The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Variables
2.3. Definition of Overweight and Sarcopenic Obesity
2.4. Definition of Metabolic Syndrome Index
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schienkiewitz, A.; Brettschneider, A.-K.; Damerow, S.; Schaffrath Rosario, A. Übergewicht und Adipositas im Kindes- und Jugendalter in Deutschland—Querschnittergebnisse aus KiGGS Welle 2 und Trends. J. Health Monit. 2018, 3, 16–23. [Google Scholar]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 1289 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Korean Disease Control and Prevention (KDCA). 2020 Korea National Health&Nurtition Examination Survey; Centers for Disease Control and Prevention: Cheongju, Republic of Korea, 2022; (Korean).
- Jung, Y.; Ko, S.; Lim, H. The Socioeconomic Cost of Adolescent Obesity. Health Soc. Welf. Rev. 2010, 30, 195–219. [Google Scholar] [CrossRef]
- de Onis, M.; Blössner, M.; Borghi, E. Global prevalence and trends of overweight and obesity among preschool children. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [CrossRef]
- Singh, A.S.; Mulder, C.; Twisk, J.W.; van Mechelen, W.; Chinapaw, M.J. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a disease. Med. Clin. N. Am. 2018, 102, 13–33. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef]
- Cauley, J.A. An Overview of Sarcopenic Obesity. J. Clin. Densitom. 2015, 18, 499–505. [Google Scholar] [CrossRef]
- Park, B.S.; Yoon, J.S. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab. J. 2013, 37, 458–464. [Google Scholar] [CrossRef]
- Steffl, M.; Chrudimsky, J.; Tufano, J. Using relative handgrip strength to identify children at risk of sarcopenic obesity. PLoS ONE 2017, 12, e0177006. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.C.; Torode, M.E.; Singh, M.A. Muscular Strength and Cardiorespiratory Fitness is Associated with Higher Insulin Sensitivity in Children and Adolescents. Int. J. Pediatr. Obes. 2006, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Steene-Johannessen, J.; Anderssen, S.A.; Kolle, E.; Andersen, L.B. Low Muscle Fitness is Associated with Metabolic Risk in Youth. Med. Sci. Sports Exerc. 2009, 41, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Dalamaga, M.; Kokkinos, A. Sarcopenic Obesity: Epidemiologic Evidence, Pathophysiology, and Therapeutic Perspectives. Curr. Obes. Rep. 2019, 8, 458–471. [Google Scholar] [CrossRef]
- Zembura, M.; Matusik, P. Sarcopenic Obesity in Children and Adolescents: A Systematic Review. Front. Endocrinol. 2022, 13, 914740. [Google Scholar] [CrossRef]
- Vukovic, R.; Milenkovic, T.; Stojan, G.; Vukovic, A.; Mitrovic, K.; Todorovic, S.; Soldatovic, I. Pediatric siMS score: A new, simple and accurate continuous metabolic syndrome score for everyday use in pediatrics. PLoS ONE 2017, 12, e0189232. [Google Scholar] [CrossRef]
- Heshmat, R.; Heidari, M.; Ejtahed, H.-S.; Motlagh, M.E.; Mahdavi-Gorab, A.; Ziaodini, H.; Taheri, M.; Shafiee, G.; Beshtar, S.; Qorbani, M.; et al. Validity of a continuous metabolic syndrome score as an index for modeling metabolic syndrome in children and adolescents: The CASPIAN-V study. Diabetol. Metab. Syndr. 2017, 9, 89. [Google Scholar] [CrossRef]
- Paulmichl, K.; Hatunic, M.; Højlund, K.; Jotic, A.; Krebs, M.; Mitrakou, A.; Porcellati, F.; Tura, A.; Bergsten, P.; Forslund, A.; et al. Modification and validation of the Triglyceride-to–HDL cholesterol ratio as a surrogate of insulin sensitivity in White juveniles and adults without diabetes mellitus: The single point insulin sensitivity estimator (SPISE). Clin. Chem. 2016, 62, 1211–1219. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, B.; Min, J.; Choi, E.J.; Kim, U.J.; Park, H.J.; Park, E.A.; Cho, S.J.; Kim, H.S.; Lee, H.; et al. Cohort profile: The Ewha Birth and Growth Study. Epidemiol. Health 2021, 43, e2021016. [Google Scholar] [CrossRef]
- Korea Center for Disease Control and Prevention. Korean Children and Adolescents 2017 Growth Chart Commentary. Available online: https://knhanes.kdca.go.kr/knhanes/sub08/sub08_01.do (accessed on 8 November 2022).
