Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques
Abstract
:1. Introduction
2. Results and Discussion
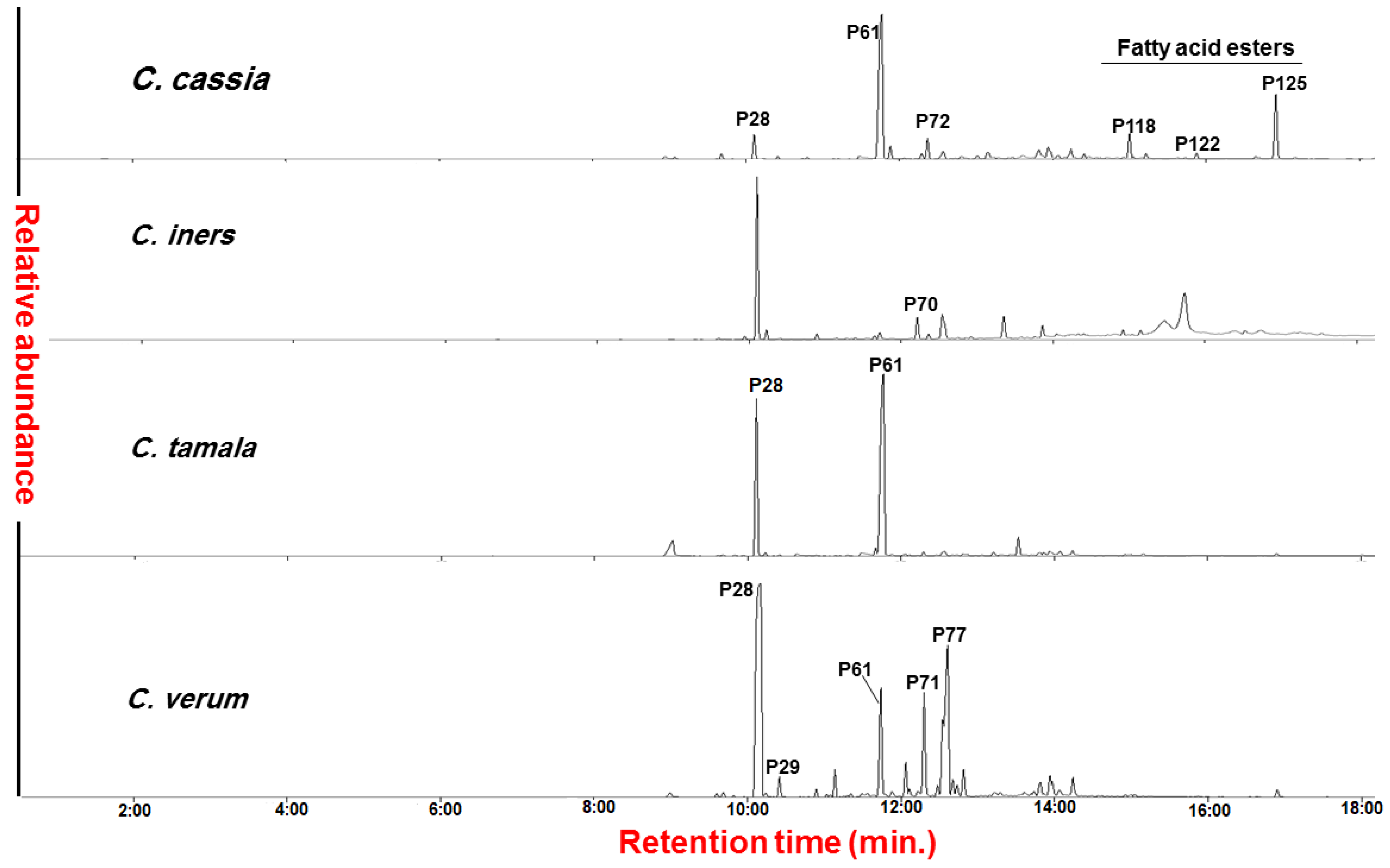
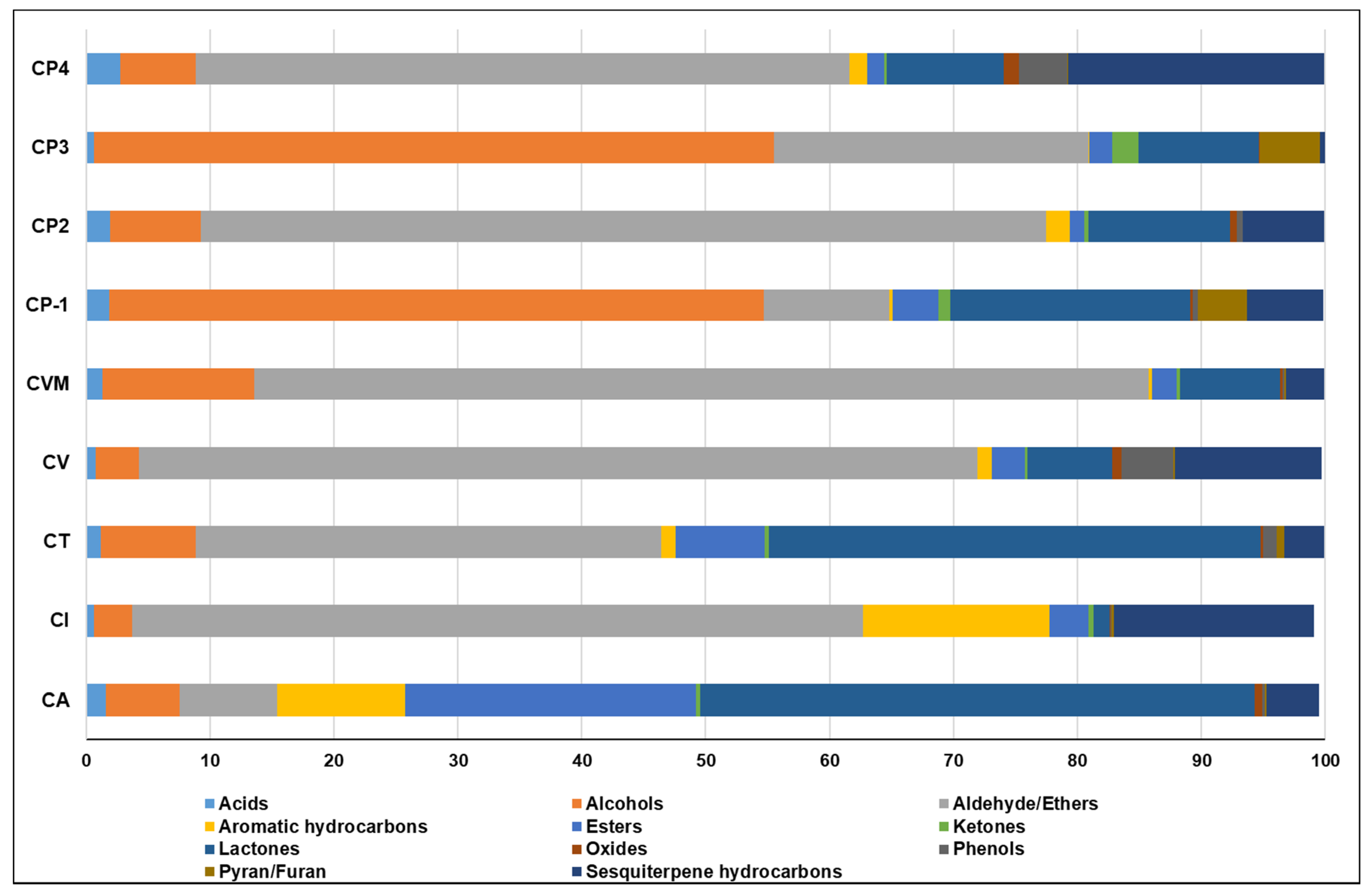
2.1. Analysis of Cinnamon Volatiles by SPME/GC–MS
2.1.1. Aldehydes/Ethers
2.1.2. Alcohols
2.1.3. Sesquiterpene Hydrocarbons
2.1.4. Esters
2.1.5. Lactones
2.1.6. Miscellaneous
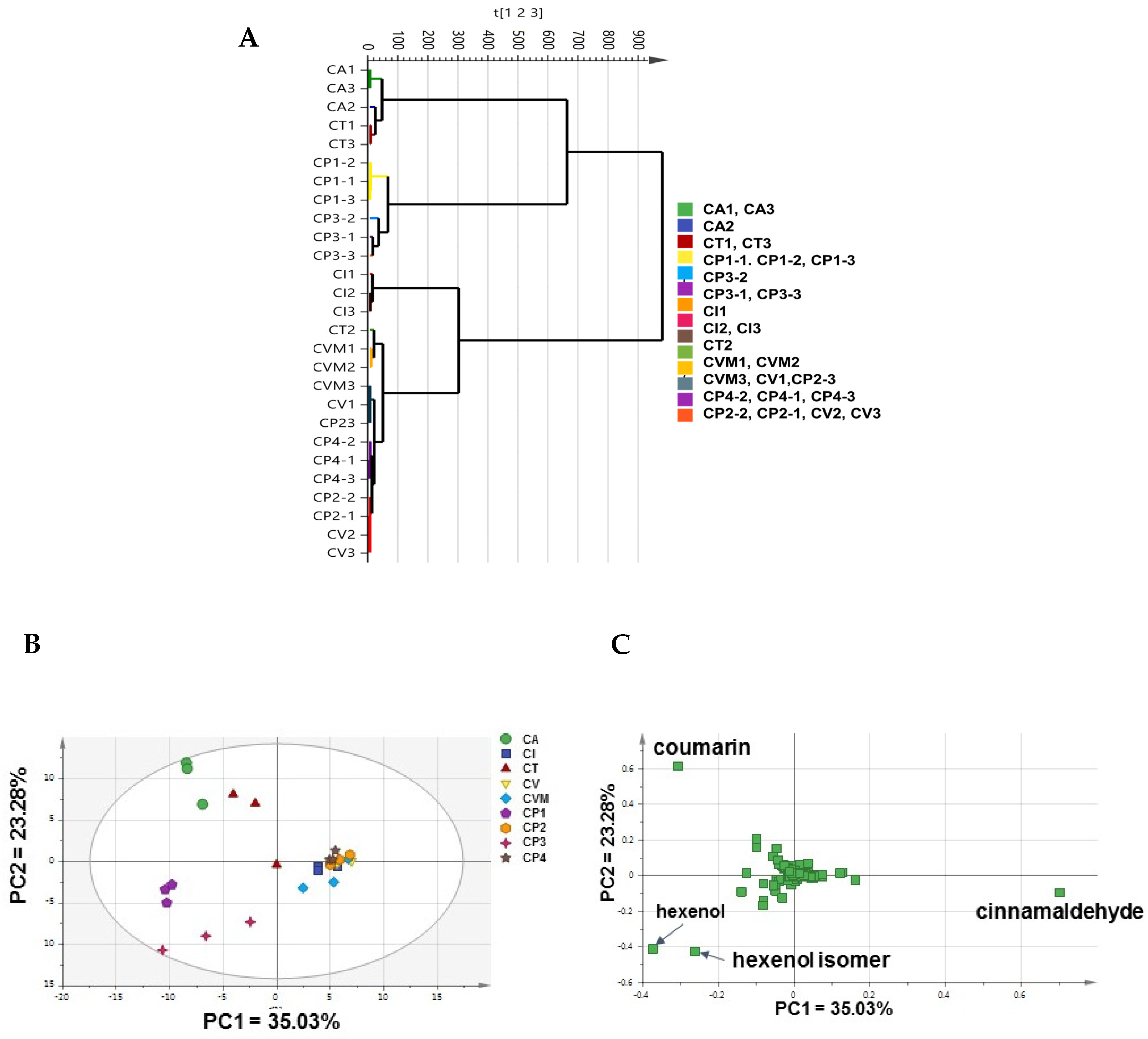
2.2. Unsupervised Analyses of Cinnamon by SPME/GC–MS
2.2.1. Analysis of the Cinnamon Authenticated Drugs and Commercial Preparations VOCs Dataset
2.2.2. Analysis of Authenticated Cinnamon Drugs VOCs Dataset
2.2.3. Analysis of the Commercial Cinnamon Products VOCs Dataset
2.3. Supervised Analyses of Cinnamon VOCs Dataset by SPME/GC–MS
2.3.1. OPLS-DA of Authenticated Cinnamon Drugs
2.3.2. OPLS-DA Analysis for Adulteration and Markers Detection
2.4. Fingerprinting of Cinnamon NMR Dataset
2.4.1. Primary Metabolites
2.4.2. Secondary Metabolites
2.4.3. Quantification of Major Metabolites via 1H-NMR
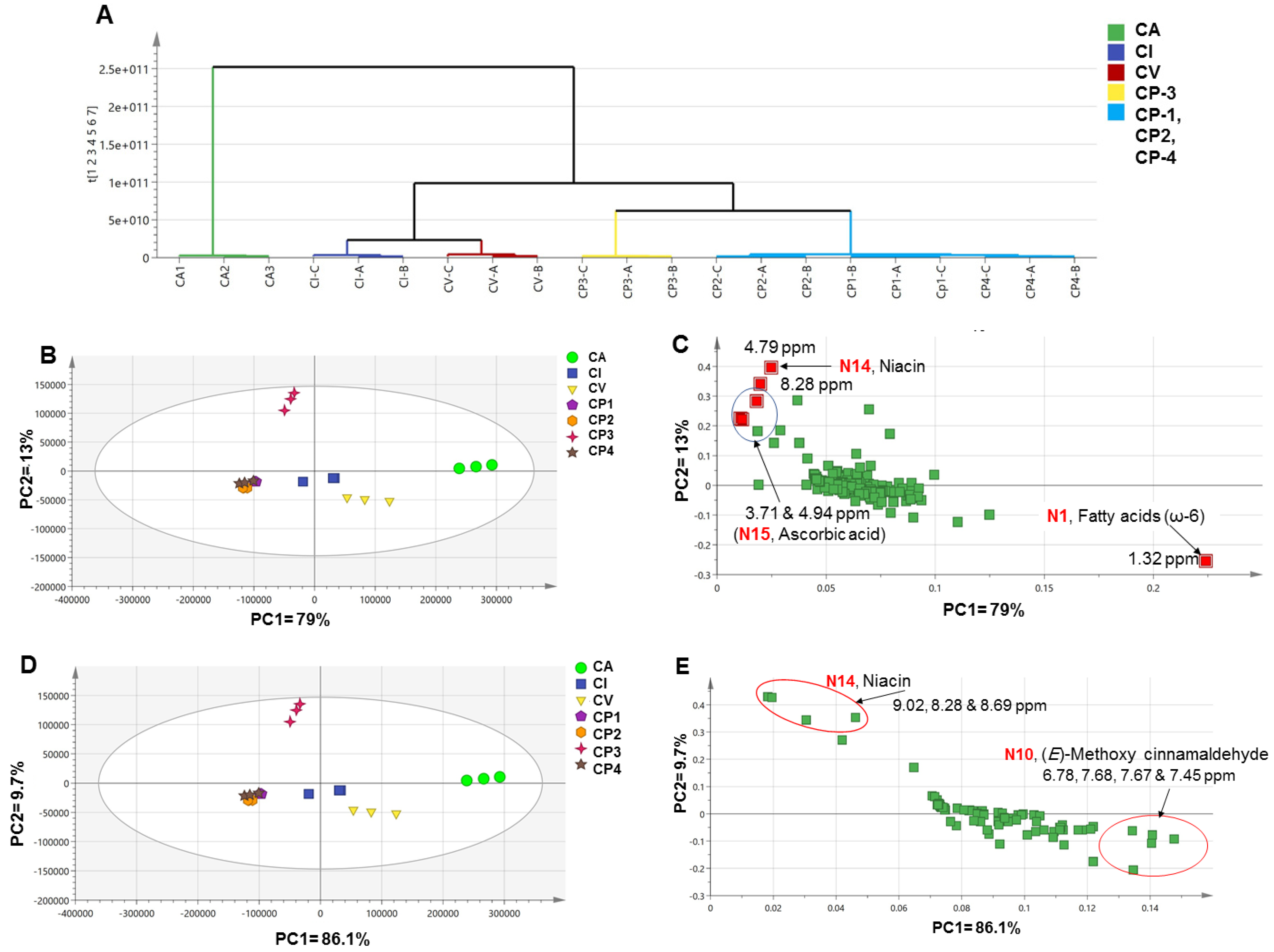
2.4.4. Unsupervised Analyses of Cinnamon Dataset by NMR
Analysis of the Whole Samples’ NMR Dataset
Analysis of Commercial Samples’ NMR Dataset
2.4.5. Supervised Analysis of Cinnamon NMR Dataset
2.5. Fingerprinting of Cinnamon Drugs Using UV/Vis
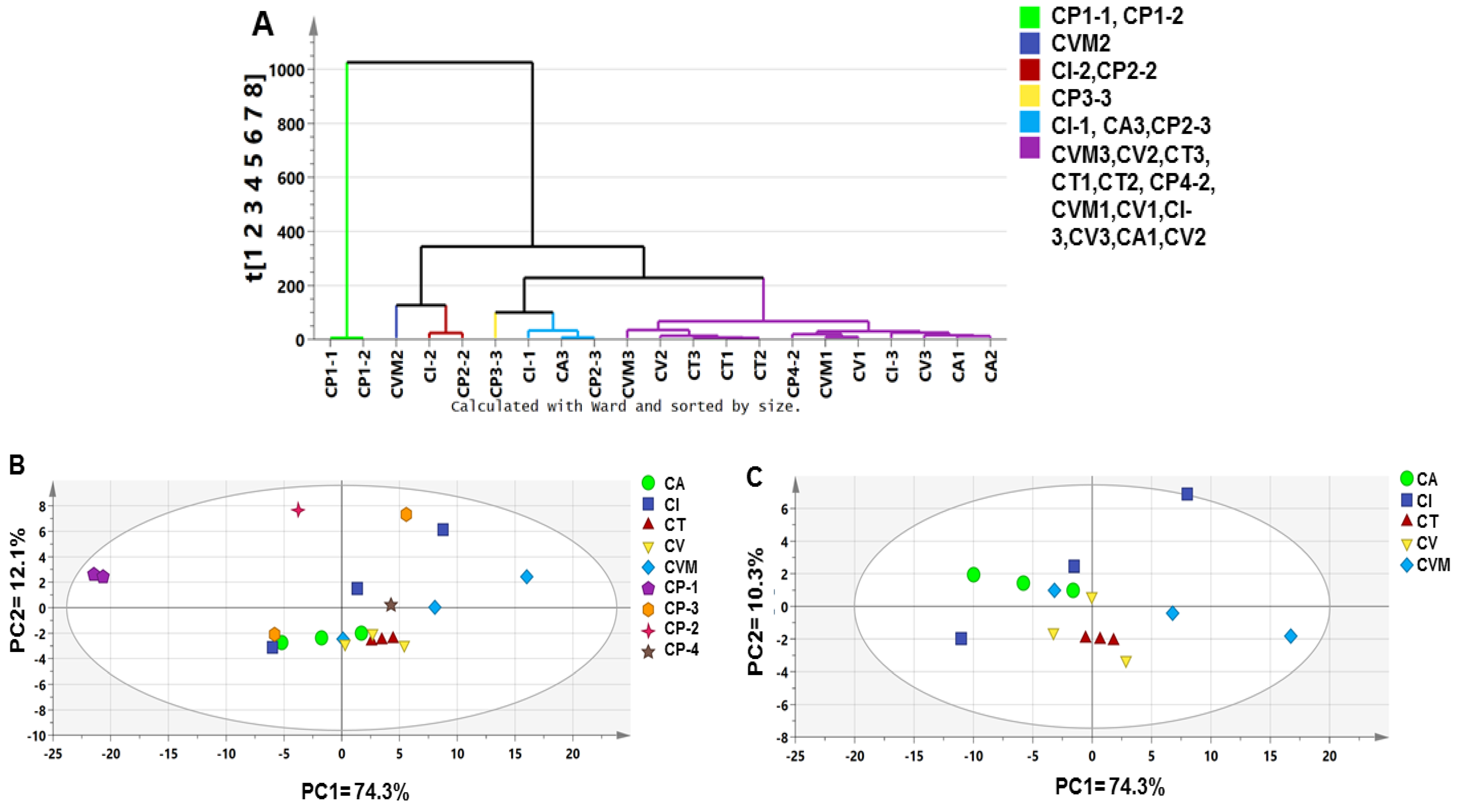
2.5.1. Unsupervised Multivariate Data Analyses of Cinnamon UV/Vis Dataset
2.5.2. Supervised Multivariate Data Analysis of Cinnamon UV/Vis Dataset
3. Materials and Methods
3.1. Analyzed Cinnamon Samples and Origins
3.2. Chemicals, Fibers, and Volatiles Analysis by SPME Coupled to GC/MS
3.3. NMR Analysis and Sample Extraction
