Levels of Acylcarnitines and Branched-Chain Amino Acids in Antipsychotic-Treated Patients with Paranoid Schizophrenia with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population and Sample Collection
2.2. Laboratory Measurements
2.3. Statistical Analysis
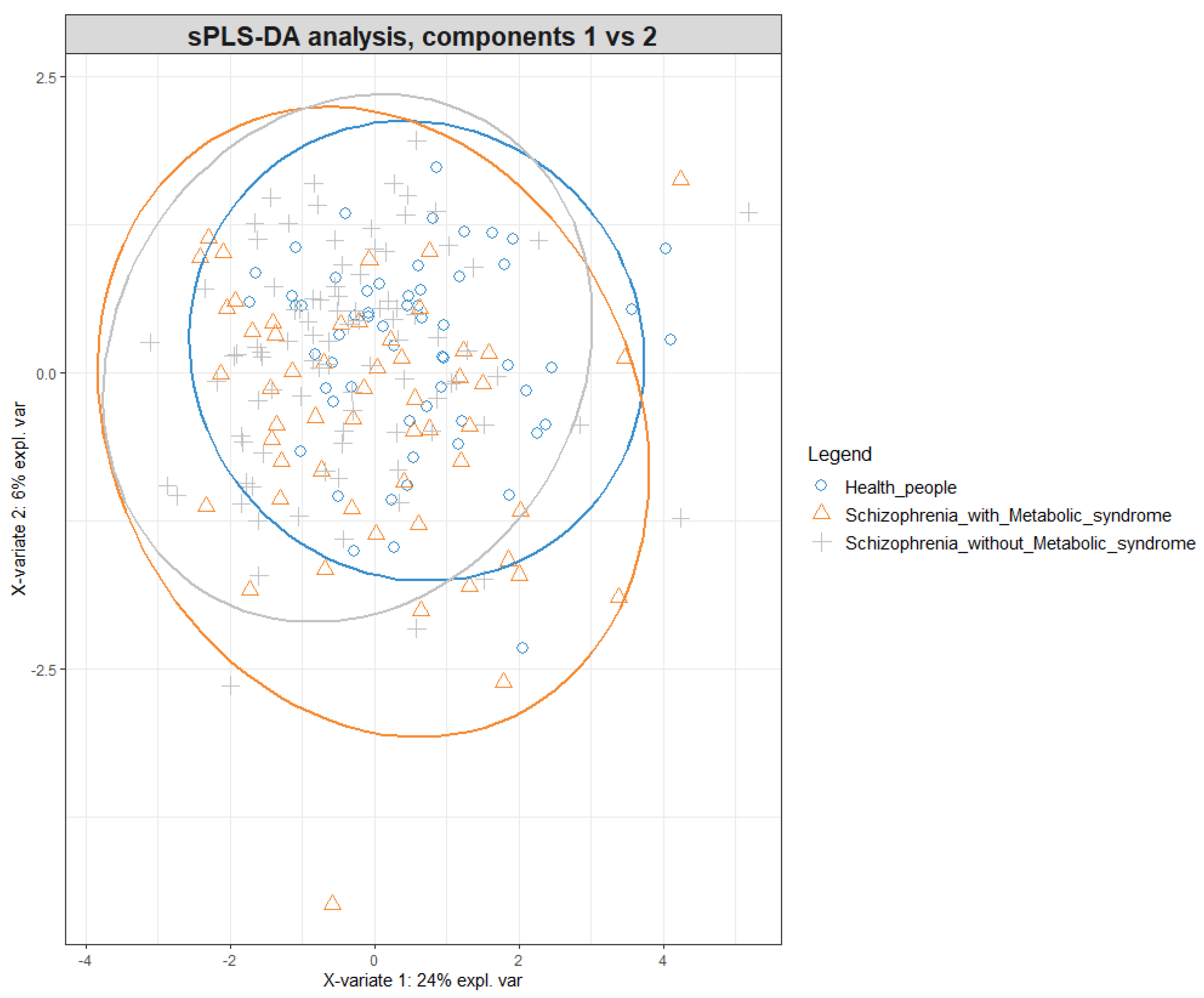
3. Results
3.1. Basic Population Characteristics
3.2. Acylcarnitine and Amino Acid Levels in the Studied Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henriksen, M.G.; Nordgaard, J.; Jansson, L.B. Genetics of schizophrenia: Overview of methods, findings and limitations. Front. Hum. Neurosci. 2017, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Chatterjee, I. Role of neurotransmitters in schizophrenia: A comprehensive study. Kuwait J. Sci. 2021, 48, 1–27. [Google Scholar] [CrossRef]
- Roberts, R.C. Mitochondrial dysfunction in schizophrenia: With a focus on postmortem studies. Mitochondrion 2021, 56, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Melamud, M.M.; Buneva, V.N.; Ivanova, S.A. Immune System Abnormalities in Schizophrenia: An Integrative View and Translational Perspectives. Front. Psychiatry 2022, 13, 880568. [Google Scholar] [CrossRef]
- Mongan, D.; Ramesar, M.; Föcking, M.; Cannon, M.; Cotter, D. Role of inflammation in the pathogenesis of schizophrenia: A review of the evidence, proposed mechanisms and implications for treatment. Early Interv. Psychiatry 2020, 14, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Chadda, R.K.; Ramshankar, P.; Deb, K.S.; Sood, M. Metabolic syndrome in schizophrenia: Differences between antipsychotic-naïve and treated patients. J. Pharmacol. Pharmacother. 2013, 4, 176. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.D.; Avila, S.; Rubio, G.; López-Muñoz, F. Metabolomic Connections between Schizophrenia, Antipsychotic Drugs and Metabolic Syndrome: A Variety of Players. Curr. Pharm. Des. 2021, 27, 4049–4061. [Google Scholar] [CrossRef]
- Deng, C. Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol. Metab. Clin. 2013, 42, 545–563. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Z.; Lian, J. Time-dependent changes and potential mechanisms of glucose-lipid metabolic disorders associated with chronic clozapine or olanzapine treatment in rats. Sci. Rep. 2017, 7, 2762. [Google Scholar] [CrossRef]
- Mednova, I.A.; Boiko, A.S.; Kornetova, E.G.; Parshukova, D.A.; Semke, A.V.; Bokhan, N.A.; Loonen, A.J.M.; Ivanova, S.A. Adipocytokines and Metabolic Syndrome in Patients with Schizophrenia. Metabolites 2020, 10, 410. [Google Scholar] [CrossRef]