- McCarthy, H.D.; Samani-Radia, D.; Jebb, S.A.; Prentice, A.M. Skeletal muscle mass reference curves for children and adolescents. Pediatr. Obes. 2014, 9, 249–259. [Google Scholar] [CrossRef]
- Lee, Y.J.; Seo, M.Y.; Kim, S.H.; Park, M.J. Validity of the pediatric simple metabolic syndrome score. Obes. Res. Clin. Pract. 2020, 14, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, J.C.; Laurson, K.R.; DuBose, K.D.; Smith, B.K.; Donnelly, J.E. Construct validity of a continuous metabolic syndrome score in children. Diabetol. Metab. Syndr. 2010, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Dudi, P.; Goyal, B.; Saxena, V.; Rabari, K.; Mirza, A.A.; Naithani, M.; Kumar, T.; Goyal, R. Single point insulin sensitivity estimator as an index for insulin sensitivity for metabolic syndrome: A study in North Indian population. J. Lab. Physicians 2019, 11, 244–248. [Google Scholar] [CrossRef]
- Correa-Burrows, P.; Blanco, E.; Gahagan, S.; Burrows, R. Validity assessment of the single-point insulin sensitivity estimator (spise) for diagnosis of cardiometabolic risk in post-pubertal hispanic adolescents. Sci. Rep. 2020, 10, 14399. [Google Scholar] [CrossRef]
- Eisenmann, J. Aerobic fitness, fatness and the metabolic syndrome in children and adolescents. Acta Paediatr. 2007, 96, 1723–1729. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Shan, X.; Cheng, H.; Hou, D.; Zhao, X.; Wang, T.; Zhao, D.; Mi, J. Association between Childhood Obesity and Metabolic Syndrome: Evidence from a Large Sample of Chinese Children and Adolescents. PLoS ONE 2012, 7, e47380. [Google Scholar] [CrossRef]
- Barchetta, I.; Dule, S.; Bertoccini, L.; Cimini, F.A.; Sentinelli, F.; Bailetti, D.; Marini, G.; Barbonetti, A.; Loche, S.; Cossu, E.; et al. The single-point insulin sensitivity estimator (SPISE) index is a strong predictor of abnormal glucose metabolism in overweight/obese children: A long-term follow-up study. J. Endocrinol. Investig. 2022, 45, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Wang, P.; Glueck, C.J. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008, 152, 201–206. [Google Scholar] [CrossRef]