3.4. NMR Data Acquisition
3.5. Quantification of Major Metabolites via 1H-NMR
- MT: Molecular weight of the target compound (g/mol),
- IT: Relative integral value of the 1H-NMR signal of the target compound,
- ISt: Relative integral value of the 1H-NMR signal of the standard compound,
- XSt: Number of protons belonging to the 1H-NMR signal of the standard compound,
- XT: Number of protons belonging to the 1H-NMR signal of the target compound,
- CS: Concentration of the standard compound in the solution used for 1H-NMR measurement (mmol/L), and
- VSt: Volume of solution used for 1H-NMR measurement (mL).
3.6. UV/Vis Analysis
3.7. Metabolites Identification, Data Processing and Multivariate Analysis
3.7.1. SPME Coupled to GC/MS Analysis
3.7.2. NMR Dataset Modeling
3.7.3. UV/Vis Dataset Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, P.; Nirmal-Babu, K.; Shylaja, M. Cinnamon and Cassia: The Genus Cinnamomum; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complementary Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 139. [Google Scholar] [CrossRef]
- Husain, I.; Ahmad, R.; Chandra, A.; Raza, S.T.; Shukla, Y.; Mahdi, F. Phytochemical characterization and biological activity evaluation of ethanolic extract of Cinnamomum zeylanicum. J. Ethnopharmacol. 2018, 219, 110–116. [Google Scholar] [CrossRef]
- Chen, P.; Sun, J.; Ford, P. Differentiation of the four major species of cinnamons (C. burmannii, C. verum, C. cassia, and C. loureiroi) using a flow injection mass spectrometric (FIMS) fingerprinting method. J. Agric. Food Chem. 2014, 62, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Stevens, N.; Allred, K. Antidiabetic Potential of Volatile Cinnamon Oil: A Review and Exploration of Mechanisms Using In Silico Molecular Docking Simulations. Molecules 2022, 27, 853. [Google Scholar] [CrossRef]
