New Markers for Cardiovascular Disease in Psoriatic Patients: Preliminary Study on Monocyte Phenotype, ADAMTS7, and mTOR Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. PBMC Extraction and Flow Cytometry
2.3. ELISA Assay
2.4. Flow Cytometry and Gating Strategy
2.5. Statistical Analysis
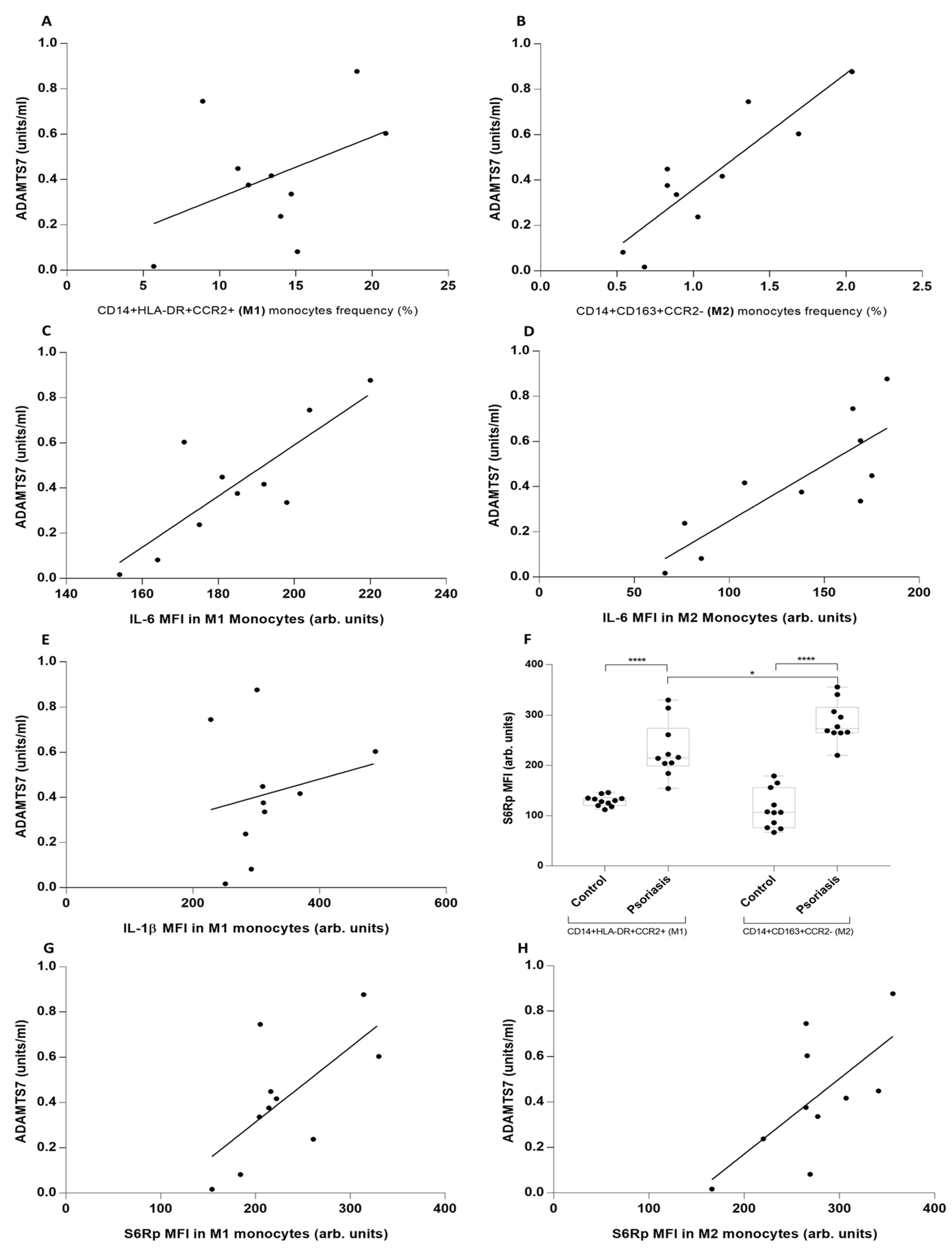
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mediated Cross Talk? Front. Immunol. 2022, 13, 868277.
