Abstract
Psoriasis, vitiligo and lichen planus (LP) are autoimmune skin diseases associated with metabolic syndrome. Angiopoietin-like 4 (ANGPTL4) is a member of angiopoietin-like proteins, which play an important role in lipid metabolism, and its serum concentration has been proposed as a biomarker of cardiometabolic complications, especially coronary artery disease (CAD). The study involved 56 patients with abovementioned dermatoses and 29 sex- and age-matched volunteers without dermatoses. ANGPTL4 serum concentration was measured by ELISA. ANGPTL4 concentration was statistically significantly higher in patients with LP compared to the control group (p < 0.01); moreover, it was significantly higher than in patients with psoriasis and vitiligo (p < 0.001, p < 0.01, respectively). There was no statistically significant difference in ANGPTL4 concentration between patients with psoriasis or vitiligo and controls. There was no correlation between ANGPTL4 concentration and age or BMI in all study groups. There was a positive correlation between ANGPTL4 concentration and fasting glucose (R = 0.43) and AST activity (R = 0.39) in psoriatic patients and ALT activity in patients with vitiligo (R = 0.44). ANGPTL4 could be a potential marker of metabolic complications in patients with LP, especially CAD. Perhaps patients with LP are more prone to CAD compared to the other two dermatoses, which requires further research.
1. Introduction
Psoriasis is one of the most common, chronic skin diseases. It occurs with a frequency of 2–4% on average worldwide [1]. It is genetically determined, but also modified, by environmental factors [2] and even despite extensive research on this matter, its pathogenesis is still not fully understood. Immunological disturbances are also key components of psoriasis pathology. Autoimmunity has been postulated, and specific autoantibodies have been recently detected [2,3]. There are many types of psoriasis, but the most frequent, which is plaque psoriasis, presents with erythematous-papular lesions and psoriatic plaques with a scaly surface [4].
Lichen planus (LP) is a dermatosis of uncertain but surely complex etiology, which occurs with unknown frequency, estimated to be slightly over 1% of the population [5]. It usually affects adults, with no gender predilection, but some sources report on higher prevalence in women than men [5]. Many factors are taken into account in the pathogenesis of LP. First, there is evidence of potential genetic predisposition for LP occurrence, since some gene polymorphisms of immune-, inflammation- and oxidative stress-related genes were detected [5]. Second, several factors have been proved to trigger LP lesions. These are, among others, hepatitis virus C (HCV) and other viral infections, stressful events or medications [5]. Last, but not least, LP is obviously associated with immune disturbances and is considered a T-cell-mediated autoimmune disorder [5]. LP involves especially glabrous skin, in approximately half of the patients, mucosal membranes and less frequently, nails or scalp. Its predominant manifestation is red-violaceus polygonal papules with a white shiny surface called Wickham’s striae [6].
Vitiligo is a skin disease of not fully understood etiology, which is characterized by the presence of well-defined depigmented macules caused by the loss or dysfunction of melanocytes [7]. Its frequency is estimated to be up to 4% of the population, with no sex predominance [7]. The onset may occur at any age, but vitiligo usually appears for the first time in children or young adults [7]. Vitiligo is classified into two types—segmental and non-segmental, of which the latter is predominant [7]. The pathogenesis of vitiligo includes genetic background, autoimmune mechanisms, oxidative stress, inflammation and melanocyte detachment mechanism [8].
Angiopoietin-like 4 (ANGPTL4) is a member of angiopoietin-like proteins, which play an important role in lipid metabolism. They regulate the function of lipoprotein lipase, which in turn is responsible for the hydrolysis of fatty acids from triglyceride-rich lipoproteins and management of their distribution to peripheral tissues [9]. ANGPTL4 is predominantly expressed in adipose tissue and is particularly engaged in the inhibition of lipoprotein lipase [9]. ANGPTL4 regulation is dependent on food intake (it is upregulated during fasting and downregulated during fed conditions) and oxygen supply (induced by hypoxia) [9]. Induction of ANGPTL4 inhibits LPL activity and increases circulating triglycerides concentration; hence, this protein exerts an influence on the development of metabolic disorders [9]. The role of ANGPTL4 in these processes is presented in Figure 1 [9,10,11,12].
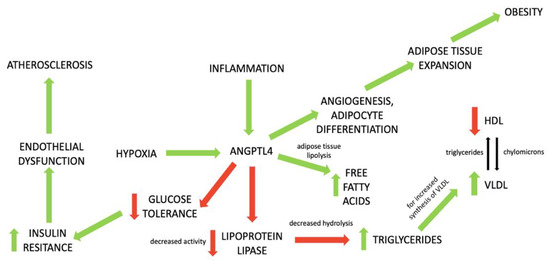
Figure 1.