- Boiko, A.S.; Mednova, I.A.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Loonen, A.J.M.; Ivanova, S.A. Apolipoprotein serum levels related to metabolic syndrome in patients with schizophrenia. Heliyon 2019, 5, e02033. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines. Clin. Pharm. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Famularo, G.; De Simone, C.; Trinchieri, V.; Mosca, L. Carnitines and its congeners: A metabolic pathway to the regulation of immune response and inflammation. Ann. N. Y. Acad. Sci. 2004, 1033, 132–138. [Google Scholar] [CrossRef]
- Waagsbø, B.; Svardal, A.; Ueland, T.; Landrø, L.; Øktedalen, O.; Berge, R.K.; Damås, J.K. Low levels of short-and medium-chain acylcarnitines in HIV-infected patients. Eur. J. Clin. Investig. 2016, 46, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Bene, J.; Márton, M.; Mohás, M.; Bagosi, Z.; Bujtor, Z.; Oroszlán, T.; Melegh, B. Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann. Nutr. Metab. 2013, 62, 80–85. [Google Scholar] [CrossRef]
- Sun, L.; Liang, L.; Gao, X.; Zhang, H.; Yao, P.; Hu, Y.; Wu, J. Early prediction of developing type 2 diabetes by plasma acylcarnitines: A population-based study. Diabetes Care 2016, 39, 1563–1570. [Google Scholar] [CrossRef]
- Rizza, S.; Copetti, M.; Rossi, C.; Cianfarani, M.A.; Zucchelli, M.; Luzi, A.; Federici, M. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis 2014, 232, 260–264. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Lynch, C.; Adams, S. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory metabolomics of metabolic syndrome: A status report. World J. Diabetes 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Noland, R.C.; Koves, T.R.; Seiler, S.E.; Lum, H.; Lust, R.M.; Ilkayeva, O.; Muoio, D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009, 284, 22840–22852. [Google Scholar] [CrossRef] [PubMed]
- Rauschert, S.; Uhl, O.; Koletzko, B.; Hellmuth, C. Metabolomic biomarkers for obesity in humans: A short review. Ann. Nutr. Metab. 2014, 64, 314–324. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, e1378–e1387. [Google Scholar] [CrossRef]
- Dambrova, M.; Liepinsh, E. Risks and benefits of carnitine supplementation in diabetes. Exp. Clin. Endocrinol. Diabetes 2015, 123, 95–100. [Google Scholar] [CrossRef]
- McCoin, C.; Knotts, T.; Adams, S. Acylcarnitines—Old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system—From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef]
