-fruiting R. idaeus cultivars. Notes: Means of the energy value expressed in kJ/100 g and in kcal/100 g marked with the same letter do not differ significantly between the cultivars at a significance level of α = 0.05.
3.2. Antioxidants: Vitamin C, Anthocyanins, and Flavonoids
The total vitamin C content in the fruits of the R. idaeus cultivars was ranked as follows (mg/100 g): ‘Polana’ (27) < ‘Polka’ (25.1) < ‘Pokusa’ (22.8). The differences between the cultivars were statistically confirmed. In turn, the concentration of anthocyanins in the fruit ranged from 25.5 in ‘Pokusa’ to 30.7 mg/100 g in ‘Polana’. The value of this parameter in ‘Polana’ and ‘Polka’ did not differ significantly and was markedly higher than in ‘Pokusa’. The flavonoid contents in Rubi fructus from the ‘Polana’, ‘Pokusa’, and ‘Polka’ cultivars were 0.5; 1.9, and 2.6 mg/100 g, respectively. The value of this parameter in ‘Pokusa’ was significantly lower than in ‘Polka’ but higher than in ‘Polana’ (Figure 4).
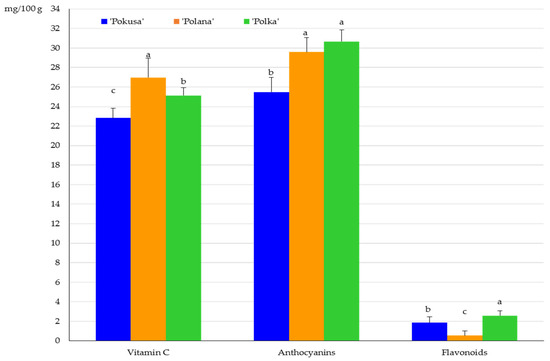
Figure 4.
Total vitamin C, anthocyanin, and flavonoid content in the fruits of the repeat-fruiting R. idaeus cultivars. Notes: Means of vitamin C, anthocyanins, and total flavonoids marked with the same letter do not differ between the cultivars at a significance level of α = 0.05.
The polyphenolic compound content in the analyzed fruits was determined with the Folin–Ciocalteu method. The concentration of polyphenols in Rubi fructus was similar in the three cultivars and ranged from 1.65 in ‘Polka’ to 1.7 mg/100 g in ‘Pokusa’ (Table 1). In turn, the fresh juice from the fruits of the three raspberry cultivars ‘Polana’, Pokusa’, and ‘Polka’ had substantially higher phenolic compound contents, i.e., 224, 251, and 260 mg/100 mL, respectively. The value of this parameter in ‘Polana’ was significantly higher than in ‘Pokusa’ and lower than in ‘Polka’ (Table 1).

Table 1.
Total phenolic compound contents in the fresh fruits and fresh juice of the repeat-fruiting R. idaeus cultivars.
3.3. Protein Amino Acids
The fruits of the analyzed cultivars contained 15 protein amino acids. In the total pool, the content of exogenous amino acids ranged from 56% in ‘Polana’ to 62% in ‘Pokusa’. They were dominated by leucine (from 6.2 in ‘Pokusa’ to 7.4% in ‘Polana’), phenylalanine (from 5.5 in ‘Pokusa’ to 6.8 in ‘Polana’), and lysine (from 5.7 in ‘Pokusa’ to 6.3% in ‘Polana). In the group of endogenous amino acids, the highest content was exhibited by glutamic acid (from 17 in ‘Polana’ to 20.1% in ‘Pokusa’), aspartic acid (from 12.5 in ‘Polana’ to 16.7% in ‘Pokusa’), and alanine (from 7.7 in ‘Pokusa’ to 8.5% in ‘Polana’) (Figure 5).

Figure 5.
Amino acid content in the fruits of the repeat-fruiting R. idaeus cultivars. Notes: Means of each amino acid marked with the same letter do not differ between the cultivars at a significance level of α = 0.05.
3.4. Fatty Acids
In the fruits of the analyzed cultivars, the content of saturated fatty acids (SFAs) in the total fatty acid pool ranged from 40% in ‘Polka’ to 45.8% in ‘Pokusa’. The SFA content in ‘Polana’ was significantly lower than in ‘Pokusa’ and markedly higher than in ‘Polka’. The levels of monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids were in a range from 13.2 in ‘Polana’ to 19.2% in ‘Polka’ and from 20.1 in ‘Polka’ to 43.7% in ‘Polana’, respectively (Figure 6). The MUFA content in ‘Pokusa’ and ‘Polka’ did not differ significantly and was markedly higher than in ‘Polana’. In turn, the PUFA content in ‘Pokusa’ markedly exceeded the value of this parameter determined in ‘Polka’ but was lower than in ‘Polana’.
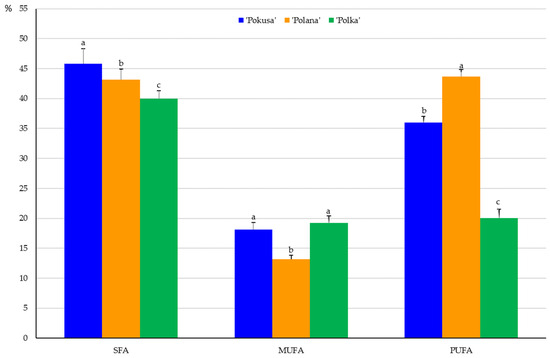
Figure 6.
Percentage of saturated fatty acids (SFAs), monounsaturated acids (MUFAs), and polyunsaturated fatty acids (PUFAs) in the fruits of the repeat-fruiting R. idaeus cultivars. Notes: Means marked with the same letter for SFA, MUFA, and PUFA do not differ between the cultivars at a significance level of α = 0.05.
In the monounsaturated fatty acid group, the sum of the oleic and elaidic acids dominated in the three cultivars and accounted for 16.8% (‘Pokusa’), 11.5% (‘Polana’), and 13.2% (‘Polka’). Palmitic acid in the cultivars ‘Pokusa’ and ‘Polana’ and erucic acid in ‘Polka’ were the second most abundant acids. The most abundant polyunsaturated acids were α-linolenic acid (19.8%) in ‘Pokusa’, linoleic acid (21.5%) in ‘Polana’, and eicosapentaenoic acid (8.3%) in ‘Polka’, as well as linoleic acid (15%) in ‘Pokusa’ and α-linolenic acid in ‘Polana’ (21.4%) and ‘Polka’ (6.2%). In turn, the most abundant saturated fatty acids in the fruits of the three cultivars were palmitic acid ranging from 12.7% in ‘Pokusa’ to 14% ‘Polana’; arachidic acid in ‘Pokusa’ (8.7%) and ‘Polana (8.8%); and tricosanoic acid in ‘Polka’ (5.5%) (Figure 7).