- Zayed, A.; Sobeh, M.; Farag, M.A. Dissecting dietary and semisynthetic volatile phenylpropenes: A compile of their distribution, food properties, health effects, metabolism and toxicities. Crit. Rev. Food Sci. Nutr. 2022; 1–20, in press. [Google Scholar] [CrossRef]
- Wang, Y.H.; Avula, B.; Nanayakkara, N.P.; Zhao, J.; Khan, I.A. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470–4476. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chao, J.; Sun, Z.; Chang, R.C.-C.; Tse, I.; Li, E.T.S.; Chen, F.; Wang, M. Beneficial Effects of Cinnamon Proanthocyanidins on the Formation of Specific Advanced Glycation Endproducts and Methylglyoxal-Induced Impairment on Glucose Consumption. J. Agric. Food Chem. 2010, 58, 6692–6696. [Google Scholar] [CrossRef]
- Wariyapperuma, W.; Kannangara, S.; Wijayasinghe, Y.S.; Subramanium, S.; Jayawardena, B. In Vitro anti-diabetic effects and phytochemical profiling of novel varieties of Cinnamomum zeylanicum (L.) extracts. PeerJ 2020, 8, e10070. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Galappaththy, P.; Constantine, G.R.; Jayawardena, R.; Weeratunga, H.D.; Premakumara, S.; Katulanda, P. Cinnamomum zeylanicum (Ceylon cinnamon) as a potential pharmaceutical agent for type-2 diabetes mellitus: Study protocol for a randomized controlled trial. Trials 2017, 18, 446. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Jakubowska, M.; Łukaszyk, J.; Krzymińska, J. Cinnamon as a Useful Preventive Substance for the Care of Human and Plant Health. Molecules 2021, 26, 5299. [Google Scholar] [CrossRef] [PubMed]
