Circulating Exosomal CircRNAs as Diagnostic Biomarkers for Chronic Coronary Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Blood Collection, Plasma Separation, and Coronary Artery Angiography
2.3. Exosomal Isolation
2.4. Exosome Identification
2.5. Exosomal RNA Extraction
2.6. RNA Sequencing
2.7. Real Time Quantitative PCR
2.8. Statistical Analysis
3. Results
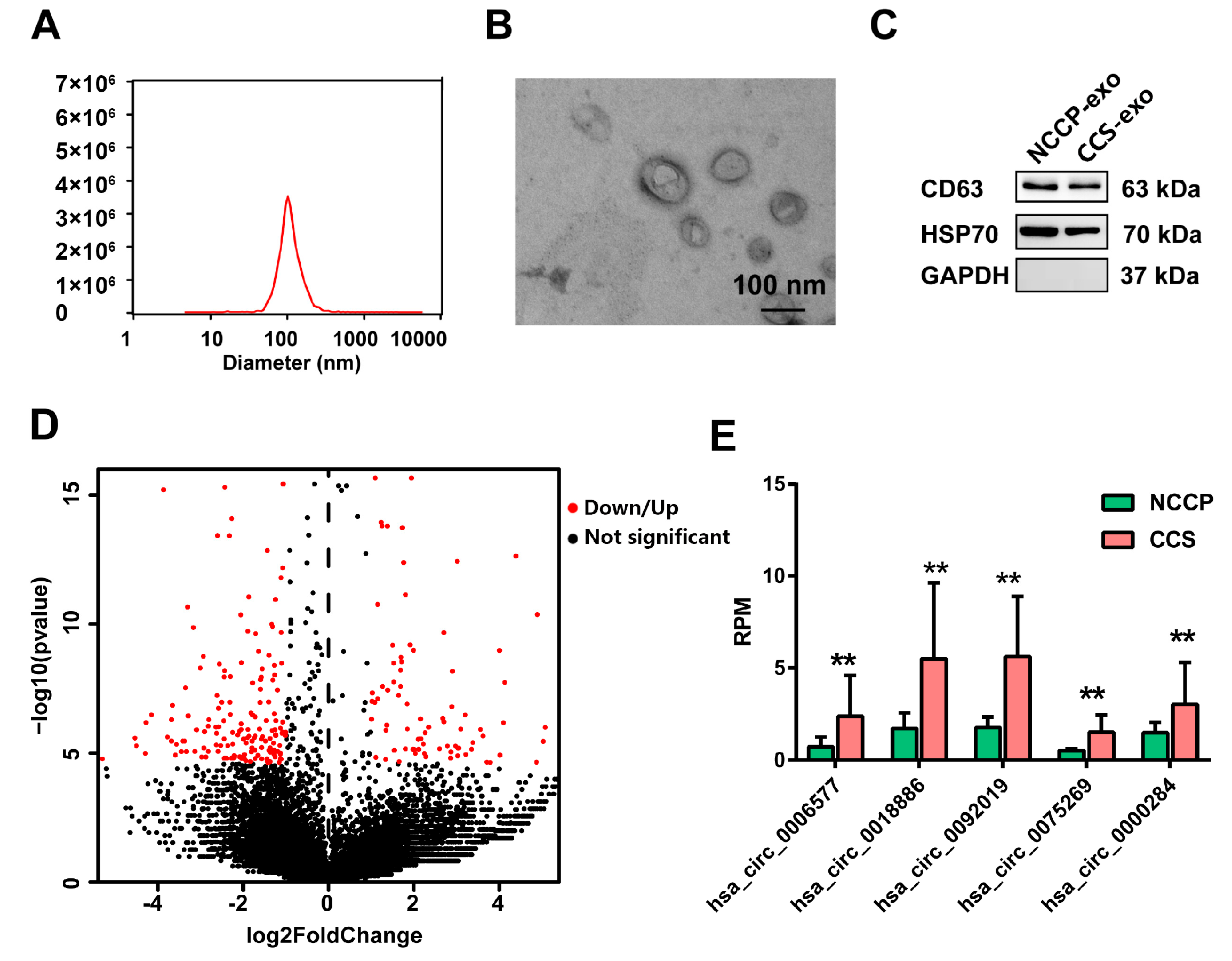
3.1. Identification of Exosomes
3.2. Sequencing Profiles of Circulating Exosomal circRNAs
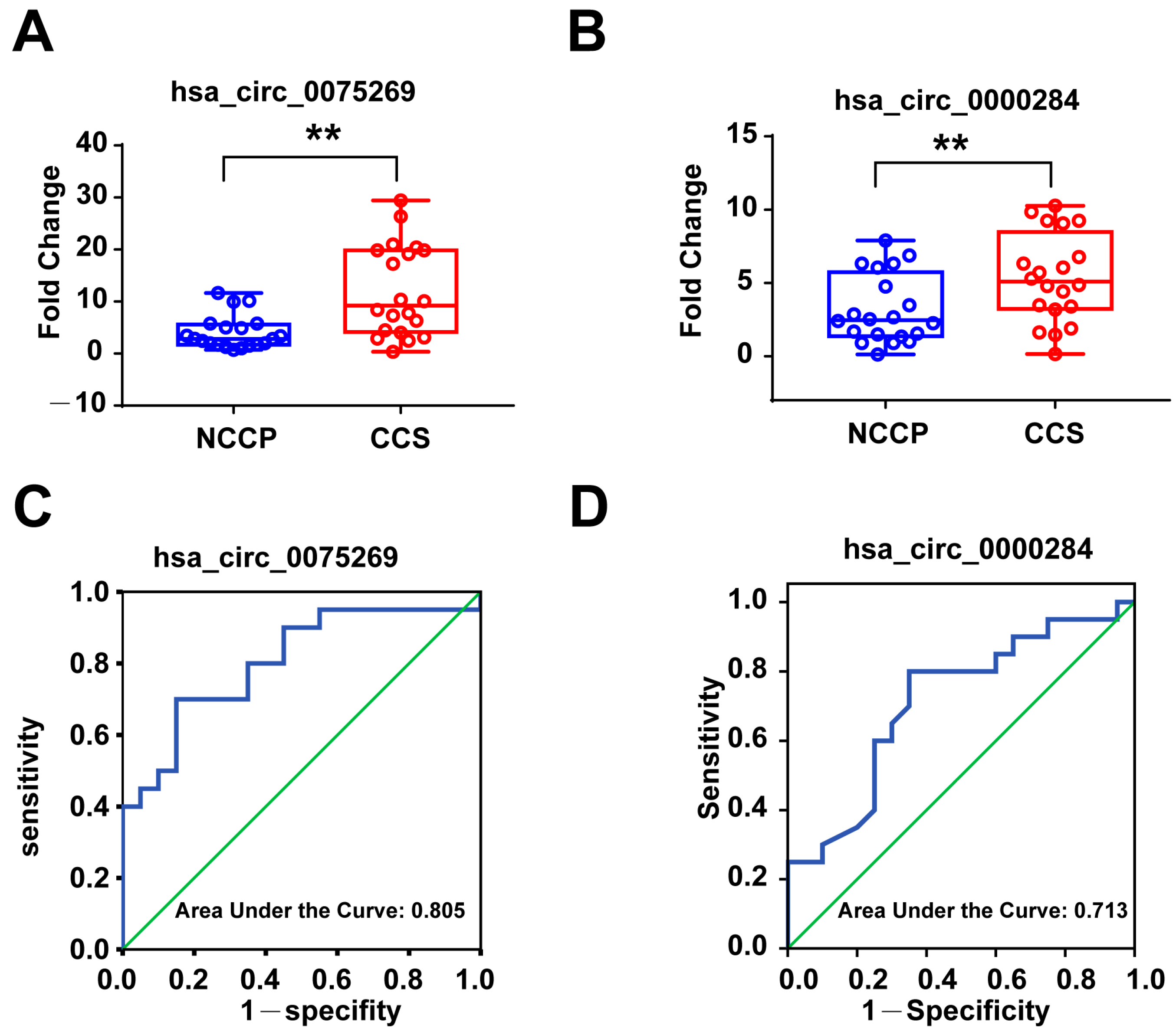
3.3. Validation of Candidate Exosomal circRNAs in the First Validation Cohort
3.4. Diagnostic Power of Plasma Exosomal circRNAs in the First Validation Cohort
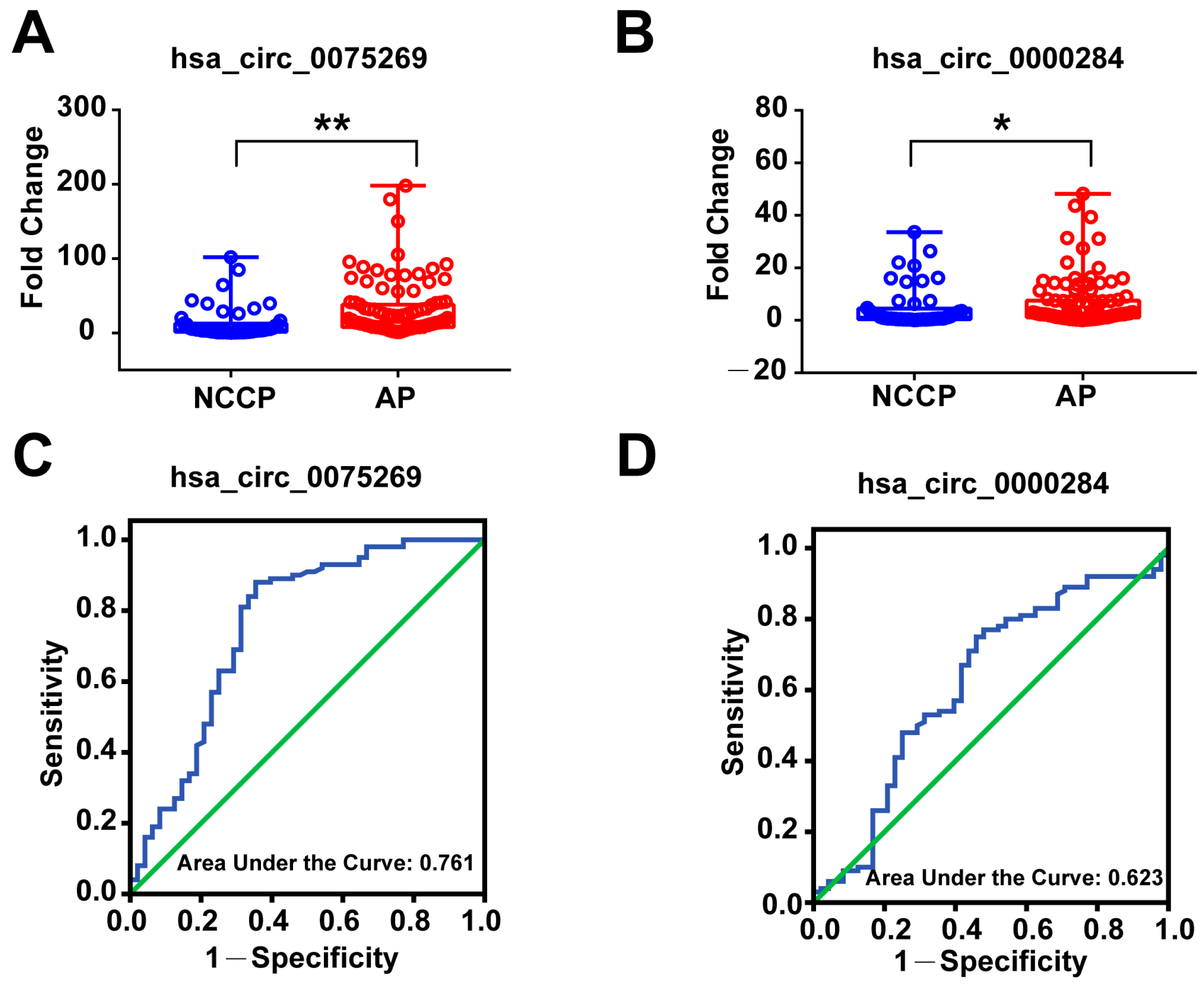
3.5. Validation of Derived Circulating Exosomal circRNAs in the Second Validation Cohort
3.6. Diagnostic Power of Plasma Exosomal circRNAs in the Second Validation Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, L.L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Teupser, D. Recent studies of the human chromosome 9p21 locus, which is associated with atherosclerosis in human populations. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 196–206. [Google Scholar] [CrossRef]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.M.; Meitinger, T.; Engelhardt, S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell. Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Wu, X.; Zhou, X. Regulatory Roles of Circular RNAs in Coronary Artery Disease. Mol. Ther. Nucl. Acids. 2020, 21, 172–179. [Google Scholar] [CrossRef]