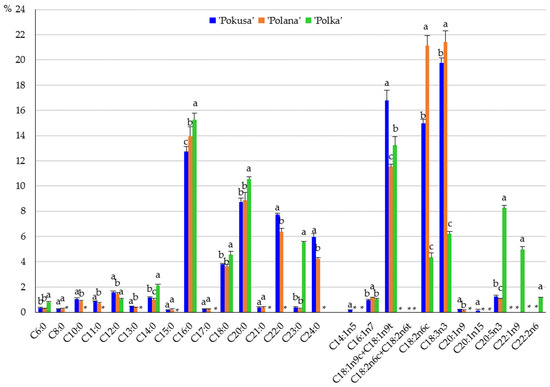
Figure 7.
Content of saturated and unsaturated fatty acids in the fruits of the repeat-fruiting R. idaeus cultivars: ‘Pokusa’, ‘Polana’, ‘Polka’. Notes: C6:0 (caproic acid), C8:0 (caprylic acid), C10:0 (capric acid), C11:0 (undecanoid acid), C12:0 (lauric acid), C13:0 (tridecanoid acid), C14:0 (myristic acid), C15:0 (pentadecanoic acid), C16:0 (palmitic acid), C17:0 (margaric acid), C18:0 (stearic acid), C20:0 (arachidic acid), C21:0 (heneicosanoic acid), C22:0 (docosanoic acid, behenic acid), C23:0 (tricosanoic acid), C24:0 (tetracosanoic acid, lignoceric acid), C14:1n5 (9-tetradecenoic acid, myristoleic acid), C16:1n-7 (palmitoleic acid), C18:1n9c (oleic acid), C18:1n9t (elaidic acid), C18:3n6 gamma (gamma-linoleic acid), C18:3n3 (alpha-linolenic acid), C20:1n9 (11-eicosenoic acid), C20:1n15 (cis-5-eicosenoic acid), C20:5n3 (eicosapentaenoic acid), C22:2n-6 (docosadienoic acid). Means of a single fatty acid included in Figure 6 followed by the same letter do not differ between the cultivars at a significance level of α = 0.05. *—not detected.
Omega-3 acids dominated the fruits of the analyzed cultivars. The total content of these acids ranged from 14.5% in ‘Polka’ to 22.5% in ‘Polana’. This group was represented by linolelaidic, α-linolenic, eicosapentaenoic, and cis-13,16-docosadienoic acids. The omega-6 fatty acid content in the raspberry fruits ranged from 5.6% in ‘Polka’ to 21.2% in ‘Polana’. Linoleic acid was one of the fatty acids from this group. In turn, the omega-9 fatty acid content ranged from 12% in ‘Polana’ to 18.2% in ‘Polka’. This group of acids was represented by erucic, oleic, and elaidic acids. The raspberry fruits also contained palmitoleic acid from the omega-7 group (Figure 7 and Figure 8). The omega-3 and omega-6 fatty acid content in the ‘Pokusa’ fruits was substantially higher than in ‘Polka’ but lower than in ‘Polana’. In turn, the level of omega-9 fatty acid in ‘Pokusa’ was markedly higher than in ‘Polana’ and lower than in ‘Polka’ (Figure 8).
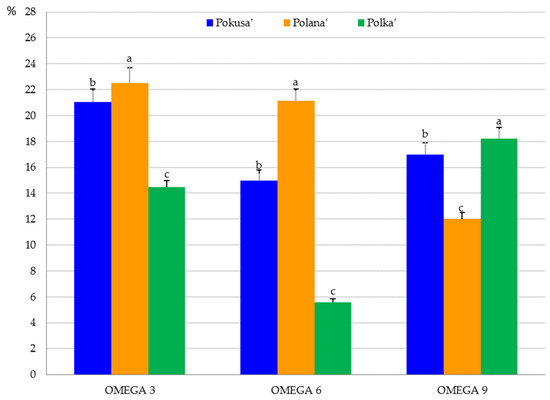
Figure 8.
Omega-3, omega-6, and omega-9 fatty acid content in the fruits of the repeat-fruiting R. idaeus cultivars. Notes: Means of each omega fatty acid group (omega-3, omega-6, and omega-9) marked with the same letter do not differ between the cultivars at a significance level of α = 0.05.
3.5. Antioxidant Activity
3.5.1. Fruits
Using the FRAP method, the highest value of the iron ion reduction capacity of the fresh fruits was exhibited by ‘Polana’ (20.27 μmol/g), followed by ‘Pokusa’ (12.58 μmol/g) and ‘Polka’ (12.79 μmol/g), and the values of this parameter in ‘Polana’ were significantly higher than in the other two cultivars. The results of the DPPH radical reduction assay calculated into Trolox equivalents revealed that the fruits of the three raspberry cultivars had comparable antioxidant activity. The shortest time required for 50% DPPH radical reduction (TEC50 [s]) and indicating the highest antioxidant activity was the same in all three cultivars, i.e., 600 s.
Another parameter of the antioxidant capacity of the fresh fruits was the antioxidant efficiency (AE) factor. This coefficient ranged between 0.0014 (‘Pokusa’) and 0.0019 dm3/µmol × s (‘Polka’). The value of this parameter in ‘Polana’ was significantly higher than in ‘Pokusa’ but lower than in ‘Polka’ (Table 2, Figure 9).

Table 2.
Antioxidant activity of fresh fruits of the repeat-fruiting Rubus idaeus cultivars.
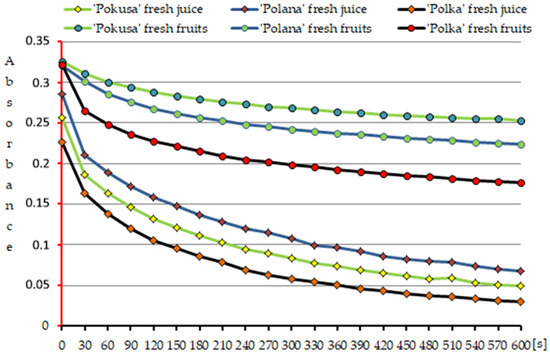
Figure 9.
Kinetics of the color reaction of DPPH• radical neutralization by polyphenols contained in the fresh fruits and in the fresh juice from the fruits of the repeat-fruiting Rubus idaeus cultivars.
Additionally, since the fruits of the analyzed cultivars contained a mixture of different concentrations of various polyphenols and their derivatives, the kinetics of the neutralization of the DPPH• radical by these bioactive chemical compounds was determined (Figure 9).
3.5.2. Fresh Juice
As shown by the FRAP analysis of the antioxidant activity of the chemical compounds contained in the fresh juice from the fruits of the analyzed cultivars, the highest iron (II)-to-iron (III) reducing power was exhibited by ‘Pokusa’, i.e., 12317 μmol/100 mL. The juice from the cultivar ‘Polana’ reduced iron ions at a level of 12276 μmol/100 mL, and the lowest antioxidant activity was exhibited by ‘Polka’ (11138 μmol/100 mL).
As determined using the DPPH method, ‘Polana’ (the cultivar with the lowest antioxidant activity) required the longest time for a 50% reduction in the DPPH radical, i.e., 291 s. ‘Polka’ exhibited the greatest antioxidant capacity, with an average reduction time of 141 s, which indicated the highest antioxidant activity. The AE coefficient reflecting the antioxidant efficacy had the highest and lowest values in ‘Polka’ (0.008071 dm3/µmol × s) and ‘Polana’ (0.003479 dm3/µmol × s), respectively (Table 3, Figure 9).