The role of ANGPTL4 in the development of metabolic disorders. Green arrows mean stimulation; red arrows mean inhibition of process.
As it happens, the aforementioned skin diseases are related to metabolic syndrome (MS). Obviously, the tightest association is the one with psoriasis, but LP and vitiligo have also been linked to MS [13,14,15]. Our team has been exploring these interrelations for years. In a recently published paper, we demonstrated that mucosal addressin cell adhesion molecule-1 (MAdCAM-1) might play a role as an inflammation indicator in psoriasis, but also exerts a beneficial impact on the lipid profile in LP [16].
The aim of our study was to investigate the role of ANGPTL4 in patients with selected dermatoses of autoimmune origin, additionally related to MS. To the best of our knowledge, this is the first research analyzing ANGPTL4 in psoriasis, lichen planus and vitiligo.
2. Materials and Methods
The participants were selected from the Bialystok+ Polish Longitudinal University Study, which started in 2016 and is supposed to enroll 10,000 randomly chosen participants in total. As of now, 56 patients (30 females and 26 males), 23 with plaque-type psoriasis, 15 with lichen planus (including several subjects from the Department of Dermatology of the same University) and 18 with vitiligo were enrolled in our study. They were all adults of Caucasian ethnicity originating from one city. They were compared with 29 sex- and age-matched volunteers without skin diseases. All participants signed informed written consents before initiation. Body mass index (BMI) was calculated as weight/height2(kg/m2). All subjects were subdivided into groups according to BMI: BMI 1 was related to normal-weight (BMI 18.5–24.9), second group—BMI 2—overweight (BMI 25–29.9) and BMI 3, obesity (BMI > 30). Laboratory tests including C-reactive protein (CRP), complete blood count (CBC), serum glucose, total cholesterol (Chol), triglycerides (TG) and asparagine and alanine aminotransferases (AST, ALT) were performed before treatment. The study was approved by the Bioethical Committee of Medical University in Bialystok, Bialystok, Poland (number: R-I-002/108/2016) and was in accordance with the principle of the Helsinki Declaration.
2.1. Serum Collection
Fasting blood samples were received from subjects and volunteers without dermatoses using vacutainer tubes with a clot activator. Samples were centrifuged at 2000× g for 10 min and preserved at −80 °C until analyses. ANGPTL4 concentrations were measured using an enzyme immunoassay kit supplied by Cloud Clone® Houston, Texas, USA (SEB019Hu). Optical density was read at a wavelength of 450 nm. The concentrations were measured by interpolation from calibration curves prepared with standard samples supplied by the manufacturer. All the tests were performed by the same investigator in standardized laboratory settings.
2.2. Statistical Analysis
Normality of distribution was tested using the Shapiro–Wilk W test. The non-Gaussian data were presented as median (full range) and analyzed using the non-parametric Kruskal–Wallis test. The correlations were analyzed using Spearman’s Rank correlation analysis. Statistical analysis was conducted using the GraphPad Prism 9.20 software (GraphPad Prism 9.20 Software, USA). The differences were deemed statistically significant when p < 0.05.
3. Results
Basic characteristics of patients and controls are presented in Table 1. The study involved 56 patients with autoimmune dermatoses: 23 with plaque psoriasis, 15 with lichen planus and 18 with vitiligo, and 29 subjects without dermatoses who served as a control group. There was no significant difference between patients and controls in terms of age, gender or BMI (NS). Moreover, there were no significant differences between all four groups in terms of basic results of laboratory investigations (NS).

Table 1.
Basic characteristics of patients and controls.
Serum concentrations of ANGPTL4 in patients and controls are presented in Figure 2. (raw data in Table S1.).
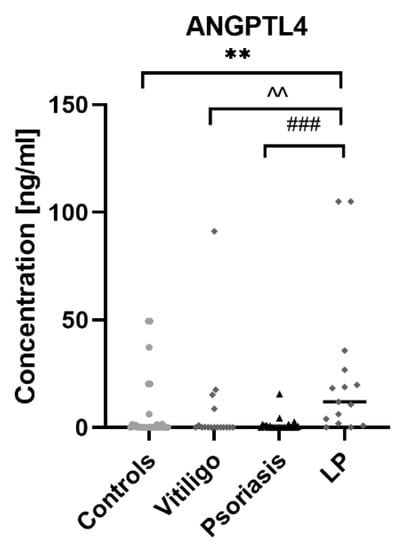
Figure 2.