- Suzuki, H.; Tsukahara, T. A view of pre-mRNA splicing from RNase R resistant RNAs. Int. J. Mol. Sci. 2014, 15, 9331–9342. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef]
- Seimiya, T.; Otsuka, M.; Iwata, T.; Shibata, C.; Tanaka, E.; Suzuki, T.; Koike, K. Emerging Roles of Exosomal Circular RNAs in Cancer. Front. Cell Dev. Biol. 2020, 8, 568366. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Han, R.; Yuan, W.; Chi, H.; Zhang, Y.; Sun, K.; Zhong, J.; Liu, X.; Yang, X. Circulating exosomal lncRNAs in patients with chronic coronary syndromes. Arch. Med. Sci. 2023, 19, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.J.; Faxon, D.P.; Audet, A.M.; Carabello, B.; Dehmer, G.J.; Eagle, K.A.; Legako, R.D.; Leon, D.F.; Murray, J.A.; Nissen, S.E.; et al. ACC/AHA guidelines for coronary angiography: Executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation 1999, 99, 2345–2357. [Google Scholar] [PubMed]
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, X.; Han, R.; Yuan, W.; Sun, K.; Zhong, J.; Yang, X. Circulating exosomal long non-coding RNAs in patients with acute myocardial infarction. J. Cell. Mol. Med. 2020, 24, 9388–9396. [Google Scholar] [CrossRef]
- Gao, X.F.; Wang, Z.M.; Wang, F.; Gu, Y.; Zhang, J.J.; Chen, S.L. Exosomes in Coronary Artery Disease. Int. J. Biol. Sci. 2019, 15, 2461–2470. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Bi, S.; Wang, C.; Jin, Y.; Lv, Z.; Xing, X.; Lu, Q. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int. J. Clin. Exp. Med. 2015, 8, 4275–4280. [Google Scholar]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Zhang, C.Y.; Zhang, S.; Chang, M.; Wang, H.Y. Microarray Expression Profile of Circular RNAs in Heart Tissue of Mice with Myocardial Infarction-Induced Heart Failure. Cell Physiol. Biochem. 