Table 3.
Antioxidant activity of the fresh juice of the repeat-fruiting Rubus idaeus cultivars.
4. Discussion
4.1. Phenolic Compounds
The Folin–Ciocalteu method revealed that the phenolic compound contents in the fresh raspberry fruits and juice were 1.65–1.71 mg/g and 223.7–260.1 mg/100 mL, respectively, expressed as caffeic acid equivalents. Cervantes et al. [51] reported that fresh raspberry fruit contained 140 mg gallic acid equivalents/100 g f.w. The anti-inflammatory activity of these compounds is related to their ability to interfere with oxidative stress signaling and the inhibition of pro-inflammatory signal transduction [2,52]. Viuda-Martos et al. [53] showed that the bioavailability of phenolic compounds, including phenolic acids and flavonoids, is determined by, e.g., the type of dietary fiber.
4.2. Fiber
The total fiber content in the R. idaeus fruits (4.7–5.8 g/100 g f.w.) was similar to the value of this parameter in common raspberry fruits reported by Baenas et al. [54] (6.1 g/100 g f.w.) and Noratto et al. [55] (6.5%). Raspberry fruit fiber consists mainly of insoluble compounds, is rich in phenolic compounds and antioxidants, and has antioxidant properties. It is characterized by good swelling properties, water and fat retention, glucose diffusion, and prebiotic activity associated with polyphenols. Fiber fractions from raspberry fruits can serve as the functional and prebiotic ingredients of food products [54]. They also adsorb cholesterol and glucose; hence, they can be a component of supplements used for the treatment of obesity [56]. Dietary fiber exerts a negative effect on the bioavailability of polyphenols, and pectins substantially reduce the total content of bioavailable phenolic compounds and monomeric anthocyanins. The interactions between phenols and pectins should be considered in designing diets, as the inclusion of pectins in red raspberry purée has been found to reduce the number of bioavailable polyphenols [57].
4.3. Anthocyanins
The anthocyanin content in the fruits analyzed in the present study was in a range of 25.5–30.7 mg/100 g. These values were higher than the concentration of anthocyanins in R. idaeus ‘Polana’ (19.3 mg/100 g f.w.) reported in the literature [58] and lower than in the common raspberry cultivars ‘Pokusa’, ‘Polana’, and ‘Polka’ (75.5–83.5 mg/100 g) [19]. Anthocyanins have therapeutic properties. These compounds contained in wild blackberry extracts are used as part of therapies improving the survival of glioblastoma patients [59]. Anthocyanins in R. coreanus fruits, i.e., cyanidin 3-O-sambubioside, cyanidin 3-O-glucoside, cyanidin 3-O-xylosylrutinoside, and cyanidin 3-O-rutinoside, are responsible for 47–55% of the total antioxidant capacity of phenolic compounds. These compounds exert a neuroprotective effect on PC-12 neuronal cells exposed to oxidative stress [60]. Additionally, anthocyanin fractions from R. eubatus ‘Hull’ fruits reduce free radicals and oxidative damage in keratinocytes. They also increase the expression of antioxidant enzymes, thereby protecting keratinocytes from UV-mediated oxidative damage [61]. The prophylactic anticancer dose of anthocyanins is approx. 2400 mg/kg per day [62]. In red, yellow, black, hybrid, and BR (blackberry) fruits of many varieties from different Rubus species, the color is determined by the composition of flavonoids, including anthocyanins. Differences in the concentration of cyanidin-3-sambubioside in the anthocyanin pathway have been found between these fruits [63].
4.4. Flavonoids
In the present study, the flavonoid content was 1.9 mg RUE/g−1 in ‘Pokusa’ and 2.6 mg RUE/g−1 in ‘Polka’. The values of this parameter were similar to those in the cultivars ‘Tulameen’ (1.98 mg RUE/g−1) and ‘Polka’ (2.16 mg RUE/g−1) [64] and higher than their content in R. idaeus (4.4–5.0 mg RUE/g 1) [65,66]. Flavonoids isolated from R. fairholmianus fruits have analgesic properties and can be used in the phytotherapy of free-radical-induced diseases [67]. The analgesic properties of phenolic-rich fractions from R. occidentalis are associated with antinociceptive effects on postoperative pain and their ability to bind to α2-adrenergic, nicotinic, cholinergic, and opioid receptors [68]. R. idaeus extract, which is rich in phenolic compounds, e.g., flavonoids, relieves pain in knee osteoarthritis patients; hence, it may be a component of non-steroidal anti-inflammatory drugs [69]. He et al. [20] found that triterpenoids, flavonoids, alkaloids, and phenolic acids present in R. chingii are involved in antioxidant, anti-inflammatory, and anticancer effects. The anticancer activity of flavonoids consists of regulating enzymes involved in scavenging ROS, cell cycle arrest, the induction of apoptosis, and the inhibition of tumor cell proliferation and invasion. Flavonoids act as antioxidants in cancer cells, participate in apoptotic pathways, and downregulate pro-inflammatory signaling pathways [70]. Genes involved in flavonoid biosynthesis and genes associated with plant hormone signal transduction are two key elements of the adaptation of Rubus corchorifolius to the environment. Flavonoids protect plants against ultraviolet-light- and high-temperature-induced stresses [71]. Using the favorable R. coreanus traits for selection and breeding programs and the R. occidentalis genome for reference-guided assembly, Chen et al. [72] found that several biological pathways are disrupted and sixteen pathways are enriched during fruit development and ripening, with the involvement of genes responsible for flavonoid biosynthesis; phenylpropanoid biosynthesis; plant hormone signal transduction; and cutin, suberin, and wax biosynthesis.
4.5. Vitamin C
In the present study, the vitamin C content in the fresh fruits of the analyzed cultivars (mg/100 g), i.e., 22.8 in ‘Pokusa’; 25.13 in ‘Polka’; and 27.0 in ‘Polana, was similar to that in the fruits of 11 preselected wild-grown common raspberry genotypes and the cultivar ‘Heritage’ (21–36 mg/100 g) described in the literature, with the wild raspberry fruit samples having, in general, higher ascorbic acid content than the cultivated fruits [73]. Similar vitamin C content in fresh R. idaeus fruits (22.1–31.2 mg/100 g) was shown in a study conducted by de Ancos et al. [18], where the values of this parameter in the late cultivars ‘Zeva’ and ‘Rubi’ were higher than in the early cultivars ‘Autumn Bliss’ and ‘Heritage’. Kazimierczak et al. [74] reported higher vitamin C content in the cultivars ‘Polka’ (42.2–47.8 mg/100 g) and ‘Polana’ (34.6–43.6 mg/100 g) than the values obtained in the present study. In contrast to ‘Polka’, ‘Polana’ fruits from the conventional cultivation system exhibited a higher value of this parameter than those from the organic plantation. Ponder and Hallmann [75] reported that fruits from four raspberry cultivars of ‘Polka’ collected in autumn had 30.2 mg/100 g of this substance. The fruits of the conventionally grown raspberries contained significantly higher vitamin C content than organic raspberries, i.e., 40.5 and 33.7 mg/100 g, respectively. The ascorbic acid content of ‘Meeker’ raspberries was 20.1 mg/100 g. In ‘Heritage’, ‘Autumn Bliss’, and ‘Fallgold’ twice-bearing fruit, it was in a range of 31.0–32.4, 31.0–37.7, and 16.8–18.5 mg/100 g, and fruits collected in the summer contained higher levels of ascorbic acid than those harvested in autumn. The ascorbic acid content in red-black Rubus fructicosus fruits was between 14.3 and 17.5 mg/100 g. In turn, the red-colored raspberry × blackberry hybrid ‘Tayberry Sunberry’ and the black-colored ‘Silvan’ contained 19.7, 28.0, and 18.4 mg/100 g of ascorbic acid [76]. Akimov et al. [77] reported that the vitamin C content in 18 promising raspberry varieties was in the range of 13.6–31.1 mg/100 g. Surya et al. [78] found that ripe R. fraxinifolius fruits had high vitamin C content (82.97 mg/100 g), whereas a lower amount of this substance was found in R. rosifolius (54.30 mg/100 g).