- Kallel, I.; Hadrich, B.; Gargouri, B.; Chaabane, A.; Lassoued, S.; Gdoura, R.; Bayoudh, A.; Ben Messaoud, E. Optimization of cinnamon (Cinnamomum zeylanicum Blume) essential oil extraction: Evaluation of antioxidant and antiproliferative effects. Evid. Based Complementary Altern. Med. 2019, 2019, 6498347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, M.A.; Dokalahy, E.U.; Eissa, T.F.; Kamal, I.M.; Zayed, A. Chemometrics-Based Aroma Discrimination of 14 Egyptian Mango Fruits of Different Cultivars and Origins, and Their Response to Probiotics Analyzed via SPME Coupled to GC–MS. ACS Omega 2022, 7, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, E.A.; Zayed, A.; Laub, A.; Modolo, L.V.; Wessjohann, L.; Farag, M.A. How Does LC/MS Compare to UV in Coffee Authentication and Determination of Antioxidant Effects? Brazilian and Middle Eastern Coffee as Case Studies. Antioxidants 2022, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Abdelwareth, A.; Mohamed, T.A.; Fahmy, H.A.; Porzel, A.; Wessjohann, L.A.; Farag, M.A. Dissecting coffee seeds metabolome in context of genotype, roasting degree, and blending in the Middle East using NMR and GC/MS techniques. Food Chem. 2022, 373, 131452. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Shiekh, R.A.; Fernie, A.R.; Alseekh, S.; Zayed, A. Metabolomics-based profiling for quality assessment and revealing the impact of drying of Turmeric (Curcuma longa L.). Sci. Rep. 2022, 12, 10288. [Google Scholar] [CrossRef]