2016, 39, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Siede, D.; Rapti, K.; Gorska, A.A.; Katus, H.A.; Altmuller, J.; Boeckel, J.N.; Meder, B.; Maack, C.; Volkers, M.; Muller, O.J.; et al. Identification of circular RNAs with host gene-independent expression in human model systems for cardiac differentiation and disease. J. Mol. Cell. Cardiol. 2017, 109, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yu, J.W. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem. Biophys. Res. Commun. 2017, 487, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Gao, C.; Jian, D.; Hao, P.; Rao, L.; Li, M. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. SCI. REP-UK. 2017, 7, 39918. [Google Scholar] [CrossRef]
- Shang, X.; Li, G.; Liu, H.; Li, T.; Liu, J.; Zhao, Q.; Wang, C. Comprehensive Circular RNA Profiling Reveals That hsa_circ_0005075, a New Circular RNA Biomarker, Is Involved in Hepatocellular Crcinoma Development. Medicine 2016, 95, e3811. [Google Scholar] [CrossRef]
- Lu, L.; Sun, J.; Shi, P.; Kong, W.; Xu, K.; He, B.; Zhang, S.; Wang, J. Identification of circular RNAs as a promising new class of diagnostic biomarkers for human breast cancer. Oncotarget 2017, 8, 44096–44107. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, X.; Zhou, W.; Kosiba, A.A.; Shi, H.; Gu, J.; Qin, Z. The circular RNA HIPK3 (circHIPK3) and its regulation in cancer progression: Review. Life Sci. 2020, 254, 117252. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Shan, K.; Liu, C.; Liu, B.H.; Chen, X.; Dong, R.; Liu, X.; Zhang, Y.Y.; Liu, B.; Zhang, S.J.; Wang, J.J.; et al. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation. 2017, 136, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.A.; Hatfield, S.A.; Brug, A.; Brooks, A.J.; Lightell, D.J.; Woods, T.C. Carotid Plaque Rupture Is Accompanied by an Increase in the Ratio of Serum circR-284 to miR-221 Levels. Circ. Cardiovasc. Genet. 2017, 10, e001720. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, R.; Lin, S.; Xie, X.; Ye, H.; Zheng, F.; Lin, J.; Huang, Q.; Huang, S.; Ruan, Q.; et al. Association of circular RNAs and environmental risk factors with coronary heart disease. BMC Cardiovasc. Disord. 2019, 19, 223. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence |
|---|---|
| hsa_circ_0006577-Forward Primer | 5′-CGCTGCCTAAGGAAATCGG-3′ |
| hsa_circ_0006577-Reverse Primer | 5′-TATGGCTGAGGACCAGTTGTG-3′ |
| hsa_circ_0018886-Forward Primer | 5′-AAGATTGAGCAAGCACAGCG-3′ |
| hsa_circ_0018886-Reverse Primer | 5′-CACATCCAGACCCTGAGATACC-3′ |
| hsa_circ_0092019-Forward Primer | 5′-GATCTTTACGGCAGGCATCG-3′ |
| hsa_circ_0092019-Reverse Primer | 5′-CACAGTGAACTCTGCCCGCT-3′ |
| hsa_circ_0075269-Forward Primer | 5′-TGATGGTGGCAAGGAGACAG-3′ |
| hsa_circ_0075269-Reverse Primer | 5′-CTCAGGCTCATAGAACTCGCTTA-3′ |
| hsa_circ_0000284-Forward Primer | 5′-TCCTGTTCGGCAGCCTTAC-3′ |
| hsa_circ_0000284-Reverse Primer | 5′-GACTTGTGAGGCCATACCTGTAG-3′ |
| hsa-18S-Forward Primer | 5′-CGCTCGCTCCTCTCCTACTT-3′ |
| hsa-18S-Reverse Primer | 5′-CGGGTTGGTTTTGATCTGATAA-3′ |
| Exo-circRNAs | AUC | 95%CI | p Value | Sensitivity | Specificity | Youden Index | Cut Off Value | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| has_circ_0075269 | 0.805 | 0.667–0.943 | 0.001 | 0.70 | 0.85 | 0.55 | 6.05 | 0.65 | 0.85 |