Vitamin C plays a role in the prophylaxis of cardiovascular diseases, dilates and seals blood vessels, and regulates blood pressure and cholesterol levels, thereby reducing the risk of atherosclerosis and coronary heart disease. It supports the immune system through the stimulation of cell activity. In physiological conditions, ascorbic acid increases the absorption of calcium, iron, and copper and helps to alleviate allergic reactions. It serves as a co-factor and a proton donor in the activation of fundamental enzymes in the biosynthesis of collagen, steroid hormones, and L-carnitine and is involved in the amidation of some peptide hormones and the formation and metabolism of amino acids or hydroxyphenyl alanine [79].
Vitamin C is a powerful antioxidant involved in the first line of defense offering protection for lipid membranes and proteins. It inhibits the oxidation of carbohydrates and nucleic acids and is an excellent source of high-energy electrons for free radicals [80].
Vitamin C is an electron donor for ascorbate peroxidase involved in the glutathione-ascorbate cycle, which operates in the cytosol, mitochondria, chloroplast stroma, and peroxisomes. Vitamin C plays a role in plant responses to abiotic stresses, including osmotic stress, drought, chilling, heat, and heavy-metal toxicity. Vitamin C has been found to be responsible for 3% of the total antioxidant power of R. idaeus fruits [81]. It is estimated that the minimum daily requirement of vitamin C in adults is 40–60 mg. Both fresh and processed common raspberry fruits are an excellent source of vitamin C in the diet. The consumption of 100 g of fresh raspberry fruit covers the daily requirement for vitamin C in approximately 50% of female and 30% of male adolescents [79,82].
4.6. Amino Acids
In the fruits of the tested cultivars, exogenous amino acids accounted for 56–62% of the total pool, with a dominance of leucine, phenylalanine, and lysine. Leucine, i.e., the main component of the anabolic response, is a measure of protein quality in the nutrition of athletes. A 2–3 g leucine dose is sufficient to increase muscle protein synthesis [83]. As demonstrated in clinical studies, leucine regulates muscle protein synthesis and has an impact on phosphorylation via the mechanistic target of rapamycin complex 1 and protein–protein interaction, thus facilitating the anabolic process [84]. Additionally, a leucine-restricted diet alleviates obesity-related problems, improves insulin resistance, promotes white adipose tissue browning, upregulates the expression of neurotrophic factors, and inhibits neural inflammation in the brain region associated with memory functions. It also changes the intestinal microflora by reducing the number of inflammation-associated bacteria and increasing the number of short-chain fatty acids associated with the growth of some bacteria; consequently, it may alleviate some obesity-related problems [85].
4.7. Fatty Acids
The fruits of ‘Pokusa’ and ‘Polana’ were dominated by linoleic acid (15–21.5%), linolenic acid (19.8–21.4%), and palmitic acid (12.7–15.3%). These values were higher than in the common raspberry fruits (9.2%) analyzed by Zorzi et al. [86]. In turn, the linolenic and palmitic acid content, i.e., 27.2 and 17.9%, respectively, was lower than the values shown by these authors. Similarly, a study of fruits of 11 raspberry genotypes and one cultivar showed the dominance of linoleic acid (42.2–52.6%), linolenic acid (17.8–24.1%), and palmitic acid (4.9–9.1%) [87]. In the present study, SFAs accounted for 40–45.8% of the total pool of fatty acids contained in the fruits of the three R. idaeus cultivars. These values are similar to those determined by other authors in the fruits of R. idaeus ‘Kweli’ (41.7%) and the common raspberry (46%) [86,88]. The monounsaturated fatty acid content shown in the present study (13–19%) was similar to that of the fruits of six other R. idaeus cultivars (14.7–18.5%) [89] and lower than in common raspberry fruits (22.7%) [86] and R. idaeus ‘Kweli’ (29.2%) [89]. The polyunsaturated fatty acid content in the fruits of the analyzed cultivars (from 20% in ‘Polka’ to 44% in ‘Polana’) was lower than in the fruits of six other R. idaeus cultivars (62.9–78.7%) [89]. The PUFA content in ‘Pokusa’ was similar to the amount detected in common raspberry and R. idaeus ‘Kweli’ fruits (29.1–31.3%) [86,88]. The PUFA/SFA ratios in ‘Pokusa’, ‘Polana’, and ‘Polka’ calculated in the present study were 0.79, 1.01, and 2.0, respectively. In comparison with the ratio in R. idaeus ‘Kweli’ (0.7) [88], the present values were similar for ‘Pokusa’ but higher for ‘Polana’ and ‘Polka’. The concentrations of ω-3, ω-6, and ω-9 fatty acids in the fruits of ‘Pokusa’, ‘Polana’, and ‘Polka’ were in a range of 14.5–22.5, 5.6–21.2, and 12–18%, respectively. The ω-6/ω-3 ratios in the fruits were 0.7, 0.9, and 0.4, respectively. These results were lower than in R. idaeus ‘Kweli’ fruits (1.4) [88]. PUFA/SFA and ω-6/ω-3 PUFA ratios are important indicators of the pro-health quality and nutritional value of food products. Their values (>0.45 and <4) evidence the high nutritional quality and health-promoting properties of the raspberries described as nutritional products in the present study and in other literature reports [86,88].