Concentrations of ANGPTL4 in patients with psoriasis, vitiligo and lichen planus, as well as in control group. ** means statistically significant difference between patients with LP and controls with p < 0.01; ^^ means statistically significant difference between patients with LP and vitiligo with p < 0.01; ### means statistically significant difference between patients with LP and psoriasis with p < 0.001.
ANGPTL4 concentration was statistically significantly higher in patients with LP compared to the control group (p < 0.01); moreover, it was significantly higher than in psoriatic patients (p < 0.001) and patients with vitiligo (p < 0.01). There was no statistically significant difference in ANGPTL4 concentration between patients with psoriasis and controls or in patients with vitiligo and controls (both NS). There was no correlation between ANGPTL4 concentration and age or BMI. Correlations between ANGPTL4 concentrations and laboratory parameters in particular groups of patients and controls are presented in Figure 3.
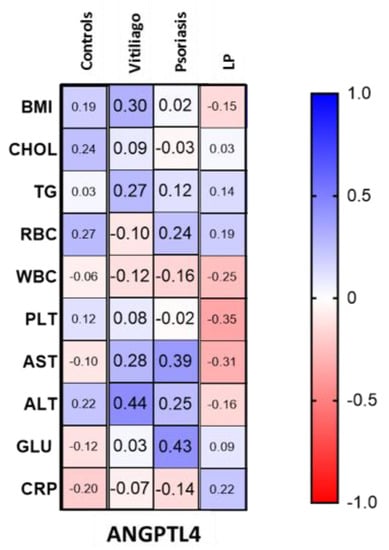
Figure 3.
Correlations between ANGPTL4 concentrations and laboratory parameters in patients with vitiligo, psoriasis and lichen planus, as well as in controls.
There was a positive correlation between ANGPTL4 concentration and fasting glucose (R = 0.43) and AST activity (R = 0.39) in patients with psoriasis. Moreover, there was a positive correlation between ANGPTL4 concentration and ALT activity in patients with vitiligo (R = 0.44). In the LP group, there was a downward trend for PLT and AST activity and ANGPTL4 concentration (R= −0.35, R= −0.31, respectively).
4. Discussion
Psoriasis is particularly well-related to metabolic disorders and many of these associations are bidirectional. Psoriatic patients are at an increased risk of cardiovascular disorders and have, therefore, a shorter life duration [13]. LP has also been described to be associated with MS [15]. In a big meta-analysis by Ying et al., it was determined that patients with LP have probably a 2-fold higher frequency of MS, which may be additionally dependent on its clinical type [15]. In recent years, publications regarding the connection between MS and vitiligo also appeared [14].
Research has shown the role of ANGPTL4 in the development of metabolic disorders based on observation of humans with loss-of-function mutations [17]. Such subjects tended to have elevated concentrations of HDL and decreased concentrations of triglyceride-rich lipoproteins [17]. Moreover, loss of ANGPTL4 leads to increased insulin sensitivity and improvement of glucose metabolism [9]. In the mouse model, it was observed that hepatocyte-specific depletion of ANGPTL4 prevents diet-induced obesity, leads to a decrease in circulating triglycerides, increases insulin sensitivity and glucose tolerance and inhibits the progression of atherosclerosis [18]. Thus, it seems that increased ANGPTL4 blood concentration could be considered a marker of metabolic complications. Nevertheless, ANGPTL4 has been also reported to have two-faced properties in relation to atherosclerosis [9]. Plasma concentrations of ANGPTL4 have been proved to be a predictive factor for future cardiovascular events, but of contradictory nature. For instance, in one study by Muendlein et al., its increased concentration exacerbated the risk of coronary artery disease (CAD) [19], and in the other, by Sun et al., decreased ANGPTL4 was significantly related to CAD, regardless of lipid concentrations, and presented as a CAD biomarker [20]. Georgiadi et al. suggested in their study that ANGPTL4 has additional influence on atherosclerotic plaque progression, beyond the impact on plasma lipids. They postulated that ANGPTL4 is capable of slowing down the development of plaques by reducing the inflammatory response to saturated fats [21].
In our study, we observed a significantly higher concentration of ANGPTL4 in patients with LP compared to controls and two other groups with dermatoses. Since all of these diseases have been associated with MS, it seems that ANGPTL4 could become a marker of metabolic complications only in subjects with LP. Apparently, it should be investigated in larger groups to establish its definite application. Another conclusion that could be drawn from this observation is that, since ANGPTL4 has been established especially as a CAD marker, patients with LP are potentially more prone to CAD in particular, compared to the other two dermatoses.