- Cao, B.; Wang, D.; Pan, Z. Characterizing acyl-carnitine biosignatures for schizophrenia: A longitudinal pre- and post-treatment study. Transl. Psychiatry 2019, 9, 19. [Google Scholar] [CrossRef]
- Cao, B.; Chen, Y.; McIntyre, R.S.; Yan, L. Acyl-Carnitine plasma levels and their association with metabolic syndrome in individuals with schizophrenia. Psychiatry Res. 2020, 293, 113458. [Google Scholar] [CrossRef]
- Kriisa, K.; Leppik, L.; Balõtšev, R.; Ottas, A.; Soomets, U.; Koido, K.; Zilmer, M. Profiling of acylcarnitines in first episode psychosis before and after antipsychotic treatment. J. Proteome Res. 2017, 16, 3558–3566. [Google Scholar] [CrossRef] [PubMed]
- Leppik, L.; Parksepp, M.; Janno, S. Profiling of lipidomics before and after antipsychotic treatment in first-episode psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Mednova, I.A.; Chernonosov, A.A.; Kasakin, M.F.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Koval, V.V.; Ivanova, S.A. Amino Acid and Acylcarnitine Levels in Chronic Patients with Schizophrenia: A Preliminary Study. Metabolites 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Le Cao, K.-A. mixOmics: An R package for ’omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Kornetova, E.G.; Kornetov, A.N.; Mednova, I.A.; Goncharova, A.A.; Gerasimova, V.I.; Pozhidaev, I.V.; Boiko, A.S.; Semke, A.V.; Loonen, A.J.M.; Bokhan, N.A.; et al. Comparative Characteristics of the Metabolic Syndrome Prevalence in Patients With Schizophrenia in Three Western Siberia Psychiatric Hospitals. Front. Psychiatry 2021, 12, 661174. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Vancampfort, D.; Sweers, K.; van Winkel, R.; Yu, W.; De Hert, M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr. Bull. 2013, 39, 306–318. [Google Scholar] [CrossRef]
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 220. [Google Scholar] [CrossRef]
- Sugawara, N.; Yasui-Furukori, N.; Sato, Y.; Umeda, T.; Kishida, I.; Yamashita, H.; Saito, M.; Furukori, H.; Nakagami, T.; Hatakeyama, M.; et al. Prevalence of metabolic syndrome among patients with schizophrenia in Japan. Schizophr. Res. 2010, 123, 244–250. [Google Scholar] [CrossRef]
- Walss-Bass, C.; Weintraub, S.T.; Hatch, J.; Mintz, J.; Chaudhuri, A.R. Clozapine causes oxidation of proteins involved in energy metabolism: A possible mechanism for antipsychotic-induced metabolic alterations. Int. J. Neuropsychopharmacol. 2008, 11, 1097–1104. [Google Scholar] [CrossRef][Green Version]