4.8. Antioxidant Activity
In the present study, the iron ion reduction capacity (FRAP method) of the fresh fruits and juice ranged from 10.3 in ‘Polana’ to 12.8 μmol/g in ‘Polka’ and from 11138 in ‘Polka’ to 12317 μmol/100 mL in ‘Pokusa’. Marić et al. [5] reported that the antioxidant activity of R. idaeus seed extract (FRAP) was in a range of 72–118 µmol Fe2+/g. Cekiç and Özgen [90] showed a level of iron ion reduction of 11.2–19.8 µmol Fe2+/g in their study of wild and cultivated R. idaeus fruits. The kinetics of DPPH radical reduction via the antioxidant compounds contained in R. idaeus fresh fruits and juice was determined using three parameters; the first one showed the percentages of remaining unreduced radicals (DPPH rem %), which were estimated at 83.2–86.6 and 142–292, respectively. The second parameter indicated the time (s) required to reduce the initial radical concentration by 50% (TEC50) (600 s—fruits; 142–292 s—juice). The third parameter, i.e., the antioxidant efficacy (AE), was 0.0014–0.0019 dm3/µmol × s and 0.004–0.008 dm3/µmol × s, respectively. Gramza-Michałowska et al. [91] determined the same parameters in five berry fruit species, Fragaria ananasa, Punica granatum, Rubus fruticosus, Rubus ideaus, and Vaccinium macrocarpon, and found that the common raspberry and cranberry exhibited moderate antioxidant activity. Gülçin et al. [92] performed DPPH, ABTS+, DMPD+, and O2− assays and found that lyophilized aqueous fruit extracts from domesticated and wild ecotypes of raspberries were effective antioxidants and free radical scavengers. This indicated that the phenolic-rich extract was a readily available source of natural antioxidants. R. chingii raspberry fruit extract inhibits the expression of TGF-β1 antibodies and reduces the expression of p-Smad2/3 signaling molecules and Smad4 protein. Concurrently, it attenuates TGF-β1 signal transduction and reduces the activation and proliferation of hepatic stellate cells (HSCs), thereby alleviating liver fibrosis in mice [93]. Martins et al. [94] investigated the health-promoting properties of Rubus fruticosus and R. ulmifolius and found that reactive species are products of normal cellular metabolism. Biologically active compounds, mainly phenolic compounds, are involved in signal transduction, growth regulation, gene expression, and immune response pathways. Exogenous antioxidants, e.g., flavonoids, stilbenes, and tannins, scavenge and detoxify radical oxygen species and prevent their generation through the modulation of signal transduction and their impact on metabolic processes, cell cycle, and apoptosis.
4.9. Applications
With their low calorific value and total sugar content, favorable profiles of amino acids and fatty acids, high concentrations of total fiber and antioxidants (polyphenols, flavonoids, anthocyanins, and vitamins), and free radical scavenging potential, the fruits of the R. idaeus ‘Pokusa’, ‘Polana’, and ‘Polka’ cultivars can be used in many industries. Given their antioxidant potential, PUFA indices, and ω-3 and ω-6 content, the analyzed Rubi fructus can serve as functional food products, superfoods, and nutraceuticals, as their polyphenolic compounds, flavonoids, anthocyanins, and vitamins contribute to free-radical scavenging and the inhibition of oxidative stress. Hence, the consumption of raspberry fruits should be recommended and encouraged. This suggests that researchers, dieticians, and pharmaceutical and cosmetic industries should develop strategies to use this raw material in the prophylaxis and adjuvant therapies of many diseases, as raspberry fruits have an appropriate nutritional and chemical profile and antioxidant compounds mitigating the harmful effects of oxidative stress.
4.10. Future Research
There is a need for further studies of many other biennial fruiting and repeat-fruiting cultivars commonly grown in commercial production, as well as wild genotypes, in order to analyze the pro-health metabolites involved in the total antioxidant capacity of this raw material and to elucidate the mechanisms of metabolic pathways and relevant interrelations between certain bioactive chemical compounds. An important role in metabolism is played by appropriate amino acid profiles regulating the nutritional value of Rubi idaei fructus. The present results can serve as a theoretical basis for the improvement of the quality of Rubus fruits and their more frequent use in the food, pharmaceutical, and cosmetic industries. Future research should be focused on a detailed analysis of natural anthocyanin pigments, which can be used as one of the main natural coloring ingredients, and on the safety of their use in food, pharmaceutical, and biocosmetological products. Another issue to be addressed in future studies is the utilization of fruit processing waste material in accordance with sustainable production and consumption principles, e.g., oil extraction from raspberry seeds. The knowledge of primary and secondary metabolites can help to determine fruit quality and prompt the choice of appropriate raspberry cultivars with high nutritional value enhanced by the flavonoid, polyphenolic compound, anthocyanin, and vitamin content. A precisely determined metabolome may provide additional information about similarities and differences in the chemical profile of primary and secondary metabolites between cultivars. It will also help to assess fruit quality and apply genetic engineering for the development of new raspberry cultivars with the best genomes to encode desired traits. Additionally, an important aspect is the use of wild raspberry genotypes, which are an important source of health-promoting metabolites and have high nutritional metabolite contents, to develop new varieties with a high concentration of phytochemicals responsible for the antioxidant capacity of raspberry fruits. Clinical trials should be conducted to determine the safety of use, tolerance, and intake doses in agreement with the prophylaxis and therapy requirements of specific diseases. Future studies are also expected to provide important data on the mechanisms of the metabolic pathways of free-radical scavenging and oxidative stress inhibition via bioactive antioxidants. Another area of research may be focused on the assessment of the expression of genes regulating enzyme activity, anthocyanin biosynthesis, and pigment metabolism with the use of structural genes and regulatory transcription factors.
5. Conclusions
Given their low calorific value, available carbohydrate and total sugar content, and glycemic index, as well as high dietary fiber, vitamin C, anthocyanin, nutritional unsaturated fatty acid, and important exogenous amino acid content, fruits of repeat-fruiting R. idaeus cultivars can be a valuable additive to the human diet and a component of superfoods recommended for pro-health and slimming diets. In the fruits of the examined cultivars, exogenous amino acids account for 56–62%, with a dominance of leucine, phenylalanine, and lysine. The endogenous amino acid group was dominated by glutamic acid, aspartic acid, and alanine. Unsaturated fatty acids represented 35–60%, and the MUFA group was dominated by oleic, elaidic, palmitic, and erucic acids. The acids of this group are known to prevent oxidative modifications of lipoproteins. In turn, α-linolenic, eicosapentaenoic, and linoleic acids were the most abundant PUFAs, and palmitic, arachidic, and tricosanoic acids dominated in the SFA group. MUFAs from the ω-9 group accounted for 12–18%, and the levels of ω-3 and ω-6 PUFAs were estimated at 15–23 and 6–21%, respectively. It is recommended that the daily dietary intake of these compounds should be below 10% of total energy. These acids supplement nutrients and regulate cholesterol and glucose metabolism. The content of polyphenolic compounds in the analyzed fruits was similar. The following order of the total polyphenolic content was established in the fresh fruit juice from the analyzed cultivars: ‘Pokusa’ < ‘Polana’ < ‘Polka’. The different antioxidant capacity assays used in the study confirmed the high antioxidant power of the fruits and fresh juice from R. idaeus ‘Pokusa’, ‘Polana’, and ‘Polka’ and indicated the potential use of Rubi fructus as a ‘fruit life’ ingredient of healthy food, supplements, and pharmaceutical and cosmetic products. The antioxidant activity of many raw materials of various species is associated with the presence of, e.g., phenolic compounds, vitamins, anthocyanins, and carotenoids and depends on the cultivar, species, and soil and environmental factors.