- Asghari, G.; Hasheminia, M.; Heidari, A.; Mirmiran, P.; Guity, K.; Shahrzad, M.K.; Azizi, F.; Hadaegh, F. Adolescent metabolic syndrome and its components associations with incidence of type 2 diabetes in early adulthood: Tehran lipid and glucose study. Diabetol. Metab. Syndr. 2021, 13, 1. [Google Scholar] [CrossRef]
- Chen, W.; Srinivasan, S.R.; Li, S.; Xu, J.; Berenson, G.S. Metabolic syndrome variables at low levels in childhood are beneficially associated with adulthood cardiovascular risk: The Bogalusa Heart Study. Diabetes Care 2005, 28, 126–131. [Google Scholar] [CrossRef]
- Koskinen, J.; Magnussen, C.G.; Sinaiko, A.; Woo, J.; Urbina, E.; Jacobs, D.R., Jr.; Steinberger, J.; Prineas, R.; Sabin, M.A.; Burns, T.; et al. Childhood age and associations between childhood metabolic syndrome and adult risk for metabolic syndrome, type 2 diabetes mellitus and carotid intima media thickness: The international childhood cardiovascular cohort consortium. J. Am. Heart Assoc. 2017, 6, e005632. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Doreswamy, S.; Narra, L.R.; Patel, P.; Guarecuco, J.E.; Baig, A.; Lahori, S.; Heindl, S.E. Childhood Obesity as a Predictor of Coronary Artery Disease in Adults: A Literature Review. Cureus 2020, 12, e11473. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hong, S.; Kim, E.Y. Reference Values of Skeletal Muscle Mass for Korean Children and Adolescents Using Data from the Korean National Health and Nutrition Examination Survey 2009–2011. PLoS ONE 2016, 11, e0153383. [Google Scholar] [CrossRef] [PubMed]
- Burrows, R.; Correa-Burrows, P.; Reyes, M.; Blanco, E.; Albala, C.; Gahagan, S. High Cardiometabolic Risk in Healthy Chilean Adolescents: Associations with Anthropometric, Biological and Lifestyle Factors. Public Health Nutr. 2016, 19, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J.H.; Yoon, J.W.; Kang, S.M.; Choi, S.H.; Park, Y.J.; Kim, K.W.; Lim, J.Y.; Park, K.S.; Jang, H.C. Sarcopenic Obesity: Prevalence and Association with Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010, 33, 1652–1654. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Kang, H.-T.; Lee, D.-C.; Lee, H.-R.; Lee, Y.-J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch Gerontol. Geriatr. 2013, 56, 270–278. [Google Scholar] [CrossRef]
