NMR-Based Metabolomic Analysis of Plasma in Patients with Adult Congenital Heart Disease and Associated Pulmonary Arterial Hypertension: A Pilot Study
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Population
2.2. Blood Samples Collection and Preparation
2.3. NMR Measurements
2.4. NMR Data Processing
2.5. Multivariate and Univariate Statistical Analyses
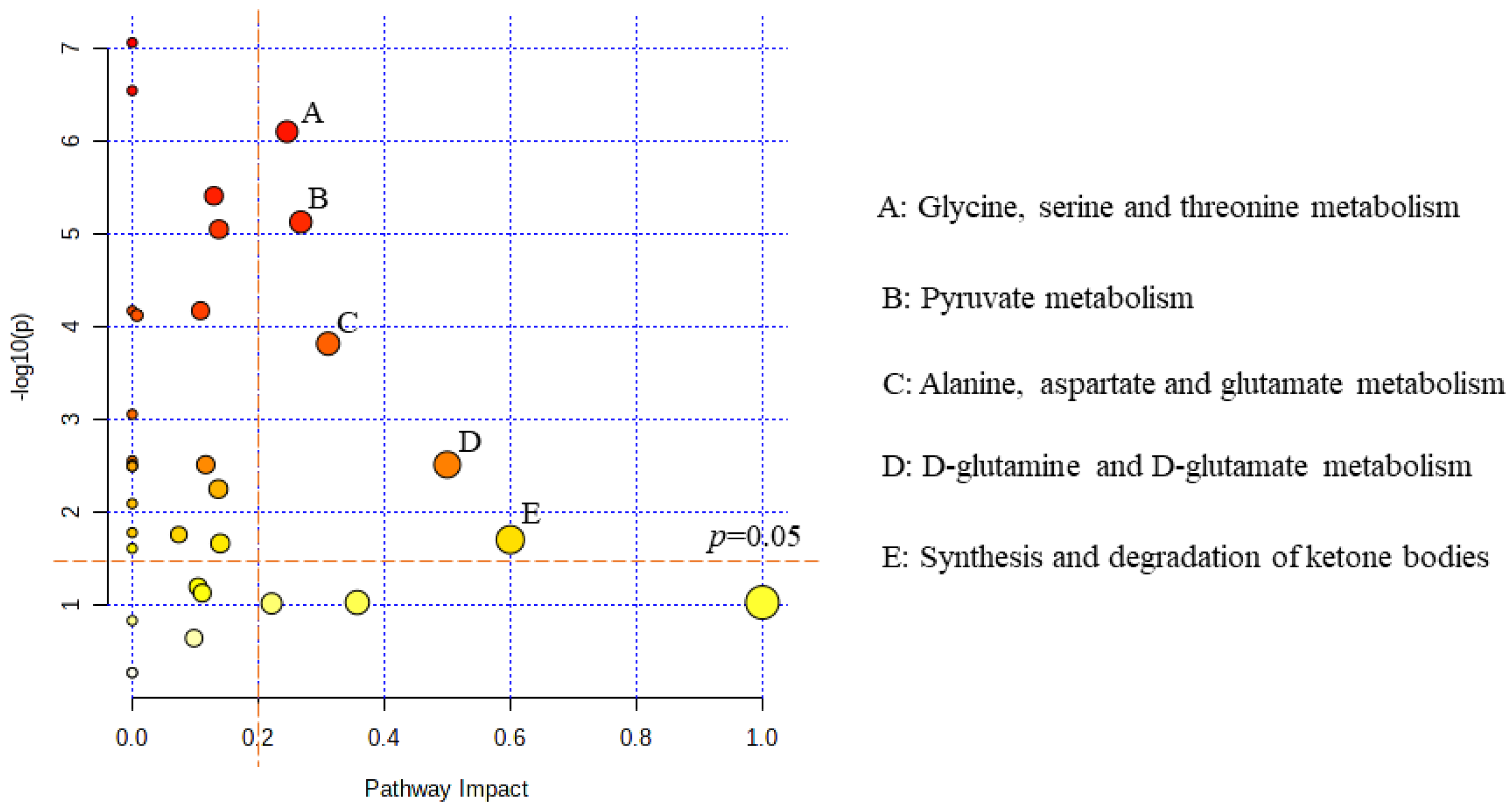
2.6. Metabolic Pathway Analysis
2.7. General Statistical Analysis
3. Results
3.1. Characterization of Subjects in Three Cohorts
3.2. Resonance Assignments of Metabolites
3.3. Metabolic Shifting Induced by Increased Pulmonary Blood Flow
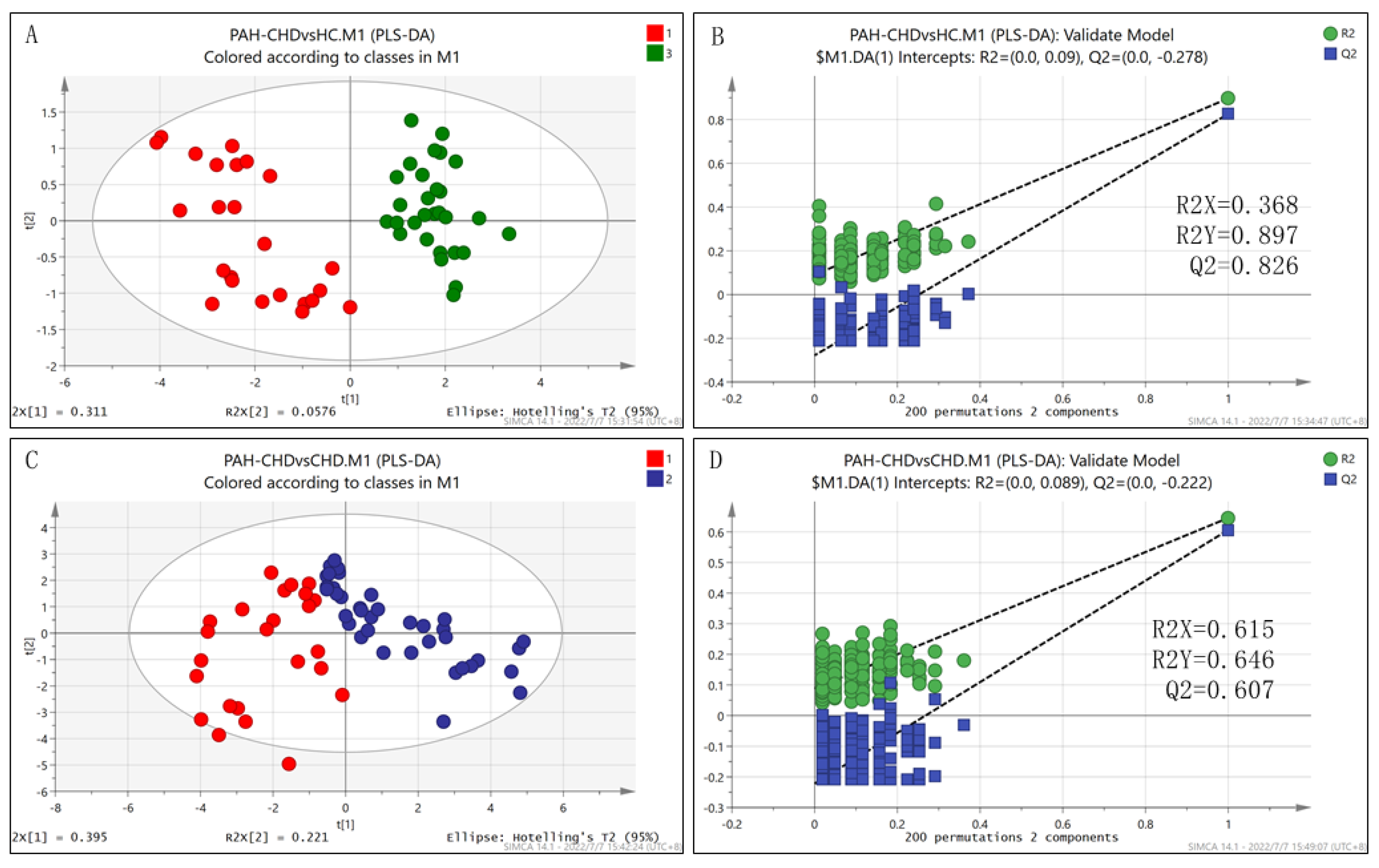
3.4. The Differential Metabolic Pattern Distinguishing PAH-CHD from CHD
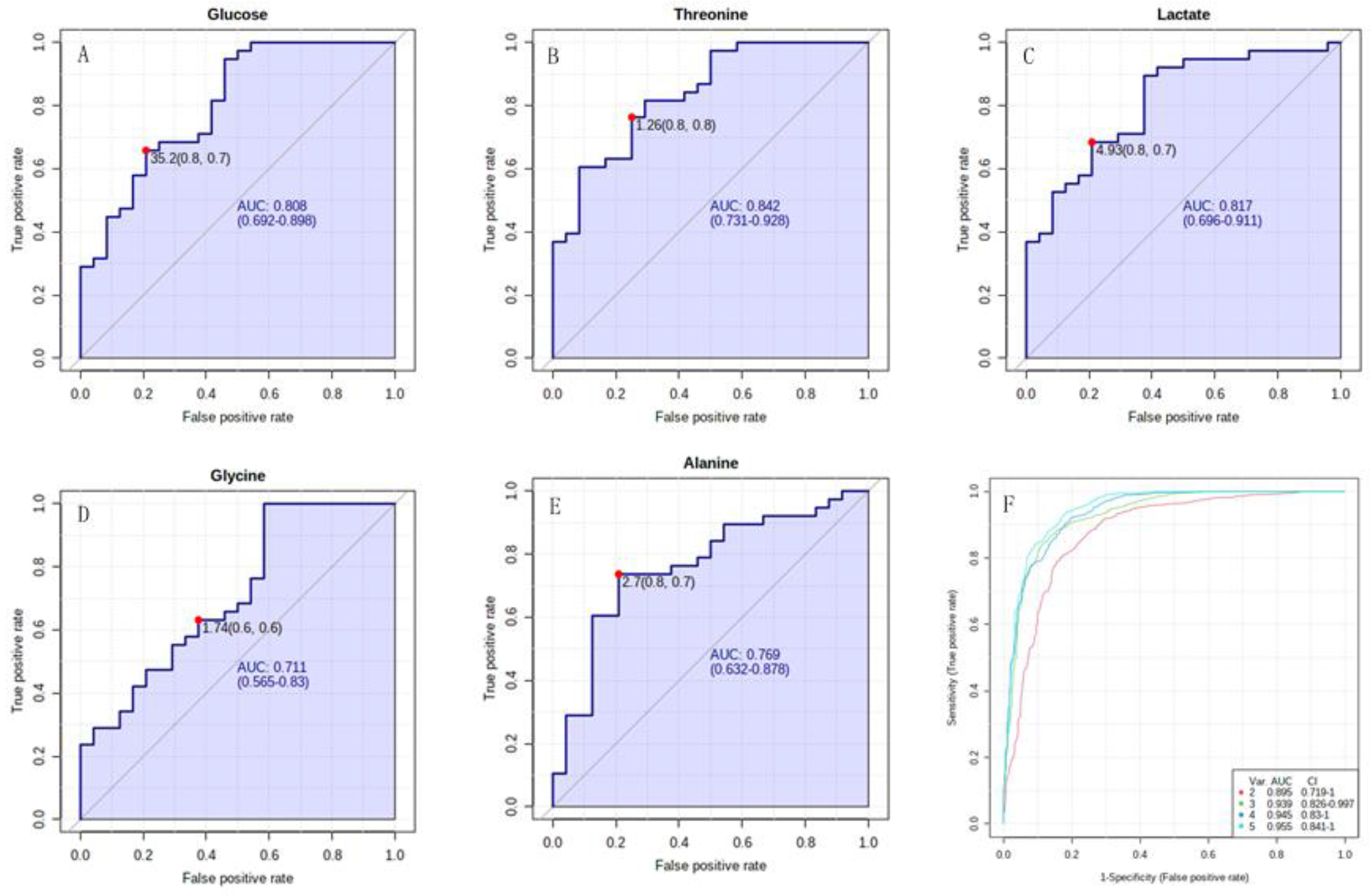
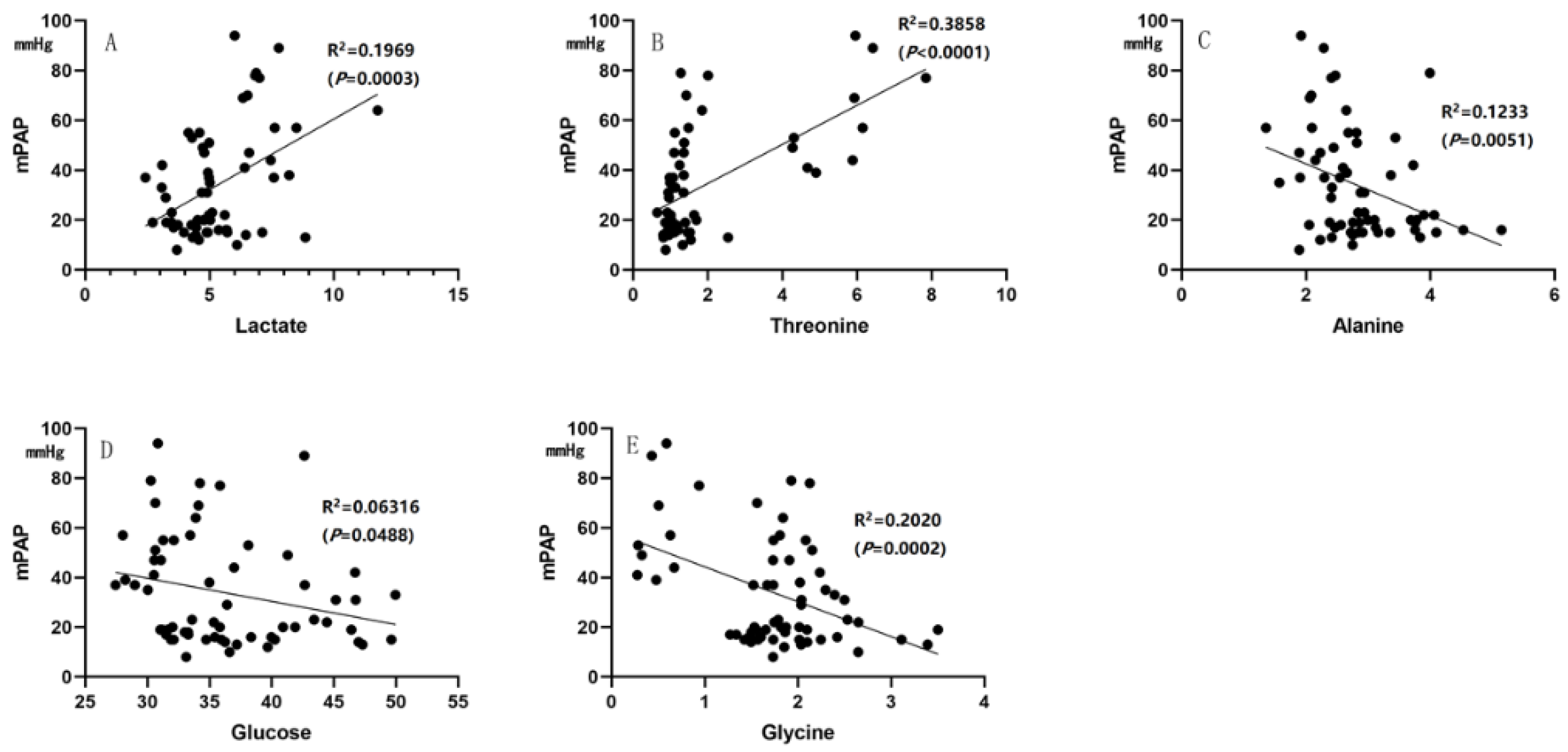
3.5. The Characteristic Metabolites Were Correlated with Diagnosis and Prognosis of PAH-CHD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Johnson Kameny, R.; Datar, S.A.; Boehme, J.B.; Morris, C.; Zhu, T.; Goudy, B.D.; Johnson, E.G.; Galambos, C.; Raff, G.W.; Sun, X.; et al. Ovine models of congenital heart disease and the consequences of hemodynamic alterations for pulmonary artery remodeling. Am. J. Respir. Cell Mol. Biol. 2019, 60, 503–514. [Google Scholar] [CrossRef]
- van der Feen, D.E.; Bartelds, B.; de Boer, R.A.; Berger, R.M.F. Pulmonary arterial hypertension in congenital heart disease: Translational opportunities to study the reversibility of pulmonary vascular disease. Eur. Heart J. 2017, 38, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Favoccia, C.; Constantine, A.H.; Wort, S.J.; Dimopoulos, K. Eisenmenger syndrome and other types of pulmonary arterial hypertension related to adult congenital heart disease. Expert. Rev. Cardiovasc. Ther. 2019, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Manes, A.; Palazzini, M.; Leci, E.; Bacchi Reggiani, M.L.; Branzi, A.; Galiè, N. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: A comparison between clinical subgroups. Eur. Heart J. 2014, 35, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Boehme, J.; Sun, X.; Tormos, K.V.; Gong, W.; Kellner, M.; Datar, S.A.; Kameny, R.J.; Yuan, J.X.; Raff, G.W.; Fineman, J.R.; et al. Pulmonary artery smooth muscle cell hyperproliferation and metabolic shift triggered by pulmonary overcirculation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H944–H957. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, F.; Wu, P.; Huang, Y.; Das, A.; Chen, S.; Chen, J.; Hu, X.; Li, F.; Fang, Z.; et al. Metabolomics reveals metabolite changes of patients with pulmonary arterial hypertension in China. J. Cell Mol. Med. 2020, 24, 2484–2496. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Ghataorhe, P.; Wharton, J.; Rue-Albrecht, K.C.; Hadinnapola, C.; Watson, G.; Bleda, M.; Haimel, M.; Coghlan, G.; Corris, P.A.; et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017, 135, 460–475. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, J.; Lu, C.; Hsin, M.; Mura, M.; Wu, L.; Chu, L.; Zamel, R.; Machuca, T.; Waddell, T.; et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS ONE 2014, 9, e88727. [Google Scholar] [CrossRef]
- Sakao, S.; Kawakami, E.; Shoji, H.; Naito, A.; Miwa, H.; Suda, R.; Sanada, T.J.; Tanabe, N.; Tatsumi, K. Metabolic remodeling in the right ventricle of rats with severe pulmonary arterial hypertension. Mol. Med. Rep. 2021, 23, 227. [Google Scholar] [CrossRef]