Author Contributions
Conceptualization, M.C. and R.M.-G.; experimental design, M.C and R.M.-G.; methodology, M.C. and R.M.-G.; software and statistical analysis, M.C. and M.K.; validation, M.C.; formal analysis, M.C. and R.M.-G.; investigation, M.C., R.M.-G. and M.K.; resources, M.C; data curation, M.C.; writing—original draft preparation, M.C., R.M.-G. and M.K.; writing—review and editing, M.C. and R.M.-G.; visualization, M.C., R.M.-G. and M.K.; supervision, M.C.; graphic design, M.C., R.M.-G. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Mannino, G.; Serio, G.; Gaglio, R.; Busetta, G.; La Rosa, L.; Lauria, A.; Settanni, L.; Gentile, C. Phytochemical profile and antioxidant, antiproliferative, and antimicrobial properties of Rubus idaeus seed powder. Foods 2022, 11, 2605. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin accumulation in berry fruits and their antimicrobial and antiviral properties: An overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Marić, B.; Abramović, B.; Ilić, N.; Bodroža-Solarov, M.; Pavlić, B.; Oczkowski, M.; Wilczak, J.; Četojević-Simin, D.; Šarić, L.; Teslić, N. UHPLC-Triple-TOF-MS Characterization, antioxidant, antimicrobial and antiproliferative activity of raspberry (Rubus idaeus L.) seed extracts. Foods 2023, 12, 161. [Google Scholar] [CrossRef]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals determination and antioxidant, antimicrobial, anti-inflammatory and anticancer activities of blackberry fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef]
- Sławińska, N.; Prochoń, K.; Olas, B. A review on berry seeds—A special Emphasis on their chemical content and health-promoting properties. Nutrients 2023, 15, 1422. [Google Scholar] [CrossRef]
- Kim, K.J.; Jeong, E.S.; Lee, K.H.; Na, J.R.; Park, S.; Kim, J.S.; Na, C.S.; Kim, Y.R.; Kim, S. Unripe Rubus coreanus Miquel extract containing ellagic acid promotes lipolysis and thermogenesis in vitro and in vivo. Molecules 2020, 25, 5954. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Naeem, A.; Zou, J.; Yu, C.; Wang, Y.; Chen, J.; Ping, Y. Isolation of phenolic compounds from raspberry based on molecular imprinting techniques and investigation of their anti-Alzheimer’s disease properties. Molecules 2022, 27, 6893. [Google Scholar] [CrossRef] [PubMed]
- Gu, I.; Brownmiller, C.; Howard, L.; Lee, S.O. Chemical composition of volatile extracts from black raspberries, blueberries, and blackberries and their antiproliferative effect on A549 non-small-cell lung cancer cells. Life 2022, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Salim, G.M.; El-Dakdouki, M.H.; Abdallah, H.; Nasser, H.M.; Arnold-Apostolides, N. Antioxidative and hepatoprotective effects of Rubus canescens DC. growing wild in Lebanon. J. Nat. Prod. 2021, 11, 44–56. [Google Scholar] [CrossRef]
- Rambaran, T.F.; Nembhard, N.; Bowen-Forbes, C.S.; Alexander-Lindo, R.L. Hypoglycemic effect of the fruit extracts of two varieties of Rubus rosifolius. J. Food Biochem. 2020, 44, 13365. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Leporini, M.; D’Urso, G.; Candela, R.G.; Falco, T.; Piacente, S.; Bruno, M.; Sottile, F. Almond (Prunus dulcis cv. Casteltermini) skin confectionery by-products: New opportunity for the development of a functional blackberry (Rubus ulmifolius Schott) Jam. Antioxidants 2021, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Yu, J.; Du, J.; Wu, X.; Chen, L.; Wang, R.; Wu, Y.; Li, Y. Constituents of the fruits of Rubus chingii Hu and their neuroprotective effects on human neuroblastoma SH-SY5Y cells. Food Res. Int. 2023, 173, 113255. [Google Scholar] [CrossRef] [PubMed]
- Land Lail, H.; Feresin, R.G.; Hicks, D.; Stone, B.; Price, E.; Wanders, D. Berries as a treatment for obesity-induced inflammation: Evidence from preclinical models. Nutrients 2021, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Papaioanou, M.; Chronopoulou, E.G.; Ciobotari, G.; Efrose, R.C.; Sfichi-Duke, L.; Chatzikonstantinou, M.; Pappa, E.; Ganopoulos, I.; Madesis, P.; Nianiou-Obeidat, I. Cosmeceutical properties of two cultivars of red raspberry grown under different conditions. Cosmetics 2018, 5, 20. [Google Scholar] [CrossRef]
- Huo, Y.; Zhao, X.; Zhao, J.; Kong, X.; Li, L.; Yuan, T.; Xu, J. Hypoglycemic effects of Fu-Pen-Zi (Rubus chingii Hu) fruit extracts in streptozotocin-induced type 1 diabetic mice. J. Funct. Foods 2021, 87, 104837. [Google Scholar] [CrossRef]
- De Ancos, M.; González, B.E.; Cano, M.P. Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Dai, L.; Jin, L.; Liu, Y.; Li, X.; Luo, M.; Wang, Z.; Kai, G. Bioactive components, pharmacological effects, and drug development of traditional herbal medicine Rubus chingii Hu (Fu-Pen-Zi). Front. Nutr. 2023, 9, 1052504. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Kim, D.L.; Lee, T.B.; Kim, K.J.; Kim, S.H.; Kim, N.H.; Kim, H.Y.; Choi, D.S.; Kim, S.G. Effect of Rubus occidentalis extract on metabolic parameters in subjects with prediabetes: A proof-of-concept, randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2016, 30, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.M.; Sibianu, M.; Hutanu, A.; Beresescu, F.G.; Sala, D.T.; Flavius, M.; Rosca, A.; Constantin, C.; Scurtu, A.; Moriczi, R.; et al. A comprehensive summary of the current understanding of the relationship between severe obesity, metabolic syndrome, and inflammatory status. J. Clin. Med. 2023, 12, 3818. [Google Scholar] [CrossRef] [PubMed]
- Stepan, M.D.; Vintilescu, Ș.B.; Streață, I.; Podeanu, M.A.; Florescu, D.N. The role of vitamin D in obese children with non-alcoholic fatty liver disease and associated metabolic syndrome. Nutrients 2023, 15, 2113. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Ning, C.; Zhang, M.; Fan, P.; Lei, D.; Du, J.; Gale, M.; Ma, Y.; Yang, Y. Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocr. J. 2019, 66, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.Y.; Kim, H.L.; Park, J.; An, H.J.; Kim, S.H.; Kim, S.J.; So, H.S.; Park, R.; Um, J.Y.; Hong, S.H. Rubi fructus (Rubus coreanus) inhibits differentiation to adipocytesc in 3T3-L1 cells. Evid. Based Complement. Altern. Med. 2013, 2013, 475386. [Google Scholar] [CrossRef]
- Tu, L.; Sun, H.; Tang, M.; Zhao, J.; Zhang, Z.; Sun, X.; He, S. Red raspberry extract (Rubus idaeus L shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem. Toxicol. 2019, 133, 110796. [Google Scholar] [CrossRef]
- Ke, H.; Bao, T.; Chen, W. Polysaccharide from Rubus chingii Hu affords protection against palmitic acid-induced lipotoxicity in human hepatocytes. Int. J. Biol. Macromol. 2019, 133, 1063–1071. [Google Scholar] [CrossRef]