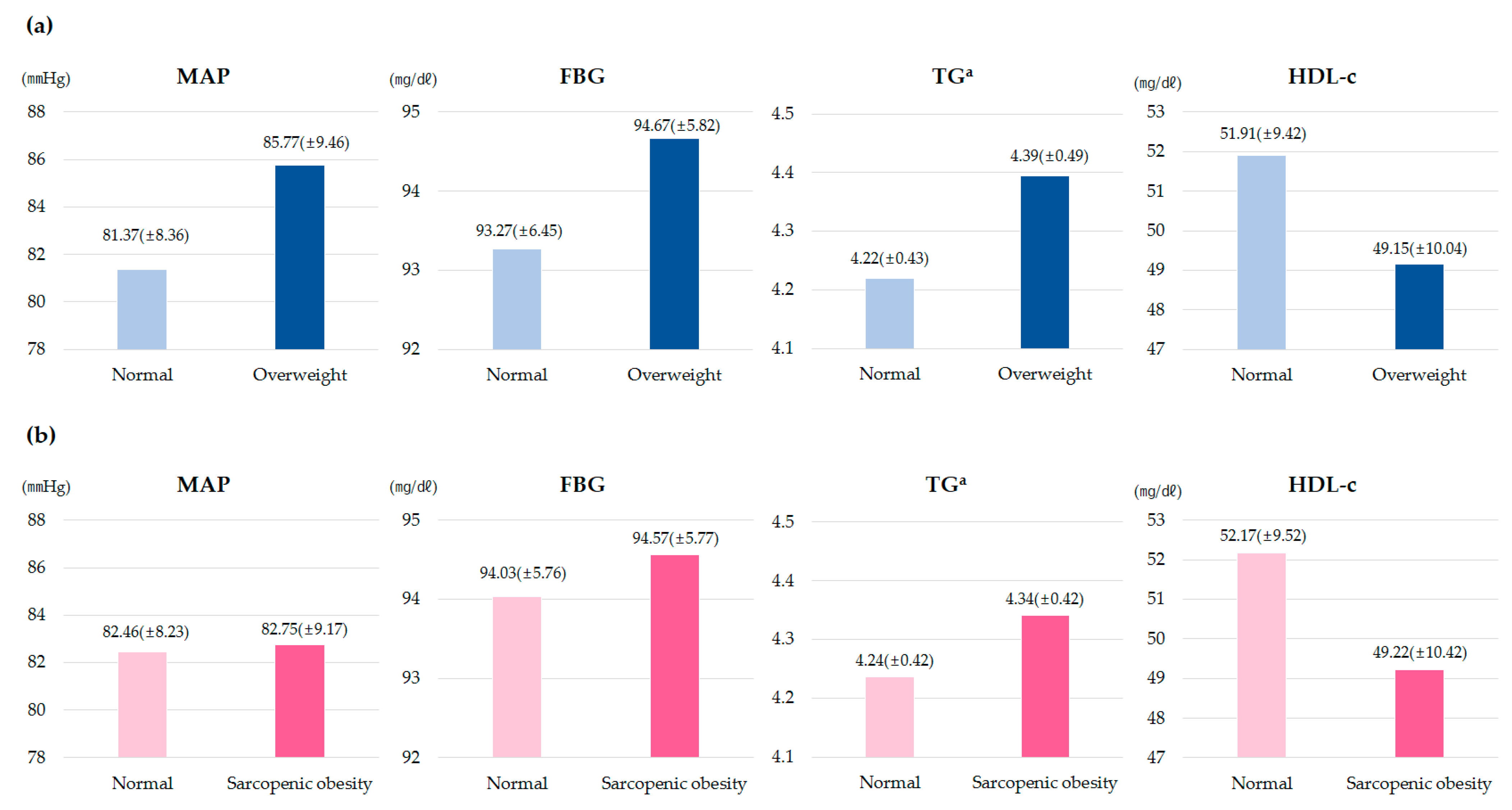
| Variables | 7–9 Years (n = 582) | 13–15 Years (n = 248) |
|---|---|---|
| Height (cm) | 126.69 (±6.83) | 160.41 (±6.59) |
| Weight (kg) | 26.77 (±6.04) | 53.42 (±10.62) |
| WC (cm) | 56.28 (±7.01) | 70.46 (±8.95) |
| BMI (kg/m2) | 16.5 (±2.44) | 20.69 (±3.34) |
| Overweight a, n (%) | 87 (15.0) | 38 (15.4) |
| SMM (kg) b | 10.04 (±1.90) | NA |
| BFM (kg) b | 6.48 (±3.59) | NA |
| MFR (kg/kg) b | 1.90 (±0.90) | NA |
| Sarcopenic obesity c, n (%) | 78 (19.5) | NA |
| Variable | Total (n = 227) | Boys (n = 111) | Girls (n = 116) | p-Value |
|---|---|---|---|---|
| WC (cm) | 70.42 (±9.02) | 72.7 (±10.35) | 68.24 (±6.89) | <0.001 |
| BMI (kg/m2) | 20.71 (±3.41) | 20.92 (±3.83) | 20.51 (±2.95) | 0.37 |
| MAP (mmHg) | 81.89 (±8.59) | 82.92 (±8.95) | 80.91 (±8.15) | 0.08 |
| SBP (mmHg) | 110.44 (±11.55) | 112.24 (±11.83) | 108.72 (±11.04) | 0.02 |
| DBP (mmHg) | 67.62 (±8.36) | 68.26 (±8.90) | 67.00 (±7.80) | 0.26 |
| FBG (mg/dL) | 93.44 (±6.38) | 93.65 (±5.72) | 93.23 (±6.97) | 0.62 |
| log TG a | 4.24 (±0.44) | 4.18 (±0.47) | 4.30 (±0.41) | 0.05 |
| HDL-C (mg/dL) | 51.58 (±9.51) | 51.55 (±8.97) | 51.6 (±10.05) | 0.97 |
| MetS index | ||||
| PsiMS | 1.89 (±0.52) | 1.90 (±0.53) | 1.88 (±0.52) | 0.83 |
| cMets | 0.01 (±3.05) | 0.00 (±3.15) | 0.01 (±2.97) | 0.99 |
| SPISE | 9.70 (±2.49) | 9.80 (±2.73) | 9.62 (±2.25) | 0.59 |
| Household income, n (%) | ||||
| <3 million KRW | 16 (7.2) | 7 (3.2) | 9 (8.1) | 0.89 |
| 3~5 million KRW | 64 (28.8) | 33 (14.9) | 31 (14.0) | |
| ≥5 million KRW | 142 (64.0) | 70 (31.5) | 72 (32.4) | |
| Mother’s education level b, n (%) | ||||
| Low | 43 (19.6) | 18 (8.2) | 25 (11.4) | 0.40 |
| High | 177 (80.5) | 89 (40.5) | 88 (40.0) | |
| Parental history of hypertension, n (%) | ||||
| No | 168 (74.0) | 87 (38.3) | 81 (35.7) | 0.17 |
| Yes | 59 (26.0) | 24 (10.6) | 35 (15.4) | |
| Frequency of overeating, n (%) | ||||
| None | 115 (51.3) | 51 (22.8) | 64 (28.6) | 0.19 |
| 1~2 times/week | 89 (39.7) | 46 (20.5) | 43 (19.2) | |
| ≥3 times/week | 20 (8.9) | 13 (5.8) | 7 (3.1) | |
| Moderate physical activity, n (%) | ||||
| Never | 42 (18.8) | 14 (6.3) | 28 (12.5) | 0.01 |
| 1~2 times/week | 101 (45.1) | 46 (20.5) | 55 (24.6) | |