As for the association with age and BMI of patients, the literature data suggest that they are positively correlated with ANGPTL4 concentrations [9], but we did not observe such a tendency in any of the studied groups.
Analyzing the relationship between the protein and laboratory parameters, we found a positive correlation between ANGPTL4 concentration and fasting glucose, but only in patients with psoriasis, which is consistent with the available literature [9]. Although such correlations have been proved, the actual role of ANGPTL4 in type 2 diabetes mellitus (DM) remains unclear due to inconsistent data from medical research—in some of them, patients with DM presented elevated ANGPTL4 serum concentrations and in the other, decreased [22]. Perhaps increased serum concentration of ANGPTL4 in patients with psoriasis could indicate an increased risk of carbohydrate metabolism disorders, but obviously, other factors must take part in this interplay considering the insignificant difference in ANGPTL4 concentration between such patients and controls. We found no correlations between ANGPTL4 and TGs, and the lack of such an evident relationship has been reported in the medical literature as well [9].
Moreover, there was a positive correlation between ANGPTL4 concentration and ALT activity in patients with vitiligo and AST activity in patients with psoriasis. In the LP group, there was a downward trend for PLT and AST activity and ANGPTL4 concentration. In the study on subjects with hepatic steatosis, Altun et al. observed no significant correlation between ANGPTL4 and ALT, but they did find a significant negative correlation with AST [23]. It should be further investigated in the future whether ANGPTL4 could become a marker of liver complications in subjects with vitiligo and psoriasis.
ANGPTL4 has been investigated as a therapeutic target, although there have been some obstacles in drug development [9]. So far, there have been no such medications finally introduced, but it could be interesting to investigate them in patients with LP in relation to the improvement of skin conditions.
The limitation of our study is that it involved subjects of only Caucasian ethnicity coming from one city. The number of participants is relatively low, although as this longitudinal study from which we selected our subjects is still going on, we expect this number to increase and to expand our investigation. As for this study, we used only one ELISA method.
5. Conclusions
This is the first study to report on the role of ANGPTL4 in patients with psoriasis, lichen planus and vitiligo. Although they are all associated with metabolic disorders, it seems that only in subjects with LP, ANGPTL4 could serve as a marker of metabolic complications, especially coronary artery disease. Perhaps patients with LP are more prone to coronary artery disease compared to the other two dermatoses, which requires further research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo12090877/s1, Table S1. Raw data.
Author Contributions
A.B.: Conceptualization, data curation, project administration, writing—original draft preparation, writing—review and editing and funding acquisition; J.N.: conceptualization, writing—original draft preparation, writing—review and editing, data curation and resources; J.M.: investigation; J.M.H.: formal analysis, software, visualization and writing—review and editing; K.A.K., M.D. and M.K.: resources and data curation; D.P.: supervision; I.F.: project administration and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Medical University of Bialystok, Poland no SUB/1/DN/20/001/1149 and by the Ministry of Education under the project ‘Excellence Initiative—Research University’.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical University of Bialystok, Poland, no R-I-002/108/2016.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available upon request.