- Takami, G.; Ota, M.; Nakashima, A. Effects of atypical antipsychotics and haloperidol on PC12 cells: Only aripiprazole phosphorylates AMP-activated protein kinase. J. Neural Transm. 2017, 117, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Park, J.; Lee, S.Y.; Hwang, I.; Kim, J.B.; Park, T.S.; Koo, S.H. Atypical antipsychotic drugs perturb AMPK-dependent regulation of hepatic lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E624–E632. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Quevedo, J.; Velligan, D.; Roberts, D.L.; Raventos, H.; Walss-Bass, C. Second generation antipsychotic-induced mitochondrial alterations: Implications for increased risk of metabolic syndrome in patients with schizophrenia. Eur. Neuropsychopharmacol. 2018, 28, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Cuturic, M.; Abramson, R.K.; Moran, R.R.; Hardin, J.W. Carnitine and metabolic correlates in hospitalized psychiatric patients: A follow-through report. J. Psychiatr. Pract. 2011, 17, 35–40. [Google Scholar] [CrossRef]
| Parameter | Patients with MetS n = 39 | Patients without MetS n = 73 | Healthy Individuals n = 61 | |
|---|---|---|---|---|
| Gender | Female, n (%) | 19 (48,8%) | 38 (52.1%) | 34 (55.7%) 27 (44.3%) |
| Male, n (%) | 20 (51.2%) | 35 (47.9%) | ||
| Age, years | 40 (30; 48.5) *** | 31 (26; 36.3) | 35 (29; 46.2) ** | |
| Age of manifestation | 26 (19.5; 30.5) | 23 (20; 27) | Na | |
| Duration of disorder, years | 15 (8.5; 20) *** | 7 (3; 12) | Na | |
| PANSS, total score | 92 (82.5; 107.5) | 95 (86; 104) | Na | |
| Duration of antipsychotic therapy, years | 9 (4; 15) * | 5 (2.5; 9.5) | ||
| Total CPZeq | 300 (175; 600) | 324 (262.5; 600) | ||
| Waist, cm | 106 (96.5; 113.5) *** | 83 (76; 90) | Na | |
| Glucose, mmol/L | 5 (4.65; 5.65) *** | 4.7 (4.3; 5.1) | Na | |
| Triglycerides, mmol/L | 2.0 (1.77; 2.35) *** | 1.1 (0.8; 1.4) | Na | |
| HDL-C, mmol/L | 0.82 (0.7; 1.0) *** | 1.1 (0.9; 1.3) | Na | |
| BP is above 130/85 mmHg (or with treatment of hypertension), n (%) | 27 (69.2%) *** | 14 (19.2%) | ||
| Parameter, µM | Patients with MetS n = 73 | Patients without MetS n = 39 | Healthy Individuals n = 61 | p-Value |
|---|---|---|---|---|
| C0 | 14.21 (12.95; 17.43) | 14.29 (11.60; 16.61) | 15.31 (12.54; 17.70) | p0–1 = 0.819 p0–2 = 0.080 p1–2 = 0.282 |
| C2 | 0.687 (0.407; 1.307) | 0.823 (0.679; 1.228) | 0.764 (0.444; 1.136) | p0–1 = 0.276 p0–2 = 0.985 p1–2 = 0.241 |
| C3 | 0.083 (0.068; 0.134) | 0.088 (0.070; 0.115) | 0.103 (0.062; 0.127) | p0–1 = 0.846 p0–2 = 0.176 p1–2 = 0.471 |