- Xu, W.; Janocha, A.J.; Erzurum, S.C. Metabolism in Pulmonary Hypertension. Annu. Rev. Physiol. 2021, 83, 551–576. [Google Scholar] [CrossRef]
- Tulevski, I.I.; Groenink, M.; van Der Wall, E.E.; van Veldhuisen, D.J.; Boomsma, F.; Stoker, J.; Hirsch, A.; Lemkes, J.S.; Mulder, B.J. Increased brain and atrial natriuretic peptides in patients with chronic right ventricular pressure overload: Correlation between plasma neurohormones and right ventricular dysfunction. Heart 2001, 86, 27–30. [Google Scholar] [PubMed]
- Brindle, J.T.; Antti, H.; Holmes, E.; Tranter, G.; Nicholson, J.K.; Bethell, H.W.; Clarke, S.; Schofield, P.M.; McKilligin, E.; Mosedale, D.E.; et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 2002, 8, 1439–1444. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, J.; Bao, Y.; Chen, T.; Zhang, Y.; Zhao, A.; Qiu, Y.; Xie, G.; Wang, C.; Jia, W.; et al. Serum metabolic signatures of fulminant type 1 diabetes. J. Proteome. Res. 2012, 11, 4705–4711. [Google Scholar]
- Lima, A.R.; Pinto, J.; Bastos, M.L.; Carvalho, M.; Guedes de Pinho, P. NMR-based metabolomics studies of human prostate cancer tissue. Metabolomics 2018, 14, 88. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e81–e192. [Google Scholar] [CrossRef]
- Xu, W.; Lin, D.; Huang, C. NMR-based metabolomic analysis for the effects of creatine supplementation on mouse myoblast cell line C2C12. Acta Biochim. Biophys. Sin. (Shanghai) 2017, 49, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, C.; Liu, Y.; Lin, D.; Zhao, Y. NMR-based metabolomic analysis of the effects of alanyl-glutamine supplementation on C2C12 myoblasts injured by energy deprivation. RSC Adv. 2018, 8, 16114–16125. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, R.; Huang, C.; Lin, D. Taurine Protects C2C12 myoblasts from impaired cell proliferation and myotube differentiation under cisplatin-induced ROS exposure. Front. Mol. Biosci. 2021, 8, 685362. [Google Scholar] [CrossRef]