Acknowledgments
We thank all the Executors of the Bialystok+ Polish Longitudinal University Study from the Medical University of Bialystok, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, M.A.; Simpson, F.C.; Gupta, A.K. Psoriasis and sleep disorders: A systematic review. Sleep Med. Rev. 2016, 29, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Hugh, J.M.; Weinberg, J.M. Update on the pathophysiology of psoriasis. Cutis 2018, 102, 6–12. [Google Scholar] [PubMed]
- Ten Bergen, L.L.; Petrovic, A.; Aarebrot, A.K.; Appel, S. Current knowledge on autoantigens and autoantibodies in psoriasis. Scand. J. Immunol. 2020, 92, e12945. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Gorouhi, F.; Davari, P.; Fazel, N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci. World J. 2014, 2014, 742826. [Google Scholar] [CrossRef]
- Tziotzios, C.; Lee, J.Y.W.; Brier, T.; Saito, R.; Hsu, C.K.; Bhargava, K.; Stefanato, C.M.; Fenton, D.A.; McGrath, J.A. Lichen planus and lichenoid dermatoses: Clinical overview and molecular basis. J. Am. Acad. Dermatol. 2018, 79, 789–804. [Google Scholar] [CrossRef]
- Czajkowski, R.; Placek, W.; Flisiak, I.; Krasowska, D.; Maj, J.; Marchlewicz, M.; Reich, A.; Wolska, H.; Rudnicka, L. Vitiligo. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Dermatol. Rev. 2019, 106, 1–15. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Aryal, B.; Price, N.L.; Suarez, Y.; Fernández-Hernando, C. ANGPTL4 in Metabolic and Cardiovascular Disease. Trends Mol. Med. 2019, 25, 723–734. [Google Scholar] [CrossRef]
- Yamazaki, F. Psoriasis: Comorbidities. J. Dermatol. 2021, 48, 732–740. [Google Scholar] [CrossRef]
- Barja-Fernandez, S.; Moreno-Navarrete, J.M.; Folgueira, C.; Xifra, G.; Sabater, M.; Castelao, C.; Fern, Ø.J.; Leis, R.; Diéguez, C.; Casanueva, F.C.; et al. Plasma ANGPTL-4 is Associated with Obesity and Glucose Tolerance: Cross-Sectional and Longitudinal Findings. Mol. Nutr. Food Res. 2018, 62, 1800060. [Google Scholar] [CrossRef] [PubMed]
- Dikker, O.; Çetin, D.N.; Şahin, M.; Türkkan, E.; Dağ, H. The association of angiopoietin-like peptide 4 levels with obesity and hepatosteatosis in adolescents. Cytokine 2020, 125, 154802. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Kirby, B. Psoriasis Is a Systemic Disease with Multiple Cardiovascular and Metabolic Comorbidities. Dermatol. Clin. 2015, 33, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Hussain, K.; Namiq, K.S.; Firoz, A.; Bouchama, M.; Raza, M.; Haris, M.; Khan, S. Vitiligo: The Association with Metabolic Syndrome and the Role of Simvastatin as an Immunomodulator. Cureus 2021, 13, e14029. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Xiang, W.; Qiu, Y.; Zeng, X. Risk of metabolic syndrome in patients with lichen planus: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238005. [Google Scholar] [CrossRef]
- Baran, A.; Nowowiejska, J.; Kaminski, T.W.; Krahel, J.A.; Flisiak, I. Circulating MAdCAM-1 and ITGB7 in Patients with Plaque Psoriasis and Eruptive Lichen Planus—Preliminary Data. Biology 2021, 10, 1129. [Google Scholar] [CrossRef]
- Ruscica, M.; Zimetti, F.; Adorni, M.P.; Sirtori, C.R.; Lupo, M.G.; Ferri, N. Pharmacological aspects of ANGPTL3 and ANGPTL4 inhibitors: New therapeutic approaches for the treatment of atherogenic dyslipidemia. Pharmacol. Res. 2020, 153, 104653. [Google Scholar] [CrossRef]
- Singh, A.K.; Chaube, B.; Zhang, X.; Sun, J.; Citrin, K.M.; Canfrán-Duque, A.; Aryal, B.; Rotllan, N.; Varela, L.; Lee, R.G.; et al. Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. J. Clin. Investig. 2021, 131, e140989. [Google Scholar] [CrossRef]
- Muendlein, A.; Saely, C.H.; Leiherer, A.; Fraunberger, P.; Kinz, E.; Rein, P.; Vonbank, A.; Zanolin, D.; Malin, C.; Drexel, H. Angiopoietin-like protein 4 significantly predicts future cardiovascular events in coronary patients. Atherosclerosis 2014, 237, 632–638. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, W.; Wei, L.; Xu, Z.; Fan, L.; Zhuo, Y.; Wang, C.; Zhang, J. Circulating ANGPTL3 and ANGPTL4 levels predict coronary artery atherosclerosis severity. Lipids Health Dis. 2021, 20, 154. [Google Scholar] [CrossRef]
- Georgiadi, A.; Wang, Y.; Stienstra, R.; Tjeerdema, N.; Janssen, A.; Stalenhoef, A.; van der Vliet, J.A.; de Roos, A.; Tamsma, J.T.; Smit, J.W.; et al. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C.; Suárez, Y. ANGPTL4: A multifunctional protein involved in metabolism and vascular homeo-stasis. Curr. Opin. Hematol. 2020, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Altun, Ö.; Dikker, O.; Arman, Y.; Ugurlukisi, B.; Kutlu, O.; Ozgun Cil, E.; Aydin Yoldemir, S.; Akarsu, M.; Ozcan, M.; Kalyon, S.; et al. Serum Angiopoietin-like peptide 4 levels in patients with hepatic steatosis. Cytokine 2018, 111, 496–499. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).