- Salem, M.A.; Zayed, A.; Alseekh, S.; Fernie, A.R.; Giavalisco, P. The integration of MS-based metabolomics and multivariate data analysis allows for improved quality assessment of Zingiber officinale Roscoe. Phytochemistry 2021, 190, 112843. [Google Scholar] [CrossRef]
- Farag, M.A.; Zayed, A.; Sallam, I.E.; Abdelwareth, A.; Wessjohann, L.A. Metabolomics-based approach for coffee beverage improvement in the context of processing, brewing methods, and quality attributes. Foods 2022, 11, 864. [Google Scholar] [CrossRef]
- Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative metabolite fingerprinting of four different cinnamon species analyzed via UPLC-MS and GC-MS and chemometric tools. Molecules 2022, 27, 2935. [Google Scholar] [CrossRef]
- Zouaoui, N.; Chenchouni, H.; Bouguerra, A.; Massouras, T.; Barkat, M. Characterization of volatile organic compounds from six aromatic and medicinal plant species growing wild in North African drylands. NFS J. 2020, 18, 19–28. [Google Scholar] [CrossRef]
- Abdelwareth, A.; Zayed, A.; Farag, M.A. Chemometrics-based aroma profiling for revealing origin, roasting indices, and brewing method in coffee seeds and its commercial blends in the Middle East. Food Chem. 2021, 349, 129162. [Google Scholar] [CrossRef] [PubMed]
- Szelényi, M.O.; Erdei, A.L.; Jósvai, J.K.; Radványi, D.; Sümegi, B.; Vétek, G.; Molnár, B.P.; Kárpáti, Z. Essential oil headspace volatiles prevent invasive box tree moth (Cydalima perspectalis) oviposition-insights from electrophysiology and behaviour. Insects 2020, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Sun, A.; Liu, X. Chemical compound identification and antibacterial activity evaluation of cinnamon