- Harper, G.M. Geriatrics experts explore relationship between heart disease and cancer, the top two leading causes of death. J. Gerontol. Nurs. 2022, 48, 55–56. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ye, B.; Sun, Z.; Mao, Z.; Wang, W. Reactive oxygen species-mediated pyroptosis with the help of nanotechnology: Prospects for cancer therapy. Adv. NanoBiomed Res. 2023, 3, 2200077. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, T.; Ding, J.; Gu, H.; Wang, Q.; Wang, Y.; Zhang, D.; Gao, C. Reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact. Mater. 2023, 19, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Sójka, M.; Klewicka, E.; Lipinska, L.; Klewicki, R.; Kołodziejczyk, K. Ellagitannins from Rubus idaeus L. exert geno- and cytotoxic effects against human colon adenocarcinoma cell line Caco-2. J. Agric. Food Chem. 2017, 65, 2947–2955. [Google Scholar] [CrossRef]
- Assad, N.K.; Dheeba, B.I.; Mohammad, F.I.; Hamad, A.I. Anti-cancer activity of the Rubus idaeus extracts against HepG2 and L20B cell lines using tissue culture technique. Egypt. Acad. J. Biol. Sci. 2015, 7, 19–23. [Google Scholar] [CrossRef]
- Chen, T.; Shi, N.; Afzali, A. Chemopreventive effects of strawberry and black raspberry on colorectal cancer in inflammatory bowel disease. Nutrients 2019, 11, 1261. [Google Scholar] [CrossRef]
- Danek, J. ‘Polka’ and ‘Pokusa’—New primocane fruiting raspberry cultivars from Poland. Acta Hortic. 2002, 585, 197–198. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Ber, C. Kinetics and mechanism of antioxidant activity using the DPPH• free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Dekker, K.D. The Luff-Schoorl method for determination of reducing sugar in juices, molasses and sugar. S. Afr. Sugar J. 1950, 34, 157–171. [Google Scholar]
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljeströem, M. Rapid enzymic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- PN75/A-04018:1975/Az3:2002; Agricultural Food Products: Nitrogen Contents Determination with Kjeldahl’s Method and Recalculation into Protein. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
- Rabie, A.L.; Wells, J.D.; Dent, L.K. The nitrogen content of pollen protein. J. Apic. Res. 1983, 22, 119–123. [Google Scholar] [CrossRef]
- Davies, M.G.; Thomas, A.J. An investigation of hydrolytic techniques for the amino acid analysis of foodstuffs. J. Sci. Food Agric. 1973, 24, 1525–1540. [Google Scholar] [CrossRef]
- PN-EN ISO 5508:1996; Animal and Vegetable Fats and Oils: Analysis by Gas Chromatography of Methyl esters of Fatty Acids. Polish Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- PN-EN ISO 12966-1:2014; Animal and Vegetable Fats and Oils: Gas Chromatography of Fatty Acids Methyl Esters: Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2014.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.E.; Favret, E.A. Anthocyanin synthesis and lengthening in the first leaf of barley isogenic lines. Plant Sci. 1990, 71, 35–43. [Google Scholar] [CrossRef]
- PN-EN 14130:2004; Foodstuffs—Determination of Vitamin C by HPLC. Polish Committee for Standardization: Warsaw, Poland, 2004.
- Lamaison, J.L.C.; Carnet, A. Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D.C) en function de la vegetation. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- European Commission. UE Commission Delegated Regulation No 78/2014. Regulation of the European Parliament and the European Council (EU) no. 1169/2011 from 25 October 2011 with amendments. Regulation of the European Council (EU) no. 1155/2013 from 21 August 2013, Regulation of the European Council (EU) no. 78/2014 from 22 November 2013 (applied from 19 February 2014); European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Cervantes, L.; Martínez-Ferri, E.; Soria, C.; Ariza, M.T. Bioavailability of phenolic compounds in strawberry, raspberry and blueberry: Insights for breeding programs. Food Biosci. 2020, 37, 100680. [Google Scholar] [CrossRef]
- Dobani, S.; Latimer, C.; McDougall, G.J.; Allwood, J.W.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Ternan, N.G.; Pourshahidi, L.K.; Lawther, R.; Tuohy, K.M.; et al. Ex vivo fecal fermentation of human ileal fluid collected after raspberry consumption modifies (poly) phenolics and modulates genoprotective effects in colonic epithelial cells. Redox Biol. 2021, 40, 101862. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Álvarez, J.A.; Muñoz, L.A.; Fernández-López, J. Evaluation of protective effect of different dietary fibers on polyphenolic profile stability of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Food Funct. 2018, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry dietary dibre: Chemical properties, functional evaluation and prebiotic in vitro effect. LWT Food Sci. Technol. 2020, 134, 110140. [Google Scholar] [CrossRef]
- Noratto, G.D.; Chew, B.P.; Antieza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Yang, G.; Sun, L.; Song, X.; Chen, Q.; Bao, Y.; Luo, T.; Wang, J. Microstructure, physicochemical properties, and adsorption capacity of deoiled red raspberry pomace and its total dietary fiber. LWT Food Sci. Technol. 2022, 153, 112478. [Google Scholar] [CrossRef]
- Tomas, M. Effect of dietary fiber addition on the content and in vitro bioaccessibility of antioxidants in red raspberry puree. Food Chem. 2022, 375, 131897. [Google Scholar] [CrossRef]
- Ivanović, M.; Pavlović, A.; Mitić, M.; Pecev-Marinković, E.; Krstić, J.; Mrmošanin, J. Determination of total and individual anthocyanins in raspberries grown in South Serbia. In Proceedings of the XXI 21: Savetovanje o Biotehnologiji sa Međunarodnim Učešćem, Čačak, Serbia, 11–12 March 2016; Volume 21, pp. 263–267. [Google Scholar]
- Sánchez-Velaázquez, O.A.; Cortes-Rodriguez, M.; Milán-Carrillo, J.; Montes-Ávila, J.; Robles-Bañuelos, B.; del Ángel, A.S.; Cuevas-Rodríguez, E.O.; Rangel-López, E. Anti-oxidant and anti-proliferative effect of anthocyanin enriched fractions from two Mexican wild blackberries (Rubus spp.) on HepG2 and glioma cell lines. J. Berry Res. 2020, 10, 513–529. [Google Scholar] [CrossRef]
- Im, S.E.; Nam, T.G.; Lee, H.; Han, M.W.; Heo, H.J.; Koo, S.I.; Lee, C.Y.; Kim, D.O. Anthocyanins in the ripe fruits of Rubus coreanus Miquel and their protective effect on neuronal PC-12 cells. Food Chem. 2013, 139, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Murapa, P.; Dai, J.; Chung, M.; Mumper, R.J.; D’Orazio, J. Anthocyanin-rich fractions of blackberry extracts reduce UV-induced free radicals and oxidative damage in keratinocytes. Phytother. Res. 2012, 26, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Grabek-Lejko, D.; Miłek, M.; Sidor, E.; Puchalski, C.; Dżugan, M. Antiviral and antibacterial effect of honey enriched with Rubus spp. as a functional food with enhanced antioxidant properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, J.; Li, W.; Wu, W. The color difference of Rubus fruits is closely related to the composition of flavonoids including anthocyanins. LWT Food Sci. Technol. 2021, 149, 111825. [Google Scholar] [CrossRef]