| hsa_circ_0000284 | 0.713 | 0.550–0.875 | 0.021 | 0.80 | 0.65 | 0.45 | 3.02 | 0.80 | 0.65 |
| Exo-circRNAs | AUC | 95%CI | p Value | Sensitivity | Specificity | Youden Index | Cut Off Value | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|
| has_circ_0075269 | 0.761 | 0.669–0.852 | <0.001 | 0.88 | 0.646 | 0.526 | 5.66 | 0.85 | 0.65 |
| hsa_circ_0000284 | 0.623 | 0.522–0.724 | 0.015 | 0.75 | 0.542 | 0.292 | 1.97 | 0.63 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zheng, M.; Han, R.; Yu, Z.; Yuan, W.; Xie, B.; Zhang, Y.; Zhong, J.; Wang, L.; Wang, L.; et al. Circulating Exosomal CircRNAs as Diagnostic Biomarkers for Chronic Coronary Syndrome. Metabolites 2023, 13, 1066. https://doi.org/10.3390/metabo13101066
Liu X, Zheng M, Han R, Yu Z, Yuan W, Xie B, Zhang Y, Zhong J, Wang L, Wang L, et al. Circulating Exosomal CircRNAs as Diagnostic Biomarkers for Chronic Coronary Syndrome. Metabolites. 2023; 13(10):1066. https://doi.org/10.3390/metabo13101066
Chicago/Turabian StyleLiu, Xiaoyan, Meili Zheng, Ruijuan Han, Ziyang Yu, Wen Yuan, Boqia Xie, Yeping Zhang, Jiuchang Zhong, Lefeng Wang, Lixia Wang, and et al. 2023. "Circulating Exosomal CircRNAs as Diagnostic Biomarkers for Chronic Coronary Syndrome" Metabolites 13, no. 10: 1066. https://doi.org/10.3390/metabo13101066
APA StyleLiu, X., Zheng, M., Han, R., Yu, Z., Yuan, W., Xie, B., Zhang, Y., Zhong, J., Wang, L., Wang, L., & Liu, X. (2023). Circulating Exosomal CircRNAs as Diagnostic Biomarkers for Chronic Coronary Syndrome. Metabolites, 13(10), 1066. https://doi.org/10.3390/metabo13101066