- Arvanitaki, A.; Giannakoulas, G.; Baumgartner, H.; Lammers, A.E. Eisenmenger syndrome: Diagnosis, prognosis and clinical management. Heart 2020, 106, 1638–1645. [Google Scholar] [CrossRef]
- Moons, P.; Canobbio, M.M.; Budts, W. Eisenmenger syndrome: A clinical review. Eur. J. Cardiovasc. Nurs. 2009, 8, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Gu, J.; Huang, C.; Zheng, S.; Lin, X.; Xie, L.; Lin, D. (1) H NMR-based analysis of serum metabolites in monocrotaline-induced pulmonary arterial hypertensive rats. Dis. Markers 2016, 2016, 5803031. [Google Scholar] [CrossRef] [PubMed]
- da Veiga Moreira, J.; De Staercke, L.; César Martínez-Basilio, P.; Gauthier-Thibodeau, S.; Montégut, L.; Schwartz, L.; Jolicoeur, M. Hyperosmolarity triggers the Warburg effect in chinese hamster ovary cells and reveals a reduced mitochondria horsepower. Metabolites 2021, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Saygin, D.; Highland, K.B.; Farha, S.; Park, M.; Sharp, J.; Roach, E.C.; Tang, W.H.W.; Thomas, J.D.; Erzurum, S.C.; Neumann, D.R.; et al. Metabolic and functional evaluation of the heart and lungs in pulmonary hypertension by gated 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Pulm. Circ. 2017, 7, 428–438. [Google Scholar] [CrossRef]
- Zhao, L.; Ashek, A.; Wang, L.; Fang, W.; Dabral, S.; Dubois, O.; Cupitt, J.; Pullamsetti, S.S.; Cotroneo, E.; Jones, H.; et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: Potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation 2013, 128, 1214–1224. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Hernandez-Saavedra, D.; Sanders, L.; Freeman, S.; Reisz, J.A.; Lee, M.H.; Mickael, C.; Kumar, R.; Kassa, B.; Gu, S.; D’ Alessandro, A.; et al. Stable isotope metabolomics of pulmonary artery smooth muscle and endothelial cells in pulmonary hypertension and with TGF-beta treatment. Sci. Rep. 2020, 10, 413. [Google Scholar] [CrossRef]
- Egnatchik, R.A.; Brittain, E.L.; Shah, A.T.; Fares, W.H.; Ford, H.J.; Monahan, K.; Kang, C.J.; Kocurek, E.G.; Zhu, S.; Luong, T.; et al. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Fang, Y.-H.; Parikh, K.; Ryan, J.J.; Toth, P.T.; Archer, S.L. Cardiac glutaminolysis: A maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J. Mol. Med. (Berl.) 2013, 91, 1185–1197. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Chinopoulos, C. Can the mitochondrial metabolic theory explain better the origin and management of cancer than can the somatic mutation theory? Metabolites 2021, 11, 572. [Google Scholar] [CrossRef]
- Koppula, P.; Zhang, Y.; Zhuang, L.; Gan, B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. (Lond) 2018, 38, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ericksen, R.E.; Lim, S.L.; McDonnell, E.; Shuen, W.H.; Vadiveloo, M.; White, P.J.; Ding, Z.; Kwok, R.; Lee, P.; Radda, G.K.; et al. Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 2019, 29, 1151–1165.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, J. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem. Biophys. Res. Commun. 2017, 486, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Tsunoda, M.; Konuma, T.; Kobayashi, M.; Nagy, T.; Glushka, J.; Tayyari, F.; McSkimming, D.; Kannan, N.; Tojo, A.; et al. Cancer progression by reprogrammed BCAA metabolism in myeloid leukaemia. Nature 2017, 545, 500–504. [Google Scholar] [CrossRef]