| C3-DC | 0.0216 (0.0137; 0.0292) | 0.0231 (0.0137; 0.0304) | 0.0245 (0.0170; 0.0349) | p0–1 = 0.804 p0–2 = 0.402 p1–2 = 0.689 |
| C4 | 0.0543 (0.0464; 0.1046) | 0.0632 (0.0479; 0.0856) | 0.0758 (0.0579; 0.0933) | p0–1 = 0.412 p0–2 = 0.445 p1–2 = 0.974 |
| C4-OH | 0.0035 (0.0015; 0.0064) | 0.0029 (0.0019; 0.0062) | 0.0027 (0.0021; 0.0067) | p0–1 = 0.755 p0–2 = 0.957 p1–2 = 0.974 |
| C4-DC | 0.0093 (0.0062; 0.0106) | 0.0080 (0.0055; 0.0093) | 0.0089 (0.0076; 0.0096) | p0–1 = 0.214 p0–2 = 0.257 p1–2 = 0.141 |
| C5 | 0.028 (0.021; 0.038) | 0.022 (0.017; 0.029) | 0.030 (0.022; 0.038) | p0–1 = 0.557 p0–2 = 0.0001 * p1–2 = 0.033 * |
| C5-OH | 0.0044 (0.0034; 0.0078) | 0.0057 (0.0044; 0.0075) | 0.0060 (0.0048; 0.0077) | p0–1 = 0.145 p0–2 = 0.662 p1–2 = 0.263 |
| C5:1 | 0.0027 (0.0020; 0.0045) | 0.0025 (0.0018; 0.0036) | 0.0045 (0.0037; 0.0065) | p0–1 = 0.126 p0–2 = 0.003 * p1–2 = 0.343 |
| C5-DC | 0.034 (0.020; 0.041) | 0.029 (0.021; 0.035) | 0.033 (0.027; 0.040) | p0–1 = 0.970 p0–2 = 0.779 p1–2 = 0.867 |
| C6 | 0.0110 (0.0065; 0.0194) | 0.0101 (0.0078; 0.0146) | 0.0131 (0.0106; 0.0208) | p0–1 = 0.815 p0–2 = 0.066 p1–2 = 0.172 |
| C8 | 0.0275 (0.0188; 0.0473) | 0.0260 (0.0146; 0.0529) | 0.0373 (0.0234; 0.0500) | p0–1 = 0.240 p0–2 = 0.138 p1–2 = 0.324 |
| C8:1 | 0.0092 (0.0064; 0.0145) | 0.0076 (0.0049; 0.0099) | 0.0082 (0.0064; 0.0102) | p0–1 = 0.465 p0–2 = 0.146 p1–2 = 0.073 |
| C10 | 0.027 (0.015; 0.055) | 0.031 (0.014; 0.045) | 0.045 (0.029; 0.066) | p0–1 = 0.018 * p0–2 = 0.006 * p1–2 = 0.990 |
| C10:1 | 0.026 (0.014; 0.043) | 0.028 (0.015; 0.037) | 0.037 (0.027; 0.053) | p0–1 = 0.009 * p0–2 = 0.003 * p1–2 = 0.995 |
| C12 | 0.014 (0.008; 0.017) | 0.014 (0.007; 0.018) | 0.016 (0.12; 0.24) | p0–1 = 0.046 * p0–2 = 0.033 * p1–2 = 0.886 |
| C14-OH | 0.0017 (0.0032; 0.0021) | 0.0016 (0.0014; 0.0020) | 0.0022 (0.0020; 0.0034) | p0–1 = 0.749 p0–2 = 0.075 p1–2 = 0.110 |
| C14:1 | 0.0154 (0.0108; 0.0267) | 0.0163 (0.0094; 0.0255) | 0.0178 (0.0111; 0.0239) | p0–1 = 0.565 p0–2 = 0.544 p1–2 = 0.989 |
| C14:2 | 0.0101 (0.0060; 0.0163) | 0.0089 (0.0067; 0.0194) | 0.0123 (0.0071; 0.0194) | p0–1 = 0.061 p0–2 = 0.127 p1–2 = 0.631 |
| C16 | 0.0295 (0.0227; 0.0370) | 0.0278 (0.0247; 0.0322) | 0.0292 (0.0236; 0.0335) | p0–1 = 0.966 p0–2 = 0.146 p1–2 = 0.269 |
| C16-OH | 0.0014 (0.0012; 0.0022) | 0.0013 (0.0010; 0.0016) | 0.0015 (0.0011; 0.0019) | p0–1 = 0.708 p0–2 = 0.275 p1–2 = 0.273 |
| C16:1 | 0.0107 (0.0061; 0.0151) | 0.0097 (0.0081; 0.0132) | 0.0116 (0.0066; 0.0161) | p0–1 = 0.954 p0–2 = 0.099 p1–2 = 0.136 |