extracts obtained by subcritical n-butane and ethanol extraction. Food Sci. Nutr. 2019, 7, 2186–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.D.; Qiao, C.F.; Han, Q.B.; Cheng, C.L.; Xu, H.X.; Jiang, R.W.; But, P.P.; Shaw, P.C. Authentication and quantitative analysis on the chemical profile of cassia bark (cortex cinnamomi) by high-pressure liquid chromatography. J. Agric. Food Chem. 2005, 53, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zong, S.-B.; Li, J.-C.; Lv, Y.-Z.; Liu, L.-N.; Wang, Z.-Z.; Zhou, J.; Cao, L.; Kou, J.-P.; Xiao, W. The essential oil from the twigs of Cinnamomum cassia Presl alleviates pain and inflammation in mice. J. Ethnopharmacol. 2016, 194, 904–912. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Wang, R.; Wang, R.; Yang, B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. Technol. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum zeylanicum Bark Essential Oil. Evid. Based Complementary Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Nie, C.-n.; Gao, Y.; Du, X.; Bian, J.-l.; Li, H.; Zhang, X.; Wang, C.-m.; Li, S.-y. Characterization of the effect of cis-3-hexen-1-ol on green tea aroma. Sci. Rep. 2020, 10, 15506. [Google Scholar] [CrossRef]
- Farias, A.P.P.; Monteiro, O.D.S.; da Silva, J.K.R.; Figueiredo, P.L.B.; Rodrigues, A.A.C.; Monteiro, I.N.; Maia, J.G.S. Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil. J. Food Sci. Technol. 2020, 57, 3176–3183. [Google Scholar] [CrossRef]
- FAO. Online Edition: “Specifications for Flavourings”. Available online: https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-flav/details/en/c/814/ (accessed on 1 March 2022).