- Xu, W.; Comhair, S.A.A.; Chen, R.; Hu, B.; Hou, Y.; Zhou, Y.; Mavrakis, L.A.; Janocha, A.J.; Li, L.; Zhang, D.; et al. Integrative proteomics and phosphoproteomics in pulmonary arterial hypertension. Sci. Rep. 2019, 9, 18623. [Google Scholar] [CrossRef]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov. 2014, 4, 1371–1373. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T.; et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef]
- Brittain, E.L.; Talati, M.; Fessel, J.P.; Zhu, H.; Penner, N.; Calcutt, M.W.; West, J.D.; Funke, M.; Lewis, G.D.; Gerszten, R.E.; et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016, 133, 1936–1944. [Google Scholar] [CrossRef]
- Talati, M.H.; Brittain, E.L.; Fessel, J.P.; Penner, N.; Atkinson, J.; Funke, M.; Grueter, C.; Jerome, W.G.; Freeman, M.; Newman, J.H.; et al. Mechanisms of Lipid Accumulation in the Bone Morphogenetic Protein Receptor Type 2 Mutant Right Ventricle. Am. J. Respir. Crit. Care Med. 2016, 194, 719–728. [Google Scholar] [CrossRef]
- Graham, B.B.; Kumar, R.; Mickael, C.; Sanders, L.; Gebreab, L.; Huber, K.M.; Perez, M.; Smith-Jones, P.; Serkova, N.J.; Tuder, R.M. Severe pulmonary hypertension is associated with altered right ventricle metabolic substrate uptake. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L435–L440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]



| Group | PAH-CHD | CHD | HC |
|---|---|---|---|
| n | 24 | 38 | 29 |
| Gender (female), n (%) | 15 (62.5) | 28 (73.7) | 16 (55.2) |
| Age, yrs | 34.5 (27.0) | 34.0 (17.0) | 25.2 (7.2) * |
| BMI, kg/m2 | 19.4 ± 3.0 | 21.5 ± 3.2 * | 21.1 ± 1.5 ** |
| Pre-tricuspid defect, n (%) | 13 (54.2) | 25 (65.8) | NA |
| mPAP, mmHg | 54 (28) | 18 (7) *** | NA |