- Zejak, D.; Glisic, I.; Spalevic, V.; Maskovic, P.; Dudic, B. Sustainable management of fruit growing in rural areas of Montenegro: The impact of location on the phonological and nutritional properties on raspberry (Rubus idaeus L.). Agronomy 2021, 11, 1663. [Google Scholar] [CrossRef]
- Veljković, B.; Djordjević, N.; Dolićanin, Z.; Ličina, B.; Topuzović, M.; Stanković, M.; Zlatić, N.; Dajić-Stevanović, Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 359–367. [Google Scholar] [CrossRef]
- Veljković, B.; Jakovljević, V.; Stanković, M.; Dajić-Stevanović, Z. Phytochemical and antioxidant properties of fresh fruits and some traditional products of wild grown raspberry (Rubus idaeus L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 565–573. [Google Scholar] [CrossRef]
- George, B.P.; Parimelazhagan, T.; Chandran, R. Evaluation of total phenolic content, antioxidant and analgesic potential of Rubus fairholmianus gard. Int. J. Pharm. Pharm. Sci. 2013, 5 (Suppl. 3), 484–488. [Google Scholar]
- Choi, G.J.; Kang, H.; Lee, O.H.; Kwon, J.W. Effect of immature Rubus occidentalis on postoperative pain in a rat model. Medicina 2023, 59, 264. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Cozannet, R.L.; Fança-Berthon, P.; Truillet, R.; Cohen-Solhal, M.; DunnGalvin, G.; Grouin, J.M.; Doolan, A. Rubus idaeus extract improves symptoms in knee osteoarthritis patients: Results from a phase II double-blind randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 650. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Xiao, Y.; Zhang, L.; Huang, Y.; Li, X.; Chen, S.; Peng, Y.; Yang, S.; Liu, Y.; et al. Genome assembly and population resequencing reveal the geographical divergence of shanmei (Rubus corchorifolius). Genom. Proteom. Bioinform. 2022, 20, 1106–1118. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Hu, Y.; Sun, B.; Hu, Y.; Wang, X.; Tang, H.; Wang, Y. Transcriptomic profiling of fruit development in black raspberry Rubus coreanus. Int. J. Genom. 2018, 2018, 8084032. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Karlidag, H.; Sengul, M. Characterization of red raspberry (Rubus idaeus L.) genotype for their physiochemical properties. J. Food Sci. 2009, 74, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, R.; Hallmann, E.; Kowalska, K.; Rembiałkowska, E. Biocompounds content in organic and conventional raspberry fruits. Acta Fytotech. Zootech. 2016, 18, 40–42. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E. The nutritional value and vitamin C content of different raspberry cultivars from organic and conventional production. J. Food Compos. Anal. 2020, 87, 103429. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Akimov, M.Y.; Koltsov, V.A.; Zhbanova, E.V.; Akimova, O.M. Nutritional value of promising raspberry varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022078. [Google Scholar] [CrossRef]
- Surya, M.I.; Suhartati, S.; Ismaini, L.; Lusini, Y.; Anggraeni, D.; Normasiwi, S.; Asni, N.; Bakar Sidiq, M.A. Fruit nutrients of five species of wild raspberries (Rubus spp.) from Indonesian mountain’s forests. J. Trop. Life Sci. 2018, 8, 75–80. [Google Scholar] [CrossRef]
- Shad, Z.; Arsalan, A.; Bano, R.; Khan, M.F.; Ahmed, I. Physicochemical, biochemical and antioxidant properties of ascorbic acid. J. Baqai Med. Univ. 2011, 14, 33–40. [Google Scholar]
- Pehlivan, F.E. Vitamin C: An antioxidant agent. In Vitamin C; Hamza, A.H., Ed.; IntechOpen: London, UK, 2017; Chapter 2; pp. 23–35. [Google Scholar]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticancerogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Zhang, M.; Jativa, D.F. Vitamin C supplementation in the critically ill: A systematic review and meta-analysis. SAGE Open Med. 2018, 6, 2050312118807615. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Nishisaka, M.M.; McGrath, A.F.; Kristo, A.S.; Sikalidis, A.K.; Reaves, S.K. Protein intake in NCAA Division 1 soccer players: Assessment of daily amounts, distribution patterns, and leucine levels as a quality indicator. Sports 2023, 11, 45. [Google Scholar] [CrossRef]
- Holowaty, M.N.; Lees, M.J.; Abou Sawan, S.; Paulussen, K.J.M.; Jäger, R.; Purpura, M.; Paluska, S.A.; Burd, N.A.; Hodson, N.; Moore, D.R. Leucine ingestion promotes mTOR translocation to the periphery and enhances total and peripheral RPS6 phosphorylation in human skeletal muscle. Amino Acids 2023, 55, 253–261. [Google Scholar] [CrossRef]
- Wang, B.; Du, M. Increasing adipocyte number and reducing adipocyte size: The role of retinoids in adipose tissue development and metabolism. Crit. Rev. Food Sci. Nutr. 2023, 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive compounds and antioxidant capacity of small berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef]
- Celik, F.; Ercisli, S. Lipid and fatty acid composition of wild and cultivated red raspberry fruits (Rubus idaeus L.). J. Med. Plant Res. 2009, 3, 583–585. Available online: http://www.academicjournals.org/JMPR (accessed on 11 June 2023).
- Vara, A.L.; Pinela, J.; Dias, M.I.; Petrović, J.; Nogueira, A.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Compositional features of the “Kweli” red raspberry and its antioxidant and antimicrobial activities. Foods 2020, 9, 1522. [Google Scholar] [CrossRef]
- Kafkas, E.; Özgen, M.; Özoğul, Y.; Türemiş, N. Phytochemical and fatty scid profile of selected red raspberry cultivars: A comparative study. J. Food Qual. 2008, 31, 67–78. [Google Scholar] [CrossRef]
- Cekiç, C.; Özgen, M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compos. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Bueschke, M.; Kulczyński, B.; Gliszczyńska-Świgło, A.; Kmiecik, D.; Bilska, A.; Purłan, M.; Wałęsa, L.; Ostrowski, M.; Filipczuk, M.; et al. Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas. Charact. 2019, 13, 1739–1747. [Google Scholar] [CrossRef]
- Gülçin, I.; Topal, F.; Çakmakçı, R.; Bilsel, M.; Gören, A.C.; Erdogan, U. Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, D.; Zhu, B.; Wang, S.; Xu, Y.; Zhang, C.; Yang, H.; Wang, S.; Liu, P.; Qin, L.; et al. Rubus chingii Hu. unripe fruits extract ameliorates carbon tetrachloride-induced liver fibrosis and improves the associated gut microbiota imbalance. Chin. Med. 2022, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.S.; Gonçalves, A.C.; Alves, G.; Silva, L.R. Blackberries and mulberries: Berries with significant health-promoting properties. Int. J. Mol. Sci. 2023, 24, 12024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).