- Wei, L.; Lin, M.; Han, B.; Deng, X.; Hou, W.; Liao, Q.; Xie, Z. The Comparison of Cinnamomi Cortex and Cinnamomum burmannii Blume Using 1H NMR and GC-MS Combined with Multivariate Data Analysis. Food Anal. Methods 2016, 9, 2419–2428. [Google Scholar] [CrossRef]
- Anju, R.; Sunitha, M.C.; Nevin, K.G. Cinnamon extract enhances the mitochondrial reactive oxygen species production and arrests the proliferation of human colon cancer cell line, HCT-116. J. Herbs Spices Med. Plants 2018, 24, 293–301. [Google Scholar] [CrossRef]
- Saeed, N.M.; El-Demerdash, E.; Abdel-Rahman, H.M.; Algandaby, M.M.; Al-Abbasi, F.A.; Abdel-Naim, A.B. Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicol. Appl. Pharmacol. 2012, 264, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ramadan, N.S.; Shorbagi, M.; Farag, N.; Gad, H.A. Profiling of primary metabolites and volatiles in apricot (Prunus armeniaca L.) seed kernels and fruits in the context of its different cultivars and soil type as analyzed using chemometric tools. Foods 2022, 11, 1339. [Google Scholar] [CrossRef]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Buzjak Služek, V.; Cindrić, I.; Molnar, M. Coumarins in Food and Methods of Their Determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A. Single Origin Coffee Aroma: From Optimized Flavor Protocols and Coffee Customization to Instrumental Volatile Characterization and Chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Shawky, E.; Selim, D.A. Rapid Authentication and Quality Evaluation of Cinnamomum verum Powder Using Near-Infrared Spectroscopy and Multivariate Analyses. Planta Med. 2018, 84, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Afifi, S.M.; Rasheed, D.M.; Khattab, A.R. Revealing compositional attributes of Glossostemon bruguieri Desf. root geographic origin and roasting impact via chemometric modeling of SPME-GC-MS and NMR metabolite profiles. J. Food Compos. Anal. 2021, 102, 104073. [Google Scholar] [CrossRef]
- Farag, M.A.; Labib, R.M.; Noleto, C.; Porzel, A.; Wessjohann, L.A. NMR approach for the authentication of 10 cinnamon spice accessions analyzed via chemometric tools. LWT 2018, 90, 491–498. [Google Scholar] [CrossRef]
- Castellano, S.; Sun, C.; Kostelnik, R. Analysis of the NMR spectrum of pyridine. J. Chem. Phys. 1967, 46, 327–330. [Google Scholar] [CrossRef]
- Eiff, J.; Monakhova, Y.B.; Diehl, B.W.K. Multicomponent analysis of fat- and water-soluble vitamins and auxiliary substances in multivitamin preparations by qNMR. J. Agric. Food Chem. 2015, 63, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Vaysse, J.; Balayssac, S.; Gilard, V.; Desoubdzanne, D.; Malet-Martino, M.; Martino, R. Analysis of adulterated herbal medicines and dietary supplements marketed for weight loss by DOSY 1H-NMR. Food Addit. Contaminants. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 903–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawardena, D.; Karunaweera, N.; Lee, S.; van Der Kooy, F.; Harman, D.G.; Raju, R.; Bennett, L.; Gyengesi, E.; Sucher, N.J.; Münch, G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts-identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015, 6, 910–919. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; He, F.; Zhang, Y.; Wang, F.; Zheng, X.; Dai, Z.; Ma, S. Formation of cinnamaldehyde dimethyl acetal in methanol during analysis. J. Essent. Oil Res. 2021, 33, 308–313. [Google Scholar] [CrossRef]
- Khalil, M.N.A.; Fekry, M.I.; Farag, M.A. Metabolome based volatiles profiling in 13 date palm fruit varieties from Egypt via SPME GC–MS and chemometrics. Food Chem. 2017, 217, 171–181. [Google Scholar] [CrossRef]

| Authenticated Cinnamon Drugs | Cinnamon Commercial Products | |||||