| SvO2, % | 65.6 ± 9.9 | 71.8 ± 6.1 * | NA |
| SaO2, % | 95.0 (13.0) | 97.0 (3.0) *** | NA |
| PVR, Wood Units | 7.55 (10.45) | 0.95 (0.68) *** | NA |
| CI, L/min/m2 | 2.83 ± 0.96 | 2.98 ± 1.00 | NA |
| RVSWI, g.m/m2 | 3.11 (1.69) | 1.38 (0.91) *** | NA |
| Targeted therapy, n (%) | 4 (16.7) | NA | NA |
| Chemical Shift (ppm) | Metabolites | CHD vs. HC | PAH-CHD vs. CHD | PAH-CHD vs. HC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VIP | Vary | p | VIP | Vary | p | VIP | Vary | p | ||
| 0.93 | Leucine | ↑↑ | 0.000 | ↓↓ | 0.000 | — | ns | |||
| 0.98 | Isoleucine | ↑↑ | 0.000 | — | ns | ↑ | 0.019 | |||
| 1.02 | Valine | ↑↑ | 0.000 | ↓↓ | 0.006 | ↑↑ | 0.008 | |||
| 1.04 | Isobutyrate | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 1.45 | Alanine | — | ns | 1.10 | ↓↓ | 0.000 | — | ns | ||
| 1.90 | Acetate | ↑↑ | 0.000 | ↑↑ | 0.001 | ↑↑ | 0.000 | |||
| 2.32 | Glutamate | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 2.35 | Pyruvate | ↑↑ | 0.009 | — | ns | — | ns | |||
| 2.43 | Glutamine | 1.94 | ↑↑ | 0.000 | ↓↓ | 0.003 | 1.56 | ↑↑ | 0.000 | |
| 2.50 | Citrate | ↑↑ | 0.000 | ↑↑ | 0.007 | 1.04 | ↑↑ | 0.000 | ||
| 2.61 | Methionine | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 2.90 | Trimethylamine | ↑↑ | 0.000 | ↑ | 0.037 | ↑↑ | 0.000 | |||
| 2.98 | Lysine | 1.77 | ↑↑ | 0.000 | — | ns | 1.74 | ↑↑ | 0.000 | |
| 3.25 | GPC | ↑↑ | 0.005 | ↓ | 0.024 | — | ns | |||
| 3.26 | Carnitine | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 3.36 | Methanol | 2.04 | ↑↑ | 0.000 | ↓↓ | 0.004 | 1.79 | ↑↑ | 0.000 | |
| 3.39 | Glucose | 2.80 | ↑ | 0.018 | 3.35 | ↓↓ | 0.000 | 1.49 | — | ns |
| 3.42 | Acetoacetate | ↑↑ | 0.000 | ↑ | 0.022 | ↑↑ | 0.000 | |||
| 3.53 | Glycine | ↑ | 0.011 | 1.15 | ↓↓ | 0.002 | — | ns | ||