| 3~4 times/week | 61 (27.2) | 34 (15.2) | 27 (12.0) | |
| ≥5 times/week | 20 (8.9) | 15 (6.7) | 5 (2.2) |
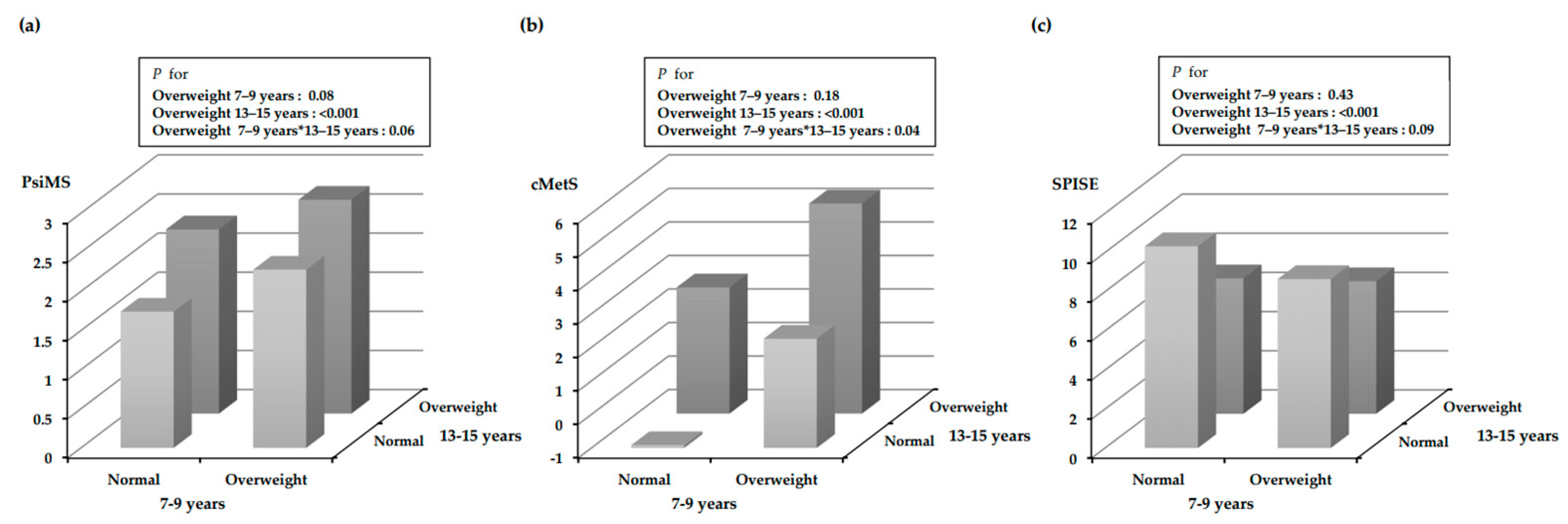
| Metabolic Syndrome Index | ||||
|---|---|---|---|---|
| Index | PsiMS | cMetS | SPISE | |
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| By BMI a | Normal (n = 200) | 1.84 ± 0.50 * | −0.33 ± 2.86 ** | 10.05 ± 2.38 ** |
| Overweight (n = 27) | 2.21 ± 0.59 * | 2.49 ± 3.32 ** | 7.10 ± 1.69 ** | |
| By MFR b | Normal (n = 131) | 1.85 ± 0.46 * | −0.12 ± 2.55 * | 10.08 ± 2.40 ** |
| Sarcopenic obesity (n = 23) | 2.13 ± 0.56 * | 1.84 ± 3.27 * | 7.50 ± 2.01 ** | |
| MetS Index | Overweight (Ref. Normal) | Sarcopenic Obesity (Ref. Normal) | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted a | Crude | Adjusted a | |||||
| β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | β (95% CI) | p-Value | |
| PsiMS | 0.37 (0.16, 0.58) | 0.001 | 0.32 (0.11, 0.53) | 0.003 | 0.28 (0.06, 0.49) | 0.011 | 0.30 (0.08, 0.52) | 0.009 |
| cMetS | 2.82 (1.64, 4.00) | <0.001 | 2.63 (1.45, 3.82) | <0.001 | 1.96 (0.77, 3.15) | 0.002 | 1.96 (0.73, 3.20) | 0.002 |
| SPISE | −2.95 (−3.88, −2.02) | <0.001 | −2.64 (−3.52, −1.75) | <0.001 | −2.59 (−3.63, −1.54) | <0.001 | −2.37 (−3.44, −1.31) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Jun, S.; Lee, H.-A.; Kim, H.S.; Hong, Y.S.; Park, H. The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study. Metabolites 2023, 13, 133. https://doi.org/10.3390/metabo13010133
Park H, Jun S, Lee H-A, Kim HS, Hong YS, Park H. The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study. Metabolites. 2023; 13(1):133. https://doi.org/10.3390/metabo13010133
Chicago/Turabian StylePark, Hyunjin, Seunghee Jun, Hye-Ah Lee, Hae Soon Kim, Young Sun Hong, and Hyesook Park. 2023. "The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study" Metabolites 13, no. 1: 133. https://doi.org/10.3390/metabo13010133
APA StylePark, H., Jun, S., Lee, H.-A., Kim, H. S., Hong, Y. S., & Park, H. (2023). The Effect of Childhood Obesity or Sarcopenic Obesity on Metabolic Syndrome Risk in Adolescence: The Ewha Birth and Growth Study. Metabolites, 13(1), 133. https://doi.org/10.3390/metabo13010133