|---|---|---|---|---|---|---|
| Sample Code | Botanical Origin | Geographical Origin | Sample Code | Trade Name | Dose (mg)/Capsule | Cinnamon Composition |
| CA | Cinamomum cassia | Malaysia | CP-1 | Diabetruw® | 112 | C. cassia |
| CVM | Cinamomum verum | CP-2 | Cinnamon Bark (GNC) | 500 | C. burmannii & C. cassia | |
| CI | Cinnamomum iners | CP-3 | Superfoods Κανέλα Extra | 110 | - C. cassia cortex - Vitamin C (60 mg) - Vitamin E (13.7 mg) - Vitamin B3 (Niacin) (18.1 mg) - Vitamin B6 (1.0 mg) - Others (Zinc, Manganese, Chromium) (7.1 mg) | |
| CT | Cinnamomum tamala | Pakistan | CP-4 | Spring Valley Cinnamon | 500 | Organic cinnamon (Cinnamomum sp. is not stated) |
| CV | Cinnamomum verum | |||||
| Metabolite Name | Protons Used for Quantification | Authenticated Cinnamon Products (μg/mg Dry Powder ± S.D) | Commercial Cinnamon Preparations (μg/mg Dry Powder ± S.D) | |||||
|---|---|---|---|---|---|---|---|---|
| CV | CA | CI | CP-1 | CP-2 | CP-3 | CP-4 | ||
| Glycerol (N2) | H1/3 | 2.4 ± 0.1 b | 4.9 ± 0.2 a | 4.7 ± 0.5 a | 0.0 d | 0.8 ± 0.1 c | 0.0 d | 0.9 ± 0.1 c |
| β-glucose (N3) | H-1 | 4.5 ± 0.2 b | 8.1 ± 0.8 a | 4.9 ± 0.4 b | 2.3 ± 0.1 c | 2.0 ± 0.1 c | 2.2 ± 0.1 c | 2.2 ± 0.3 c |
| α-glucose (N4) | H-1 | 3.0 ± 0.3 b | 5.2 ± 0.5 a | 3.0 ± 0.4 b | 2.2 ± 0.1 c | 1.4 ± 0.2 d | nd | 1.5 ± 0.1 d |
| Fructose (N5) | H-5 | 6.2 ± 0.4 c | 13.1 ± 0.4 a | 8.0 ± 0.8 b | 3.4 ± 0.1 d | 2.7 ± 0.1 d | nd | 3.3 ± 0.3 d |
| Sucrose (N6) | H-1 | nd | 5.1 ± 0.3 b | 3.7 ± 0.3 c | 9.0 ± 0.7 a | nd | nd | nd d |
| Total sugars | 16.1 ± 0.9 c | 36.3 ± 2.3 a | 24.4 ± 2.5 b | 16.8 ± 0.8 c | 6.9 ± 0.9 d | 2.2 ± 0.1 e | 7.8 ± 0.7 d | |
| (Z)-Cinnamic acid (N7) | H-2 | 4.2 ± 0.5 b | 7.0 ± 0.1 a | 6.9 ± 0.5 a | 2.6 ± 0.1 c | 2.0 ± 0.1 d | nd | 2.4 ± 0.1 c d |
| (E)-Cinnamic acid (N8) | H-2 | 6.4 ± 0.6 b | 7.5 ± 0.2 a | 6.0 ± 0.6 b | 3.1 ± 0.2 c | 2.5 ± 0.1 d | nd | 2.8 ± 0.1 c d |
| (E)-Cinnamaldehyde (N9) | H-2 | 13.7 ± 0.7 b | 19.3 ± 1.0 a | 11.0 ± 1.0 c | 6.4± 0.1 d | 4.9 ± 0.2 e | nd | 7.3 ± 0.7 d |
| (E)-Methoxy cinnamaldehyde (N10) | H-2 | 11.2 ± 0.9 a | nd | nd | nd | nd | nd | nd |
| Cinnamaldehyde dimethyl acetal (N11) | H-2 | 10.1 ± 3.1 a | 9.6 ± 0.3 a | 6.5 ± 0.7 b | nd | nd | nd | nd |
| Total cinnamates | 45.7 ± 5.8 a | 43.5 ± 1.4 a | 30.4 ± 2.7 b | 12.1 ± 0.2 c | 9.3 ± 0.3 c | nd | 12.5 ± 0.9 c | |
| Protocatechuic acid (N12) | H-6 | 2.9 ± 0.6 b | 4.2 ± 0.4 a | 4.2 ± 0.1 a | nd | nd | nd | nd |
| Coumarin (N13) | H-5 | nd | 10.4 ± 0.6 b | 13.8 ± 1.5 a | 3.2 ± 0.1 c | 2.7 ± 0.1 c | nd | 3.0 ± 0.3 c |
| Vitamin B3 (Niacin, N14) | H-2 | nd | nd | nd | nd | nd | 14.2 ± 1.6 a | nd |
| Vitamin C (Ascorbic acid, N15) | H-6 | nd | nd | nd | nd | nd | 44.5 ± 3.4 a | nd |
| Vitamin E (α-Tocopherol, N16) | C5-CH3 | nd | nd | nd | nd | nd | 5.4 ± 0.2 a | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Khaled, S.E.; El Gingeehy, Z.; Shamma, S.N.; Zayed, A. Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques. Metabolites 2022, 12, 614. https://doi.org/10.3390/metabo12070614
Farag MA, Khaled SE, El Gingeehy Z, Shamma SN, Zayed A. Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques. Metabolites. 2022; 12(7):614. https://doi.org/10.3390/metabo12070614
Chicago/Turabian StyleFarag, Mohamed A., Sally E. Khaled, Zeina El Gingeehy, Samir Nabhan Shamma, and Ahmed Zayed. 2022. "Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques" Metabolites 12, no. 7: 614. https://doi.org/10.3390/metabo12070614
APA StyleFarag, M. A., Khaled, S. E., El Gingeehy, Z., Shamma, S. N., & Zayed, A. (2022). Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques. Metabolites, 12(7), 614. https://doi.org/10.3390/metabo12070614