| 3.56 | Threonine | ↑↑ | 0.002 | 2.19 | ↑↑ | 0.000 | 2.09 | ↑↑ | 0.000 | |
| 3.91 | Creatine | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 3.95 | Serine | ↑↑ | 0.000 | ↑↑ | 0.003 | 1.47 | ↑↑ | 0.000 | ||
| 4.05 | Choline | ↑↑ | 0.001 | ↓↓ | 0.001 | — | ns | |||
| 4.09 | Lactate | 1.08 | — | ns | 2.02 | ↑↑ | 0.000 | 2.22 | ↑↑ | 0.000 |
| 6.87 | Tyrosine | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 7.30 | Phenylalanine | ↑↑ | 0.000 | — | ns | ↑↑ | 0.000 | |||
| 7.73 | Histidine | ↑↑ | 0.000 | ↓ | 0.019 | — | ns | |||
| 8.43 | Formate | ↑↑ | 0.000 | ↑↑ | 0.000 | ↑↑ | 0.000 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Huang, C.; Zhang, C.; Lin, D.; Wu, W. NMR-Based Metabolomic Analysis of Plasma in Patients with Adult Congenital Heart Disease and Associated Pulmonary Arterial Hypertension: A Pilot Study. Metabolites 2022, 12, 845. https://doi.org/10.3390/metabo12090845
Xu B, Huang C, Zhang C, Lin D, Wu W. NMR-Based Metabolomic Analysis of Plasma in Patients with Adult Congenital Heart Disease and Associated Pulmonary Arterial Hypertension: A Pilot Study. Metabolites. 2022; 12(9):845. https://doi.org/10.3390/metabo12090845
Chicago/Turabian StyleXu, Beizhu, Caihua Huang, Caojin Zhang, Donghai Lin, and Weifeng Wu. 2022. "NMR-Based Metabolomic Analysis of Plasma in Patients with Adult Congenital Heart Disease and Associated Pulmonary Arterial Hypertension: A Pilot Study" Metabolites 12, no. 9: 845. https://doi.org/10.3390/metabo12090845
APA StyleXu, B., Huang, C., Zhang, C., Lin, D., & Wu, W. (2022). NMR-Based Metabolomic Analysis of Plasma in Patients with Adult Congenital Heart Disease and Associated Pulmonary Arterial Hypertension: A Pilot Study. Metabolites, 12(9), 845. https://doi.org/10.3390/metabo12090845