- Gisondi, P.; Farina, S.; Giordano, M.V.; Girolomoni, G. Usefulness of the framingham risk score in patients with chronic psoriasis. Am. J. Cardiol. 2010, 106, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Pourani, M.R.; Abdollahimajd, F.; Zargari, O.; Shahidi Dadras, M. Soluble biomarkers for diagnosis, monitoring, and therapeutic response assessment in psoriasis. J. Dermatol. Treat. 2022, 33, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Colaco, K.; Lee, K.A.; Akhtari, S.; Winer, R.; Welsh, P.; Sattar, N.; McInnes, I.B.; Chandran, V.; Harvey, P.; Cook, R.J.; et al. Association of Cardiac Biomarkers With Cardiovascular Outcomes in Patients With Psoriatic Arthritis and Psoriasis: A Longitudinal Cohort Study. Arthritis Rheumatol. 2022, 74, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Hojman, L.; Karsulovic, C. Cardiovascular Disease-Associated Skin Conditions. Vasc. Health Risk Manag. 2022, 18, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sukhorukov, V.N.; Zhuravlev, A.; Orekhov, N.A.; Kalmykov, V.; Orekhov, A.N. Modulating mTOR Signaling as a Promising Therapeutic Strategy for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Krueger, J.G. The immunopathogenesis of psoriasis. Dermatol. Clin. 2015, 33, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zekavat, S.M.; Haidermota, S.; Bernardo, R.; MacDonald, B.T.; Libby, P.; Finucane, H.K.; Natarajan, P. Genome-wide pleiotropy analysis of coronary artery disease and pneumonia identifies shared immune pathways. Sci.Adv. 2022, 8, eabl4602. [Google Scholar] [CrossRef] [PubMed]
- Ten Bergen, L.L.; Petrovic, A.; Aarebrot, A.K.; Appel, S. Current knowledge on autoantigens and autoantibodies in psoriasis. Scand. J. Immunol. 2020, 92, e12945. [Google Scholar] [CrossRef] [PubMed]
- Karsulovic, C.; Lopez, M.; Tempio, F.; Guerrero, J.; Goecke, A. mTORC inhibitor Sirolimus deprograms monocytes in “cytokine storm” in SARS-CoV2 secondary hemophagocytic lymphohistiocytosis- like syndrome. Clin. Immunol. 2020, 218, 108539. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Bernhagen, J.; Heitman, L.H.; Weber, C.; Dichgans, M. Targeting the CCL2-CCR2 axis for atheroprotection. Eur. Heart J. 2022, 43, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, T.; MacDonald, B.T.; Bhandary, B.; Popp, N.R.; Laprise, D.; Arduini, A.; Lai, D.; Zhu, Q.M.; Xing, Y.; Kaushik, V.K.; et al. Coronary Disease Association With ADAMTS7 Is Due to Protease Activity. Circ. Res. 2021, 129, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Karsulovic, C.; Tempio, F.; Lopez, M.; Guerrero, J.; Goecke, A. In vitro Phenotype Induction of Circulating Monocytes: CD16 and CD163 Analysis. J. Inflamm. Res. 2021, 14, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, G.; Conte, S.; Morello, M.; Pellegrino, G.; Marra, L.; Morello, A.; Nicoletti, G.; De Rosa, G.; Golino, P.; Cirillo, P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? J. Cardiovasc. Dev. Dis. 2022, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, G.; Nader, D.; Watkin, R.; McCoy, C.E.; Curley, G.F.; Kerrigan, S.W. Human endothelial cell-derived exosomal microRNA-99a/b drives a sustained inflammatory response during sepsis by inhibiting mTOR expression. Front. Cell. Infect. Microbiol. 2022, 12, 854126. [Google Scholar] [CrossRef] [PubMed]

| Group 1 | Group 2 | ||
|---|---|---|---|
| Healthy Subjects (N = 11) | Psoriasis (N = 10) | p Value | |
| Demographic | |||
| Age, years ± SD | 36.4 ± 5.7 | 39.1 ± 13 | NS |
| Men, n | 6 | 6 | |
| Female, n | 5 | 4 | |
| Psoriasis Characteristics | |||
| Diagnosis, years ± SD | n/a | 6.1 ± 9.1 | |
| PASI, score ± SD | n/a | 27.3 ± 9.1 | |
| Extracutaneous Involvement, n | |||
| Ungueal | n/a | 8 | |
| Articular | n/a | 0 | |
| Treatment, n | |||
| In use | n/a | 0 | |
| Suspended + 12 months | n/a | 2 | |
| Biologics in use, n | n/a | 0 | |
| CVD Characteristics | |||
| Myocardial Infarction, n | 0 | 0 | |
| Stroke, n | 0 | 0 | |
| Cardiovascular Risk Factors | |||
| HBP, n | 1 | 3 | |
| Lipid Disorder, n | 1 | 2 | |
| Obesity, n | 1 | 2 | |
| Diabetes, n | 0 | 0 | |
| Tobacco use, (Pack/year) ± SD | 0.46 ± 1.15 | 3.53 ± 3.79 | NS |
| Medication | |||
| Statins, n | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loyola, K.; Karsulovic, C.; Cabrera, R.; Perez, C.; Hojman, L. New Markers for Cardiovascular Disease in Psoriatic Patients: Preliminary Study on Monocyte Phenotype, ADAMTS7, and mTOR Activity. Metabolites 2023, 13, 116. https://doi.org/10.3390/metabo13010116
Loyola K, Karsulovic C, Cabrera R, Perez C, Hojman L. New Markers for Cardiovascular Disease in Psoriatic Patients: Preliminary Study on Monocyte Phenotype, ADAMTS7, and mTOR Activity. Metabolites. 2023; 13(1):116. https://doi.org/10.3390/metabo13010116
Chicago/Turabian StyleLoyola, Khanty, Claudio Karsulovic, Raúl Cabrera, Claudio Perez, and Lía Hojman. 2023. "New Markers for Cardiovascular Disease in Psoriatic Patients: Preliminary Study on Monocyte Phenotype, ADAMTS7, and mTOR Activity" Metabolites 13, no. 1: 116. https://doi.org/10.3390/metabo13010116
APA StyleLoyola, K., Karsulovic, C., Cabrera, R., Perez, C., & Hojman, L. (2023). New Markers for Cardiovascular Disease in Psoriatic Patients: Preliminary Study on Monocyte Phenotype, ADAMTS7, and mTOR Activity. Metabolites, 13(1), 116. https://doi.org/10.3390/metabo13010116