| C16:1-OH | 0.0013 (0.0010; 0.0024) | 0.0012 (0.0007; 0.0017) | 0.0012 (0.0010; 0.0016) | p0–1 = 0.236 p0–2 = 0.942 p1–2 = 0.190 |
| C18 | 0.0124 (0.0098; 0.0143) | 0.0112 (0.0107; 0.0134) | 0.0159 (0.0130; 0.0182) | p0–1 = 0.003 * p0–2 = 0.0001 * p1–2 = 0.864 |
| C18-OH | 0.0014 (0.0011; 0.0017) | 0.0015 (0.0014; 0.0019) | 0.0016 (0.0010; 0.0019) | p0–1 = 0.319 p0–2 = 0.733 p1–2 = 0.127 |
| C18:1 | 0.057 (0.016; 0.065) | 0.069 (0.045; 0.076) | 0.059 (0.022; 0.084) | p0–1 = 0.649 p0–2 = 0.247 p1–2 = 0.724 |
| C18:1-OH | 0.0031 (0.0020; 0.0037) | 0.0029 (0.0022; 0.0038) | 0.0034 (0.0029; 0.0041) | p0–1 = 0.944 p0–2 = 0.985 p1–2 = 0.927 |
| C18:2-OH | 0.0025 (0.0011; 0.0029) | 0.0013 (0.0008; 0.0022) | 0.0017 (0.0011; 0.0023) | p0–1 = 0.207 p0–2 = 0.199 p1–2 = 0.061 |
| Alanine | 158.75 (131.80; 177.80) | 138.84 (116.97; 168.80) | 142.09 (114.92; 165.82) | p0–1 = 0.044 * p0–2 = 0.780 p1–2 = 0.019 * |
| Valine | 88.36 (73.86; 96.57) | 79.26 (69.24; 90.19) | 83.4 (75.78; 95.18) | p0–1 = 0.456 p0–2 = 0.065 p1–2 = 0.096 |
| Leucine/Isoleucine | 49.58 (42.45; 58.58) | 44.32 (32.81; 50.23) | 44.72 (36.64; 51.67) | p0–1 = 0.098 p0–2 = 0.742 p1–2 = 0.019 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mednova, I.A.; Chernonosov, A.A.; Kornetova, E.G.; Semke, A.V.; Bokhan, N.A.; Koval, V.V.; Ivanova, S.A. Levels of Acylcarnitines and Branched-Chain Amino Acids in Antipsychotic-Treated Patients with Paranoid Schizophrenia with Metabolic Syndrome. Metabolites 2022, 12, 850. https://doi.org/10.3390/metabo12090850
Mednova IA, Chernonosov AA, Kornetova EG, Semke AV, Bokhan NA, Koval VV, Ivanova SA. Levels of Acylcarnitines and Branched-Chain Amino Acids in Antipsychotic-Treated Patients with Paranoid Schizophrenia with Metabolic Syndrome. Metabolites. 2022; 12(9):850. https://doi.org/10.3390/metabo12090850
Chicago/Turabian StyleMednova, Irina A., Alexander A. Chernonosov, Elena G. Kornetova, Arkadiy V. Semke, Nikolay A. Bokhan, Vladimir V. Koval, and Svetlana A. Ivanova. 2022. "Levels of Acylcarnitines and Branched-Chain Amino Acids in Antipsychotic-Treated Patients with Paranoid Schizophrenia with Metabolic Syndrome" Metabolites 12, no. 9: 850. https://doi.org/10.3390/metabo12090850
APA StyleMednova, I. A., Chernonosov, A. A., Kornetova, E. G., Semke, A. V., Bokhan, N. A., Koval, V. V., & Ivanova, S. A. (2022). Levels of Acylcarnitines and Branched-Chain Amino Acids in Antipsychotic-Treated Patients with Paranoid Schizophrenia with Metabolic Syndrome. Metabolites, 12(9), 850. https://doi.org/10.3390/metabo12090850





